Abstract
Low-serum concentrations of 25-hydroxyvitamin D [25(OH)D] are associated with insulin resistance in adults. Less data are available in pediatric populations. Serum 25(OH)D serum concentrations were assessed in 125 obese and 31 nonobese children (age 11.9 ± 2.7 y, range 6–16 y, 49% male) living in Bonn, Germany. The relationship between 25(OH)D, measured by liquid chromatography-tandem mass spectrometry, and measures of insulin sensitivity and adipokines adiponectin and resistin were analyzed. Seventy-six % of subjects were 25(OH)D deficient (<20 ng/mL). Higher insulin, homeostasis model assessment-insulin resistance (HOMA-IR r = −0.269, P = 0.023), and hemoglobin A1c (HbA1c) as well as lower quantitative insulin-sensitivity check index (QUICKI r = 0.264, P = 0.030) values were found in obese children with lower 25(OH)D concentrations even after adjustment for gender, age, and body mass index. Furthermore, 25(OH)D correlated significantly with adiponectin, but not with resistin. Our results suggest that hypovitaminosis D is a risk factor for developing insulin resistance independent of adiposity.
1. Introduction
Obesity is the primary risk factor for the development of impaired glucose tolerance, type 2 diabetes mellitus, and the metabolic syndrome. The prevalence of type 2 diabetes among children and adolescents has increased at an alarming rate during the last two decades, with the highest prevalence among African American adolescents [1].
Studies have shown that lifestyle factors contribute to this development. As obese children are usually sedentary and therefore less likely to play outdoors, their exposure to sunlight may be limited [2]. In addition, unhealthy high caloric food might be low in mineral and vitamin content [3, 4]. Both represent risk factors for developing vitamin D deficiency. Additionally, bioavailability of vitamin D in obese subjects might be low because of its deposition in a fat tissue [5] and higher body fat mass might be associated with a higher risk of vitamin D deficiency [6]. Vitamin D plays a central role in skeletal health. Additionally, vitamin D might also provide protection against major health problems such as autoimmune disease, cardiometabolic disease, and cancer [7, 8]. In a recent population study, subjects with cardiovascular disease had a greater frequency of vitamin D deficiency (defined as 25-hydroxyvitamin D [25(OH)D] levels <20 ng/mL) than those without [9]. Beta-cell function improves after the administration of vitamin D to animals [10–12] and humans [13] with vitamin D deficiency. In adult humans, low-serum 25(OH)D levels have been correlated with impaired glucose tolerance, the metabolic syndrome, and diabetes, independent of obesity [14–16]. Using the hyperglycemic clamp method, Chiu et al. [17] found that serum 25(OH)D levels were positively associated with insulin sensitivity and negatively associated with first- and second-phase insulin secretion. In that study, subjects with 25(OH)D deficiency (<20 ng/mL) had a higher risk of insulin resistance and metabolic syndrome. In a recent large population-based health research study, a significant inverse correlation was found between serum 25(OH)D levels and type 2 diabetes risk as well as subclinical inflammation [18]. Although these studies suggest vitamin D deficiency as a risk factor of disturbed glucose homeostasis in humans, it is still controversial, particularly, in children. Short-term supplementation studies have provided conflicting results on the effect of vitamin D on glucose tolerance and insulin sensitivity [13, 17, 19]. And it remains unclear if vitamin D deficiency in children is associated with insulin resistance and if there is a role for vitamin D replacement in the treatment of glucose intolerance in this age group. Hormones such as the adipokines adiponectin and resistin are a possible link between insulin resistance and adiposity. Adiponectin exerts anti-inflammatory effects, appetite-restraining effects, and counters insulin resistance, thereby offering protective mechanisms against the development of both T2DM and cardiovascular disease. Resistin is involved in insulin sensitivity and has been shown to modulate both glucose tolerance and lipid metabolism in vivo and in vitro [20].
To further evaluate whether vitamin D deficiency is associated with insulin resistance and changes of adipokine secretion, we measured serum 25(OH)D concentrations in 156 children and adolescents (125 obese, 31 nonobese) using liquid chromatography-tandem mass spectrometry [21]. We hypothesized that low 25(OH)D levels are associated with insulin resistance independent of adiposity.
2. Methods
2.1. Patients and Anthropometric Data
Both groups presented to and were examined at the Department of Pediatrics, University of Bonn, and consecutively recruited at the endocrine and general pediatric outpatient clinics without any further selection beside the criteria listed below. Protocols were approved by the institutional review boards at the University Bonn, Germany, as well as Seattle Children's Hospital. Written parental consent and/or patient assent obtained and investigations were conducted according to the principles expressed in the Declaration of Helsinki. We examined anthropometrical markers. Height was measured to the nearest cm using a rigid stadiometer and weight was measured in underwear to the nearest 0.1 kg using a calibrated balance scale. Body mass index (BMI) and its standard deviation score (SDS-BMI) were calculated as described previously [22]. Obesity was defined by BMI greater than the 97th percentile in a national population as described previously [22]. Pubertal developmental stage was assessed using the standards of Marshall and Tanner [23, 24]. Inclusion criteria were normal weight or obese male or female children age 6–16 years old. Exclusion criteria were current endocrine disorders, calcium metabolism disorders, syndromal obesity, premature adrenarche, diabetes mellitus, or intake of prescription medications. Thirty-one nonobese (age 12.2 ± 1.8 y, 52% male) and 125 obese (age 11.7 ± 3.0 y, 48% male) children were recruited. Twenty-six percent of all children (27% of obese, 20% of nonobese children) had a migration background (mostly Turkish or Arab-Islamic background).
2.2. Laboratory Tests
Insulin was measured by microparticle-enhanced immunometric assay (Abbott), glucose on a Vitros analyzer (Ortho Clinical Diagnostics), and HbA1c using a Variant II analyzer (Biorad). Serum total adiponectin and resistin were determined by Luminex xMAP technology (Millipore, Billerica, Man, USA) on a Luminex 200 instrument (Luminex Corp., Austin, Tex, USA). The intra-assay CV was <8% and the inter-assay CV < 14%. Insulin resistance was estimated from fasting plasma measurements using the HOMA-IR (Insulin [mU/L] × Glucose [mmol/L]/22.5) and insulin sensitivity was estimated by QUICKI (1/[log insulin (mU/L) + log baseline glucose (mg/dL)]) [25]. We measured 25(OH)D serum levels using HPLC tandem mass spectrometry [26, 27]. The intra- and interassay CVs were 2.8% and 6.2%, respectively.
2.3. Statistical Analysis
All statistical analyses were performed in Excel (Microsoft), Prism (GraphPad Software), or SPSS (IBM) and all tests of significance were two tailed. Subjects were divided into quartiles based on their serum 25(OH)D concentrations (Table 1). One-way ANOVA was used to test for a linear trend across quartiles of plasma 25(OH)D concentrations as well as for linear regression analyses. We also performed multiple linear regression analyses utilizing plasma 25(OH)D concentrations as a continuous variable.
Table 1.
Fasting glucose metabolism parameters in 125 obese children, in quartiles (n = 30–32) of plasma 25-hydroxy vitamin D concentrations.
| Quartiles based on plasma 25-hydroxyvitamin D levels | Test for Trend (P values) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| 25(OH)D (ng/mL) | 8.0 ± 2.3 | 13.6 ± 1.2 | 17.5 ± 1.2 | 25.3 ± 4.3 | |
| Age (years) | 11.6 ± 3.1 | 10.8 ± 3.4 | 10.9 ± 3.7 | 10.0 ± 3.7 | 0.114 |
| Gender (% male) | 60% | 35% | 41% | 56% | 0.879 |
| BMI-SDS | 2.7 ± 0.6 | 2.7 ± 0.5 | 2.7 ± 0.6 | 2.7 ± 0.3 | 0.710 |
| Tanner stage | 2.9 ± 1.4 | 2.4 ± 1.4 | 2.6 ± 1.7 | 2.2 ± 1.3 | 0.107 |
| Glucose (mg/dL) | 87.8 ± 8.6 | 83.5 ± 11.8 | 83.9 ± 11.2 | 81.9 ± 11.6 | 0.050 |
| Insulin (μU/mL) | 20.6 ± 16.6 | 14.2 ± 13.5 | 13.7 ± 7.4 | 12.3 ± 9.2 | 0.010 |
| HOMA-IR | 4.4 ± 3.4 | 2.9 ± 2.9 | 2.8 ± 1.6 | 2.5 ± 2.2 | 0.008 |
| QUICKI | 0.32 ± 0.04 | 0.34 ± 0.06 | 0.34 ± 0.05 | 0.36 ± 0.06 | 0.003 |
| Hemoglobin A1c (%) | 5.37 ± 0.31 | 5.55 ± 0.42 | 5.17 ± 0.36 | 5.21 ± 0.38 | 0.035 |
All data are means ± standard deviation except for gender.
3. Results and Discussion
3.1. Results in All Studied Subjects
Mean ± SD 25(OH)D concentration of all subjects was 15.8 ± 6.6 ng/mL and 25(OH)D concentrations were not statistically different in obese children (obese: 16.2 ± 6.8 ng/mL; nonobese: 13.6 ± 5.2 ng/mL). We found that 25(OH)D concentrations of 96% of all subjects were below the normal threshold (<30 ng/mL) and 76% of all studied subjects were 25(OH)D deficient (<20 ng/mL). There were significant seasonal changes of 25(OH)D concentrations with significantly higher concentrations from July to October compared to January to April (P < 0.05, Figure 1). In correlation analyses, we found that 25(OH)D levels correlated with HOMA-IR (r = −0.173, P = 0.031) and QUICKI (r = 0.157, P = 0.050). As both measures, HOMA-IR and QUICKI, depend also on the degree of adiposity, we next set out to determine if there was an association between 25(OH)D concentrations and these measures of glucose homeostasis after adjusting for age, gender, and SDS-BMI and included all obese and nonobese subjects in this analysis. Even after these adjustments, 25(OH)D correlated significantly with HOMA-IR (r = −0.269, P = 0.023) and QUICKI (r = 0.264, P = 0.030).
Figure 1.
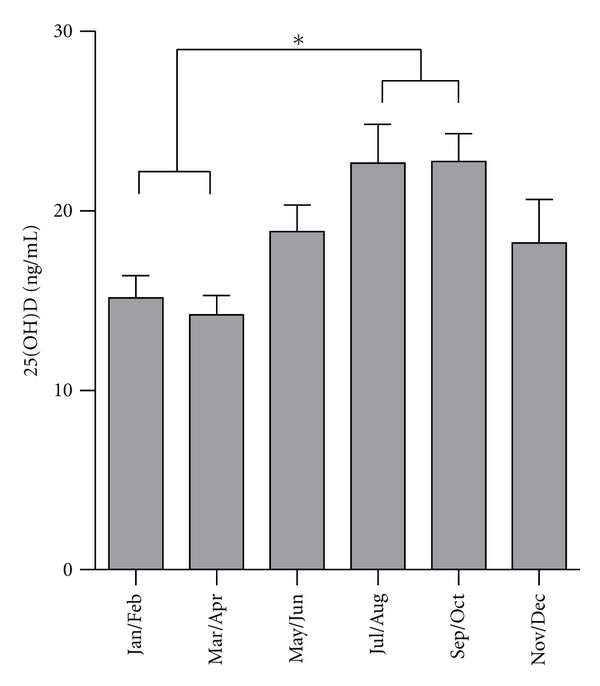
Seasonal changes of 25(OH) serum concentrations in all studied subjects showing significantly higher levels in summer and early fall (July–October), compared to winter and early spring (January–April, *P < 0.05).
3.2. Results in Obese Subjects
Obese subjects were divided into quartiles based on their serum 25(OH)D concentrations. Using one-way ANOVA tests for trend and linear regression, there were no associations between 25(OH)D levels and age, gender, or Tanner stage for pubertal development (Table 1). In addition, there was no association between 25(OH)D concentration and SDS-BMI by ANOVA, but there was a significant trend towards higher insulin concentrations, insulin resistance HOMA-IR, hemoglobin A1c values, and lower insulin sensitivity QUICKI in subjects with lower 25(OH)D concentration (Table 1, Figure 2). In obese subjects, we also analyzed adiponectin and resistin levels. Serum adiponectin was significantly lower in the lowest compared to the highest 25(OH)D quartile (Figure 3(a)), whereas no significant differences between the quartiles were found for resistin. Serum 25(OH)D concentrations correlated significantly with adiponectin (Figure 3(b)). Even after adjusting for SDS-BMI, 25(OH)D correlated with adiponectin (r = 0.192, P = 0.031), but not with resistin (r = −0.156, P = 0.078).
Figure 2.
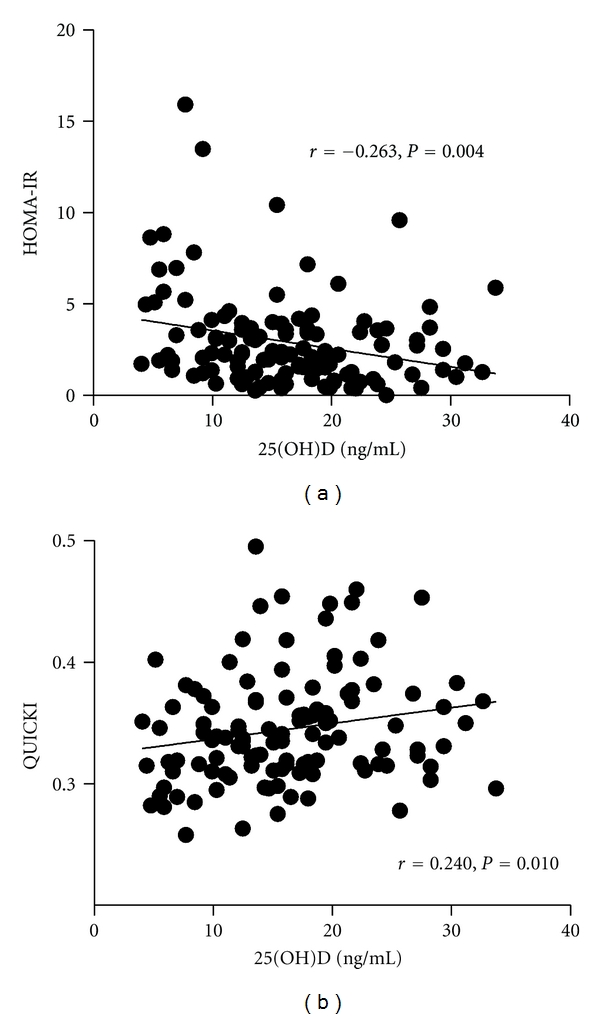
Insulin resistance HOMA-IR (a) and insulin sensitivity QUICKI (b) concentration in relation to 25(OH)D serum levels in obese children.
Figure 3.
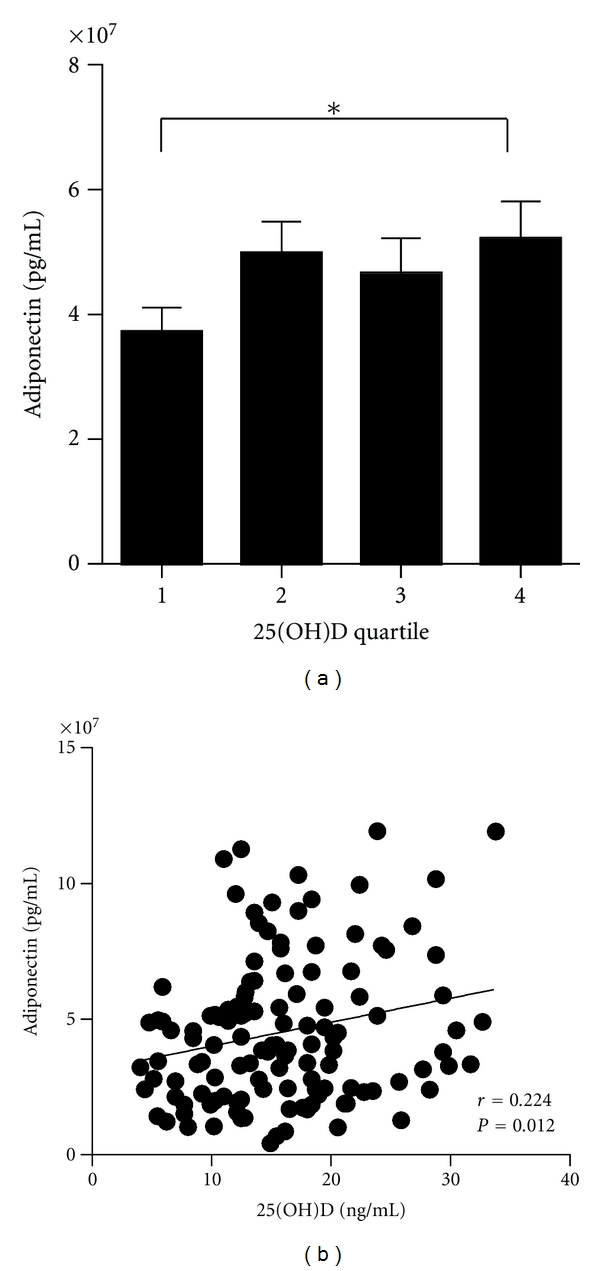
Serum adiponectin in levels in different quartiles (a) for serum 25(OH)D obese children (Q1: 8.0 ± 2.3; Q2: 13.6 ± 1.2; Q3: 17.5 ± 1.2; Q4: 25.3 ± 4.3 ng/mL). *P < 0.05. Adiponectin concentration in relation to 25(OH)D serum levels (b) in obese children.
3.3. Discussion of Results
To summarize, low 25(OH)D serum levels were associated with low adiponectin concentrations and higher insulin resistance. Ninety-six % of all studied subjects had suboptimal 25(OH)D levels (<30 ng/mL) and 76% of all studied subjects were vitamin D deficient (defined as 25(OH)D <20 ng/mL), which is higher than the reported rate from the USA [28] and similar to a recent study in French-Canadian children and adolescents [29]. In a recent study investigating vitamin D status in Norwegian children, 50% of subjects had 25(OH)D levels below 30 ng/mL [30].
These findings are important as it is becoming increasingly recognized that vitamin D deficiency continues to be largely undertreated in children and adults worldwide [31]. In general, 25(OH)D concentrations below 20 ng/mL are considered to indicate vitamin D deficiency, whereas levels between 20 to 30 ng/mL indicate a relative insufficiency, and levels of 30 ng/mL or greater indicate sufficient vitamin D [13, 31]. Obese children are more likely to be sedentary with reduced sunlight exposure [2]. In addition, children often consume high caloric foods low in mineral and vitamin content [3]. These lifestyle factors increase the risk of vitamin D deficiency; furthermore, a higher body fat mass as well as limited bioavailability of vitamin D caused by trapping vitamin D in adipose tissue may further increase the risk of vitamin D deficiency among obese children compared to normal weight, active children [5, 6]. Black American children and adolescents have a higher risk for low vitamin D status as demonstrated in a recent pediatric study performed in the USA in which low levels of 25(OH)D were associated with higher body mass index and fat mass, and lower levels of HDL [32]. In a large study investigating 25(OH)D levels in German children and adolescents, higher prevalence of vitamin D deficiency was found among immigrants from Turkey, Arab-Islamic countries, Asia, or Africa [33].
Vitamin D receptors (VDRs) are ubiquitously expressed in several tissues, including gut, liver, adipose tissues, cardiac and skeletal muscles, β-cells, and immune cells, such as lymphocytes, dentritic cells, and monocytes/macrophages [34]. It has therefore been hypothesized that low-serum vitamin D status could play a significant role in the pathogenesis of metabolic syndrome [35], type 1 diabetes [36], and T2DM [13, 15, 37–39]. Vitamin D can also have diverse effects on the immune system, particularly on the function of monocytes, macrophages, and T cells [7], and it has been observed to inhibit cytokine production [40]. Adipocytes and adipose tissue macrophages secrete proinflammatory molecules, which have been proposed to play a major role in the development of insulin resistance in obesity [41, 42]. It is therefore possible that vitamin D deficiency contributes to insulin resistance and the development of metabolic syndrome secondary to inflammation.
Administration of vitamin D to deficient humans and animals improves β-cell function [10, 17], whereas low 25(OH)D levels are associated with insulin resistance [5]. Pancreatic β-cell impairment due to vitamin D deficiency would cause increased average glucose concentrations. As a result, the inverse correlation between vitamin D status and HbA1c concentrations in our study supports the hypothesis that vitamin D deficiency is associated with poorer glycemic control which can be caused by β-cell dysfunction as well as insulin resistance. The relationship between HbA1c and 25(OH)D concentrations was independent of body mass, further establishing an independent association between vitamin D status and glucose homeostasis in obese adolescents. In addition to the effects of vitamin D deficiency on β-cell function, other pathophysiological mechanisms include unfavorable effects on liver and reduced immunomodulation. In a recent study performed in rats, we demonstrated that vitamin D deficiency can contribute to nonalcoholic steatohepatitis, insulin resistance, and increased cytokine secretion [43].
The present study confirms a previous investigation that also demonstrated that 25(OH)D was associated with insulin sensitivity in obese children [44]. However, lower insulin sensitivity could also be related to an increased body fat mass in obesity. Therefore, we adjusted our correlation analyses to SDS-BMI as a clinical surrogate marker for adiposity. In the present study, the relationship between 25(OH)D and measures of insulin sensitivity and resistance persisted even after adjustment for body mass, which supports the hypothesis that low 25(OH)D may in fact be directly associated with insulin resistance irrespective of body fat mass.
Recently in a small study of 34 African-American adolescents, low 25(OH)D levels correlated with low adiponectin levels and obesity [45]. We found a negative correlation between serum 25(OH)D and adiponectin levels even after correction for SDS-BMI. As mentioned above, vitamin D can also have diverse effects on the immune system. The coexistence of obesity and a low-grade inflammatory state is well described in children, and previous studies showed that low adiponectin levels are associated with higher concentrations of inflammatory makers in blood [46, 47]. Low adiponectin levels are associated with a higher insulin resistance, higher levels of c-reactive protein, and lower HDL levels of in children [46]. Therefore, the higher degree of insulin resistance and inflammation and lower HDL levels in obese children with low-vitamin-D status might be related to low adiponectin levels.
Resistin was initially identified as an adipokine released from adipose tissue and thought to play a role in insulin resistance [48]. In humans, resistin is secreted by adipocytes and by macrophages in adipose tissue and liver, likely stimulating the secretion of proinflammatory molecules [49, 50]. Some studies showed that serum-resistin levels are elevated in obesity, however, the role of resistin in insulin resistance and type 2 diabetes still remains controversial [51, 52]. In the present study, we did not detect a correlation between 25(OH)D levels and serum resistin in obese children.
In a pediatric population, Reinehr et al. [53] examined obese children and found a significant increase in 25(OH)D levels after a lifestyle intervention induced weight loss. In a more recent study in adult women, weight loss was also associated with an increase of 25(OH)D levels and was accompanied by improved insulin resistance [54]. For treatment of vitamin D deficiency, it is important to note that the American Academy of Pediatrics (AAP) recommended that daily intake of vitamin D of 400 IU is insufficient to correct vitamin D deficiency in obese African American children [2, 55]. New guidelines recommend much higher doses to treat vitamin D deficiency in children and adolescents, which is in particular important in obese subjects [56].
As a strength of this study, 25(OH)D concentrations were LC-MS/MS method to some previous studies, where 25(OH)D levels have been quantified by immunoassays or competitive protein-binding assays that are known to have variable cross-reactivity with other vitamin D metabolites [29, 30, 32, 44, 57, 58]. For that reason, the LC-MS/MS method is now considered as the gold-standard for vitamin D measurements [27, 59].
This study has a few limitations. First, BMI percentiles were used to classify overweight. Although BMI is a good measure for assessing overweight in children and adolescents [60], one needs to be aware of its limitation because of its unreliability as an indicator of fat mass. A high BMI may be an indication of a high fat mass but it could also be caused by a larger mass of skeletal muscle, and the relation of BMI for age to body fatness among children and adolescents also differs by race/ethnicity [61]. Second, we could study vitamin D status only in relatively fewer nonobese children. Third, we did not assess parathyroid hormone levels, which were related to BMI in a previous pediatric study [53]. Fourth, the interpretation of the data is limited due to the cross-sectional study design. Future vitamin D treatment studies are required to investigate the effectiveness of vitamin D administration in the treatment of the metabolic syndrome or insulin resistance, in particular to test whether vitamin D treatment can lead to increased insulin sensitivity and adiponectin secretion.
4. Conclusion
In conclusion, the prevalence of hypovitaminosis D is high among obese and nonobese children living in Germany, and low-serum concentrations of 25(OH)D are associated with impaired insulin sensitivity and low-adiponectin serum levels. To investigate the impact of vitamin D treatment in obese children and the role of vitamin D on glucose homeostasis and low-grade inflammation, future studies should use highly sensitive measures of insulin sensitivity and β-cell serum markers well as adiponectin.
Acknowledgments
The study was supported by the Bonfor Research Foundation, University of Bonn, Germany. The authors thank the patients and their families for participating in the obesity study in Bonn.
References
- 1.Kaufman FR, Shaw J. Type 2 diabetes in youth: rates, antecedents, treatment, problems and prevention. Pediatric Diabetes. 2007;8(9):4–6. doi: 10.1111/j.1399-5448.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- 2.Rajakumar K, Fernstrom JD, Holick MF, Janosky JE, Greenspan SL. Vitamin D status and response to vitamin D3 in obese vs. non-obese African American children. Obesity. 2008;16(1):90–95. doi: 10.1038/oby.2007.23. [DOI] [PubMed] [Google Scholar]
- 3.Bradlee ML, Singer MR, Qureshi MM, Moore LL. Food group intake and central obesity among children and adolescents in the Third National Health and Nutrition Examination Survey (NHANES III) Public Health Nutrition. 2010;13(6):797–805. doi: 10.1017/S1368980009991546. [DOI] [PubMed] [Google Scholar]
- 4.Muscogiuri G, Sorice GP, Prioletta A, et al. 25-hydroxyvitamin D concentration correlates with insulin-sensitivity and BMI in obesity. Obesity. 2010;18(10):1906–1910. doi: 10.1038/oby.2010.11. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Bertolini L, Scala L, et al. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutrition, Metabolism and Cardiovascular Diseases. 2007;17(7):517–524. doi: 10.1016/j.numecd.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Lenders CM, Feldman HA, Von Scheven E, et al. Relation of body fat indexes to vitamin D status and deficiency among obese adolescents. American Journal of Clinical Nutrition. 2009;90(3):459–467. doi: 10.3945/ajcn.2008.27275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. American Journal of Physiology. 2005;289(1):F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 8.de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. Journal of the American Society of Nephrology. 2009;20(8):1805–1812. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendrick J, Targher G, Smits G, Chonchol M. 25-hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205(1):255–260. doi: 10.1016/j.atherosclerosis.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. Journal of Clinical Investigation. 1984;73(3):759–766. doi: 10.1172/JCI111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billaudel B, Barakat L, Faure-Dussert A. Vitamin D3 deficiency and alterations of glucose metabolism in rat endocrine pancreas. Diabetes and Metabolism. 1998;24(4):344–350. [PubMed] [Google Scholar]
- 12.Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. The FASEB Journal. 2003;17(3):509–511. doi: 10.1096/fj.02-0424fje. [DOI] [PubMed] [Google Scholar]
- 13.Ashraf A, Alvarez JA. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. International Journal of Endocrinology. 2010;2010:18 pages. doi: 10.1155/2010/351385. Article ID 351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Need AG, O’Loughlin PD, Horowitz M, Nordin BEC. Relationship between fasting serum glucose, age, body mass index and serum 25 hydroxyvitamin D in postmenopausal women. Clinical Endocrinology. 2005;62(6):738–741. doi: 10.1111/j.1365-2265.2005.02288.x. [DOI] [PubMed] [Google Scholar]
- 15.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 16.Hyppönen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 british birth cohort. Diabetes. 2008;57(2):298–305. doi: 10.2337/db07-1122. [DOI] [PubMed] [Google Scholar]
- 17.Chiu KC, Chu A, Go VLW, Saad MF. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. American Journal of Clinical Nutrition. 2004;79(5):820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 18.Thorand B, Zierer A, Huth C, et al. Effect of serum 25-hydroxyvitamin D on risk for type 2 diabetes may be partially mediated by subclinical inflammation: results from the monica/kora augsburg study. Diabetes Care. 2011;34(10):2320–2322. doi: 10.2337/dc11-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gedik O, Akalin S. Effects of vitamin D deficiency and repletion on insulin and glucagon secretion in man. Diabetologia. 1986;29(3):142–145. doi: 10.1007/BF02427083. [DOI] [PubMed] [Google Scholar]
- 20.Roth CL, Kratz M, Ralston MM, Reinehr T. Changes in adipose-derived inflammatory cytokines and chemokines after successful lifestyle intervention in obese children. Metabolism. 2011;60:445–452. doi: 10.1016/j.metabol.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Hoofnagle AN. Quantitative clinical proteomics by liquid chromatography—tandem mass spectrometry: assessing the platform. Clinical Chemistry. 2010;56(2):161–164. doi: 10.1373/clinchem.2009.134049. [DOI] [PubMed] [Google Scholar]
- 22.Reinehr T, Roth CL. A new link between skeleton, obesity and insulin resistance: relationships between osteocalcin, leptin and insulin resistance in obese children before and after weight loss. International Journal of Obesity. 2010;34(5):852–858. doi: 10.1038/ijo.2009.282. [DOI] [PubMed] [Google Scholar]
- 23.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conwell LS, Trost SG, Brown WJ, Batch JA. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care. 2004;27(2):314–319. doi: 10.2337/diacare.27.2.314. [DOI] [PubMed] [Google Scholar]
- 26.Saenger AK, Laha TJ, Bremner DE, Sadrzadeh SMH. Quantification of serum 25-hydroxyvitamin D2 and D3 using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. American Journal of Clinical Pathology. 2006;125(6):914–920. doi: 10.1309/J32U-F7GT-QPWN-25AP. [DOI] [PubMed] [Google Scholar]
- 27.Hoofnagle AN, Laha TJ, Donaldson TF. A rubber transfer gasket to improve the throughput of liquid-liquid extraction in 96-well plates: application to vitamin D testing. Journal of Chromatography B. 2010;878(19):1639–1642. doi: 10.1016/j.jchromb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 2009;124(3):e362–e370. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delvin EE, Lambert M, Levy E, et al. Vitamin D status is modestly associated with glycemia and indicators of lipid metabolism in French-Canadian children and adolescents. Journal of Nutrition. 2010;140(5):987–991. doi: 10.3945/jn.109.112250. [DOI] [PubMed] [Google Scholar]
- 30.Lagunova Z, Porojnicu AC, Lindberg FA, Aksnes L, Moan J. Vitamin D status in Norwegian children and adolescents with excess body weight. Pediatric Diabetes. 2011;12(2):120–126. doi: 10.1111/j.1399-5448.2010.00672.x. [DOI] [PubMed] [Google Scholar]
- 31.Holick MF. Medical progress: vitamin D deficiency. The New England Journal of Medicine. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 32.Rajakumar K, de Las Heras J, Chen TC, Lee S, Holick MF, Arslanian SA. Vitamin D status, adiposity, and lipids in black American and Caucasian children. Journal of Clinical Endocrinology and Metabolism. 2011;96(5):1560–1567. doi: 10.1210/jc.2010-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hintzpeter B, Scheidt-Nave C, Müller MJ, Schenk L, Mensink GBM. Higher prevalence of vitamin D deficiency is associated with immigrant background among children and adolescents in Germany. Journal of Nutrition. 2008;138(8):1482–1490. doi: 10.1093/jn/138.8.1482. [DOI] [PubMed] [Google Scholar]
- 34.Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-dihydroxyvitamin D3 works as anti-inflammatory. Diabetes Research and Clinical Practice. 2007;77(1):47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Kayaniyil S, Vieth R, Harris SB, et al. Association of 25(OH)D and PTH with metabolic syndrome and its traditional and nontraditional components. Journal of Clinical Endocrinology and Metabolism. 2011;96(1):168–175. doi: 10.1210/jc.2010-1439. [DOI] [PubMed] [Google Scholar]
- 36.Hyppönen E, Läärä E, Reunanen A, Järvelin M-R, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. The Lancet. 2001;358(9292):1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 37.Palomer X, González-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes, Obesity and Metabolism. 2008;10(3):185–197. doi: 10.1111/j.1463-1326.2007.00710.x. [DOI] [PubMed] [Google Scholar]
- 38.Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D3 on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010;53(10):2112–2119. doi: 10.1007/s00125-010-1838-1. [DOI] [PubMed] [Google Scholar]
- 39.Kayaniyil S, Vieth R, Retnakaran R, et al. Association of vitamin D with insulin resistance and β-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care. 2010;33(6):1379–1381. doi: 10.2337/dc09-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zarrabeitia MT, Riancho JA, Amado JA, Olmos JM, Gonzalez-Macias J. Effect of calcitriol on the secretion of prostaglandin E2, interleukin 1, and tumor necrosis factor α by human monocytes. Bone. 1992;13(2):185–189. doi: 10.1016/8756-3282(92)90010-t. [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Yang S, Lin H, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37(2):343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 42.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. Journal of Clinical Investigation. 2006;116(6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roth CL, Elfers CT, Figlewicz DP, et al. Vitamin D deficiency in obese rats exacerbates NAFLD and increases hepatic resistin and toll-like receptor activation. doi: 10.1002/hep.24737. Hepatology. In press. [DOI] [PubMed] [Google Scholar]
- 44.Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism. 2008;57(2):183–191. doi: 10.1016/j.metabol.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 45.Nunlee-Bland G, Gambhir K, Abrams C, Abdul M, Vahedi M, Odonkor W. Vitamin D deficiency and insulin resistance in obese African-American adolescents. Journal of Pediatric Endocrinology and Metabolism. 2011;24(1-2):29–33. doi: 10.1515/jpem.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winer JC, Zern TL, Taksali SE, et al. Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. Journal of Clinical Endocrinology and Metabolism. 2006;91(11):4415–4423. doi: 10.1210/jc.2006-0733. [DOI] [PubMed] [Google Scholar]
- 47.Reinehr T, Roth C, Menke T, Andler W. Adiponectin before and after weight loss in obese children. Journal of Clinical Endocrinology and Metabolism. 2004;89(8):3790–3794. doi: 10.1210/jc.2003-031925. [DOI] [PubMed] [Google Scholar]
- 48.Vidal-Puig A, O’Rahilly S. Resistin: a new link between obesity and insulin resistance? Clinical Endocrinology. 2001;55(4):437–438. doi: 10.1046/j.1365-2265.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 49.Qatanani M, Szwergold NR, Greaves DR, Ahima RS, Lazar MA. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. Journal of Clinical Investigation. 2009;119(3):531–539. doi: 10.1172/JCI37273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szalowska E, Elferink MGL, Hoek A, Groothuis GMM, Vonk RJ. Resistin is more abundant in liver than adipose tissue and is not up-regulated by lipopolysaccharide. Journal of Clinical Endocrinology and Metabolism. 2009;94(8):3051–3057. doi: 10.1210/jc.2008-2787. [DOI] [PubMed] [Google Scholar]
- 51.Kusminski CM, McTernan PG, Kumar S. Role of resistin in obesity, insulin resistance and type II diabetes. Clinical Science. 2005;109(3):243–256. doi: 10.1042/CS20050078. [DOI] [PubMed] [Google Scholar]
- 52.Roth CL, Reinehr T. Roles of gastrointestinal and adipose tissue peptides in childhood obesity and changes after weight loss due to lifestyle intervention. Archives of Pediatrics and Adolescent Medicine. 2010;164(2):131–138. doi: 10.1001/archpediatrics.2009.265. [DOI] [PubMed] [Google Scholar]
- 53.Reinehr T, de Sousa G, Alexy U, Kersting M, Andler W. Vitamin D status and parathyroid hormone in obese children before and after weight loss. European Journal of Endocrinology. 2007;157(2):225–232. doi: 10.1530/EJE-07-0188. [DOI] [PubMed] [Google Scholar]
- 54.Tzotzas T, Papadopoulou FG, Tziomalos K, et al. Rising serum 25-hydroxy-vitamin D levels after weight loss in obese women correlate with improvement in insulin resistance. Journal of Clinical Endocrinology and Metabolism. 2010;95(9):4251–4257. doi: 10.1210/jc.2010-0757. [DOI] [PubMed] [Google Scholar]
- 55.Dong Y, Stallmann-Jorgensen IS, Pollock NK, et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. Journal of Clinical Endocrinology and Metabolism. 2010;95(10):4584–4591. doi: 10.1210/jc.2010-0606. [DOI] [PubMed] [Google Scholar]
- 56.Ross AC, Manson JE, Abrams SA, et al. The 2011 dietary reference intakes for calcium and vitamin D: what dietetics practitioners need to know. Journal of the American Dietetic Association. 2011;111(4):524–527. doi: 10.1016/j.jada.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 58.Ding S, Schoenmakers I, Jones K, Koulman A, Prentice A, Volmer DA. Quantitative determination of vitamin D metabolites in plasma using UHPLC-MS/MS. Analytical and Bioanalytical Chemistry. 2010;398(2):779–789. doi: 10.1007/s00216-010-3993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yetley EA, Pfeiffer CM, Schleicher RL, et al. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. Journal of Nutrition. 2010;140(11):2030S–2045S. doi: 10.3945/jn.110.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Himes JH. Challenges of accurately measuring and using BMI and other indicators of obesity in children. Pediatrics. 2009;124(1):S3–S22. doi: 10.1542/peds.2008-3586D. [DOI] [PubMed] [Google Scholar]
- 61.Freedman DS, Wang J, Thornton JC, et al. Racial/ethnic differences in body fatness among children and adolescents. Obesity. 2008;16(5):1105–1111. doi: 10.1038/oby.2008.30. [DOI] [PubMed] [Google Scholar]


