Abstract
As the number of products containing nanomaterials increase, human exposure to nanoparticles (NPs) is unavoidable. Presently, few studies focus on the potential long-term consequences of developmental NP exposure. In this study, zebrafish embryos were acutely exposed to three gold NPs that possess functional groups with differing surface charge. Embryos were exposed to 50 μg/mL of 1.5 nm gold nanoparticles (AuNPs) possessing negatively charged 2-mercaptoethanesulfonic acid (MES) or neutral 2-(2-(2-mercaptoethoxy)ethoxy)ethanol (MEEE) ligands or 10 μg/mL of the AuNPs possessing positively charged trimethylammoniumethanethiol (TMAT). Both MES- and TMAT-AuNP exposed embryos exhibited hypo-locomotor activity, while those exposed to MEEE-AuNPs did not. A subset of embryos that were exposed to 1.5 nm MES- and TMAT-AuNPs during development from 6–120 hours post fertilization were raised to adulthood. Behavioral abnormalities and the number of survivors into adulthood were evaluated at 122 days post fertilization. We found that both treatments induced abnormal startle behavior following a tap stimulus. However, the MES-AuNPs exposed group also exhibited abnormal adult behavior in the light and had a lower survivorship into adulthood. This study demonstrates that acute, developmental exposure to 1.5 nm MES- and TMAT- AuNPs, two NPs differing only in the functional group, affects larval behavior, with behavioral effects persisting into adulthood.
Keywords: Acute exposure, gold nanoparticles, zebrafish, behavior, long-term effects
1. Introduction
With the number of nanotechnology-enabled products entering the consumer world increasing steadily (Scholars et al. 2011) exposure to nanoparticles (NPs) is inevitable. At present, there are many unknowns about how NPs affect human and environment health. Most studies have focused on understanding how acute nanoparticle exposure affects organ system development or causes mortality using both in vitro and in vivo models (Powers et al. 2010; Zhu et al. 2010; Gao et al. 2011). While these studies are critical and provide informative data, there are other areas of potential concern regarding nanoparticles that have yet to be explored, including potential long-term effects following short-term developmental exposure. Because many NPs are designed with metal cores, some of which are known neurotoxicants (e.g., lead), there is particular interest in the long-term effects of NP exposure on the nervous system. While some groups are beginning to address this data gap by either investigating the effects of NPs on brain development following in utero exposure (Gao et al. 2011) or behavior following long-term exposure as adults (Oszlanczi et al. 2011) few groups have coupled these endpoints and tested the behavior of adults following developmental exposure. Additionally, because metal cores are often the focus of toxicity studies, few groups have considered the potential effects of surface functional groups on nervous system development and function, even when the metal NP core is apparently benign (e.g., gold). The paucity of research in these two areas leaves a data gap regarding potential long-term effects of NP exposure on the developing nervous system. The first objective of this research was to investigate the short- and long-term behavioral effects of developmental NP exposure. The second objective was to investigate whether surface functional groups surrounding a benign metal core (i.e., gold) affect nervous system development and long-term behavior.
We conducted this study using the zebrafish model. The zebrafish is the most appropriate model for this type of study because embryos develop externally, all of their organs have formed within 5 days (Amacher 2001), and the fish mature to adulthood in just 3 months (Brand et al. 2002). Due to their small size and external development, an entire cohort of embryos can be exposed using just ~1 mg of nanoparticles, while the traditional rodent model would require gram quantities. Furthermore, due to their predictable swimming habits, both larval and adult behavior tests using locomotor activity as the endpoint can be quickly and efficiently conducted.
Based on a previous study (Truong et al. Submitted), we elected to use three types of gold nanoparticles with a core diameter of 1.5 nm and functionalized with either 2-mercaptoethanesulfonic acid (MES), trimethylammoniumethanethiol (TMAT) or 2-(2-(2-mercaptoethoxy)ethoxy)ethanol (MEEE). The primary difference between these three NPs is the charge of their surface functional groups. MES has a negative charge, TMAT has a positive charge, and MEEE has a neutral charge. These different surface charges greatly influence biological responses. Previously, we found that the positively charge surface functional group (TMAT) induced embryo lethality, the negatively charge (MES) induced sublethal toxic effects, while the neutral group (MEEE) caused no adverse biological response (Harper et al. 2011). Our goal was to identify whether acute exposure to MES- and TMAT-AuNPs during development would lead to deleterious effects that persist into adulthood. Specifically, we wanted to detect whether the charged surface functional groups on these gold nanoparticles would impact development of the central nervous system leading to abnormal behavior or survivorship in adulthood.
2. Materials and Methods
2.1. Preparation of TMAT-, MES- and MEEE-AuNPs
1.5 nm gold particles were synthesized using published procedures (Woehrle et al. 2005). All reagents were purchased from Sigma (St. Louis, MO, USA) or Strem (Newburyport, MA, USA) and used as received. Dichloromethane was distilled over phosphorous pentoxide, and chloroform was filtered through a plug of basic alumnia prior to use. 2-[2-(2-mercaptoethoxy)ethoxy]ethanol (Woehrle et al. 2004) and thiocholine (N,N,N-trimethylaminoethanethiol iodide) (Warner et al. 2003) were synthesized according to known procedures.
2.2. Nanoparticle Characterization and Analytical Procedures
Proton NMR spectra, UV-visible spectra and transmission electron microscopy (TEM) images were collected for each nanoparticle to confirm the size, composition and purity of the samples. Varian Unity Inova 300 MHz was used to collect proton NMR spectra in D2O. A Hewlett-Packard 8453 diode array instrument was used to obtain UV-visible spectra in a 1-cm quartz cuvette. TEM images were obtained on an FEI Titan at 300 kV using a Cs aberration corrector. Amine functionalized SMART grids (Dune Sciences, Inc.) were used for TEM imaging. SMART grids were soaked in the nanoparticle solution and then in nanopure water for 2 min each to produce samples for TEM with an even distribution of particles across the grid. Characterization data for the batch of MES-, TMAT- and MEEE-AuNPs used in this study can be found in a study conducted in parallel (Harper et al. 2011).
2.3. Zebrafish
Adult Tropical 5D strain of zebrafish (Danio rerio) were reared at Oregon State University - Sinnhuber Aquatic Research Laboratory (SARL). Fish were kept at standard laboratory conditions of 28 °C on a 14h light/10h dark photoperiod in fish water (FW) consisting of reverse osmosis water supplemented with a commercially available salt (Instant Ocean®).
2.4. Exposure Protocol
Adult zebrafish were group spawned, and their embryos were collected and staged according to Kimmel et al. (1995). At 4 h post fertilization (hpf), the embryonic chorion was enzymatically removed with pronase to increase bioavailability using protocols previously published (Truong et al. 2011). Dechorionated embryos were rested for 30 min prior to nanoparticle exposure. For both the larval behavior assessment and the adult studies, embryos were exposed from 6 to 120 hpf in the individual wells of a 96-well plate with 100 μL of either embryo media (Kimmel et al. 1995), 10 μg/mL of TMAT-AuNPs, 50 μg/mL of MES-AuNPs, or 50 μg/mL of MEEE-AuNPs. The NP exposure concentrations were selected as the most appropriate for behavior testing based on a previous study showing that developmental exposure to these concentrations does not lead to significant morphological defects (e.g., yolk sac edema) which could affect swimming behavior and confound the results of the behavior tests (Truong et al. submitted). Additionally, TMAT-AuNPs induced 100 % embryo lethality at 50 μg/mL, therefore a lower concentration was selected that did not induce mortality or sublethal effects. For the larval behavior assessment, 24 embryos were exposed per treatment (3 replicates); for the adult assessments, 96 embryos were exposed per treatment, but only 50 were selected to be raised into adulthood. Note that embryo media was used as the control for the adult study based on the results of the larval behavior tests, which showed no statistical behavioral difference between embryos exposed to MEEE or embryo media (see Figure 1b). Embryos for the adult study were thoroughly washed at 120 hpf then raised under standard conditions until adulthood. A subset of embryos with intact chorions was kept to monitor inherent clutch quality. Exposure plates were sealed to prevent evaporation and wrapped with aluminum foil to eliminate potential light degradation of the NPs during exposure.
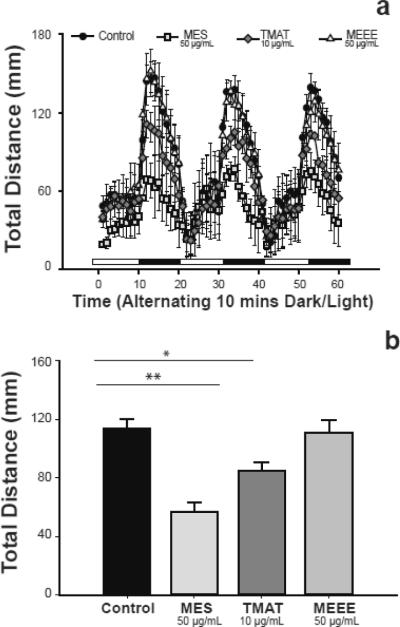
Figure 1. Transient AuNP exposure results in abnormal behavior at 120 hpf.
(a) Distance moved for embryos exposed to 50 μg/mL of MES- and MEEE- AuNP or 10 μg/mL TMAT-AuNPs. (b) Analysis of the total distance traveled during the dark cycle for the sum of three consecutive cycles. Data with * denotes statistical significance determined by one-way ANOVA, Dunnet's post-test. *p<0.05, **p<0.01. Error bars represent standard error.
2.5. Larval Behavior Assay
At 120 hpf, exposed embryos were tested in the original 96-well exposure plate by placing the plate in a Viewpoint ZebraBox (software version 3.0, Viewpoint Life Sciences, Lyon, France) and measuring locomotor activity using the tracking setting during alternating periods of light and dark. This test is a modification of that described by MacPhail et al. (2009). Larvae subjected to this test typically move less during the light periods and more during dark periods (Figure 1a) and behavioral differences following developmental exposures can be determined by comparing distances moved during the light or dark periods. Briefly, the test consisted of acclimating embryos in the light for 20 min, after which a cycle of 10 minutes in the light, then 10 min in the dark, was repeated three times over a course of one hour. Raw data files were processed using a custom perl script to average total distance traveled for each of the three dark periods. Twenty-four embryos were used for each replicate, with a total of three replicates for each of the three AuNPs or embryo media controls. A two way ANOVA-Dunnett's Post Hoc Test using conditions of cycle and treatment was used to compare whether larval locomotor activity differed across each of the three dark cycles.
2.6. Survivorship Measurements
Ninety-six embryos were exposed from 6 – 120 hpf to embryo media control, TMAT- and MESAuNPs. At 120 hpf, 50 phenotypically normal larvae were selected from each treatment and washed with fish water prior to being raised to adulthood on a recirculating FW system. The larvae were initially reared at a density of 50 per tank. At 22 days post fertilization (dpf), larvae were split to a density of 25 per tank. On 44 dpf, each treatment group was evenly distributed between two tanks to control for possible density effects. The number of surviving larvae was recorded at 22, 44 and 122 dpf. These evaluation dates correspond to when juvenile zebrafish transition to different sized food (based on the mouth size). A Fisher Exact Test (p<0.05) was applied to the raw data (the number of survivors into adulthood). For ease of visualization, percent survivors was graphed rather than number of individuals.
2.7. Adult Behavior Assay
One day prior to behavioral assessment, five fish (n=5) were randomly selected from each treatment group and placed into individual 2 L tanks in the behavior testing room. The behavior room was temperature controlled on a 14 h light/10h dark cycle and had a custom built shelf that held 15 tanks at a time, and at each location, had a solenoid that was manually triggered from a remote location to create a brief tap on the outside of the tank. A custom light setup with a sheet diffuser was placed behind the shelf system and controlled remotely using a light switch placed in a different part of the room. A Sony High Definition camcorder (HDR-SR11) was set up on a tripod 15 feet away from the tank arrays to capture movement of the fish. After capturing the videos, Noldus Ethovision XT version 7 software (Leesburg, VA, USA) was used to quantify total distance moved and velocity. All data obtained were normalized by averaging the total velocity or distance travelled among the five fish per treatment and dividing this value by the average length for the same five fish for each treatment (see section 2.8). A one way ANOVA and Dunnett's post hoc test was used to assess total distance travelled after a light and startle stimulus and the velocity after the light stimulus for each nanoparticle-treated group for the adult behavior assessments. An ANOVA on ranks with Dunn post hoc test was used to assess average velocity after startle assay for control compared to each treatment.
2.7.1. Light Stimulus
After moving fish to the behavior testing room, they were left in the dark for 20 min to acclimate to their new environment; afterwards, the light stimulus was produced by turning on the lights. The camcorder was used to record the movement of the fish for 10 min following the light stimulus, after which the fish were allowed to settle down in the dark.
2.7.2. Startle Stimulus
Approximately 60 min after the light stimulus test was conducted, the same fish underwent a startle stimulus assay. The test began when the tap trigger was manually initiated and lasted 10 min during which the fish movements were recorded.
2.8. Adult Weight and Length Measurements
On 117 days post exposure, and after behavioral analysis was complete, all adult zebrafish were humanely euthanized using MS-222. Body mass was measured using a digital scale length was measured from the snout to the end of the caudal fin using digital calipers. Condition factor (K) indices was calculated for each treatment to quantify the condition of the fish [K=mass (g)× 100/length3 (mm)] (Jones et al. 1999). An ANOVA on ranks with Dunn post hoc test was used to assess average weight, and condition factor indices for control compared to each treatment. A one way ANOVA and Dunnett's post hoc test was used to average length for control compared to each treatment.
3. Results
3.1. Surface functionalization of 1.5nm AuNPs impacts larval behavior
After exposure to the three types of gold nanoparticles, we conducted a 1-hour larval behavior test consisting of recording distance moved during three cycles of alternating 10-minute light and dark periods. After determining that there was no statistical difference between the distances moved during each of the three dark periods (data not shown), total distance traveled in the three dark periods was averaged and distance moved was compared across treatments (Figure 1b). Embryos exposed to MES- and TMAT- AuNPs swam 50% and 33% less distance in the dark compared to the control, respectively, while those exposed to MEEE-AuNPs showed no difference in distance traveled compared to the control. This data suggests that the MES- and TMAT surface functionalizations (i.e., charge) impacted development leading to altered locomotor behavior. Note that since the larval test showed no difference in behavior between MEEE-exposed embryos and those raised in embryo media, the embryo media exposed were used as the control for the adult tests rather than adding an additional NP control (MEEE). As a control, embryo media exposed embryos was used rather than fish water because the embryo media composition is known, while fish water chemistry is unknown due to the use of a commercial product with proprietary ingredients.
3.2. Acute exposure to AuNPs impacts survivorship into adulthood
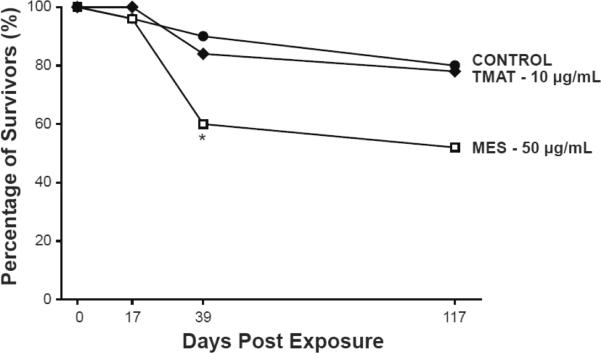
Embryos that were exposed to TMAT-AuNPs at 10 μg/mL, MES-AuNPs at 50 μg/mL or an embryo media control from 0 to 5 dpf were raised in fresh water until 122 dpf. By 117 days post exposure (dpe) to the nanoparticles or the control, there was a statistically significant decrease in the percentage of MES-AuNP-exposed survivors, while survivorship of fish that had been exposed to TMAT-AuNPs was not statistically different from the control survival rate. At 117 dpe, both the control and TMAT-AuNPs exposed groups had 80% survivors, while MES-AuNPs had only 50%. Figure 2 illustrates that both control and TMAT-AuNP-exposed fish had similar rates of mortality throughout the experiment, while the majority of the death to the MES-AuNP-exposed fish occurred between 17 to 39 dpe. The MES-AuNP treated group's survivorship dropped from 90% at 17 dpe to 60%, 22 days later. Overall, this data shows that acute exposure to 1.5nm MES-AuNPs decreases the number of survivors into adulthood.
Figure 2. AuNPs effect on development into adulthood.
Percent of adult survivorship of embryos exposed to 1.5 nm MES- or TMAT- AuNPs, or embryo media control from 0 to 5 days post fertilization (dpf), and were rinsed prior to being raised in fresh water until 122 dpf. Statistical significance was determined using a Fisher Exact Test. *p<0.05.
3.3. Surviving adults exhibit higher condition factor indices
To determine if acute exposure to MES- and TMAT-AuNPs affects zebrafish development, we measured the length and weight of all survivors for each of the three groups. The surviving fish in both MES- and TMAT-AuNPs treatment groups were statistically longer than the control (34 ± 0.22 mm) with an average length of 38 ± 0.32 and 36 ± 0.31 mm, respectively. MES-AuNP treated fish weighed more than the control (0.53 +/− 0.03 g vs 0.42 +/− 0.02 g). To account for the different number of survivors per treatment, we calculated a condition factor index (K) to evaluate the quality of the fish. The condition factor index (K= mass × 100 / length3) for the control group was 0.97 +/− 0.049, while both the MES- and TMAT-AuNPs fish had an average K of 1.12 (Table 1). These results demonstrate the importance of taking into consideration the different number of survivors per treatment and that exposure to either positively or negatively charged AuNPs during development leads to an increase in both weight and length of adult zebrafish.
Table 1. Mean growth, survival and condition factor indices (± SEM) of adult zebrafish exposed to embryo media, MES- or TMAT- AuNP.
Means with different superscript letter designations within columns are statistically significantly different from the embryo media control (p<0.05).
| Exposure | Survival (%) | Length (mm) | Weight (g) | Condition factor (K) |
|---|---|---|---|---|
| Embryo media | 80 | 34.78 (0.22) | 0.50 (0.011) | 0.97 (0.049) |
| MES-AuNPs | 52 | 38.25 (0.32)a | 0.63 (0.021)b | 1.12 (0.032) |
| TMAT-AuNPs | 78 | 36.27 (0.31)a | 0.54 (0.016) | 1.12 (0.022) |
3.4. Behavioral abnormality detected in acutely exposed zebrafish
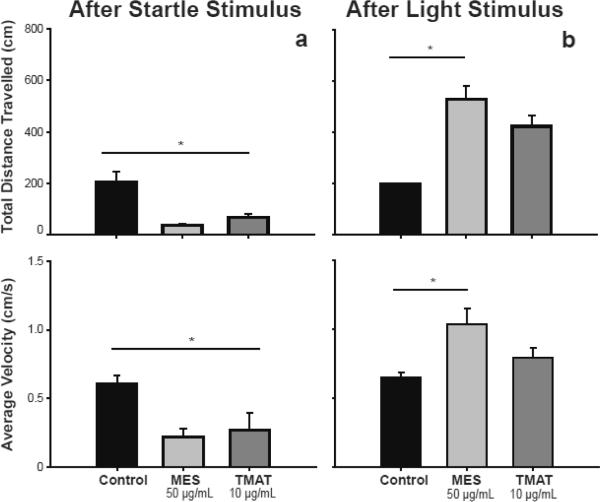
Zebrafish exposed during development to MES- and TMAT-AuNPs exhibited behavioral abnormalities at 5 dpf. We wanted to evaluate whether these behavioral abnormalities persistent into adulthood. To assess this, we performed two adult assays of locomotor activity. Since the NP-exposed adults were bigger than the controls, and bigger fish may be expected to travel larger distances than smaller fish, all locomotor data was normalized to the average body length. The first assay was to measure the total distance travelled and the average velocity for 10 minutes following a tap on the tank. As Figure 3a illustrates, after the startle stimulus, the control fish moved a total distance of 207 cm, while both MES and TMAT-AuNP treated fish travelled a statically significant shorter distance, 35 and 69 cm, respectively. The velocity for the control and TMAT-AuNPs exposed fish were 0.60 and 0.27 cm/s, respectively, while the velocity of MES-AuNP exposed fish was lower than the control (0.22 cm/s). The second assay consisted of measuring the total distance travelled and the average velocity during 10 minutes in the light following a light stimulus (Figure 3b). For this assay, the control fish travelled a total distance of 197 cm, while TMATAuNP-exposed fish travelled 421 cm). MES-AuNPs exposed fish travelled a statistically significant greater distance (527 cm than the control. The velocity of the fish followed the same trend, where MES-AuNP exposed fish had an average velocity that was greater than the control (1,04 vs 0.65 cm/s, respectively), while both the control and TMAT-AuNP-exposed fish had an average velocity of 0.79 cm/s. These results demonstrate that acute exposure to both MES- and TMAT-AuNPs cause behavioral abnormalities that persists into adulthood. However, the negatively charged MES-AuNPs have a more severe impact on both larval and adult behavior compared to the effects of the positively charged TMAT-AuNPs.
Figure 3. Total distanced travelled and velocity following a stimulus.
Immediately after a tap on the tank (a) or a light stimulus (b), total distance travelled and velocity was recorded for 10 minutes for 5 fish (n=5). To account for size difference in the treated versus control fish, movement data were normalized to fish size using the average morphometric index determined for each treatment group. Statistical significance (p<0.05) relative to control was determined using One way ANOVA with a Dunnett's Post Hoc Test. Error bars represent standard error.
4. Discussion
In this study, we report for the first time that acute exposure to 1.5 nm MES- and TMAT- AuNPs during embryonic development results in larval behavioral abnormalities that persist into adulthood. Additionally, developmental exposure to both NPs resulted in larger adults, and exposure to 1.5 nm MES-AuNPs at 50 μg/mL resulted in reduced adult survivorship. Although there is a lack of significant differences in condition factor index (K), the data suggests that the fish are compositionally similar when adjusted for length and weight. Historically, the condition factor index is considered a good indicator of health in fish; however, it has only been applied to one zebrafish study where the average K was 1, which is similar to our finding in this study (Siccardi et al. 2009). Applying a condition factor index to adult zebrafish studies is appropriate as it illustrates the health of the fish and allows comparison to other studies.
Ours is the first study where embryonic zebrafish were exposed to nanoparticles only during development, then raised in freshwater to adulthood. Previous studies have tested chemicals using this same method and model (Görge et al. 1990; Gerlai et al. 2006). What we are learning from these studies is that even brief exposure during development can result in potentially maladaptive effects later in life. As others have suggested, embryonic development is the most critical and sensitive life stage (Aparicio et al. 2002; Rubinstein 2003) and if any molecular pathways are perturbed during this period, permanent derailment of development may occur. Effects on development can be minute or cause a cascade of effects that result in either mortality or inhibition of growth and possibly central nervous system damage. While behavior tests are a comprehensive assessment of potential impacts on nervous system development, follow-up studies are required to determine the “window of exposure” and the mode of action underlying the observed effects.
While altered locomotor activity suggests an impact on nervous system development, it does not exclude the possibility that the NP is impacting other target organs such as the eye (i.e., ability to detect light) or neuromuscular system (i.e., ability to swim). By utilizing both a light and tap stimulus tests, we tested the effects of the NPs on both vision and sensory perception. The ability to sense a tap is achieved through the hair cells on the lateral line of teleosts (Crispino 1983). Our results suggest that while MES may impact both vision and sensory perception, TMAT is unlikely to have an impact on vision. The next step in determining whether these NPs impact nervous system, vision, sensory-motor perception, or all three target organ systems, is to begin to look at the molecular events underlying the observed changes in behavior. The zebrafish is an excellent model for investigating mode of action underlying observed phenotypes mainly due to being genetically tractable. The shared genomic homology between humans and zebrafish (Barbazuk et al. 2000) makes studies using developing zebrafish highly relevant to human health. We can use this model to investigate gene expression changes underlying a given phenotype, and begin to identify which genes or pathways in any organ of interest (e.g., brain, eye, or somites) are being misexpressed following developmental NP exposure.
In a previous study, global gene expression data were collected on embryos exposed to 1.5 nm TMAT- and MES-AuNPs from 6 – 24 and 6 – 48 hpf (Truong et al. Submitted). Analysis of this data using Ingenuity Pathway Analysis software (Ingenuity® Systems, www.ingenuity.com), found that for both exposure time points, nervous system development and function was one of the biological processes most perturbed. Although the gene expression profiling was conducted using samples collected during the most rapid development of the zebrafish central nervous system (suggesting that our results could be merely reflective of normal developmental patterns), pathways related to cellular function and maintenance, and nervous system development and function were significantly impacted compared to their time-matched control. The gene expression data corroborates our hypothesis that exposure to TMAT- and MES-AuNPs during development perturbs neurophysiological processes by 5 dpf leading to permanent damage to the central nervous system that persists into adulthood. Further analyses will be required to determine the extent to which the central nervous system or other target organs contribute to the observed behavioral phenotypes.
An additional clue to the molecular impact of the tested NPs comes from the NPs, themselves. Our study demonstrates that exposure to gold nanoparticles functionalized with MES or TMAT, induces unexpected behavioral effects that are driven by the surface functionalities.. The behavioral abnormalities detected cannot be generalized to every gold nanoparticle. For example, MEEE-AuNP exposed embryos did not exhibit any behavior defects, but MES- and TMAT-AuNP exposed embryos did. These results suggest that surface coatings, and in this case the charge specifically, either positive or negative, has a significant impact on development. Further studies are currently being conducted by our group to begin to determine how NP surface charge impacts development.
5. Conclusions
In summary, evaluation of adult zebrafish after acute exposure to nanoparticles during embryonic development provides critical information for predicting potential long-term effects of specific NPs. From this study, we have learned that exposure to NPs during development can have lasting impacts on the central nervous system. More follow-up studies using different nanoparticles must be conducted to determine what physicochemical properties are driving these responses. Collectively, all data point to surface functionalization of a nanoparticle being a critical driver to adverse response since the effects observed are isolated to only certain functional groups. Utilizing the zebrafish model for acute NP exposure and long-term development studies provides insight into potential human health implications and allows for follow-up investigation at the molecular level to address the mode of action underlying these undesired effects. Studies using this model will help regulatory agencies establish safety precautions to minimize detrimental effects from nanoparticles and provide data that will aid NP manufacturers in developing safer NPs that are both effective and nontoxic.
Acknowledgements
We thank Sinnhuber Aquatic Research Laboratory for the embryos and Dr. Tatiana Zaikova for her assistance in preparation of the materials. These studies were partially supported by National Institute of Environmental Health Sciences (NIEHS), R01ES016896, P3000210, F31 ES019445-02, the Air Force Research Laboratory (AFRL) under agreement number FA8650-05-1-5041, and Environmental Protection Agency (EPA) RD-833320. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of NIEHS, AFRL, EPA, or the U.S. Government. Further support was provided by the W.M Keck Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amacher SL. Zebrafish Embryo as a Developmental System. John Wiley & Sons, Ltd.; 2001. [Google Scholar]
- Aparicio S, Chapman J, Stupka E, Putnam N, Chia J.-m., Dehal P, Christoffels A, Rash S, Hoon S, Smit A, Gelpke MDS, Roach J, Oh T, Ho IY, Wong M, Detter C, Verhoef F, Predki P, Tay A, Lucas S, Richardson P, Smith SF, Clark MS, Edwards YJK, Doggett N, Zharkikh A, Tavtigian SV, Pruss D, Barnstead M, Evans C, Baden H, Powell J, Glusman G, Rowen L, Hood L, Tan YH, Elgar G, Hawkins T, Venkatesh B, Rokhsar D, Brenner S. Whole-Genome Shotgun Assembly and Analysis of the Genome of Fugu rubripes. Science. 2002;297:1301–1310. doi: 10.1126/science.1072104. [DOI] [PubMed] [Google Scholar]
- Barbazuk WB, Korf I, Kadavi C, Heyen J, Tate S, Wun E, Bedell JA, McPherson JD, Johnson SL. The syntenic relationship of the zebrafish and human genomes. Genome Res. 2000;10:1351–1358. doi: 10.1101/gr.144700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Granato M, Nusslein-Volhard C. Keeping and raising zebrafish. In: Nusslein-Volhard CD,R, editor. In Zebrafish: A Practical Approach. Oxford University Press; Oxford: 2002. pp. 7–37. [Google Scholar]
- Crispino L. Modification of responses from specific sensory systems in midbrain by cerebellar stimulation: experiments on a teleost fish. J Neurophysiol. 1983;49:3–15. doi: 10.1152/jn.1983.49.1.3. [DOI] [PubMed] [Google Scholar]
- Gao X, Yin S, Tang M, Chen J, Yang Z, Zhang W, Chen L, Yang B, Li Z, Zha Y, Ruan D, Wang M. Effects of developmental exposure to TiO(2) nanoparticles on synaptic plasticity in hippocampal dentate gyrus area: An in vivo study in anesthetized rats. Biol Trace Elem Res. 2011 doi: 10.1007/s12011-011-8990-4. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Lee V, Blaser R. Effects of acute and chronic ethanol exposure on the behavior of adult zebrafish (Danio rerio) Pharmacol Biochem Behav. 2006;85:752–761. doi: 10.1016/j.pbb.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görge G, Nagel R. Toxicity of lindane, atrazine, and deltamethrin to early life stages of zebrafish (Brachydanio rerio) Ecotoxicol Environ Safe. 1990;20:246–255. doi: 10.1016/0147-6513(90)90004-o. [DOI] [PubMed] [Google Scholar]
- Harper SL, Carriere JL, Miller JM, Hutchison JE, Maddux BLS, Tanguay RL. Systematic evaluation of nanomaterial toxicity: Utility of standardized materials and rapid assays. ACS Nano. 2011;5:4688–7697. doi: 10.1021/nn200546k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RE, Petrell RJ, Pauly D. Using modified length-weight relationships to assess the condition of fish. Aquacult Engineer. 1999;20:261–276. [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- MacPhail RC, Brooks J, Hunter DL, Padnos B, Irons TD, Padilla S. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology. 2009;30:52–58. doi: 10.1016/j.neuro.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Oszlanczi G, Papp A, Szabo A, Nagymajtenyi L, Sapi A, Konya Z, Paulik E, Vezer T. Nervous system effects in rats on subacute exposure by lead-containing nanoparticles via the airways. Inhal Toxicol. 2011;23:173–181. doi: 10.3109/08958378.2011.553248. [DOI] [PubMed] [Google Scholar]
- Powers CM, Badireddy AR, Ryde IT, Seidler FJ, Slotkin TA. Silver nanoparticles compromise neurodevelopment in PC12 cells: Critical contributions of silver ion, particle size, coating and composition. Environ Health Perspect. 2011;119:37–44. doi: 10.1289/ehp.1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein AL. Zebrafish: from disease modeling to drug discovery. Curr Opin Drug Discov Devel. 2003;6:218–223. [PubMed] [Google Scholar]
- Scholars W.W.I.C.f., PEW . Woodrow Wilson International Center for Scholars. vol. 2011 2011. Project on Emerging Nanotechnologies. [Google Scholar]
- Siccardi AJ, Garris HW, Jones WT, Moseley DB, D'Abramo LR, Watts SA. Growth and survival of zebrafish (Danio rerio) fed different commercial and laboratory diets. Zebrafish. 2009;6:275–280. doi: 10.1089/zeb.2008.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Harper SL, Tanguay R. Evaluation of embryotoxicity using the zebrafish model. Humana Press; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Tilton SC, Zaikova T, Richman E, Waters KM, Hutchison JE, Tanguay RL. surface functionalities of gold nanoparticles (AuNPS) impact gene expression in the developing zebrafish. Small. doi: 10.3109/17435390.2011.648225. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner MG, Hutchison JE. Linear assemblies of nanoparticles electrostatically organized on DNA scaffolds. Nat Materials. 2003;2:272–277. doi: 10.1038/nmat853. [DOI] [PubMed] [Google Scholar]
- Woehrle GH, Brown LO, Hutchison JE. Thiol-functionalized, 1.5-nm gold nanoparticles through ligand exchange reactions: Scope and mechanism of ligand exchange. J Am Chem Soc. 2005;127:2172–2183. doi: 10.1021/ja0457718. [DOI] [PubMed] [Google Scholar]
- Woehrle GH, Warner MG, Hutchison JE. Molecular-level control of feature separation in one-dimensional nanostructure assemblies formed by biomolecular nanolithography. Langmuir. 2004;20:5982–5988. doi: 10.1021/la049491h. [DOI] [PubMed] [Google Scholar]
- Zhu Z-J, Carboni R, Quercio MJ, Yan B, Miranda OR, Anderton DL, Arcaro KF, Rotello VM, Vachet RW. surface properties dictate uptake, distribution, excretion, and toxicity of nanoparticles in fish. Small. 2010;6:2261–2265. doi: 10.1002/smll.201000989. [DOI] [PMC free article] [PubMed] [Google Scholar]