Abstract
Background. Nonalcoholic fatty liver disease (NAFLD) is one of the metabolic disorders presented in liver. The relationship between severity of NAFLD and coronary atherosclerotic burden remains largely unknown. Methods and Materials. We analyzed subjects undergoing coronary calcium score evaluation by computed tomography (MDCT) and fatty liver assessment using abdominal ultrasonography. Framingham risk score (FRS) and metabolic risk score (MRS) were obtained in all subjects. A graded, semiquantitative score was established to quantify the severity of NAFLD. Multivariate logistic regression analysis was used to depict the association between NAFLD and calcium score. Results. Of all, 342 participants (female: 22.5%, mean age: 48.7 ± 7.0 years) met the sufficient information rendering detailed analysis. The severity of NAFLD was positively associated with MRS (X 2 = 6.12, trend P < 0.001) and FRS (X 2 = 5.88, trend P < 0.001). After multivariable adjustment for clinical variables and life styles, the existence of moderate to severe NAFLD was independently associated with abnormal calcium score (P < 0.05). Conclusion. The severity of NAFLD correlated well with metabolic abnormality and was independently predict coronary calcification beyond clinical factors. Our data suggests that NAFLD based on ultrasonogram could positively reflect the burden of coronary calcification.
1. Introduction
Atherosclerosis is the most common vascular pathology in patients with cardiovascular events, which leads to mortality in developed countries. There are various tools to evaluate the diseased populations for prediction, diagnosis, and risk stratification. Among asymptomatic patients, the predictive and prognostic values of calcium scores via electron beam computed tomography (EBCT) were proved in previous studies, either as an addition to the FRS or to C-reactive protein [1], or used alone [2]. Because coronary calcification is not uncommon in patients with myocardial ischemia, multidetected computed tomography (MDCT) has become a more useful modality for coronary heart disease evaluation. On the other hand, evolving evidence showed that NAFLD may actually represent metabolic syndrome in liver [3]. The existence and severity of NAFLD by liver biopsy as a gold standard also proved to be association with MRS [4, 5]. Recent studies demonstrated that NAFLD could further predict cardiovascular diseases or even involved the pathophysiologic process of atherosclerosis [4].
In our study, we sought to define the correlation between the extent of NAFLD, cardiovascular risks, and MDCT-acquired calcium score. We also tried to examine the role of NAFLD beyond other clinical factors in the prediction and identification of coronary calcification.
2. Materials and Methods
2.1. Study Subjects
We subsequently enrolled 342 non-alcoholism from outpatient clinics or subjects underwent health evaluation who had both MDCT examination for cardiovascular risk stratification as well as abdominal ultrasonography for the detection of fatty liver disease. This study was proved by the ethics committee of Mackay Memorial Hospital, Taipei, Taiwan (09MMHIS038), in accordance with the Declaration of Helsinki.
2.2. Data Collection and Laboratory Parameters
Anthropometric measurements including body height, body weight, waist circumference, and blood pressures at rest were taken by a registered technician who was blinded to the other test results. Body mass index (BMI) was derived from the ratio of weight to height squared. Body surface area (BSA) was calculated according to formula of DuBois. Review of medical history, physical examination, 12-lead electrocardiogram, and chest plain film were all performed by study physicians. High-sensitivity C-reactive protein (hs-CRP) levels were determined by a latex particle-enhanced immunoassay using Elecsys 2010 method (Roche, Mannheim, Germany). The other laboratory data, including a lipid profile and a renal function test, was obtained by a Hitachi 7170 Automatic Analyzer (Hitachi Corp. Hitachinaka Ibaraki, Japan). Immunoreactive insulin was measured by radioimmunoassay (PerkinElmer Automatic Gamma Counter 1470; PerkinElmer, Waltham, MA, USA). Insulin resistance was further defined as using the following homeostasis model equation: insulin resistance method (HOMA-IR) = fasting glucose (mmol/L) × fasting insulin (μU/mL)/22.5. HbA1c level was obtained by high-performance liquid chromatography (Bio-rad Variant II; Bio-Rad Laboratories, Hercules, CA, USA). Body fat composition percentage was assessed by using a commercialized foot-to-foot bioelectrical impedance scale (Tanita Inc., Tokyo, Japan, models TBF 410GS).
2.3. Abdominal Ultrasonography for NAFLD Grading
Abdominal ultrasonography is a validated tool to diagnose fatty liver rather than liver biopsy. The presence of fatty liver disease was detected using abdominal ultrasonography done by an experienced gastroenterologist who has no reference of the participants' other data. Three levels adopted for evaluating the severity of fatty liver was based on 4 basic techniques (hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring) as described from previous studies [6]. Right kidney echogenicity was used for the contrast of liver parenchyma echogenicity. It is normal with the same kidney cortex and liver parenchyma echogenicity. The severity of NAFLD is graded as the following: mild: minimal diffuse increase in liver brightness, but diaphragm and intrahepatic vessel contours seem normal; moderate: medium grade diffuse increase in liver brightness and mild attenuation of diaphragm and intrahepatic vessels; severe: apparent increase in echogenicity. Under the aware that fatty liver interpretation by using ultrasonic assessment may be subjective, we adopted a more strict methodology in categorizing subjects with at least moderate degree fatty liver disease as significant fatty liver disease in this study.
2.4. Coronary Calcium Score Measurement
Coronary calcification of coronary arteries was quantified by using a dedicated offline workstation (Aquarius 3D Workstation, TeraRecon, San Mateo, CA, USA). A coronary calcified lesion was defined as an area with a density >130 HU and covering at least 6 pixels. Scanning was performed by a 16-slice MDCT scanner (Sensation 16, Siemens Medical Solutions, Forchheim, Germany) with 16 × 0.75 mm collimation, rotation time 420 ms, and tube voltage of 120 kV in one breath hold. From the raw data, the images were reconstructed with standard kernel in 3 mm thick axial, nonoverlapping slices and 25 cm field of view. The Agatston score method was applied by multiplying each lesion (area) by a weighted CT attenuation score in the lesion.
2.5. Definition of Framingham Risk Score
The Framingham risk score (FRS) is designed to estimate 10-year risk of developing coronary heart disease (myocardial infarction and coronary death) in adults aged 20 and older who do not have heart disease or diabetes. This tool is based on the score (Adult Treatment Panel III, ATP-III) deriving from the information of age, gender, cholesterol dysregulation, (pre) hypertension, and smoking [7].
2.6. Definition of Metabolic Risk Score
Similar to ATP-III criteria, abnormal metabolic components defined by the Bureau of Health Promotion, Department of Health, Taiwan were [8]: (1) increased waist circumference of at least 90 cm in men and of at least 80 cm in women; (2) abnormally elevated serum triglycerides (TG) of at least 150 mg /dL; (3) lower serum high-density lipoprotein (HDL) cholesterol of less than 40 mg /dL for men and less than 50 mg /dL for women; (4) higher fasting blood glucose level greater than 110 mg /dL; (5) elevated systolic blood pressure of at least 130 mmHg and/or diastolic blood pressure of at least 85 mmHg , or undergoing hypertension treatment. The scoring system was calculated and presented as the numbers of abnormal items meeting the criteria, with score 0 for the absence and score 5 for the presence of all 5 abnormal metabolic components. Metabolic risk score (MRS) of 3 or more met the definition of metabolic syndrome.
2.7. Statistics
All data was presented as mean ± SD. Continuous data between groups with and without abnormal calcium score deposition were compared by using Mann-Whitney test with abnormal distribution and by Student t-test if in normal distribution. Categorical variables were compared by Chi square and Fisher Exact tests as appropriate. Nonparametric rank sum test was used to test the graded change of cardiovascular risks estimated by using ATP III or Framingham system over different fatty liver status. Multivariable regression model was used based on Framingham system (FRS) and ATP III (MRS) with life styles or other variables not included in the Framingham system (FRS) or ATP III (MRS) model sequentially entered to identify the independent value in predicting fatty liver disease. Receiving operative characteristic (ROC) curves were used to test the hypothesis that whether the existence of NAFLD helps provide incremental value in the detection of abnormal coronary calcium deposition beyond traditional cardiovascular risk systems. P value was set at two-tailed probability, and a P value less than 0.05 was considered statistically significant. All analysis was done by the software package STATA 8.2 (StataCorp, College Station, TX, USA).
3. Results
3.1. Patients Demographics and Baseline Characteristics
Of all 342 participants, 239 subjects (mean age 47 years, 27.6% female) did not have notable calcification of all coronary territories (Group I), whereas 103 subjects (mean age 52.6 years, 10% female) were with obvious coronary calcification (Group II). Main demographic data and baseline characteristics are illustrated in Table 1. Subjects with abnormal calcium scores (Group II) were older with more male gender. In addition, they tended to have larger BMI, waist circumference, and higher blood pressure. Baseline biochemistry did not show significant differences between these two groups except a trend toward higher serum glucose level. MRS and FRS were also higher in group II. The prevalence of abnormal electrocardiographic patterns like ischemia or hypertrophy was higher in group II. Subjects in group II were also observed to have higher prevalence of hypertension, diabetes or hyperlipidemia medical history when compared with group I.
Table 1.
Baseline demographic data and clinical information in our study.
| Calcium score zero (N = 239) | Calcium score abnormal (N = 103) | P value | |
|---|---|---|---|
| Age, years | 47.0 (6.4) | 52.6 (6.6) | <0.001 |
| Gender, female % | 66 (27.6) | 11 (10.6) | |
| Height, cm | 165.9 (7.5) | 167.7 (7.5) | 0.09 |
| Weight, kg | 65.9 (11.3) | 68.9 (10.1) | 0.008 |
| BMI, Kg/m2 | 23.8 (3.1) | 24.4 (2.7) | 0.013 |
| Waist, cm | 81.4 (9.1) | 85.0 (7.9) | <0.001 |
| Waist to hip ratio | 0.88 (0.07) | 0.91 (0.06) | <0.001 |
| SBP, mmHg | 116.7 (14.9) | 125.1 (15.1) | 0.003 |
| DBP, mmHg | 74.5 (10.2) | 77.9 (9.7) | 0.002 |
| Biochemistry | |||
| Sugar (AC), mg/dL | 93.3 (21.2) | 102.0 (20.9) | 0.086 |
| Sugar (PC), mg/dL | 105.9 (31.6) | 118.5 (44.4) | 0.082 |
| HbA1c, % | 5.8 (0.6) | 6.0 (0.6) | 0.32 |
| Insulin, U/mL | 6.25 (4.24) | 6.91 (3.75) | 0.046 |
| HOMA-IR | 1.55 (1.25) | 1.76 (1.06) | 0.016 |
| Cholesterol, mg/dL | 191.1 (32.9) | 196.1 (35.1) | 0.23 |
| LDL, mg/dL | 128.0 (31.3) | 125.8 (31.7) | 0.56 |
| HDL, mg/dL | 53.0 (13.7) | 49.1 (12.8) | 0.02 |
| TG, mg/dL | 141.9 (103) | 145.8 (85.2) | 0.74 |
| BUN, mg/dL | 11.8 (3.2) | 12.6 (3.7) | 0.32 |
| Creatinine, mg/dL | 0.91 (0.18) | 0.97 (0.18) | 0.39 |
| Uric Acid, mg/dL | 5.6 (1.4) | 6.1 (1.5) | 0.003 |
| Homocystine, mg/dL | 7.3 (2.1) | 8.4 (2.3) | 0.03 |
| Hs-CRP, mg/dL | 0.2 (0.35) | 0.32 (0.7) | 0.043 |
| ECG pattern | |||
| ECG (LVH or myocardial ischemia) | 41 (7.5) | 28 (13.2) | 0.016 |
| ECG (old infarct) | 5 (1) | 9 (4.2) | 0.002 |
| Medical history | |||
| Smoking | 84 (24.9) | 42 (29.4) | 0.311 |
| HTN history | 41 (12) | 35 (23.7) | 0.001 |
| DM history | 11 (3.2) | 14 (9.5) | 0.004 |
| Hyperlipidemia | 17 (5.6) | 13 (9.6) | 0.039 |
| CVD | 12 (3.9) | 12 (8.9) | 0.033 |
| Family Hx | |||
| Family Hx HTN | 147 (43) | 62 (41.9) | 0.823 |
| Family Hx DM | 88 (25.8) | 42 (28.4) | 0.554 |
| Family Hx Stroke | 49 (14.3) | 20 (13.5) | 0.812 |
| Family Hx CVD | 57 (16.7) | 30 (20.3) | 0.345 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; sugar (AC): fasting glucose; sugar (PC): postprandial glucose; HOMA-IR: insulin resistance; ECG: electrocardiography; LVH: left ventricular hypertrophy; HTN: hypertension; DM: diabetes; NAFLD: nonalcoholic fatty liver disease; CVD: cardiovascular disease: Hx: history.
3.2. The Independent Predictive Value of NAFLD in Different Models
In Figure 1, we illustrated the relationship between coronary calcium score, cardiovascular risk scores, and the degree of fatty liver echogenicity. More severe fatty liver degree was observed to be associated with higher calcium scores, higher FRS and MRS (trend P < 0.001). We further examined the independent value of NAFLD in the prediction of abnormal coronary calcium scores by testing different clinical variables based on FRS or MRS separately into three different models (Table 2). In model 1, when other clinical variables including smoking and exercise based on the relatively insufficiency of individual scores were entered into regression model, significant fatty liver disease independently identified coronary calcium existence in either FRS group or the MRS group. In model 2, when liver function tests were together in regression model, moderate to severe fatty liver disease was still independently associated in both groups. In model 3, when body fat composition was further entered in the regression model, the presence of fatty liver disease remained statistically significantly independent in the prediction of coronary calcium existence.
Figure 1.
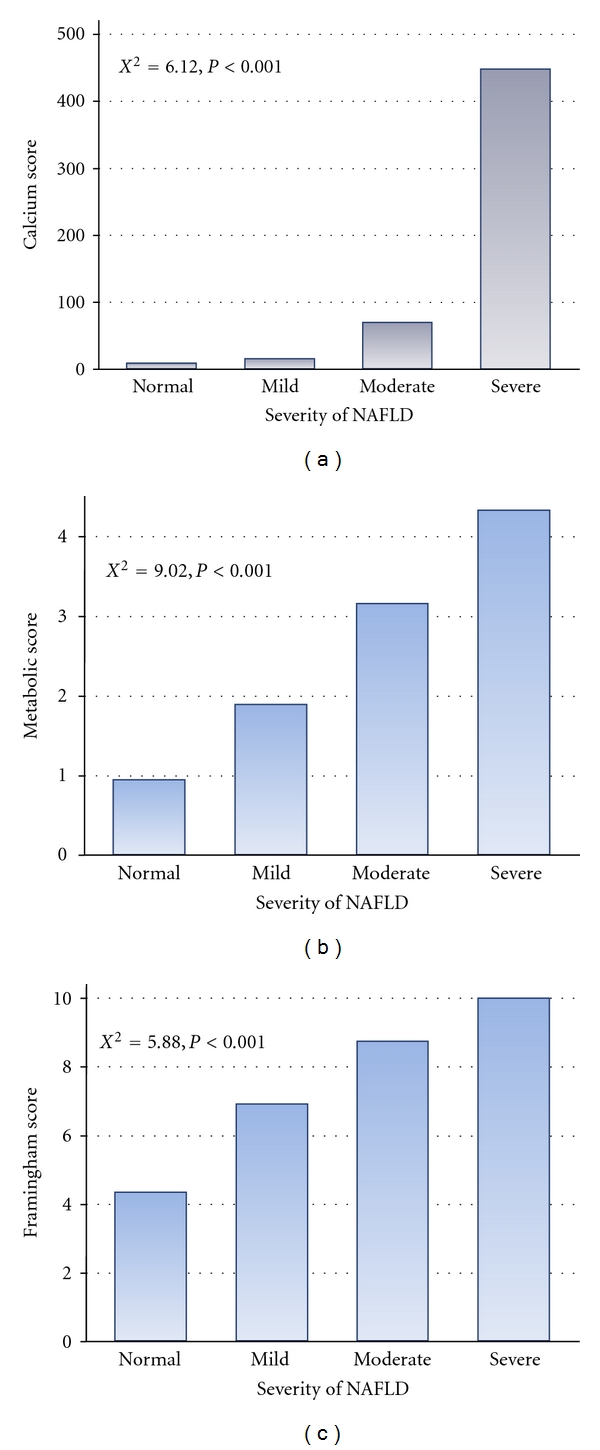
Estimated cardiovascular risk scores and coronary calcification categorized by the severity of nonalcoholic fatty liver disease. More severe fatty liver disease was associated with higher cardiovascular risk scores by either metabolic or Framingham risk scores and coronary calcium scores.
Table 2.
Multivariate logistic regression models in predicting coronary calcium deposition.
| Variables | OR | SE | Z | P | CI 95% | |
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| FRS | 1.16 | 0.05 | 3.09 | 0.002 | 1.054599 | 1.268832 |
| BMI | 0.93 | 0.06 | −1.23 | 0.218 | .8193498 | 1.04653 |
| Sugar (AC) | 0.99 | 0.01 | −0.5 | 0.618 | .9744172 | 1.015514 |
| Drinking | 0.99 | 0.39 | −0.02 | 0.981 | .4557979 | 2.153525 |
| Exercise | 0.76 | 0.63 | −0.33 | 0.742 | .1488091 | 3.882643 |
| Fatty liver (moderate to severe) | 4.47 | 2.66 | 2.52 | 0.012 | 1.393377 | 14.33824 |
| DM history | 1.92 | 2.15 | 0.58 | 0.563 | .2114827 | 17.35303 |
|
| ||||||
| MRS | 1.1 | 0.15 | 0.67 | 0.506 | .836501 | 1.436341 |
| Age | 1.16 | 0.03 | 5.16 | <0.001 | 1.097237 | 1.229447 |
| Sex | 4.84 | 2.62 | 2.92 | 0.004 | 1.677794 | 13.97886 |
| Drinking | 0.7 | 0.28 | −0.88 | 0.381 | .3176925 | 1.549465 |
| Smoking | 0.8 | 0.36 | −0.49 | 0.627 | .3329831 | 1.94089 |
| Exercise | 0.32 | 0.3 | −1.21 | 0.225 | .0488721 | 2.032724 |
| Fatty liver (moderate to severe) | 3.83 | 2.13 | 2.42 | 0.016 | 1.288897 | 11.39297 |
|
| ||||||
| Model 2 | ||||||
| FRS | 1.15 | 0.06 | 2.87 | 0.004 | 1.045555 | 1.26785 |
| BMI | 0.92 | 0.06 | −1.18 | 0.24 | .811891 | 1.053551 |
| Sugar (AC) | 0.99 | 0.01 | −0.71 | 0.478 | .9715331 | 1.013632 |
| Drinking | 0.77 | 0.33 | −0.61 | 0.544 | .3299596 | 1.794328 |
| Exercise | 0.78 | 0.67 | −0.29 | 0.772 | .145282 | 4.191667 |
| Fatty liver (moderate to severe) | 7.36 | 5.35 | 2.75 | 0.006 | 1.769904 | 30.56831 |
| DM history | 1.71 | 2 | 0.46 | 0.649 | .1713221 | 16.99859 |
| Viral hepatitis carrier | 1.14 | 0.65 | 0.23 | 0.82 | .3714342 | 3.491325 |
| GPT | 0.99 | 0.01 | −0.92 | 0.359 | .9695872 | 1.011263 |
| rGT | 1.02 | 0.01 | 1.1 | 0.272 | .9879836 | 1.043843 |
|
| ||||||
| MRS | 1.06 | 0.16 | 0.37 | 0.71 | .7835745 | 1.43072 |
| Age | 1.16 | 0.03 | 4.93 | <0.001 | 1.092158 | 1.226692 |
| Sex | 5.19 | 2.9 | 2.95 | 0.003 | 1.737443 | 15.52867 |
| Drinking | 0.58 | 0.25 | −1.26 | 0.207 | .2466988 | 1.353505 |
| Smoking | 0.8 | 0.38 | −0.47 | 0.641 | 3155051 | 2.035192 |
| Exercise | 0.29 | 0.27 | −1.31 | 0.19 | .0437895 | 1.8624 |
| Fatty liver (moderate to severe) | 7.43 | 4.95 | 3.01 | 0.003 | 2.014803 | 27.42179 |
| Viral hepatitis carrier | 1.34 | 0.77 | 0.51 | 0.613 | .4322222 | 4.144012 |
| GPT | 0.98 | 0.01 | −1.41 | 0.159 | .9619023 | 1.006359 |
| rGT | 1.01 | 0.01 | 1.01 | 0.312 | .9861562 | 1.044676 |
|
| ||||||
| Model 3 | ||||||
| FRS | 1.14 | 0.05 | 2.78 | 0.005 | 1.040021 | 1.253657 |
| BMI | 1 | 0.08 | 0.1 | 0.921 | .8635472 | 1.176196 |
| Sugar (AC) | 0.99 | 0.01 | −0.67 | 0.505 | .9722974 | 1.013941 |
| Drinking | 0.73 | 0.32 | −0.74 | 0.461 | .3102782 | 1.70035 |
| Exercise | 0.4 | 0.39 | −0.94 | 0.347 | .0574678 | 2.726738 |
| Fatty liver (moderate to severe) | 6.77 | 4.95 | 2.62 | 0.009 | 1.61448 | 28.35676 |
| DM history | 1.46 | 1.68 | 0.33 | 0.743 | .1532743 | 13.87366 |
| Viral hepatitis carrier | 1.31 | 0.77 | 0.47 | 0.641 | .418516 | 4.118456 |
| GPT | 0.99 | 0.01 | −0.87 | 0.385 | .9708703 | 1.011461 |
| rGT | 1.02 | 0.01 | 1.03 | 0.302 | .9870173 | 1.043093 |
| Body fat | 0.92 | 0.04 | −1.94 | 0.053 | .8456979 | 1.000979 |
|
| ||||||
| MRS | 1.12 | 0.18 | 0.72 | 0.473 | .8203864 | 1.532548 |
| Age | 1.16 | 0.03 | 4.97 | <0.001 | 1.092734 | 1.226381 |
| Sex | 3.1 | 2 | 1.76 | 0.078 | .8790155 | 10.95577 |
| Drinking | 0.58 | 0.25 | −1.25 | 0.211 | .2463022 | 1.363246 |
| Smoking | 0.83 | 0.4 | −0.38 | 0.702 | .3256642 | 2.129526 |
| Exercise | 0.18 | 0.18 | −1.75 | 0.08 | .0260914 | 1.227528 |
| Fatty liver (moderate to severe) | 8.58 | 6 | 3.07 | 0.002 | 2.176022 | 33.82722 |
| Viral hepatitis carrier | 1.44 | 0.84 | 0.63 | 0.528 | .4627544 | 4.488757 |
| GPT | 0.99 | 0.01 | −1.17 | 0.243 | .9656288 | 1.008901 |
| rGT | 1.02 | 0.02 | 1.04 | 0.298 | .9864816 | 1.045414 |
| Body fat | 0.94 | 0.04 | −1.57 | 0.117 | .8679529 | 1.015836 |
FRS: Framingham risk score; MRS: metabolic risk score; γ-GT: γ-glutamyl transpeptidase; GPT: alanine aminotransferase. Other abbreviations were listed in Table 1.
3.3. The Incremental Value of NAFLD beyond Traditional Cardiovascular Risks in the Prediction of Coronary Calcium Scores
In Figure 2, we tested the hypothesis that whether the presence of fatty liver disease could further expand the area under curve (AUC) for the discrimination of coronary artery calcification from normal subjects meaningfully. While FRS and MRS alone have an AUC of 0.67 and 0.64, the addition of NAFLD further significantly expanded the AUC to 0.71 and 0.69, respectively (c-statistics < 0.05).
Figure 2.
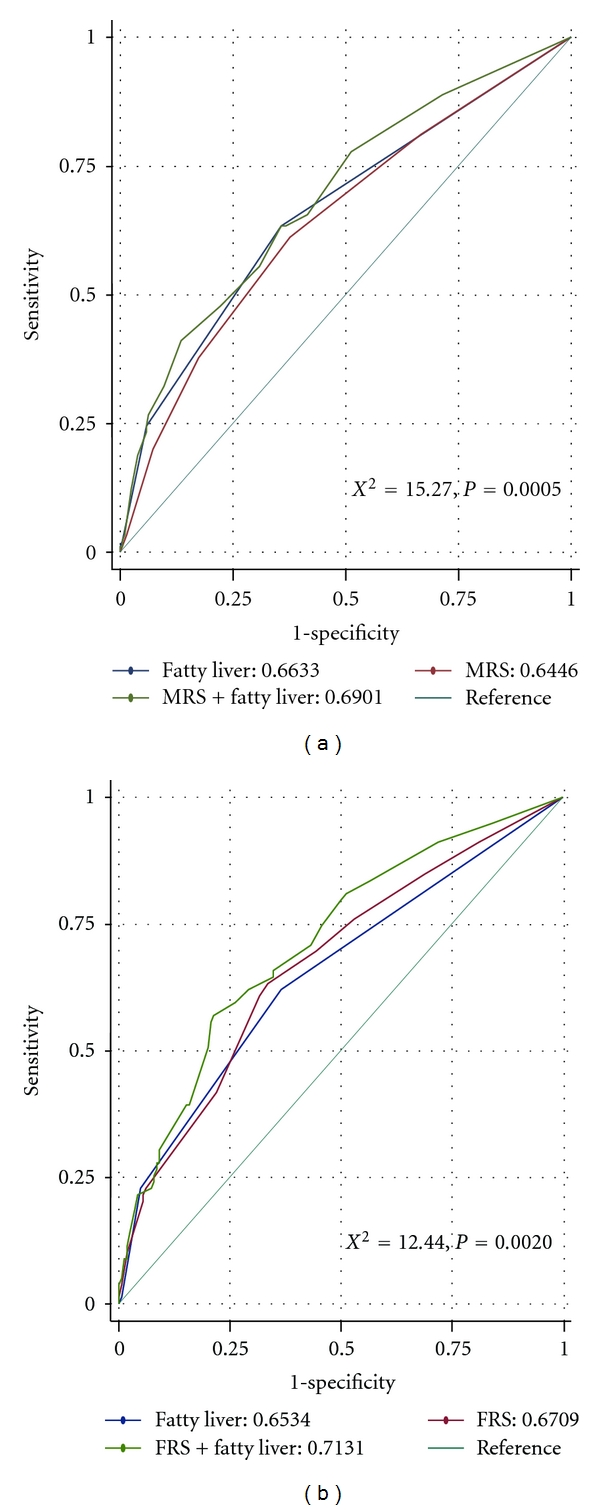
Receiver operating characteristic curves demonstrated by both metabolic or Framingham risk scores and superimposed by fatty liver disease in predicting coronary calcium deposition. When presence of fatty liver disease was added on traditional cardiovascular risk scores, there was significant increase in the area under curve.
4. Discussion
In this study, anthropometric data from asymptomatic people without known cardiovascular disease demonstrated a significantly positive correlation between coronary calcium score and traditional anthropometrics, blood pressure, insulin resistance, and systemic inflammation marker in terms of hs-CRP. Both estimated cardiovascular risk scores including FRS and MRS for the prediction of coronary calcification in our study by utilizing ROC analysis were similar to that in Wanamethee's report [9]. However, unique to this study, we found that more severe fatty liver disease, when assessed by abdominal ultrasonography, may serve as an independent factor even after adjustment of clinical variables and estimated cardiovascular risk scores. Furthermore, we also demonstrated that the presence of more severe degree fatty liver disease added incremental value beyond such traditional cardiovascular risks in the prediction of coronary artery calcification.
Atherosclerosis, conceptualized as a convergence of bone biology with vascular inflammatory pathobiology [10], can recently be assessed and quantified by extent of coronary artery calcification in terms of coronary calcification score when EBCT was clinically introduced and started to serve as a feasible surrogate marker [11–13]. Higher calcium scores are seen in most patients with myocardial ischemia, either symptomatic or silent [2, 14]. And the result of coronary calcium score derived from EBCT also predicts stress-related ischemia on stress nuclear images [14] and future cardiovascular events [2].
Metabolic syndrome as a medical disorder complex with a central key factor of obesity accompanied with insulin resistance has been proved as an antecedent of types of diabetes mellitus and several cardiovascular diseases [9, 15–20]. Another frequently used cardiovascular risk estimate such as Framingham score is also a widely accepted scoring system, using age, smoking, HDL, BP, and cholesterol instead of triglyceride, in predicting cardiovascular risks [1, 9]. The ATP III had suggested usage of both the metabolic risk factors plus Framingham score in determining the risk of cardiovascular events and targeting treatment goal of LDL. It is thus not surprising that NAFLD, defined as excess fat accumulation in the fat-laden hepatocytes by light microscopy which covers a broad clinical spectrum of liver diseases, be viewed as a component or consequence of metabolic syndrome [21, 22]. However, this “gold standard” is not clinically feasible in daily practice. In the recent years, liver ultrasound has emerged as the most common and simplest one of the alternative tools in NAFLD diagnosis [23].
In contrast to Caucasian, the Asian populations have shown higher prevalence of nonalcoholic steatohepatitis than alcoholic hepatitis with nearly 33% of NAFLD meet the complete diagnosis of metabolic syndrome [24]. In this regard, NAFLD has thus been deemed a hepatic representation of metabolic syndrome [3].
Recent studies have demonstrated that NAFLD patients had developed subclinical atherosclerosis when compared to nonsteatosis individuals. Further, cardiovascular disease is the second most common cause of death in NAFLD patients [4]. More importantly, subjects with known steatosis are associated with abnormal carotid intima-media [25], more vulnerable coronary plaques by MDCT [26], higher serum markers of inflammation [27], worse endothelial function, increased myocardial insulin resistance [28], decreased adiponectin concentrations, and abnormal lipoprotein metabolism [29–31].
Ethnic differences in MESA study, such as higher percentage of coronary calcification in Chinese than Hispanic and black groups, was suggestive of unknown mechanism influencing cardiovascular diseases [32]. So far, it remains inconclusive and controversial regarding the true causal relationship between NAFLD and cardiovascular diseases. Some studies had ever described that NAFLD is less likely a direct mediator of cardiovascular disease but an “epiphenomenon" [33]; however, our study reported the independent role of NAFLD in predicting coronary calcification beyond traditional cardiovascular scores. This finding may have an impact on cardiovascular risk stratification or even disease process evaluation. More severe form of NAFLD could thus be viewed as an independent clinical marker for higher cardiovascular risks that may benefit from a more aggressive approach. It also deserves more efforts to work out the influence of liver fat intervention on cardiovascular diseases compared to prior reports [34, 35].
5. Conclusion
The severity of NAFLD not only links to metabolic derangement and traditional cardiovascular risks but also independently identifies the burden of coronary atherosclerosis in terms of coronary calcification. Simple NAFLD grading by liver ultrasonography may serve as a clinically useful tool beyond conventional risk factors in cardiovascular risk stratification.
References
- 1.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. Journal of the American Medical Association. 2004;291(2):210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 2.Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Archives of Internal Medicine. 2004;164(12):1285–1292. doi: 10.1001/archinte.164.12.1285. [DOI] [PubMed] [Google Scholar]
- 3.Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(1):27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 4.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191(2):235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Ryan MC, Wilson AM, Slavin J, Best JD, Jenkins AJ, Desmond PV. Associations between liver histology and severity of the metabolic syndrome in subject with nonalcoholic fatty liver disease. Diabetes Care. 2005;28(5):1222–1224. doi: 10.2337/diacare.28.5.1222. [DOI] [PubMed] [Google Scholar]
- 6.Osawa H, Mori Y. Sonographic diagnosis of fatty liver using a histogram technique that compares liver and renal cortical echo amplitudes. Journal of Clinical Ultrasound. 1996;24(1):25–29. doi: 10.1002/(SICI)1097-0096(199601)24:1<25::AID-JCU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Cleeman JI. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) Journal of the American Medical Association. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Lin CC, Liu CS, Lai MM, et al. Metabolic syndrome in a Taiwanese metropolitan adult population. BMC Public Health. 2007;7, article 239 doi: 10.1186/1471-2458-7-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham risk score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Archives of Internal Medicine. 2005;165(22):2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 10.Doherty TM, Asotra K, Fitzpatrick LA, et al. Calcification in atherosclerosis: bone biology and chronic inflammation at the arterial crossroads. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(20):11201–11206. doi: 10.1073/pnas.1932554100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blankenhorn DH, Stern D. Calcification of the coronary arteries. The American Journal of Roentgenology, Radium Therapy, and Nuclear Medicine. 1959;81(5):772–777. [PubMed] [Google Scholar]
- 12.Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. Journal of the American College of Cardiology. 1998;31(1):126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 13.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 14.He ZX, Hedrick TD, Pratt CM, et al. Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000;101(3):244–251. doi: 10.1161/01.cir.101.3.244. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. Journal of Clinical Endocrinology and Metabolism. 2007;92(2):399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- 17.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 18.Hong Y, Jin X, Mo J, et al. Metabolic syndrome, its preeminent clusters, incident coronary heart disease and all-cause mortality—results of prospective analysis for the Atherosclerosis Risk in Communities study. Journal of Internal Medicine. 2007;262(1):113–123. doi: 10.1111/j.1365-2796.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- 19.Daly CA, Hildebrandt P, Bertrand M, et al. Adverse prognosis associated with the metabolic syndrome in established coronary artery disease: data from the EUROPA trial. Heart. 2007;93(11):1406–1411. doi: 10.1136/hrt.2006.113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 21.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37(5):1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 22.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003;38(2):p. 536. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 23.Karcaaltincaba M, Akhan O. Imaging of hepatic steatosis and fatty sparing. European Journal of Radiology. 2007;61(1):33–43. doi: 10.1016/j.ejrad.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 25.Völzke H, Robinson DM, Kleine V, et al. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World Journal of Gastroenterology. 2005;11(12):1848–1853. doi: 10.3748/wjg.v11.i12.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akabame S, Hamaguchi M, Tomiyasu KI, et al. Evaluation of vulnerable coronary plaques and non-alcoholic fatty liver disease (NAFLD) by 64-detector multislice computed tomography (MSCT) Circulation Journal. 2008;72(4):618–625. doi: 10.1253/circj.72.618. [DOI] [PubMed] [Google Scholar]
- 27.Haukeland JW, Damås JK, Konopski Z, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. Journal of Hepatology. 2006;44(6):1167–1174. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Lautamäki R, Borra R, Iozzo P, et al. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. American Journal of Physiology—Endocrinology and Metabolism. 2006;291(2):E282–E290. doi: 10.1152/ajpendo.00604.2005. [DOI] [PubMed] [Google Scholar]
- 29.Bugianesi E, Pagotto U, Manini R, et al. Plasma Adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. Journal of Clinical Endocrinology and Metabolism. 2005;90(6):3498–3504. doi: 10.1210/jc.2004-2240. [DOI] [PubMed] [Google Scholar]
- 30.Adiels M, Taskinen MR, Packard C, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49(4):755–765. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 31.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. American Journal of Gastroenterology. 2004;99(8):1497–1502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 32.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111(10):1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 33.McKimmie RL, Daniel KR, Carr JJ, et al. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the diabetes heart study. American Journal of Gastroenterology. 2008;103(12):3029–3035. doi: 10.1111/j.1572-0241.2008.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viljanen APM, Iozzo P, Borra R, et al. Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. Journal of Clinical Endocrinology and Metabolism. 2009;94(1):50–55. doi: 10.1210/jc.2008-1689. [DOI] [PubMed] [Google Scholar]
- 35.Hannukainen JC, Borra R, Linderborg K, et al. Liver and pancreatic fat content and metabolism in healthy monozygotic twins with discordant physical activity. Journal of Hepatology. 2011;54(3):545–552. doi: 10.1016/j.jhep.2010.07.029. [DOI] [PubMed] [Google Scholar]


