Abstract
Background
We assessed the association between sleep apnea, snoring, incident cardiovascular (CV) events and all-cause mortality in the Multi Ethnic Study of Atherosclerosis (MESA) cohort.
Methods
Out of 5338 respondents to a sleep questionnaire administered during the second MESA exam period, 208 had physician-diagnosed sleep apnea (PDSA), 1452 were habitual snorers (HS) and 3678 were neither a habitual snorer nor had PDSA (normal participants). Cox proportional hazard analysis was used to assess the associations adjusting for age, gender, race/ethnicity, smoking, diabetes mellitus, total cholesterol, HDL, triglycerides, BMI, current alcohol use, benzodiazepine use, BP medications and statin use.
Results
Over a 7.5 year average follow-up period, 310 adjudicated CV events including MI, stroke, angina, resuscitated cardiac arrest, stroke death and CVD death and 189 deaths occurred. Compared to HS, PDSA was associated with higher incident CV rates in both univariate and multivariable models [hazard ratio (95%); 1.89(1.22–2.93), p=0.004 and 1.91(1.20 –3.04), p=0.007 respectively]. PDSA was also associated with a higher death rates compared with HS [hazard ratio (95%); 2.13(1.25 – 3.63), p=0.006 and 2.70(1.52– 4.79), p=0.007 respectively]. Compared with normal participants, PDSA had higher incident CV event rates in both univariate and multivariable models [hazard ratio (95%); 2.23[1.39–3.60], p=0.001 and 2.16[1.30–3.58], p=0.003 respectively]. Similarly, PDSA had a higher death rate compared with normal participants in both the univariate and multivariable models [hazard ratio (95%CI); 2.44(1.36 – 4.37), p=0.003 and 2.71(1.45 – 5.08), p=0.002 respectively]. Habitual snorers had similar incident CV event rates and death rates in both univariate and multivariable models compared with normal participants.
Conclusion
PDSA but not habitual snoring was associated with high incident CV events and all-cause mortality in a multi-ethnic population based study of adults free of clinical CV disease at baseline.
Keywords: Obstructive sleep apnea, habitual snorers, cardiovascular events, mortality, population
Introduction
Recognized obstructive sleep apnea affects about 15 million American adults, with reported snoring occurring in an even greater number of American adults (1).While almost all individuals with sleep apnea report snoring, the majority of snorers do not have frequent apneas and hypopneas diagnostic of sleep apnea (2). The diagnosis of sleep apnea requires objective physiological monitoring and clinical interpretation, while history of ascertainment of snoring is often by self- report, which is simple but subject to various reporting biases and yields data that do not provide quantifiable information on the severity of physiological abnormalities (3).
Despite the subjective nature of self –reported snoring, recent research supports an association between both sleep apnea as well as snoring with cardiovascular risk factors, measures of subclinical atherosclerosis and cardiovascular diseases (4–10). However, data on the association between sleep apnea and /or snoring and cardiovascular disease/mortality are limited and have been collected mostly in high risk or individuals with established cardiovascular diseases (11– 17) and a few population based studies (18–21). Most research has examined cross-sectional associations, with incident data predominantly from older cohorts, from samples with limited representation of African Americans, or from studies with limited information on cardiovascular risk factors (18–21). Whether sleep apnea in individuals with established cardiovascular disease accelerates disease progression, and whether treatment of sleep apnea results in clinical improvement, fewer cardiovascular events, and reduced mortality remains unclear. It also remains unclear whether sleep apnea is important in initiating the development of cardiac and vascular diseases.
To further address the longitudinal associations between sleep apnea, snoring, cardiovascular disease and all-cause mortality in community-based samples, we assessed the association between physician-diagnosed sleep apnea (PDSA), self-reported snoring and incident cardiovascular events and all-cause mortality in an adult multi- ethnic population free of clinical cardiovascular disease at baseline. We explicitly compared models using physician diagnosed sleep apnea with self-reported snoring and explored the consistency of the associations across gender and race/ethnicity groups.
Methods
Study Population and Data Collection
The study design for MESA has been published elsewhere (22). In brief, MESA is a prospective cohort study designed to investigate the prevalence, correlates and progression of subclinical CVD in individuals without clinically recognized CVD at baseline. The cohort includes 6814 women and men aged 45–84 years old recruited from 6 US communities (Baltimore, MD.; Chicago, Ill.; Forsyth County, N.C.; Los Angeles County, Calif.; northern Manhattan, N.Y.; and St. Paul, Minn.). MESA cohort participants were 38% white, 28% black, 22% Hispanic and 12% Chinese. Individuals with a history of physician–diagnosed myocardial infarction, angina, heart failure, stroke, or transient ischemic attack, or who had undergone an invasive procedure for CVD (coronary artery bypass graft, angioplasty, valve replacement, pacemaker placement or other vascular surgeries) were excluded from participation. This study was approved by the Institutional Review Boards of each study site and written informed consent was obtained from all participants.
Demographics, medical history, anthropometric and laboratory data for the present analysis were obtained from the first examination of the MESA cohort (July 2000– August 2002). Current smoking was defined as having smoked a cigarette in the last 30 days. Antihypertensive and other medications use was assessed by reviewing participant's medication containers. As part of a personal-history questionnaire, participants were asked, “Have you ever consumed alcoholic beverages?” If yes, they were then asked, “Do you presently drink alcoholic beverages?” The answers to these 2 questions determined our categorization into never, former, and current drinkers. Resting blood pressure was measured 3 times in the seated position, and the average of the second and third readings was recorded. Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or use of medication prescribed for hypertension. Body mass index was calculated as weight (kg)/ height (m2). Total and high-density lipoprotein cholesterol were measured from blood samples obtained after a 12-hour fast. Low-density lipoprotein cholesterol was estimated by the Friedewald equation (23). Diabetes mellitus was defined as fasting glucose ≥ 126 mg/dl or the use of hypoglycemic medications.
Physician diagnosed sleep apnea and self- reported snoring data collection
During the second MESA examination, a self-administered sleep history questionnaire was obtained. Among the questions were a)”Have you ever snored (now or at any time in the past)?”; if yes, “How frequent and loudly do you snore?”; and b)”Have you ever been told by a doctor that you had sleep apnea (a condition in which breathing stops briefly during sleep)?” Participants were provided 3 potential responses to each question: yes, no and don't know. Among the 6814 MESA participants, 1476 either did not participate in the sleep history study or reported “don't know” to either the sleep apnea or snoring questions and were therefore excluded from this analysis, yielding an analytical sample of 5338. Habitual snorers are participants who reported snoring greater than or equal to 3–5 days/week. Participants who did not have PDSA and who did not report symptoms of habitual snoring were classified as “normal” participants.
Ascertainment of Cardiovascular Events
Cardiovascular events obtained over an average 7.5 year follow-up period were adjudicated by a MESA committee which included cardiologists, physician epidemiologists and neurologists. A detailed description of the cardiovascular event adjudication process has already been published (24). For the purposes of this study, we define our composite outcome (composite event) as incident myocardial infarction, definite angina, probable angina (if followed by coronary artery bypass grafting and percutaneous coronary intervention), resuscitated cardiac arrest, stroke, stroke death, CHD death or other CVD death as defined by the MESA protocol.
Statistical Analysis
Demographic characteristic of participants in each of the three exposure groups: PDSA, habitual snoring and “normal”', were compared using either using chi square analysis or ANOVA and reported as mean± standard deviation or percent for continuous and categorical variable respectively. Kaplan Meier analysis was used to assess the association between the sleep apnea/snoring exposures and incident cardiovascular events/ all -cause mortality. Cox proportional hazard regression analysis was used to assess the univariate and the multivariable association for each exposure group with the outcomes, adjusting for potential confounders or mediators such as age, gender, race/ethnicity, BMI, cigarette smoking status, diabetes mellitus, systolic blood pressure, total cholesterol, HDL, triglycerides, current alcohol and benzodiazepine use, blood pressure and statin medication use. These potential confounders or mediators were chosen based on prior published association of cardiovascular events with all -cause mortality or sleep disorders. Stratified analyses exploring the association between PDSA and incident CVD events/ all- cause mortality within gender and race/ethnic groups was also done. A 2-tailed value of P<0.05 was considered significant. All statistical analyses were performed using SAS version 9.2.2 (SAS Institute, Cary, N.C.) and figures were drawn/plotted using Graphpad.
Results
At the time of the second MESA exam, 208 participants (3.9%) reported PDSA, 1452 participants (27.2%) reported symptoms of habitual snoring and 3678 participants (68.9%) reported neither PDSA nor snoring. Over an average of 7.5 years follow-up period, 310 cardiovascular events and 189 deaths were adjudicated. In all 31.1% of the participants were taken aspirin and 78.8% of the participants with diabetes were either on insulin or an oral hypoglycemic agent. Participants with PDSA were relatively younger, more obese, were more likely to be diabetic, current cigarette smokers and alcohol users than either habitual snorers or normal participants (Table 1). In the Kaplan Meier analysis, participants with PDSA had a lower event free survival rate compared with either habitual snorers or normal participants [89.4% vs. 94.2% and 89.4% vs. 94.4%, log-rank p =0.001 and 0.003, respectively] (Figure 1). Participants with PDSA also had a lower overall survival rate compared with either habitual snorers or normal participants [92.8%vs.96.5% and 92.8% vs. 96.9%, p =0.005 and 0.007 respectively] (Figure 2). There were no significant differences between the either event free survival rates or survival rates of participants with habitual snoring and normal participants (Figure 1 and 2).
Table 1.
Demographic characteristics of self- identified Habitual Snorers, Physician Diagnosed Sleep Apnea and normal participants in MESA.
| Variables | Habitual Snorers (N=1452) (mean ± SD) | Sleep Apnea (N=208) (mean± SD) | Normal Participants (N=3678) (mean ± SD) | P value |
|---|---|---|---|---|
| Age (years) | 61.4± 9.4 | 61.8± 9.2 | 63.7± 10.2 | 0.001 |
|
| ||||
| Female (%) | 610(42.0) | 71(34.2) | 2014(54.8) | <0.0001 |
|
| ||||
| Race/ethnicity (%) | <0.001 | |||
| Caucasian | 485(33.5) | 97(46.6) | 1544(42.0) | |
| Chinese | 215(14.8) | 9(4.3) | 406(11.0) | |
| African Americans | 337(28.2) | 65(31.3) | 1021(27.7) | |
| Hispanic | 415(28.5) | 37(17.8) | 707(19.3) | |
|
| ||||
| BMI (Kg/m2) | 29.8± 5.8 | 32.2± 6.2 | 27.8± 5.8 | 0.003 |
|
| ||||
| Diabetes mellitus (%) | 245(16.9) | 42(20.4) | 492(13.4) | <0.001 |
|
| ||||
| Cholesterol(mg/dl) | 0.01 | |||
| Total | 192.0 ± 35.7 | 182.2± 33.4 | 191.0± 35.8 | |
| HDL | 48.2 ± 12.9 | 47.8± 12.7 | 47.8± 12.8 | |
| LDL | 115.9 ± 32.0 | 107.5± 31.3 | 113.0±31.8 | |
| Triglycerides | 144.0 ± 95.3 | 139.0± 83.3 | 126.7± 75.6 | |
|
| ||||
| Systolic BP(mmHg) | 124.8± 19.8 | 123.0± 18.5 | 124.1± 20.9 | 0.35 |
|
| ||||
| Diastolic BP(mmHg) | 71.9± 10.2 | 71.4± 9.9 | 70.2 ±9.9 | 0.08 |
|
| ||||
| Cigarette Smoking (%) | <0.001 | |||
| Never | 614 (42.4) | 77(37.2) | 1700(47.0) | |
| Former | 652(45.2) | 101(48.8) | 1553(42.4) | |
| Current | 179(12.4) | 29(14.0) | 391(10.6) | |
|
| ||||
| Benzodiazepine use (%) | 42(3.10) | 10(4.9) | 100(2.8) | 0.20 |
|
| ||||
| Current alcohol use (%) | 774(53.2) | 121(58.2) | 1894(51.6) | 0.001 |
|
| ||||
| BP med use (%) | 588(42.7) | 102(50.5) | 1429(40.5) | 0.004 |
|
| ||||
| Statin use (%) | 268(19.5) | 55(27.3) | 728(20.7) | 0.02 |
Figure 1.
Kaplan Meier curves showing the event free survival (without incident cardiovascular events) of participants with physician diagnosed sleep apnea, habitual snorers, and normal participants in MESA.
Figure 2.
Kaplan Meier curves showing the event free survival of participants with physician diagnosed sleep apnea, habitual snorers, and normal participants and all-cause mortality in MESA.
Comparison of incident CVD events and all-cause mortality in habitual snorers, participants with physician diagnosed sleep apnea and normal participants
As shown in Table 2, participants with PDSA (n=208) had higher incident CVD rates in both the univariate and multivariable analysis compared with habitual snorers (n=1452) [hazard ratio (95%CI); 1.89(1.22 – 2.93), p=0.004 and 1.91(1.20 –3.04), p=0.007, p=0.003, respectively]. Similarly, participants with PDSA had a higher death rate compared with habitual snorers in both the univariate and multivariable models [hazard ratio (95%CI); 2.13(1.25 – 3.63), p=0.006 and 2.70(1.52 – 4.79), p=0.007, respectively.]]. PDSA was also associated with higher incident CVD rates compared with normal participants in both the univariate and multivariable models [hazard ratio (95%CI); 2.23(1.39 – 3.60), p=0.001 and 2.16(1.30 – 3.58), p=0.003, respectively]. PDSA was additionally associated with a higher death rate in the univariate and multivariable models compared with normal participants [hazard ratio (95%CI); 2.44(1.36 – 4.37, p=0.003 and 2.71(1.45 – 5.08), p=0.002, respectively.]
Table 2.
Comparison of cardiovascular events and all- cause mortality of habitual snorers, physician diagnosed sleep apneas and normal participants.
| Comparison | # of Events | Univariate Hazard ratio(95%CI) | P value | Multivariable Hazard ratio (95%CI) | P value |
|---|---|---|---|---|---|
| Sleep apneas vs. H. Snorers | a95 | 1.89(1.22–2.93) | 0.004 | 1.91(1.20–3.04) | 0.007 |
| b60 | 2.13(1.25–3.63) | 0.006 | 2.70(1.52–4.79) | 0.007 | |
| Sleep apneas vs. Normal participants | a237 | 2.23(1.39–3.60) | 0.001 | 2.16(1.30–3.58) | 0.003 |
| b144 | 2.44(1.36–4.37) | 0.003 | 2.71(1.45–5.08) | 0.002 | |
| Normal participants vs. H.Snorers | a288 | 0.96(0.72 – 1.28) | 0.80 | 0.98(0.72 – 1.33) | 0.92 |
| b174 | 0.83(0.52–1.46) | 0.47 | 1.13(0.81–1.72) | 0.42 |
Multivariable model were adjusted for age, gender, race/ethnicity, BMI, cigarette smoking, diabetes mellitus, total cholesterol, HDL, triglycerides, systolic blood pressure, BP medication use, statin use, benzodiazepine use and current alcohol use.
denotes rows for cardiovascular events
denotes rows for all – cause mortality
All-cause mortality and incident CVD events (PDSA vs. No PDSA)
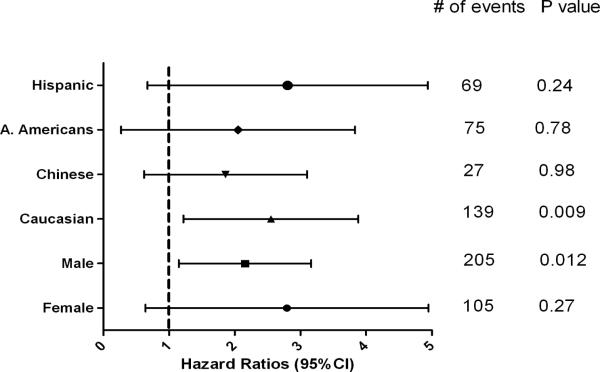
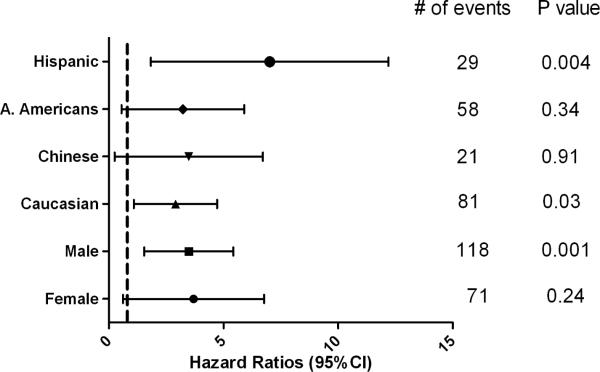
In comparison to “No PDSA” (including habitual snorers and normal participants), PDSA was associated with an increased risk for incident cardiovascular events in the univariate analysis [hazard ratio (95%CI); 2.02(1.31– 3.12), p=0.002] and also in our multivariable model that included multiple potential cofounders [hazard ratio (95%CI); 1.90(1.21–2.98], p=0.005]. PDSA was also associated with increased risk of all-cause mortality in both the univariate and multivariable Cox proportional models [hazard ratio (95%CI); 2.21(1.30 – 3.74), p=0.003 and 2.62(1.51 – 4.55), p=0.001 respectively] compared with participants with no PDSA (Table 3). In gender and race/ethnicity stratified analyses, PDSA was significantly associated with incident CV events in males but not in females [hazard ratio (95%); 1.91(1.15 –3.16), p=0.012 and 1.78(0.64 – 4.95), p=0.27 respectively]. PDSA was statistically significantly associated with incident CV events in only the Caucasian subsample. (Figure 3). Among the other race/ethnic groups, the confidence intervals were wider due to smaller numbers. PDSA was also statistically significantly associated with an increased death rate in males, Hispanics and Caucasians. However, confidence intervals again were wider for females, Chinese and African Americans (Figure 4).
Table 3.
Association of Physician Diagnosed Sleep apnea (PDSA vs. No PDSA) with cardiovascular outcomes and all-cause mortality in MESA*
| Outcome | # of events | Univariate Hazard ratio(95% CI) | P value | Multivariate Hazard ratio(95% CI) | P value |
|---|---|---|---|---|---|
| Composite | 310 | 2.02(1.31 – 3.12) | 0.002 | 1.90(1.21 – 2.98) | 0.005 |
| Hard CHD | 128 | 1.94(0.98 – 3.81) | 0.056 | 2.06(1.02 – 4.16) | 0.045 |
| MI | 107 | 1.79(0.83 – 3.85) | 0.140 | 2.02(0.91 – 4.48) | 0.061 |
| Angina | 180 | 3.08(1.92 – 4.96) | <0.001 | 2.39(1.44 – 3.97) | 0.001 |
| Stroke | 76 | 1.05(0.33 – 3.32) | 0.940 | 1.15(0.36 – 3.74) | 0.811 |
| All-cause mortality | 189 | 2.21(1.30 – 3.74) | 0.003 | 2.62(1.51 – 4.55) | 0.001 |
Multivariable model were adjusted for age, gender, race/ethnicity, BMI, cigarette smoking, diabetes mellitus, total cholesterol, HDL, triglycerides, systolic blood pressure, BP medication use, statin use, benzodiazepine use and current alcohol use.
Participants reporting physician-diagnosed sleep apnea compared to those not reporting sleep apnea.
Figure 3.
Association of physician diagnosed sleep apnea with incident cardiovascular event in stratified multivariable Cox proportional hazard regression model in MESA.
Figure 4.
Association of physician diagnosed sleep apnea and all-cause mortality in stratified multivariable Cox proportional hazard regression models in MESA.
All-cause mortality and incident CVD events (Non habitual snorers vs. Habitual snorers)
Non- habitual snorers (all participants except those with habitual snoring, n=3886) and habitual snorers (n=1452) had similar incident cardiovascular events in both the univariate [hazard ratio (95%CI); 0.85(0.65 – 1.11), p=0.23] and multivariable analysis [hazard ratio (95%CI); 0.91(0.69–1.20), p=0.49]. Habitual snoring was not associated with increased CV risk of any of the components of our composite outcome (Table 4). Non-habitual snorers and habitual snorers also had similar risk of death in both the univariate [hazard ratio (95%CI); 0.87(0.62–1.23), p=0.43] and multivariable analysis [hazard ratio (95%CI); 1.17(0.81 –1.68), p=0.40] (Table 4 and Figure 4).
Table 4.
Association of Habitual Snoring (Habitual snoring vs. No Habitual snoring) with Cardiovascular Outcomes and All-cause Mortality in MESA*
| Outcome | # of events | Univariate Hazard ratio(95%CI) | P value | Multivariate Hazard ratio(95%CI) | P value |
|---|---|---|---|---|---|
| Composite | 310 | 0.85(0.65 – 1.11) | 0.23 | 0.91(0.69–1.20) | 0.49 |
| Hard CHD | 128 | 0.77(0.51– 1.18) | 0.23 | 0.89(0.57– 1.40) | 0.63 |
| MI | 107 | 0.75(0.47– 1.19) | 0.22 | 0.87(0.53– 1.42) | 0.58 |
| Angina | 180 | 0.89(0.63–1.26) | 0.51 | 0.96(0.63–1.32 | 0.60 |
| Stroke | 76 | 1.09(0.66–1.79) | 0.74 | 1.24(0.73–2.13) | 0.42 |
| All-cause mortality | 189 | 0.87(0.62–1.23) | 0.43 | 1.17(0.81 –1.68) | 0.40 |
Multivariable model were adjusted for age, gender, race/ethnicity, BMI, cigarette smoking, diabetes mellitus, total cholesterol, HDL, triglycerides, systolic blood pressure, BP medication use, statin use, benzodiazepine use and current alcohol use.
Participants reporting snoring >3–5 days/ week compared to those reporting no snoring or snoring < 3–5days/week.
In all the above multivariable models the addition of aspirin use and hypoglycemic medication use did not significantly change either the point estimates or the confidence intervals.
Discussion
This study assessed the relationship between PDSA and habitual snoring with incident cardiovascular events and all- cause mortality in a large multi-ethnic cohort. Even after adjusting for multiple potential confounders, we observed that PDSA but not habitual snoring is significantly associated with incident cardiovascular events and all-cause mortality. The current study provides further support for sleep apnea as a novel CVD risk factor and the need for clinical trials to define the role of sleep apnea screening and treatment in cardiovascular disease reduction. Although physician-diagnosed sleep apnea and self-reported snoring each provide a limited amount of information on sleep apnea severity, our analyses indicate that PDSA is potentially more informative than snoring in predicting adverse cardiovascular health outcomes. There is likely substantial misclassification in the use of reports of physician-diagnosed sleep apnea. In fact, it is estimated that as many as 85% of individuals with sleep apnea are undiagnosed and untreated (25). It is likely that those who reported this diagnosis were referred for relatively symptomatic and more severe disease. Thus, PDSA may represent a less sensitive but relatively specific marker of moderate to severe sleep apnea.
While there have been consistent associations between sleep apnea and cardiovascular risk factors/ cardiovascular disease (7), data on the association between snoring and cardiovascular risk/ cardiovascular diseases have been rather weak and inconsistent other than reports relating snoring to stroke (12, 13, 26). Our findings show that while the majority of those with PDSA reported snoring (98%), snoring history alone is likely not of sufficient specificity to identify individuals with the most severe overnight physiological stresses who may be at greatest risk for adverse cardiovascular disease and mortality. This observation also is consistent with data from the Sleep Heart Health Study that showed that the highest risk for incident cardiovascular disease, stroke, and mortality is in individuals with moderate to severe sleep apnea (18–20). It should be noted that we analyzed a composite outcome, which only included 76 cases of stroke. Given a postulated mechanism whereby snoring per se may cause carotid vessel trauma (27), it is possible that our analyses were under-powered to detect a unique association between snoring and cerebrovascular events.
We also explored the consistency of associations in men and women, and across race/ethnic groups. Of interest, the point estimates for the relationship between PDSA and incident CVD were similar in men and women but did not reach statistical significance in women due to the lower number of reported PDSA and fewer events. This contrasts with the Sleep Heart Health Study findings which showed much stronger point estimates in men than in women when characterizing exposure using the apnea hypopnea index. This important difference may be explained by the younger age of the women or by the greater predictiveness of a simple report of physician diagnosed sleep apnea compared to an apnea hypopnea index measured at a single time point.
We also explored the consistency of associations across 4 racial/ethnic groups. Although there was limited power to detect subgroup differences, overall similar patterns were seen for Caucasians, Chinese, African Americans and Hispanics, with a suggestion that the highest relative risks for death occurred in Hispanics. Further research of larger samples of women and ethnic minorities are needed to better understand whether population differences exist in vulnerability to sleep apnea-associated CVD.
The biological plausibility of our findings has been well described and includes deleterious effect of sleep apnea and associated hypoxemia on the cardiovascular system through activation of the sympathetic nervous system, oxidative stress, endothelial dysfunction, insulin resistance and dyslipidemia. These mechanisms, in turn, increases risk for hypertension, diabetes mellitus and increased intima media thickness (5, 8, 28, 29). The results of the current analysis may underestimate the total impact of PDSA on CVD since we adjusted for many of the intermediate risk factors that may be in the causal pathway linking sleep apnea to cardiovascular disease.
The strengths of our study included the large multi ethnic nature of our cohort, rigorous adjudication procedures, availability of cardiovascular risk factors that were included in our models and relatively long follow up period.
Our study had several limitations, notably the lack of objective data on snoring and/sleep apnea severity and the use of a self-reported measure of PDSA. Despite this limitation, our findings were consistent with studies such as the Sleep Heart Health study which used objective diagnosis of sleep apnea. We also had limited power to test for subgroup differences. Although we adjusted for several potential confounders, it is possible that unmeasured residual confounding occurred. In particular, it is possible that individuals reporting a PDSA are individuals who seek medical attention for other medical conditions that we did not assess.
In summary, this study of a large multiethnic cohort showed that PDSA was associated with increased risk for incident CV events and all- cause mortality, with estimates similar across gender and race/ethnic groups. Habitual snoring was not independently associated with either incident CV events or all- cause mortality.
Acknowledgement
The authors would like to thank the investigators, the staff, and the participants of the MESA study for their valuable contributions. This research was supported by contracts N01- HC-95159 through N01-HC-95165 and N01-HC-95167 and grant NHLBI T32 HL076132. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ. Sleep apnea and cardiovascular disease: An American heart association/American college of cardiology foundation scientific statement from the American heart association council for high blood pressure research professional education committee, council on clinical cardiology, stroke council, and council on cardiovascular nursing in collaboration with the national heart, lung, and blood institute national center on sleep disorders research (national institutes of health) J.Am.Coll.Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 3.Dyken ME, Im KB. Obstructive sleep apnea and stroke. Chest. 2009;136:1668–1677. doi: 10.1378/chest.08-1512. [DOI] [PubMed] [Google Scholar]
- 4.McNicholas WT, Bonsigore MR. Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. EurRespir J. 2007;29:156–178. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 5.Kapa S, SertKuniyoshi FH, Somers VK. Sleep apnea and hypertension:interactions and implications for management. Hypertension. 2008;51:605–608. doi: 10.1161/HYPERTENSIONAHA.106.076190. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Jimenez F, SertKuniyoshi FHS, Gami A, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease: part II: contemporary reviews in sleep medicine. Chest. 2008;133:793–804. doi: 10.1378/chest.07-0800. [DOI] [PubMed] [Google Scholar]
- 7.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea:implications for cardiac and vascular disease. JAMA. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 8.Veasey SC. Molecular and physiologic basis of obstructive sleep apnea. Clin Chest Med. 2003;24:179–193. doi: 10.1016/s0272-5231(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 9.Quan SF, Gersh BJ. Cardiovascular consequences of sleep-disordered breathing: past, present and future: report of a workshop from the National Center on Sleep Disorders Research and the National Heart,Lung, and Blood Institute. Circulation. 2004;109:951–957. doi: 10.1161/01.CIR.0000118216.84358.22. [DOI] [PubMed] [Google Scholar]
- 10.Caples SM, Garcia-Touchard A, Somers VK. Sleep-disordered breathing and cardiovascular risk. Sleep. 2007;30:291–303. doi: 10.1093/sleep/30.3.291. [DOI] [PubMed] [Google Scholar]
- 11.Bradley TD, Floras JS. Sleep apnea and heart failure: part I: obstructive sleep apnea. Circulation. 2003;107:1671–1678. doi: 10.1161/01.CIR.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- 12.Palomake H. Snoring and the risk of ischemic brain infarction. Stroke. 1991;22:1021–1025. doi: 10.1161/01.str.22.8.1021. [DOI] [PubMed] [Google Scholar]
- 13.Partinen M, Palomaki H. Snoring and cerebral infarction. Lancet. 1985;2:1325–1326. doi: 10.1016/s0140-6736(85)92625-x. [DOI] [PubMed] [Google Scholar]
- 14.Arzt M, Young T, Finn L, et al. Association of sleep disordered breathing and the occurrence of stroke. Am J RespirCrit Care Med. 2005;172:1147–1151. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaggi KH, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;19:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 16.Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–2321. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 17.Valham F, Mooe T, Rabben T, et al. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation. 2008;118:955–960. doi: 10.1161/CIRCULATIONAHA.108.783290. [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010 Jul 27;122(4):352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010 Jul 15;182(2):269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009 Aug;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am J RespirCrit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapur VK. Obstructive sleep apnea: diagnosis, epidemiology and economics. Respir care. 2010;55:1155–67. [PubMed] [Google Scholar]
- 26.Palomake H, Partinen M, Juvela S, et al. Snoring as a risk factor for sleep-related brain infarction. Stroke. 1989;10:1311–1315. doi: 10.1161/01.str.20.10.1311. [DOI] [PubMed] [Google Scholar]
- 27.Hedner J. Vascular function in OSA. Sleep. 1996;10:s231–7. doi: 10.1093/sleep/19.suppl_10.s213. [DOI] [PubMed] [Google Scholar]
- 28.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemic. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 29.Drager LF, Bortolotto LA, Maki-Nunes C, Trombetta IC, Alves MJ, Fraga RF, Negrao CE, Krieger EM, Lorenzi-Filho G. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis. 2010;208:490–495. doi: 10.1016/j.atherosclerosis.2009.08.016. [DOI] [PubMed] [Google Scholar]