Abstract
The FMS-like tyrosine kinase 3 (FLT3) is highly expressed in acute myeloid leukemia (AML). Internal tandem duplications (ITD) of the juxtamembrane domain lead to the constitutive activation of the FLT3 kinase inducing the activation of multiple genes, which may result in the expression of leukemia-associated antigens (LAAs). We analyzed the regulation of LAA in FLT3-wild-type (WT)- and FLT3-ITD+ myeloid cells to identify potential targets for antigen-specific immunotherapy for AML patients. Antigens, such as PR-3, RHAMM, Survivin, WT-1 and PRAME, were upregulated by constitutively active FLT3-ITD as well as FLT3-WT activated by FLT3 ligand (FL). Cytotoxic T-cell (CTL) clones against PR-3, RHAMM, Survivin and an AML-directed CTL clone recognized AML cell lines and primary AML blasts expressing FLT3-ITD, as well as FLT3-WT+ myeloid dendritic cells in the presence of FL. Downregulation of FLT3 led to the abolishment of CTL recognition. Comparing our findings concerning LAA upregulation by the FLT3 kinase with those already made for the Bcr-Abl kinase, we found analogies in the LAA expression pattern. Antigens upregulated by both FLT3 and Bcr-Abl may be promising targets for the development of immunotherapeutical approaches against myeloid leukemia of different origin.
Keywords: acute myeloid leukemia, FLT3 kinase, leukemia-associated antigens, T-cell clones, immunotherapy
Introduction
The FMS-like tyrosine kinase 3 (FLT3) receptor tyrosine kinase is highly expressed by malignant cells in most cases of acute myeloid leukemia (AML) and acute B-lineage leukemia (ALL).1, 2 In addition, FLT3 mutations belong to the most frequent somatic alterations in AML and occur in approximately 30% of AML patients.3 The most common forms of mutant FLT3 are internal tandem duplications (ITDs) in the juxtamembrane domain. This duplication shows strong variations in length of 3 to more than 400 bp between different patients.4, 5 The FLT3-ITD leads to the constitutive activation by ligand-independent phosphorylation of the receptor.3 It has been shown in clinical studies that AML patients harboring FLT3-ITD mutations have a poor prognosis.6, 7
Several small-molecule inhibitors of FLT3 have been developed and are currently in different stages of clinical development, including Sorafenib and SU5416.8, 9, 10 Although treatment with FLT3 inhibitors results in clinical responses in relapsed AML with activating FLT3 mutations, the reduction in peripheral blood and bone marrow blasts is only transient.10, 11, 12, 13, 14, 15, 16 Combination therapies of FLT3 inhibitors and conventional chemotherapy are currently being studied.17 However, the insensitivity of quiescent leukemic stem cells towards kinase inhibitors may lead to the selective outgrowth of these cells and finally to disease relapse even after years of continuous treatment.
T lymphocytes have the potential to eliminate the AML stem cell. Proof of principle has been shown in an exceptional clinical situation where donor lymphocyte infusions can induce complete cytogenetic remissions of AML relapsed after allogeneic stem cell transplantation.18 The donor's T lymphocytes include allo-restricted T cells, which may ideally combine antigen specificity, high avidity and a superior leukemia–lytic function. However, most of the allo-restricted T cells display broad peptide specificity or even a peptide-independent human leukocyte antigen (HLA)-dominant binding, both characteristics leading to a wide reactivity and potentially to graft-versus-host disease. Besides, the risk of graft-versus-host disease rises if target antigens are widely expressed in the body. Therefore, the current immunotherapeutic concepts focus on targeting those antigens that are preferentially or even exclusively expressed by AML blasts, including the AML stem cell.
Graf et al.19 successfully generated cytotoxic T cells (CTLs) highly specific for an HLA-A*0101 (HLA-A1)-restricted epitope derived from the FLT3-ITD of one AML patient showing that the FLT3-ITD is a potential target antigen for immunotherapeutic approaches. However, the variations in the length of the ITD imply the problem of having to find an individual antigenic epitope for each individual patient.4 An alternative approach to circumvent this problem may lie in the observation that the likewise constitutively active tyrosine kinase Bcr-Abl expressed in Philadelphia+ chronic myeloid leukemia (CML) upregulates immunogenic leukemia-associated antigens (LAA).20 Some of the Bcr-Abl-regulated LAA, such as PR-3 and Wilms tumor protein (WT)-1, are also expressed in AML. Moreover, for PR-3 and WT-1, it has been shown that they spontaneously activate T cells in AML patients, indicating the immunogenicity of these antigens.21 On the basis of these findings, we asked the question as to whether LAA are also regulated by FLT3-WT and/or FLT3-ITD in AML.
In this study, we show that a panel of LAA, such as PR-3, Survivin and RHAMM, is upregulated by constitutively active FLT3-ITD. Moreover, the activation of non-mutated FLT3 upon stimulation with FLT3 ligand (FL) leads to the upregulation of the same LAA. Vice versa, treatment of FLT3-WT+ leukemia cells with FLT3-small interfering RNA (siRNA) leads to the downregulation of the LAA even in the presence of FL. LAA downregulation was also observed after treatment of FLT3-ITD+ leukemia cells with the FLT3 inhibitor Sugen SU5416. To prove the functional relevance of FLT3-regulated LAA as immunological targets, we used LAA-reactive CTL clones as effector cells. Indeed, CTL clones specific for PR-3, Survivin and RHAMM recognized various myeloid target cells harboring the activated FLT3 kinase. The strong dependence of CTL killing on the FLT3 activation status indicates the relevance of FLT3-regulated LAA as promising targets for T-cell-based immunotherapy.
Materials and methods
Cell culture
The human leukemia cell lines RS4;11 and MV4;11 (obtained from the DSMZ, Braunschweig, Germany) carry the chromosomal translocation t(4;11)(q21;q23), but express different MLL-AF4 variants owing to different break points. Further leukemic cell lines used in this study were the cell lines K562 and K562 transfected with HLA-A*0201 (HLA-A2) (generous gift from Th Wölfel, Klinikum der Johannes-Gutenberg-Universität, Mainz, Germany) and BV-173 (DSMZ). The TAP-defective HLA-A2+ T2 cell line was provided by P Cresswell (Yale University School of Medicine, New Haven, CT, USA). The B lymphoblastoid cell lines (LCLs) were generated by EBV transformation of peripheral blood B cells from healthy donors.
MV4;11, K562tA2, BV-173 and T2 cells were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA, USA). RS4;11 cells were maintained in α-minimum essential medium. All media were supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 m glutamine and 10% fetal calf serum.
The expression of HLA-A2 was confirmed by fluorescence-activated cell sorting (FACS) analyses with a fluorescein isothiocyanate-conjugated anti-HLA-A2 monoclonal antibody (BD Pharmingen, San Diego, CA, USA). For interferon (IFN)-γ treatment, the medium was supplemented with 100 U/ml IFN-γ 48 h before the functional assays.
Generation of CTL clones
Peripheral blood mononuclear cells (PBMCs) were derived from healthy donors and AML patients. Isolation of CD8+ T cells occurred via magnetic-activated cell sorting (Miltenyl Biotec, Bergisch Gladbach, Germany) after indirect antibody staining. The isolated HLA-A2− CD8+ T cells were repetitively stimulated with allogenic HLA-A2+ dendritic cells (DCs) loaded with 10 μg of the HLA-A2-restricted peptide epitope PR-3169−177 (VLQELNVTV), RHAMM165−173 (ILSLELMKL) or Survivin95−104 (ELTLGEFLKL). CD8+ T cells and peptide-pulsed DCs were co-cultured in 200 μl AIM-V medium in a stimulator to a response ratio of 1: 20 in 96-well round-bottom plates with 5% human AB-Serum (Milan Analytica, LaRoche, Switzerland), 1000 U/ml interleukin (IL)-6 and 10 ng/ml IL-12. Restimulation was set up in the presence of 5 ng/ml IL-7 and 100 U/ml IL-2. After two stimulations, the proliferating T cells were stained with HLA-A2/PR-3169−177, HLA-A2/RHAMM165−173 and HLA-A2/Survivin95−104 multimers, respectively, and the specific T cells were FACS sorted with a MoFlo cell sorter (Cytomation, Fort Collins, CO, USA)22 and cloned by limiting dilution in five 96-well plates. The expansion of the T-cell clones occurred in the presence of anti-CD3, IL-15, IL-2 and irradiated LCL and PBMC as feeder cells.
IFN-γ ELISpot assay
The production of IFN-γ was determined in an ELISpot assay. ELISpot membrane plates (Millipore, Bedford, MA, USA) were coated overnight with an IFN-γ-specific antibody (Mabtech AB, Stockholm, Sweden). Target cells (2 × 104 cells per well) and CTL (5 × 103 cells per well) were washed twice in RPMI medium (Invitrogen) before seeding them onto the ELISpot plates. After 24 h incubation at 37 °C, the spots were assessed using antibodies against IFN-γ, and then stained with AEC (3-amino-9-ethylcarbazole; Sigma-Aldrich, St Louis, MO, USA) staining solution. The number of spots was counted by using an automated ELISpot reader system (KS ELISpot, Carl Zeiss, Jena, Germany).
Enzyme-linked immunosorbent assay
To detect the IFN-γ production of the CTL clones, 2 × 104 T cells per well were co-cultured with 1 × 104 target cells in 96-well round-bottom plates at 37 °C. After 24 h, supernatants were collected and IFN-γ production was determined using a commercially available IFN-γ enzyme-linked immunosorbent assay (ELISA) kit (BD BioSciences International, San Jose, CA, USA).
Cytotoxicity assay
Cytolytic activity was analyzed in a standard 4 h chromium release assay as described.23 In short, the tumor cell lines (5 × 105 cells in 100 μl of fetal calf serum) were incubated with 100 μCi of 51Cr for 1.5 h at 37 °C, washed and used as target cells. Peptide-loaded T2 cells were first labeled with 51Cr for 1.5 h at 37 °C and then loaded with the respective peptide (10 μg/ml) for an additional hour at room temperature. As negative controls (ctrls), 51Cr-labeled T2 cells were loaded with the HLA-A2-restricted peptide HIVpol476−484 (ILKEPVHGV). The 51Cr-labeled targets were cultured with the T cells in RPMI 1640 with 10% fetal calf serum at 200 μl per well in V-bottom, 96-well tissue culture plates (Greiner, Greiner Bio-One, Frickenhausen, Germany). For evaluating the efficacy of CTL-mediated lysis, the T cells were serially diluted and then co-cultured with a fixed amount of target cells, resulting in graded E:T ratios. For testing functional T-cell receptor (TCR) avidity, the T cells were plated at a fixed E:T ratio of 30:1 while the peptide concentration was titrated.
After 4 h of co-culturing the effector and target cells at 37 °C, 100 μl of supernatant were collected and radioactivity was measured in a γ-counter. The killing was calculated as the percentage of specific 51Cr release using the equation percentage of specific lysis=((sample release–medium release)/(maximal release–medium release)).
siRNA treatment
The siRNA SMARTpool containing four pooled siRNA duplexes directed against FLT3 (catalog no. M-003137-02-0005) and a nonspecific siRNA (catalog no. D-001206-13-20) were purchased from Thermo Science and Perbio (Thermo Fisher Scientific, Waltham, MA, USA), respectively.
The transfection of siRNA was performed using the Nucleofactor system (Amaxa, Cologne, Germany) according to the manufacturer's instructions. The knockdown of the specified protein was determined by FACS analyses.
RNA isolation and reverse transcription–polymerase chain reaction
Total RNA was isolated using the RNeasy mini kit according to the manufacturer's instructions (Qiagen, Hilden, Germany). The RNA was reverse transcribed using Moloney murine leukemia virus reverse transcription with oligo(dT) (Invitrogen). The following primers were used: β-actin (sense: 5′-GGGACCTGACTGACTACCTCAT-3′ antisense: 5′-ATAGTCCGCCTAGAAGCATTTG-3′); PRAME (sense: 5′-CTGTACTCATTTCCAGAGCCAGA-3′ antisense: 5′-TATTGAGAGGGTTTCCAAGGGGTT-3′); PR-3 (sense: 5′-CGGCCACATAACATTTGCAC-3′ antisense: 5′-TGGCACATCCCCAGATCAC-3′); RHAMM (sense: 5′-CAGGTCACCCAAAGGAGTCTC-3′ antisense: 5′-CAAGCTCATCCAGTGTTTGC-3′); Survivin (sense: 5′-ACCACCGCATCTCTACATTC-3′ antisense: 5′-GCTCTTTCTCTGTCCAGTTTC-3′); and WT-1 (sense: 5′-TAACCACACAACGCCCATC-3′ antisense: 5′-AAAACCTTCGTTCACAGTCC-3′). The polymerase chain reaction (PCR) products were electrophoresed on a 2% agarose gel and stained with ethidium bromide.
Flow cytometry
Flow cytometry was performed using HLA-A2/PR-3169−177, HLA-A2/RHAMM165−173 and HLA-A2/Survivin95−104 multimers, and anti-human CD8 monoclonal antibody, fluorescein isothiocyanate conjugated (Immunotech, Marseille, France). Fluorescence analyses were performed with the Coulter Epics XL flow cytometer (Coulter Electronics, GmbH, Krefeld, Germany) and documented with the FlowJo software (Tree Star, Ashland, OR, USA).
RESULTS
The LAA PR-3, RHAMM, Survivin, WT-1 and PRAME are upregulated by the FLT3 receptor tyrosine kinase
First we analyzed whether the FLT3 kinase has an influence on the expression of the LAA PR-3, RHAMM, WT-1, PRAME and Survivin that are known to be expressed in AML.24, 25, 26, 27, 28, 29 As examples for the FL-dependent FLT3-WT and the constitutively active FLT3-ITD variant, we used the two different human acute leukemia cell lines RS4;11 and MV4;11, which endogenously express the FLT3-WT and FLT3-ITD, respectively.
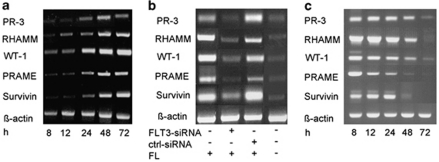
In RS4;11 leukemia cells, the administration of FL increased the expression levels of the LAA PR-3, RHAMM, WT-1, PRAME and Survivin from low/intermediate levels before FL treatment to high levels after incubation with FL (Figure 1a). This experimental finding indicates the dependency of these LAA on the activity of the FLT3 kinase. To further examine this finding, we made an approach using the RS4;11 leukemia cells transfected with siRNA against FLT3 and analyzed the expression of the LAA PR-3, RHAMM, WT-1, PRAME and Survivin in the presence of FL. Following treatment with FLT3-siRNA, the RS4;11 cells showed a marked downregulation of these LAA even in the presence of FL. The transfection of RS4;11 cells with the irrelevant ctrl-siRNA in the presence of FL did not result in any downregulation of LAA expression compared with that after exclusive treatment with FL (Figure 1b).
Figure 1.
Influence of the FLT3 kinase on the expression of the LAA PR-3, RHAMM, WT-1, PRAME and Survivin. (a) RS4;11 (FLT3-WT+) cells were treated with FL for a time period of 72 h and the expression of the LAA PR-3, RHAMM, WT-1, PRAME and Survivin was documented via RT–PCR at 8, 12, 24, 48 and 72 h after treatment. (b) RS4;11 (FLT3-WT+) cells were treated with FL, FLT3-siRNA and/or ctrl-siRNA for 72 h, and the expression of the LAA PR-3, RHAMM, WT-1, PRAME and Survivin was analyzed by RT–PCR. (Lanes 1–4 show the expression of the different tumor antigens PR-3, RHAMM, WT-1, PRAME and Survivin in RS4;11 cells treated with FL (lane 1), in RS4;11 cells treated with FLT3-siRNA (lane 2), in RS4;11 cells treated with ctrl-siRNA (lane 3) and in RS4;11 cells without FL (lane 4).) (c) MV4;11 (FLT3-ITD+) cells were treated with the FLT3 kinase inhibitor SU5614 for 72 h, and the expression of the LAA PR-3, RHAMM, WT-1, PRAME and Survivin was documented via RT–PCR at different time points after treatment. The three figures are composed of different gels.
In MV4;11 leukemia cells, in which the FLT3 kinase is constitutively active, we detected a permanently high expression of the different LAA (Figure 1c). Culturing these cells with SU5416, a known inhibitor of the FLT3-ITD activity, reduced the expression of Survivin, an antigenic protein that has already been known to be upregulated by the FLT3 kinase activity.30, 31 In addition, we documented decreasing levels of the expression of PRAME, WT-1, RHAMM and PR-3 after an incubation of 48–72 h. On the basis of these findings, we conclude that active FLT3 kinase upregulates the expression of the LAA PR-3, RHAMM, WT-1, PRAME and Survivin.
The correlation between the activity of the FLT3 kinase and the expression of different LAA underlines the significance of these antigens for the development of immunotherapeutic approaches against myeloid leukemia.
CTL clones against the LAA PR-3, RHAMM and Survivin recognize and lyse leukemia cell lines and primary AML blasts
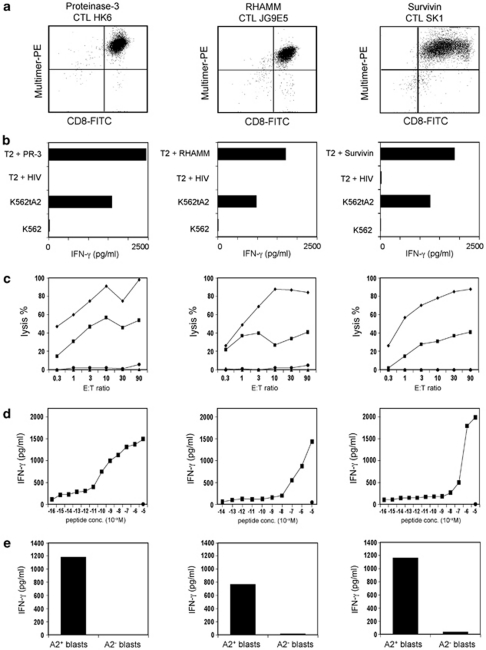
On the basis of our findings that FLT3-expressing cell lines are able to upregulate the LAA PR-3, RHAMM, WT-1, PRAME and Survivin, our aim was the generation of CTL clones directed against HLA-A2-restricted epitopes of these LAA. For this purpose, CD8+ T lymphocytes from healthy donors were stimulated with allogeneic DCs loaded with the peptides PR-3169−177, Survivin95−104, WT-1126−134, PRAME300−309 or RHAMM165−173. Following repetitive stimulations, the amount of PR-3169−177-, Survivin95−104- and RHAMM165−173-specific T cells could be increased to numbers that permitted the visualization, sorting with HLA-A2/peptide multimers and cloning by limiting dilution. Sixty-two CTL clones grew in the approach stimulated with PR-3169−177 from which after further testing two proved to be tumour reactive. In the case of Survivin95−104, one of 25 CTL clones tested was able to lyse Survivin-expressing tumor cells. For RHAMM165−173, we got three tumor-reactive out of 48 potential clones. Figure 2a exemplifies FACS staining of, in each case, one representative CTL clone with the respective multimer.
Figure 2.
CTL clones against PR-3, RHAMM, and Survivin, respectively, recognize the corresponding peptide epitopes. (a) FACS staining of the different CTL clones with the respective multimers. (b) The different CTL clones were co-cultivated with T2 cells loaded with PR-3169−177, RHAMM165−173, Survivin95−104 or HIVpol476−484, K562tA2, K562 or MCF7. The IFN-γ concentration of the supernatant was determined by ELISA. (c) Differential lytic activity of HLA-A2-restricted PR-3169−177-, RHAMM165−173- or Survivin95−104-directed CTL clones against T2 cells loaded with the respective peptide (⧫) and HLA-A2+ K562tA2 cells (▪). T2 cells loaded with the irrelevant peptide HIVpol476−484 (▴) and K562 cells (•) were used as negative ctrl. (d) Functional avidity of CTL clones was determined by the recognition of T2 cells pulsed with graded amounts of peptides. (e) The different CTL clones were co-cultivated with FLT3-ITD+ blasts of an HLA-A2+ AML patient or with blasts of an HLA-A2− AML patient. The IFN-γ concentration of the supernatant was determined by ELISA.
The peptide specificity of the respective clones was confirmed in an IFN-γ ELISA, in which T2 cells loaded with PR-3169−177, Survivin95−104, RHAMM165−173 or the irrelevant peptide HIVpol476−484 were used as target cells. As shown in Figure 2b, the different CTL clones secreted high amounts of IFN-γ after incubation with the respective relevant, but not with the irrelevant peptide. In the same test, we analyzed whether the different CTL clones were able to recognizse endogenously processed PR-3169−177, Survivin95−104 and RHAMM165−173, respectively. Therefore, the HLA-A2+ K562tA2 leukemia cells known to express PR-3, Survivin and RHAMM were used as target cells. The reactivity pattern of the different clones is shown in Figure 2b. The different CTL clones were able to recognize K562tA2 cells, whereas there was no recognition of K562 cells used as negative ctrl. The lytic activity was determined in a 51Cr release assay. Lysis of T2 cells loaded with the relevant peptide PR-3169−177 by CTL clone HK6 was between 45 and 100% at increasing E:T ratios, whereas there was no recognition of T2 cells pulsed with the irrelevant peptide HIVpol476−484. The leukemic cell line K562tA2 was lysed with a maximum of about 55% at an E:T ratio of 10:1 (Figure 2c, left). The Rhamm-specific CTL clone JG9E5 showed about 100% lysis of RHAMM165−173-loaded T2 cells at higher E:T ratios and up to 40% lysis of K562tA2 cells (Figure 2c, middle). The lysis of T2 cells loaded with Survivin95−104 by CTL clone SK1 was between 25 and 90% at increasing E:T ratios. K562tA2 cells were lysed with a maximum of about 40% (Figure 2c, right).
Furthermore, the functional avidities of the CTL clones toward the peptides PR-3169−177, Survivin95−104 and RHAMM165−173 were assessed by the recognition of serially diluted amounts of the respective peptides (10−5–10−16 ) loaded onto T2 cells in an IFN-γ ELISA. The PR-3-specific CTL clone displayed a rather high avidity toward the PR-3169−177 peptide with a half-maximum IFN-γ secretion of 10−10 (Figure 2d, left). The RHAMM- and the Survivin-reactive CTL clones have a relatively low avidity with a half-maximum IFN-γ secretion of about 10−7–10−6 (Figure 2d, middle, right).
On the basis of our findings that FLT3-expressing cell lines upregulate the LAA PR-3, RHAMM, WT-1, PRAME and Survivin, we were led to question whether our CTL clones reactive against PR-3, Survivin and RHAMM were able to recognize leukemic blasts. Hence, we co-cultivated the three CTL clones with primary blasts of an HLA-A2+ patient with FLT3-ITD+ AML. Blasts of an HLA-A2− AML patient served as negative ctrl. As shown in Figure 2e, the CTL clones performed high IFN-γ secretion only in the presence of HLA-A2+, FLT3-ITD+ blasts, but not in the presence of HLA-A2− blasts.
Leukemia-reactive CTL clones are generated by stimulation with blast-derived DC
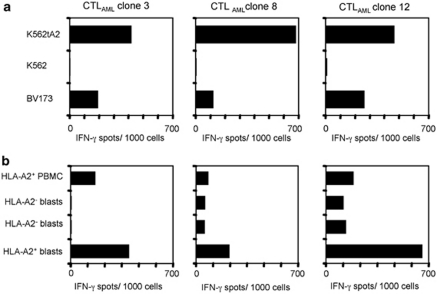
On the basis of our findings, we were led to question whether FLT3-ITD+ leukemic blast-derived DCs (DCAML) were capable of inducing a primary CTL response. In this approach, we stimulated HLA-A2− CD8+ T lymphocytes from a healthy donor with allogeneic DCAML of a 71-year-old HLA-A2+ female patient with FLT3-ITD+ AML. The expression of the LAA PR-3, Survivin, WT-1, PRAME and RHAMM by these DCAML has been documented by RT–PCR (Supplementary Figure 1A). The dependency of this expression on the FLT3 kinase activity could be shown indirectly by the downregulation of IFN-γ secretion by the respective clones following treatment of DCAML with SU5416, a known inhibitor of the FLT3 kinase activity (Supplementary Figure 1B). After two stimulations, we did an IFN-γ ELISpot assay with T2 cells pulsed with the AML-associated peptides PR-3169−177, Survivin95−104, WT-1126−134, PRAME300−309 or RHAMM165−173, HIVpol476−484, as well as with K562tA2 and K562 to test the specificity of the different T-cell populations in the 96-well plates. There were no T cells specific for one of the peptides tested, so we cloned T cells recognizing K562A2, but not K562 (data not shown). The reactivity pattern of three of the resulting CTL clones (CTLAML) is shown in Figure 3. The three CTLAML clones showed high reactivity against the HLA-A2+ leukemia cell lines K562tA2 and BV173, but no recognition of K562 (Figure 3a) in an IFN-γ ELISpot. Furthermore, we wanted to know whether the recognition of the CTLAML clones is specifically restricted to HLA-A2+ leukemic blasts and not to PBMC of a healthy donor. As depicted in Figure 3b, there was only low or no reactivity against HLA-A2+ PBMC and HLA-A2− blasts. In contrast, there was a marked IFN-γ secretion when co-cultivated with HLA-A2+ leukemic blasts (Figure 3b).
Figure 3.
CTL clones stimulated with HLA-A2+ ITD+ DCAML recognize leukemia cell lines and blasts. CD8+ T cells from a healthy HLA-A2− donor were primed with allogeneic HLA-A2+ DCAML and specific T cells were cloned. (a) In an IFN-γ ELISpot, the three CTLAML clones were co-incubated with K562tA2, K562 and HLA-A2+ BV173 cells, and the resulting spots were counted. (b) An IFN-γ ELISpot was performed using allogeneic HLA-A2− PBMC and blasts, as well as HLA-A2+ blasts as targets and the three CTLAML clones as effector cells.
DCs transfected with FLT3-ITD are recognized by CTL clones against PR-3, RHAMM and Survivin, as well as by CTLAML clones
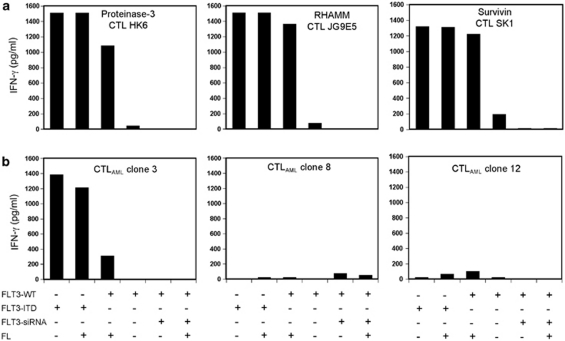
We wanted to know whether downregulation of the FLT3 kinase is also leading to a lower recognition of FLT3+ cells by our isolated CTL clones. As we do not have any FLT3+ cell lines, we used HLA-A2+ DCs from a healthy donor. For testing the FLT3-WT and the constitutively active mutant variant FLT3-ITD, the DCs were nucleofected with FLT3-WT- or FLT3-ITD-mRNA. To analyze if FLT3 was active in DCs transfected with FLT3-WT, we compared recognition of DCs either treated or not treated with FL by the CTL clones directed against PR-3, RHAMM, Survivin and the CTLAML clones. As depicted in Figure 4, there was no or only little IFN-γ secretion of the different CTL clones in the presence of untreated FLT3-WT DCs. The CTL clones directed against PR-3, RHAMM and Survivin showed high reactivity against DCs treated with FL (Figure 4a). In contrast, only one of the CTLAML clones (clone no. 3) secreted some IFN-γ when incubated with DCs treated with FL (Figure 4b). The three peptide-specific clones and the CTLAML clone no. 3 displayed high FL-independent recognition of ITD+ DCs. To see the effect of downregulation of FLT3-WT, we used the FLT3-siRNA that we already used in RS4;11 cells (Figure 1b). Following treatment of the DCs with FLT3-siRNA, no recognition could be observed by any of the clones independently of FL. With these experimental settings, we were able to further confirm our hypothesis that the expression of the LAA PR-3, RHAMM and Survivin is regulated by active FLT3.
Figure 4.
Reactivity of CTL clones directed against PR-3, RHAMM and Survivin, as well as CTLAML clones towards DCs nucleofected with FLT3-WT plus and minus FLT3-siRNA or FLT3-ITD. All approaches were carried out with and without the addition of FL. (a, b) HLA-A2+ DCs from a healthy donor were nucleofected with FLT3-WT-mRNA or FLT3-ITD-mRNA and co-cultivated with the different CTL clones with and without the addition of FL. Furthermore, the FLT3-WT+ DCs were additionally transfected with FLT3-siRNA. The supernatants were tested in an IFN-γ ELISA. (a, b) show the results of the ELISA with the CTL clones directed against PR-3, RHAMM and Survivin, as well as with the CTLAML clone nos 3, 8 and 12.
In contrast to CTLAML clone no. 3, which also seems to have an FLT3-dependent recognition, the CTLAML clone nos 8 and 12 did not show any reactivity against DCs nucleofected with FLT3-WT or FLT3-ITD.
CTL clones against PR-3, RHAMM and Survivin recognize DCs transfected with Bcr-Abl-WT, but not DCs transfected with Bcr-Abl-KD
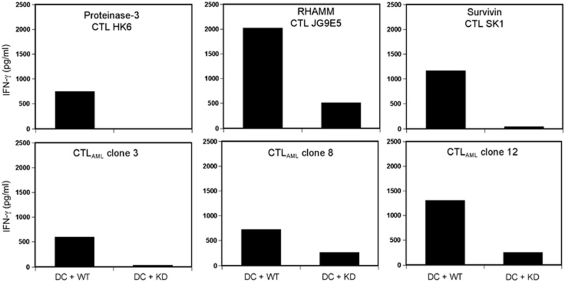
Our findings concerning the upregulation of different LAA by the active FLT3 kinase are in accordance with the observations about the immunogenicity of the Bcr-Abl kinase made by our group.20 It has been shown that the tyrosine kinase activity of Bcr-Abl leads to enhanced expression of some CML-associated antigens, for example, PR-3. In the meantime, we could also detect an upregulation of RHAMM and Survivin in Bcr-Abl+ cells. As we now have T-cell clones against these LAA, which recognize cells expressing FLT3, we wanted to know whether these clones are also reactive against Bcr-Abl-WT+ cells. For comparison of the active and inactive form of Bcr-Abl, we transfected DCs with Bcr-Abl-WT-mRNA or a kinase-deficient Bcr-Abl-mRNA (KD) as described before.20
The reactivity of the different peptide-specific T-cell clones and the CTLAML clones against the transfected DCs were documented by analyzing their IFN-γ release (Figure 5). The pattern of T-cell-derived IFN-γ release shows a high reactivity of the different peptide specific CTL clones and the CTLAML clone no. 3 against Bcr-Abl-WT+ DC, but not against Bcr-Abl-KD+ DCs. Interestingly, this was also true for the two other CTLAML clones that did not recognize FLT3-WT-transfected DCs (Figures 4b and 5).
Figure 5.
Reactivity of CTL clones directed against PR-3, RHAMM and Survivin, as well as CTLAML clones towards DCs nucleofected with Bcr-Abl-WT or Bcr-Abl-KD. The CTL clones were co-incubated with the respective DCs and an IFN-γ ELISA was performed using the resulting supernatant.
DISCUSSION
Cytotoxic T lymphocytes have the potential to eliminate malignant stem cells, even if they are quiescent. Immunotherapeutic approaches are being developed based on antigen-specific T lymphocytes, such as antigen vaccination or adoptive T-cell transfer. As FLT3-ITD is partially expressed by CD34+/CD33− AML progenitors, sequences from FLT3-ITD itself may serve as ideal leukemia-specific epitopes for leukemia-specific T lymphocytes.32 Indeed, from one patient with FLT3-ITD+, AML leukemia-specific CTL clones have been isolated that recognize an HLA-A1-restricted peptide sequence derived from the FLT3-ITD sequence.19 However, the strong variation in length of ITD duplications between AML patients implies the problem to find T-cell epitopes for every single patient to design an individual immunotherapy. In AML, several shared antigens have been identified, which can potentially serve as targets for cytotoxic T lymphocytes, such as PR-3, RHAMM, WT-1, PRAME and Survivin.24, 25, 26, 27, 28, 29 Until now, the expression pattern of these AML-associated antigens and their relevance for the CTL-mediated elimination of AML stem cells is not entirely understood. As the FLT3-ITD is active in early progenitor AML cells, we asked the question as to whether some of the shared antigens are dependent on the activated form of the mutant FLT3 kinase.
In this study, we analyzed the expression of PR-3, RHAMM, WT-1, PRAME and Survivin in an FLT3-WT+ leukemia cell line with and without the addition of FL. There was a weak basic expression of the five LAA analyzed. The addition of FL, however, resulted in a stronger LAA expression being the first hint for an existent association between FLT3 activity and LAA regulation. This could be confirmed by the downregulation of FLT3 activity, with FLT3-siRNA subsequently lowering the expression of the LAA analyzed. To test the influence of a constitutively active mutant variant of FLT3, we analyzed the LAA expression in an FLT3-ITD+ leukemia cell line. These cells showed a high level of LAA corresponding to that of the FLT3-WT+ leukemia cell line treated with FL. The inhibition of the constitutively active FLT3-ITD by SU5614, a known inhibitor of the FLT3-ITD activity,30 showed an effect analogous to that achieved after treatment of FLT3-WT+ cells with FLT3-siRNA. These data document for the first time that the expression of PR-3, RHAMM, WT-1, PRAME and Survivin is upregulated upon activation of the FLT3 kinase.
We next asked the question as to whether the upregulation of LAA by the activation status of FLT3 influenced the T-cell-mediated lysis of leukemia cells. First, we established a panel of allo-HLA-A2-restricted CTL clones specific for PR-3, RHAMM and Survivin. To effectively stimulate and select T cells against these self-antigens, we took advantage of an HLA mismatch between DCs and T cells by using DCs from an HLA-A2+ donor as stimulator cells and T cells from an HLA-A2− donor as responder cells.33, 34 For further experiments, we expanded those CTL clones that displayed a fine specificity against the respective peptides as shown by IFN-γ release and lytic activity. The selected CTL clones HK5, JG9ES and SK1 recognized the K562tA2 leukemic cell line expressing PR-3, RHAMM and Survivin. As assumed, FLT3-ITD+ AML blasts were also recognized by these CTL in an HLA-A2-restricted manner. To answer the important question as to whether T-cell recognition of myeloid cells is dependent on the FLT3-modulated expression level of their antigens, we used myeloid DCs as target cells. The herein generated monocyte-derived DCs transfected with FLT-WT were not recognized by the antigen-specific CTL clones HK5, JG9ES and SK1. FMS-like tyrosine kinase 3-WT+ DCs were recognized upon FL stimulation, indicating that the activation of the FLT3 kinase raised the expression level of PR-3, RHAMM and Survivin above a certain threshold, which further enabled the T cells to recognize their targets. Similarly, CTL recognition could be induced when FLT3-WT+ DCs were transfected with the constitutively active FLT3-ITD. The correlation between FLT3 activity and the upregulation of CTL-defined LAA points to the significance of these antigens for the physiological as well as the therapeutic relevance of immune responses against acute leukemias.35 Exemplarily, the expression of RHAMM and PRAME have been shown to be associated with a favorable clinical outcome in AML patients and to induce T-cell responses.29 Peptide vaccination trials performed with RHAMM, PR-3 and WT-1 peptides resulted in measurable immunological and clinical responses in patients with different hematological diseases.29, 36, 37, 38, 39, 40
As a second approach to prove the immunological relevance of FLT3-regulated antigens for targeting AML cells, we generated DCs from AML blasts known to be able to stimulate leukemia-reactive autologous T lymphocytes.41, 42 Following stimulation of allogeneic HLA-A2− CD8+ T cells with HLA-A2+ DCAML, we isolated three different leukemia-reactive CTL clones. All of the three established CTLAML clones recognized FLT3-ITD+ AML blasts in an HLA-A2-restricted manner. None of the HLA-A2-restricted epitopes of the known LAA PR-3, RHAMM, Survivin, PRAME and WT-1 was recognized by the CTLAML clones. Of note, one of the three CTLAML clones (CTL no. 3) secreted IFN-γ when stimulated with DCs harboring the active form of the FLT3 kinase, such as FLT3-ITD-transfected DCs or FL-stimulated FLT3-WT+ DCs. This indicates that FLT3-regulated antigens are able to stimulate primary T-cell responses towards myeloid leukemia cells.
On the basis of our previous findings that the expression level of some LAA, such as PR-3 and PRAME, is not only induced by activated FLT3, but also by the constitutively active Bcr-Abl kinase,20 we next analyzed the recognition pattern of the herein described AML-reactive CTL clones in response to monocyte-derived DCs transfected with Bcr-Abl-WT or Bcr-Abl-KD. We first analyzed the IFN-γ secretion by the PR-3-specific CTL clone HK6 in response to HLA-A2-matched Bcr-Abl-WT+ DCs versus Bcr-Abl-KD+ DCs. As postulated, the PR-3-specific CTL clone released IFN-γ in the presence of Bcr-Abl-WT+ DC, but not in the presence of Bcr-Abl-KD+ DCs. Similarly, the RHAMM- and Surivin-specific CTL clones both released high amounts of IFN-γ only in the presence of Bcr-Abl-WT+ DCs. Interestingly, all three CTLAML clones with yet undefined antigen specificity reacted strongly with Bcr-Abl-WT+ DCs and only to a low extent with Bcr-Abl-KD+ DCs. Therefore, CTL clone nos 8 and 12 seem to recognize a LAA, which is regulated by the Bcr-Abl, but not by the FLT3 kinase. In contrast, the antigen recognized by CTL clone no. 3 is regulated by both the FLT3 and the Bcr-Abl kinase. In summary, there are common as well as differential antigen patterns induced on the activation of the Bcr-Abl and FLT3 kinases. Our findings raise the question as to whether the same and/or other LAAs are upregulated by additional tyrosine kinases, for example, the NPM/Alk kinase.
In conclusion, antigens upregulated by kinases activated in leukemia cells may be promising targets for the development of T-cell-based immunotherapies against myeloid leukemia of different origins. As the broad clinical application of adoptive T-cell transfer is limited owing to the laborious procedure of T-cell isolation and characterization, we will pursue an alternative methodology whereby primary human T-cell populations are transduced with the LAA-reactive TCR of interest. The herein presented CTL clones against FLT3- and Bcr-Abl-regulated antigens, respectively, for example, PR-3 and RHAMM, may serve as a source for leukemia-reactive TCR. The TCR gene transfer is a convenient method to produce antigen-specific T cells, further allowing that an individualized therapy will be available for a mass of patients with FLT3+ or Bcr-Abl+ myeloid leukemias.
Acknowledgments
We thank Kathrin Hofer and Julia Müller for excellent technical assistance. This study was supported by grants from the José Carreras Leukemia Foundation (DJCLS R 07/34f to HB), the Deutsche Forschungsgemeinschaft (SFB-456 to HB and DHB; DFG BE 1579/4-1 to HB) and from the Helmholtz Alliance ‘Immunotherapy of Cancer' (to HB and DHB). BB and HC designed and performed research, collected and analyzed data and wrote the paper; JD and BK performed research; HK revised the paper; DHB provided vital tools; JA and CP supervised research; and HB designed and supervised research, reviewed data and wrote the paper.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Birg F, Courcoul M, Rosnet O, Bardin F, Pebusque MJ, Marchetto S, et al. Expression of the FMS/KIT-like gene FLT3 in human acute leukemias of the myeloid and lymphoid lineages. Blood. 1992;80:2584–2593. [PubMed] [Google Scholar]
- Carow CE, Levenstein M, Kaufmann SH, Chen J, Amin S, Rockwell P, et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood. 1996;87:1089–1096. [PubMed] [Google Scholar]
- Small D. FLT3 mutations: biology and treatment. Hematol Am Soc Hematol Educ Program. 2006;1:178–184. doi: 10.1182/asheducation-2006.1.178. [DOI] [PubMed] [Google Scholar]
- Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- Seedhouse CH, Pallis M, Grundy M, Shang S, Russell NH. FLT3-ITD expression levels and their effect on STAT5 in AML with and without NPM mutations. Br J Haematol. 2009;147:653–661. doi: 10.1111/j.1365-2141.2009.07901.x. [DOI] [PubMed] [Google Scholar]
- Yokota S, Kiyoi H, Nakao M, Iwai T, Misawa S, Okuda T, et al. Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia. 1997;11:1605–1609. doi: 10.1038/sj.leu.2400812. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- Tong FK, Chow S, Hedley D. Pharmacodynamic monitoring of BAY 43-9006 (Sorafenib) in phase I clinical trials involving solid tumor and AML/MDS patients, using flow cytometry to monitor activation of the ERK pathway in peripheral blood cells. Cytometry B. 2006;70:107–114. doi: 10.1002/cyto.b.20092. [DOI] [PubMed] [Google Scholar]
- Stopeck A, Sheldon M, Vahedian M, Cropp G, Gosalia R, Hannah A. Results of a phase I dose-escalating study of the antiangiogenic agent, SU5416, in patients with advanced malignancies. Clin Cancer Res. 2002;8:2798–2805. [PubMed] [Google Scholar]
- Fiedler W, Serve H, Dohner H, Schwittay M, Ottmann OG, O'Farrell AM, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105:986–993. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- O'Farrell AM, Foran JM, Fiedler W, Serve H, Paquette RL, Cooper MA, et al. An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res. 2003;9:5465–5476. [PubMed] [Google Scholar]
- Giles FJ, Stopeck AT, Silverman LR, Lancet JE, Cooper MA, Hannah AL, et al. SU5416, a small molecule tyrosine kinase receptor inhibitor, has biologic activity in patients with refractory acute myeloid leukemia or myelodysplastic syndromes. Blood. 2003;102:795–801. doi: 10.1182/blood-2002-10-3023. [DOI] [PubMed] [Google Scholar]
- Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- Knapper S, Burnett AK, Littlewood T, Kell WJ, Agrawal S, Chopra R, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108:3262–3270. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- DeAngelo DJ, Stone RM, Heaney ML, Nimer SD, Paquette RL, Klisovic RB, et al. Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome: safety, pharmacokinetics, and pharmacodynamics. Blood. 2006;108:3674–3681. doi: 10.1182/blood-2006-02-005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104:1145–1150. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- Porter DL, Collins RH, Jr, Hardy C, Kernan NA, Drobyski WR, Giralt S, et al. Treatment of relapsed leukemia after unrelated donor marrow transplantation with unrelated donor leukocyte infusions. Blood. 2000;95:1214–1221. [PubMed] [Google Scholar]
- Graf C, Heidel F, Tenzer S, Radsak MP, Solem FK, Britten CM, et al. A neoepitope generated by an FLT3 internal tandem duplication (FLT3-ITD) is recognized by leukemia-reactive autologous CD8+ T cells. Blood. 2007;109:2985–2988. doi: 10.1182/blood-2006-07-032839. [DOI] [PubMed] [Google Scholar]
- Scheich F, Duyster J, Peschel C, Bernhard H. The immunogenicity of Bcr-Abl expressing dendritic cells is dependent on the Bcr-Abl kinase activity and dominated by Bcr-Abl regulated antigens. Blood. 2007;110:2556–2560. doi: 10.1182/blood-2007-01-071001. [DOI] [PubMed] [Google Scholar]
- Scheibenbogen C, Letsch A, Thiel E, Schmittel A, Mailaender V, Baerwolf S, et al. CD8 T-cell responses to Wilms tumor gene product WT1 and proteinase 3 in patients with acute myeloid leukemia. Blood. 2002;100:2132–2137. doi: 10.1182/blood-2002-01-0163. [DOI] [PubMed] [Google Scholar]
- Neudorfer J, Schmidt B, Huster KM, Anderl F, Schiemann M, Holzapfel G, et al. Reversible HLA multimers (Streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens. J Immunol Methods. 2007;320:119–131. doi: 10.1016/j.jim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Meyer zum Buschenfelde C, Nicklisch N, Rose-John S, Peschel C, Bernhard H. Generation of tumor-reactive CTL against the tumor-associated antigen HER2 using retrovirally transduced dendritic cells derived from CD34+ hemopoietic progenitor cells. J Immunol. 2000;165:4133–4140. doi: 10.4049/jimmunol.165.7.4133. [DOI] [PubMed] [Google Scholar]
- Dengler R, Munstermann U, al-Batran S, Hausner I, Faderl S, Nerl C, et al. Immunocytochemical and flow cytometric detection of proteinase 3 (myeloblastin) in normal and leukaemic myeloid cells. Br J Haematol. 1995;89:250–257. doi: 10.1111/j.1365-2141.1995.tb03297.x. [DOI] [PubMed] [Google Scholar]
- Miwa H, Beran M, Saunders GF. Expression of the Wilms' tumor gene (WT1) in human leukemias. Leukemia. 1992;6:405–409. [PubMed] [Google Scholar]
- Miyagi T, Ahuja H, Kubota T, Kubonishi I, Koeffler HP, Miyoshi I. Expression of the candidate Wilm's tumor gene, WT1, in human leukemia cells. Leukemia. 1993;7:970–977. [PubMed] [Google Scholar]
- Rosenfeld C, Cheever MA, Gaiger A. WT1 in acute leukemia, chronic myelogenous leukemia and myelodysplastic syndrome: therapeutic potential of WT1 targeted therapies. Leukemia. 2003;17:1301–1312. doi: 10.1038/sj.leu.2402988. [DOI] [PubMed] [Google Scholar]
- Carter BZ, Milella M, Altieri DC, Andreeff M. Cytokine-regulated expression of survivin in myeloid leukemia. Blood. 2001;97:2784–2790. doi: 10.1182/blood.v97.9.2784. [DOI] [PubMed] [Google Scholar]
- Greiner J, Schmitt M, Li L, Giannopoulos K, Bosch K, Schmitt A, et al. Expression of tumor-associated antigens in acute myeloid leukemia: Implications for specific immunotherapeutic approaches. Blood. 2006;108:4109–4117. doi: 10.1182/blood-2006-01-023127. [DOI] [PubMed] [Google Scholar]
- Yee KW, O'Farrell AM, Smolich BD, Cherrington JM, McMahon G, Wait CL, et al. SU5416 and SU5614 inhibit kinase activity of wild-type and mutant FLT3 receptor tyrosine kinase. Blood. 2002;100:2941–2949. doi: 10.1182/blood-2002-02-0531. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Singh P, Moh A, Abe M, Conway EM, Boswell HS, et al. Survivin mediates aberrant hematopoietic progenitor cell proliferation and acute leukemia in mice induced by internal tandem duplication of Flt3. Blood. 2009;114:394–403. doi: 10.1182/blood-2008-11-188714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JA, Alonzo TA, Gerbing RB, Woods WG, Lange BJ, Sweetser DA, et al. FLT3 internal tandem duplication in CD34+/CD33− precursors predicts poor outcome in acute myeloid leukemia. Blood. 2006;108:2764–2769. doi: 10.1182/blood-2006-04-012260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad H, Gebhard K, Kronig H, Neudorfer J, Busch DH, Peschel C, et al. CTLs directed against HER2 specifically cross-react with HER3 and HER4. J Immunol. 2008;180:8135–8145. doi: 10.4049/jimmunol.180.12.8135. [DOI] [PubMed] [Google Scholar]
- Kronig H, Hofer K, Conrad H, Guilaume P, Muller J, Schiemann M, et al. Allorestricted T lymphocytes with a high avidity T-cell receptor towards NY-ESO-1 have potent anti-tumor activity. Int J Cancer. 2009;125:649–655. doi: 10.1002/ijc.24414. [DOI] [PubMed] [Google Scholar]
- Greiner J, Ringhoffer M, Taniguchi M, Li L, Schmitt A, Shiku H, et al. mRNA expression of leukemia-associated antigens in patients with acute myeloid leukemia for the development of specific immunotherapies. Int J Cancer. 2004;108:704–711. doi: 10.1002/ijc.11623. [DOI] [PubMed] [Google Scholar]
- Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo T, Nakajima H, et al. Induction of WT1 (Wilms' tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci USA. 2004;101:13885–13890. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop HE, Stevenson FK, Molldrem JJ. Immunotherapy of hematologic malignancy. Hematol Am Soc Hematol Educ Program. 2003;1:331–349. doi: 10.1182/asheducation-2003.1.331. [DOI] [PubMed] [Google Scholar]
- Rezvani K, Yong AS, Mielke S, Savani BN, Musse L, Superata J, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111:236–242. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M, Schmitt A, Rojewski MT, Chen J, Giannopoulos K, Fei F, et al. RHAMM-R3 peptide vaccination in patients with acute myeloid leukemia, myelodysplastic syndrome, and multiple myeloma elicits immunologic and clinical responses. Blood. 2008;111:1357–1365. doi: 10.1182/blood-2007-07-099366. [DOI] [PubMed] [Google Scholar]
- Greiner J, Dohner H, Schmitt M. Cancer vaccines for patients with acute myeloid leukemia—definition of leukemia-associated antigens and current clinical protocols targeting these antigens. Haematologica. 2006;91:1653–1661. [PubMed] [Google Scholar]
- Choudhury BA, Liang JC, Thomas EK, Flores-Romo L, Xie QS, Agusala K, et al. Dendritic cells derived in vitro from acute myelogenous leukemia cells stimulate autologous, antileukemic T-cell responses. Blood. 1999;93:780–786. [PubMed] [Google Scholar]
- Kremser A, Dressig J, Grabrucker C, Liepert A, Kroell T, Scholl N, et al. Dendritic cells (DCs) can be successfully generated from leukemic blasts in individual patients with AML or MDS: an evaluation of different methods. J Immunother. 2010;33:185–199. doi: 10.1097/CJI.0b013e3181b8f4ce. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.