Dasatinib (BMS-354825, SPRYCEL, Bristol-Myers Squibb, New York, NY, USA) is an ATP-competitive protein tyrosine kinase inhibitor (TKI), which was originally identified as a potent inhibitor of Src family kinases (including Src, Lck, Hck, Yes, Fgr, Lyn and Fyn) and was subsequently found to have activity against Abl, Kit, the macrophage colony stimulating factor receptor (Fms), the platelet-derived growth factor (PDGF) receptor (PDGFR)-α and -β and the Eph receptor family members EphB1, EphB2 and EphB4.1, 2, 3 Dasatinib is an effective therapy for chronic myeloid leukaemia (CML) in patients who are resistant to front-line imatinib mesylate therapy due to its increased affinity for the CML oncoprotein BCR-Abl and its insensitivity to mutations in the BCR-Abl kinase domain. Furthermore, recent data suggest that dasatinib may be more effective than imatinib as a front-line therapy for chronic phase CML.4
The success of TKIs for the treatment of CML has resulted in the investigation of the use of these drugs in an increasing number of paediatric haematological and solid tumours. However, although TKI treatment is generally well tolerated, there is emerging data to suggest that TKI therapy may result in decreased growth in children. Three recently published case-studies report decelerated growth in juvenile CML patients undergoing imatinib therapy.5, 6, 7 Additionally, a French phase IV trial has recently reported decreased growth in a cohort of 22 children and adolescents (age: 10 months–17 years) receiving imatinib therapy for chronic-phase CML.8 Growth rates in this group were significantly lower following imatinib treatment, with a significant decrease in height z-score (median, −0.37; range, −1.09 to +0.14) after 12 months of treatment, compared with baseline.8 In keeping with this, we have previously reported that imatinib treatment caused growth plate closure in normal rats in vivo and inhibit chondrocyte proliferation and activity in vitro,9 providing a possible explanation for the reduced longitudinal growth observed in juvenile patients treated with imatinib.
In paediatric cases that are resistant or intolerant to imatinib and where allogeneic transplants are not possible, dasatinib is recommended as a second-line therapy. In light of this increased investigation of the use of dasatinib in the treatment of paediatric cancers, we investigated whether dasatinib, like imatinib, affected the growth plate in vivo.
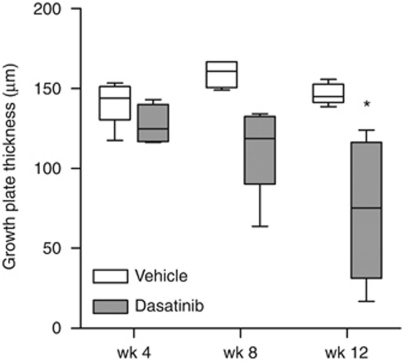
In this study, it was found that dasatinib treatment resulted in a significant decrease in cartilagenous growth plate thickness in normal rats. Sprague–Dawley rats were treated with dasatinib (5 mg/kg) or vehicle (10% DMSO/90% PEG 300) by daily oral gavage for up to 12 weeks and the proximal tibial growth plate was examined by histology. In vehicle-treated controls, the width of the cartilagenous growth plate at the proximal tibia remained constant at all time-points examined (P=0.070, one-way ANOVA; Figure 1). In contrast, the mean growth plate thickness decreased significantly over time in dasatinib-treated animals, with complete closure at the centre of the growth plate in two of five animals after 12 weeks. The width of the growth plate was significantly lower in the dasatinib-treated group than in vehicle-treated controls after 12 weeks of treatment (P<0.05; Figure 1).
Figure 1.
The proximal tibial growth plate is thinned in dasatinib-treated animals. Nine-month-old female Sprague–Dawley rats were treated for 12 weeks with dasatinib (5 mg/kg per day) or vehicle (10% DMSO/90% PEG 300). Six animals per group were humanely killed after 4, 8 and 12 weeks of treatment and right tibiae were collected for histological analysis. Samples were embedded in paraffin and 5-μm sections were stained with safranin O/fast green. Growth plate thickness was measured at five equidistant points across the centre of the growth plate. Box plots depict median, 25th and 75th percentiles±range (n=4–6). *P<0.05 (Student's t-tests).
Postnatal longitudinal bone growth in the axial and appendicular limb skeleton is controlled by the regulated proliferation and activity of chondrocytes and osteoblasts within the epiphyseal growth plate. This process of endochondral ossification involves the chondrocyte-mediated production of a cartilagenous template, which is later mineralised and remodelled to form mature lamellar bone. We postulate that the accelerated growth-plate narrowing observed in dasatinib-treated rats may be due, in part, to the inhibition of chondrocyte proliferation and activity.
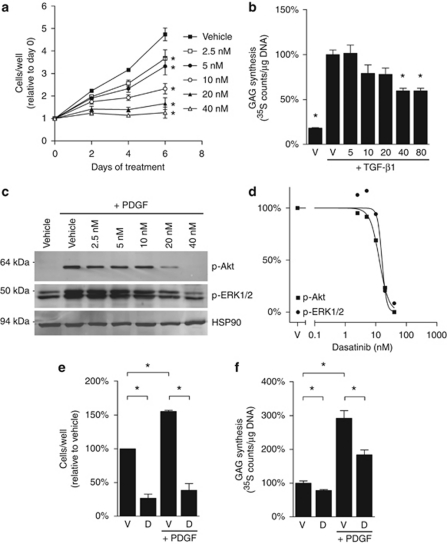
To determine whether dasatinib directly affects chondrocyte proliferation and activity, the effects of dasatinib on the murine pre-chondrocyte cell line ATDC5 were investigated in vitro. ATDC5 cells were cultured for up to 6 days with dasatinib or vehicle and the relative number of cells per well was determined by WST-1 assay. In vehicle-treated cultures, cell numbers increased fourfold during the 6 days of culture (Figure 2a). Treatment of ATDC5 cells with dasatinib significantly inhibited cell proliferation at concentrations of 2.5 n and higher after 2, 4 or 6 days, with an IC50 of 6.2 n on day 6.
Figure 2.
Dasatinib inhibits cell proliferation and activity in ATDC5 cultures. (a) ATDC5 cells (1.56 × 104 cells per cm2) were seeded in 96-well plates in α-MEM with 10% fetal bovine serum, 2 m-glutamine, 1 m sodium pyruvate, 15 m HEPES, 50 U/ml penicillin, 50 μg/ml streptomycin and 100 μ ascorbate (c-α-MEM) and were cultured overnight to allow the cells to adhere. The cells were then treated with dasatinib or vehicle (0.05% DMSO) for the indicated periods of time before the relative number of cells per well were determined by WST-1 assay. Graphs depict mean±range of two representative experiments. *P<0.05 relative to vehicle control at each time-point (one-way ANOVA with Dunnett's post-tests). (b) ATDC5 cells (1.56 × 105 cells per cm2) were treated with rhTGF-β1 (10 ng/ml) and the indicated doses of dasatinib or 0.05% DMSO vehicle for 48 h. GAG levels were then quantitated and normalised to DNA content to determine relative GAG production per cell. Graphs depict mean±s.e.m. of triplicate wells of a representative experiment, normalised to the rhTGF-β1-treated vehicle control. *P<0.05 relative to the rhTGF-β1-treated vehicle control (one-way ANOVA with Dunnett's post tests). (c) ATDC5 cells were cultured overnight at 1.56 × 105 cells per cm2 in six-well plates in c-α-MEM. The cells were serum starved by overnight incubation in serum-free α-MEM. The cells were pre-treated with dasatinib or 0.05% DMSO for 2 h before stimulation with rhPDGF-BB (10 ng/ml) for 5 min. Cell lysates (30 μg per lane) were resolved through a 10% sodium dodecyl sulphate-PAGE gel and the phosphorylated forms of Akt (p-Akt) and ERK1/2 (p-ERK1/2), as well as total HSP90, were detected using specific antibodies. Images from a representative of two experiments are shown. (d) Graphs indicate the pixel intensity for p-Akt and p-ERK1/2, relative to HSP90, normalised to vehicle controls. (e) ATDC5 cells were treated with 40 n dasatinib or vehicle (0.05% DMSO) supplemented, where indicated, with 10 ng/ml rhPDGF-BB. After 6 days, the relative number of cells per well was determined by WST-1 assay. Graphs depict mean±range of two representative experiments, normalised to vehicle control. (f) ATDC5 cells (cultured as in b) were then treated with vehicle (V) or 40 n dasatinib (D) with or without 100 ng/ml rhPDGF-BB. After 48 h, GAG levels and DNA content were quantitated and GAG production per cell was calculated. Graphs depict mean±s.e.m. of triplicate wells of a representative experiment, normalised to vehicle controls. *P<0.05, as indicated (Student's t-tests).
The effects of dasatinib on chondrocyte activity were next investigated using a GAG-synthesis assay. ATDC5 cultures were treated with 10 ng/ml rhTGF-β1, an inducer of chondrocyte differentiation and activity (Figure 2b). Dasatinib treatment for 48 h significantly decreased TGF-β1-induced GAG synthesis at 40 n concentrations and higher, with a 40% reduction in GAG synthesis at 40 and 80 n dasatinib, relative to vehicle controls (Figure 2b). These data demonstrate that dasatinib inhibits proliferation and extracellular matrix synthesis in cultures of chondrocyte-like cells in vitro, suggesting that inhibition of chondrocyte proliferation and activity may be responsible for the decreased growth plate thickness observed in the dasatinib-treated normal rats.
The dasatinib target PDGFR is an important regulator of chondrocyte proliferation and activity. PDGF-BB is a known mitogen for chondrocytes and has been reported to promote chondrocyte activity in at least some cell types,10, 11 suggesting that dasatinib treatment may have direct effects on chondrocyte proliferation and activity through inhibition of PDGFR-β. We examined whether inhibition of PDGFR-β contributed to the inhibitory effects of dasatinib on ATDC5 proliferation and GAG production. First, the effects of dasatinib on PDGFR receptor signalling through Akt and ERK1/2 were examined by western blot. Consistent with the known effects of dasatinib on the PDGFR, dasatinib inhibited the rhPDGF-BB-induced phosphorylation of Akt and ERK1/2 in a dose-dependent manner, with 40 n dasatinib completely abrogating PDGFR signalling (Figure 2c). Dasatinib inhibited PDGFR signalling through Akt and ERK1/2 at IC50 of 13.0 and 16.0 n, respectively (Figure 2d).
Consistent with the known effects of PDGF on chondrocyte proliferation, treatment of ATDC5 with PDGF-BB for 6 days increased the number of cells per well by 1.5-fold, compared with vehicle-treated controls (Figure 2e). This increase in cell proliferation was abrogated by dasatinib treatment (Figure 2e). Additionally, treatment with rhPDGF-BB for 48 h induced a threefold increase in GAG production, on a per cell basis, relative to untreated controls (Figure 2f). This stimulatory effect of PDGF-BB was partially inhibited by co-treatment with 40 n dasatinib, although levels did not reach those of unstimulated dasatinib-treated cultures (Figure 2f).
In this study, dasatinib treatment partially reversed the activating effects of PDGFR on the proliferation and GAG-synthetic properties of the murine chondrocyte cell line ATDC5, suggesting that inhibition of PDGFR signalling is likely to contribute to the inhibitory effects of dasatinib on chondrocytes. However, given the broad target specificities of dasatinib, other tyrosine kinases may also have a role in the effects of dasatinib on chondrogenesis. Inhibition of Src-family kinases may contribute to the anti-proliferative effects of dasatinib on chondrocytes, as inhibition of Src-family kinases with PP2 has previously been found to inhibit chondrocyte proliferation in vitro.12, 13, 14 However, although Src-/- mice have retarded long bone growth, the growth plates of Src-deficient mice are thicker than normal15 suggesting that inhibition of Src alone cannot explain the growth plate thinning observed in this study.
In addition to inhibiting chondrocyte proliferation and activity, dasatinib can affect osteoblast activity, inhibiting stromal-cell proliferation while, under at least some conditions, promoting osteoblast activity.16, 17, 18 Growth plate thickness is determined by rate of chondrocyte proliferation and hypertrophy and by the rate of replacement of the cartilagenous matrix with mature bone. Therefore, the stimulation of osteoblast activity by dasatinib may have additional effects on growth plate thickness by accelerating growth plate mineralisation. However, although published data suggest that dasatinib can increase Sprague–Dawley osteoblast activity in vitro, we have previously reported that treatment of normal Sprague–Dawley rats with 5 mg/kg dasatinib had no effect on osteoblast activity.16 These findings suggest that the effects of dasatinib on the growth plate in this study were independent of effects on osteoblast activity.
The putative effects of dasatinib on the growth plate may have implications for the use of dasatinib in the paediatric setting. Although these studies suggest that TKI therapy in pre-pubertal individuals may retard growth, there are currently no reports suggesting that dasatinib may also have effects on growth in paediatric patients. Our results suggest that growth plate changes should be investigated in paediatric patients who are undergoing treatment with dasatinib. The relative benefit of using dasatinib as a front-line treatment for diseases affecting children and adolescents may need to be re-evaluated, taking into account the potential effects of dasatinib on the growth plate.
Acknowledgments
We are grateful to Behzad Baradaran and the staff at Veterinary Services, IMVS, for their assistance with the animal experiments. We wish to thank Lee Anne Griffiths, Francis Lee, Richard Smykla and Kate Church from Bristol-Myers Squibb for the provision of dasatinib and for helpful discussions. This work was supported by a grant from the Leukemia and Lymphoma Society Translational Research Program.
The authors declare no conflict of interest.
References
- Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- Melnick JS, Janes J, Kim S, Chang JY, Sipes DG, Gunderson D, et al. An efficient rapid system for profiling the cellular activities of molecular libraries. Proc Natl Acad Sci USA. 2006;103:3153–3158. doi: 10.1073/pnas.0511292103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandyke K, Dewar AL, Farrugia AN, Fitter S, Bik To L, Hughes TP, et al. Therapeutic concentrations of dasatinib inhibit in vitro osteoclastogenesis. Leukemia. 2009;23:994–997. doi: 10.1038/leu.2008.356. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- Mariani S, Giona F, Basciani S, Brama M, Gnessi L. Low bone density and decreased inhibin-B/FSH ratio in a boy treated with imatinib during puberty. Lancet. 2008;372:111–112. doi: 10.1016/S0140-6736(08)61023-5. [DOI] [PubMed] [Google Scholar]
- Kimoto T, Inoue M, Kawa K. Growth deceleration in a girl treated with imatinib. Int J Hematol. 2009;89:251–252. doi: 10.1007/s12185-008-0251-8. [DOI] [PubMed] [Google Scholar]
- Schmid H, Jaeger BA, Lohse J, Suttorp M. Longitudinal growth retardation in a prepubertal girl with chronic myeloid leukemia on long-term treatment with imatinib. Haematologica. 2009;94:1177–1179. doi: 10.3324/haematol.2009.008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millot F, Baruchel A, Guilhot J, Petit A, Leblanc T, Bertrand Y, et al. Imatinib is efficient but has a negative impact on growth in children with previously untreated chronic myelogenous leukaemia (CML) in early chronic phase (CP): results of the French national phase IV trial [abstract] Blood. 2009;114:863. [Google Scholar]
- Vandyke K, Dewar AL, Fitter S, Menicanin D, To LB, Hughes TP, et al. Imatinib mesylate causes growth plate closure in vivo. Leukemia. 2009;23:2155–2159. doi: 10.1038/leu.2009.150. [DOI] [PubMed] [Google Scholar]
- Kieswetter K, Schwartz Z, Alderete M, Dean DD, Boyan BD. Platelet derived growth factor stimulates chondrocyte proliferation but prevents endochondral maturation. Endocrine. 1997;6:257–264. doi: 10.1007/BF02820501. [DOI] [PubMed] [Google Scholar]
- Weiser L, Bhargava M, Attia E, Torzilli PA. Effect of serum and platelet-derived growth factor on chondrocytes grown in collagen gels. Tissue Eng. 1999;5:533–544. doi: 10.1089/ten.1999.5.533. [DOI] [PubMed] [Google Scholar]
- Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemba T, Valbracht J, Alsalameh S, Lotz M. Focal adhesion kinase and mitogen-activated protein kinases are involved in chondrocyte activation by the 29-kDa amino-terminal fibronectin fragment. J Biol Chem. 2002;277:907–911. doi: 10.1074/jbc.M109690200. [DOI] [PubMed] [Google Scholar]
- Gill KS, Beier F, Goldberg HA. Rho-ROCK signaling differentially regulates chondrocyte spreading on fibronectin and bone sialoprotein. Am J Physiol Cell Physiol. 2008;295:C38–C49. doi: 10.1152/ajpcell.00548.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Vandyke K, Dewar AL, Diamond P, Fitter S, Schultz CG, Sims NA, et al. The tyrosine kinase inhibitor dasatinib dysregulates bone remodeling through inhibition of osteoclasts in vivo. J Bone Miner Res. 2010;25:1759–1770. doi: 10.1002/jbmr.85. [DOI] [PubMed] [Google Scholar]
- Lee YC, Huang CF, Murshed M, Chu K, Araujo JC, Ye X, et al. Src family kinase/abl inhibitor dasatinib suppresses proliferation and enhances differentiation of osteoblasts. Oncogene. 2010;29:3196–3207. doi: 10.1038/onc.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Id Boufker H, Lagneaux L, Najar M, Piccart M, Ghanem G, Body JJ, et al. The Src inhibitor dasatinib accelerates the differentiation of human bone marrow-derived mesenchymal stromal cells into osteoblasts. BMC Cancer. 2010;10:298. doi: 10.1186/1471-2407-10-298. [DOI] [PMC free article] [PubMed] [Google Scholar]