Abstract
Background
We have previously reported life-supporting kidney xenograft-survival greater than 80 days using a steroid-free antithymocyte globulin (ATG)-based induction regimen (ATG regimen) in a GalT-KO pig-to-baboon thymokidney (TK) model. We evaluated two induction regimens, a newly developed anti-monkey CD3 recombinant immunotoxin (anti-CD3 rIT) and an anti-human CD2 antibody (LoCD2), by assessing T-cell depletion (TCD) and graft survival.
Methods
Four baboons received anti-CD3 rIT; the time course of TCD was studied in two animals and the other two received GalT-KO TK transplants. Two additional baboons underwent GalT-KO TK transplantation after treatment with LoCD2. All other treatments were identical to previous TCD studies with ATG. TCD was assessed by flow-cytometry; renal function was evaluated by serum creatinine and histology.
Results
Baboons that received the anti-CD3 rIT died from pneumonia or cardiac failure on days 15 and 23. Both animals in the rIT group died with functioning grafts. Thymokidney grafts from baboons treated with the LoCD2 regimen were rejected by day 14. TCD levels in baboons receiving the anti-CD3 rIT regimen were 150 to 250 cells/μL for at least 14 days, whereas baboons receiving the LoCD2 recovered to more than 300 cells/μL by day 7.
Conclusions
The newly developed anti-CD3 rIT could be a useful TCD agent in baboons. However, optimal dosage, treatment duration, and bioactivity must be studied to avoid side effects. A LoCD2-based regimen was not effective for preventing xenogeneic rejection. Optimal TCD levels less than 250/μL during the induction period seem to be important for success of xenothymokidney transplantation.
Keywords: GalT-KO, Renal graft, Thymokidney, T-cell depletion, Xenotransplantation, Large animal model
Because of their size, physiologic compatibility, breeding characteristics, and the potential for genetic-manipulation, miniature swine organs could potentially alleviate the disparity between the supply and demand for human donor organs (1). Elimination of the swine α1,3–galactosyltransferase (Gal) gene yielded Gal knockout (GalT-KO) pigs (2, 3). Using GalT-KO organs, our group has overcome hyperacute xenorejection, and increased life-supporting xenogeneic renal graft-survival to greater than 80 days in a pig-to-baboon thymokidney model (4, 5).
In a pig-to-mouse model, data have demonstrated that complete T-cell depletion (TCD) during the induction period is essential for tolerance induction (6, 7). The anti-CD4 and anti-CD8 monoclonal antibodies (mAb) used in that study are unfortunately not available for non-human primates. However, using an anti-monkey CD3-conjugated diphtheria IT, FN18-CRM9, we have demonstrated efficient TCD in a xenogeneic thymic tissue transplantation in a pig-to-primate system (8, 9). This conjugated immunotoxin was formed by chemically cross-linking the monoclonal antibody FN18 to a diphtheria-toxin binding site mutant, CRM9. Thus, immunotoxin binding was dictated by the antibody moiety FN18. Because the linkage heterogeneity remained and the production level was extremely low (2% of toxin input), our center, in collaboration with Dr. David Neville’s laboratory (Section on Biophysical Chemistry Laboratory of Molecular Biology, NIMH), recently produced an anti-monkey CD3 recombinant immunotoxin (anti-CD3 rIT, A-dmDT390-scfbDb[C207]). Anti-CD3 rIT contains a truncated diphtheria-toxin DT390 and two identical single chain fragment variables in a foldback-diabody format derived from an anti-monkey CD3 monoclonal antibody FN18 (10).
In this study, we tested a TCD induction-regimen aimed at reducing the risk of infection. First, we evaluated the efficacy of the newly developed anti-CD3 rIT on TCD in thymectomized baboons. We then tested an anti-CD3 rIT-based TCD induction regimen and an anti-CD2-specific mAb (LoCD2) induction regimen in a GalT-KO thymokidney pig-to-baboon model.
RESULTS
Effect of Anti-CD3 rIT on TCD in Baboons (Group 1)
On the basis of a pilot study in non-human primates (10), we first attempted to characterize the effect of anti-CD3 rIT on TCD in two baboons without xenografts (group 1). In keeping with the thymokidney protocol, thymectomy was performed 2 weeks before administration of anti-CD3 rIT. Eight doses of anti-CD3 rIT (25 μg/kg) were administered over 4 days. Figure 1(A) shows the absolute number of CD3+ T-cells in the peripheral blood following the 4-day course of anti-CD3 rIT. CD3+ T-cells decreased markedly in the first 7 days. Thereafter, T-cell levels increased and returned to pre-injection levels by days 15 and 25, respectively. Figure 1(B) shows the percentage of CD3+ T-cells and CD20+ cells in LNs, by FACS analysis. Pre-rIT, 80% to 90% of LN lymphocytes were CD3+. Thirteen days after the first rIT injection, the CD3+ percentage decreased markedly and the majority of remaining lymphocytes were B-cells. One month after injection, LN lymphocytes positive for CD3 returned to 55% and 35% from 3% and 6% in animals 1 and 2 (B234 and B243), respectively.
FIGURE 1.
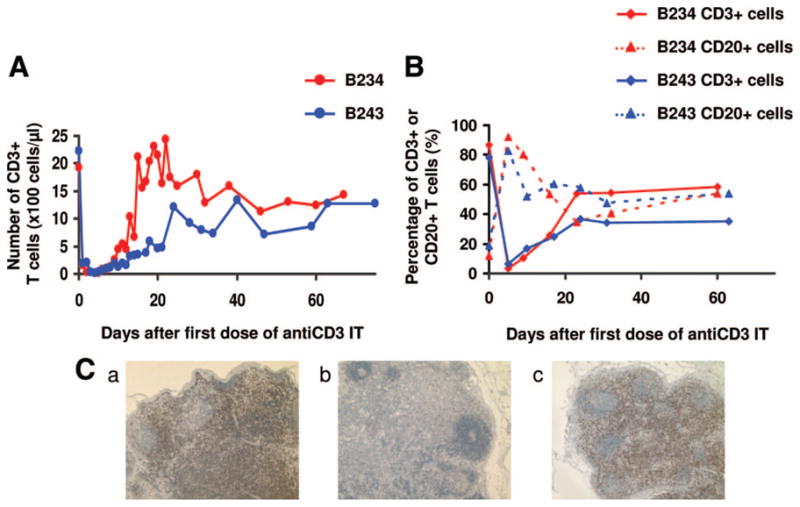
(A) Absolute number of CD3+ cells in peripheral blood after administrating of 25 μg/kg ×8 anti CD3-IT. (B) Percentage of CD3 or CD20+ cells in lymph node (LN) after administrating of 25 μg/kg ×8 anti CD3-IT. (C) Histology of lymph node after administrating of 25 μg/kg ×8 anti CD3-IT (hematoxylin-eosin staining). Pretreatment state (a), in contrast with the pretreatment state, the T-cell areas of LN decreased on day 5 (b) but recovered by day 60 (c).
Analysis of a LN biopsy corroborated the extent of TCD shown by FACS. Figure 1(C) shows LN analysis results for animal B234 on day −14 (Fig. 1C-a), day +5 (Fig. 1C-b) and day +60 (Fig. 1C-c) from administration of anti-CD3 rIT. A comparison of Figure 1(C-a, C-b) demonstrated a decrease in T-cell concentration in the LN after anti-CD3 rIT treatment. Histology also showed a late increase of CD3+ cells and CD20+ cells in the LN. Results from the second animal (not shown) were similar.
No clinically relevant adverse reactions (hematologic or allergic) were observed. However, both animals experienced elevations in liver enzymes after a 4-day course of anti-CD3 rIT. Both animals had transient increases in ALT and AST (peak of 255 and 300, respectively) during the first week. These enzyme levels resolved spontaneously within 2 weeks without treatment.
Effect of Anti-CD3 rIT-Based Induction Regimen on Thymokidney Graft Survival (Group 2)
Clinical Course
Because results from group 1 illustrated transient TCD with administration of 8 doses of anti-CD3 rIT at 25 μg/kg, we doubled the dosage of anti-CD3 rIT for thymokidney transplantation (group 2). Two baboons received eight doses of anti-CD3 rIT at 50 μg/kg/dose for 4 days before transplantation (day −4 to −1). Despite doubling the dose, we felt anti-CD3 rT alone might be insufficient to yield long-lasting TCD and increasing the dose further might induce side effects. Therefore, we gave a single dose of LoCD2 at 4 mg/kg/dose to both animals 1 day after transplantation to augment TCD. As with our previously published antithymocyte globulin (ATG) regimen (5), both group 2 animals underwent thymectomy plus splenectomy and received Rituximab, FK506, MMF, and anti-CD154 (5).
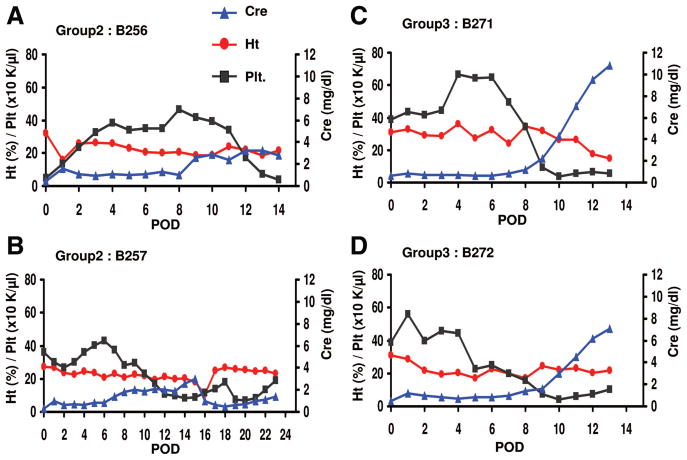
Animal no. 1 (B256) underwent thymokidney transplantation immediately after the 4-day course of anti-CD3 rIT. CD3+ T-cells were depleted to a nadir of 127 cells/μL from preoperative levels of 2788 by day 0. With the addition of LoCD2, T-cell counts remained below 20 cells/μL. B256 had stable serum creatinine (<1.7 mg/dL) until day 8. This period of stable renal function was correlated with a CD3+ count of roughly 200. Serum creatinine started to rise on day 9 and then fluctuated between 2.4 and 3.2 mg/dL (Fig. 2A) until day15, when the animal died from pneumonia. Necropsy revealed gross pin-point hemorrhages of the thymokidney graft.
FIGURE 2.
Serum creatinine levels (mg/dL) after TK transplantation with the anti-CD3 rIT regimen (A, B) and LoCD2 regimen (C, D).
Animal no. 2 (B257) from group 2 underwent transplantation 6 days after completing a 4-day course of anti-CD3 rIT. Transplantation was delayed because ALT and AST increased to over 1000 and 2000, respectively, after rIT. The animal’s absolute CD3+ count was depleted from 2220 to a nadir of 137 after eight doses anti-CD3 rIT (50 μg/kg) over 4 days. Three doses of LoCD2 were given at days −2, −1, and 1 from thymokidney transplantation because T-cell counts increased slightly during the 6-day period before transplantation. This regimen yielded an absolute CD3+ count lower than 200 between days 1 and 6. A 4th dose of LoCD2 (2 mg/kg) was given on day 7 because the T-cell level increased to 375. The animal maintained stable renal function with a creatinine less than 0.8 mg/dL through day 6. On day 7, the creatinine slowly increased and reached 3.0 mg/dL by day 15. During this period (days 7–15), the animal developed severe proteinuria, which led to generalized edema. For these reasons, we performed a graft nephrectomy and simultaneously retransplanted the animal with a second donor-matched thymokidney on day 15. The second thymokidney was well re-vascularized, produced urine immediately, and postoperative day 1 creatinine was 0.6 mg/dL, suggesting the animal was not sensitized. Despite stable renal function the animal became dyspneic on day 24, likely from systemic inflammation and residual generalized edema which had developed during the first transplant and was euthanized (Fig. 2B). At necropsy, the second thymokidney appeared grossly normal; however, the animal had cardiac ischemia and pneumonia on histology.
Histologic Findings
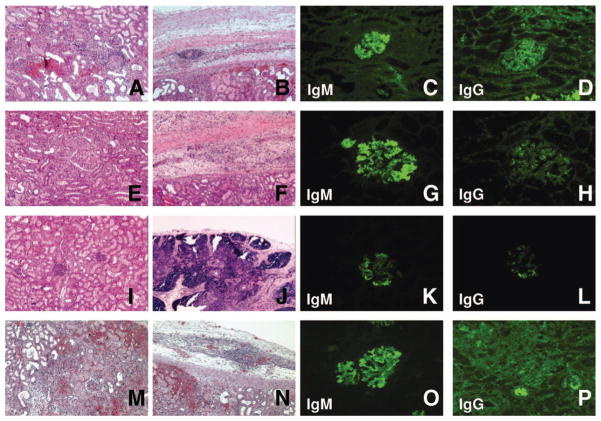
The thymokidney graft from animal no. 1 showed focal segmental mild thrombotic microangiopathy, focal mild mononuclear interstitial cell filtrates, focal hemorrhage, and focal tubular injury at day 15. The thymic portion of the thymokidney graft was thin but well-preserved, as demonstrated by the presence of Hassal bodies and lymphocyte aggregation (Fig. 3A, B). Immunohistochemistry showed IgM deposition in most glomeruli, whereas only focal IgG deposition was present (Fig. 3C, D).
FIGURE 3.
Group 2 and 3: Graft histology. Histology of B256 (group 2, animal no. 1) on POD15 revealed the TK graft was injured, but not rejected (A, B). Immunohistochemistry showed frequent glomerular anti-non-Gal IgM and scattered anti-non-Gal IgG in few glomeruli (C, D). Histology of B257 (group 2, animal no. 2) after TK transplantation with a regimen of anti CD3-IT T-cell depletion (hematoxylin-eosin staining): the TK graft had mild hemorrhage and multiple microthrombi on POD15 (E, F). Immunohistochemistry showed IgM deposition in most glomeruli (G) with weak IgG deposition in some of the glomeruli (H). The transplanted TK graft was accepted on day 8 after Re–TK transplantation (I, J). Immunohistochemistry showed deposition of IgM in many glomeruli (K) but few IgG deposits (L). Histology of B272 (group 3, animal no. 2) after TK transplantation with a regimen of LoCD2 T-cell depletion (hematoxylin-eosin staining). Cellular rejection and advanced antibody mediated rejection developed (M). Hemorrhage was also seen in the thymic tissue under the renal capsule (N). Immunohistochemistry findings demonstrated both anti-non-Gal IgM and IgG deposition in glomeruli (O, P).
The thymokidney graft from animal no. 2 (excised on POD 15 for retransplantation) showed focal cellular infiltration around the small vessels and focal segmental glomerulopathy. These findings are consistent with those we observed in animal no. 1 (B256). The thymic graft of B257 was thin, mildly hemorrhagic, and multiple microthrombi were present (Fig. 3E, F). No rejection of either the kidney or the thymic graft was observed in B257. However, there were clear signs of congestion in the peritubular capillaries and renal capsule, likely due to cardiac failure (Fig. 3I, J). Immunohistochemistry of the first kidney showed only scattered glomerular IgG deposition, but IgM deposition was evident in the majority of examined glomeruli (Fig. 3G, H). Interestingly, animal no. 2’s second graft had no IgG deposition and minimal IgM deposition: histologically, this indicated a lack of sensitization (Fig. 3K, L).
Effect of LoCD2-Based Induction Regimen on Survival of Thymokidneys (Group 3)
Clinical Course
In group 3, we tested LoCD2 anti-T-cell mAb alone for TCD in two baboon thymokidney recipients. Two IV doses of LoCD2 on 4 mg/kg/day were given on days −3 and −2, followed by a single IV dose (2 mg/kg) on day −1. In addition, horse ATG (hATG) at 50 mg/kg was given on day 1 to augment TCD. All other immunosuppression agents, except anti-CD3 rIT, were administered identically to group 2.
TCD in group 3 was less-complete than for group 2. CD3+ T-cell levels were 223 (B271-animal no. 1) and 274 (B272–animal no. 2) at the day of transplantation. Both animals received hATG on day 1. Animal no. 2 from group 3 received an additional dose of ATG on day 3. Despite multiple doses of LoCD2 and hATG supplementation, T-cell levels increased markedly to 599 and 1260 for animal nos. 1 and 2, respectively, by day 7. Both recipients exhibited stable creatinine (<1.5 mg/dL) until day 8. Beginning on day 9, the creatinine for both animals increased sharply (Fig. 2C, D), whereas the hematocrit and platelet counts decreased significantly. The creatinine reached 10.8 and 7.1 mg/dL, for animal nos. 1 and 2, respectively, by day 13. At the day 14 biopsy, both thymokidneys were enlarged with diffuse hemorrhaging, indicating complete rejection. Both animals were subsequently euthanized on day 14.
Histologic Findings
Histology of these thymokidneys revealed moderate to severe humoral and cellular rejection. The kidneys demonstrated interstitial hemorrhage, vasculitis with thrombi in small vessels, acute glomerulopathy, and mononuclear cell infiltration. Subcapsular thymic grafts also demonstrated hemorrhagic changes, yet some of the epithelial cells and Hassal’s bodies remained intact (Fig. 3M, N). Immunohistochemistry demonstrated diffuse glomerular IgM and IgG deposition (Fig. 3O, P).
Correlation Between TCD and Thymokidney Function
We attempted to determine the optimal level of TCD necessary for graft and baboon survival using the pig-to-baboon xenogeneic thymokidney model. We analyzed T-cell levels within the induction period (first 2 weeks after thymokidney transplantation) for animals treated with CD3-rIT (group 2) or LoCD2 (group 3), and compared these results with our previously published experience with rabbit ATG (rATG) (5).
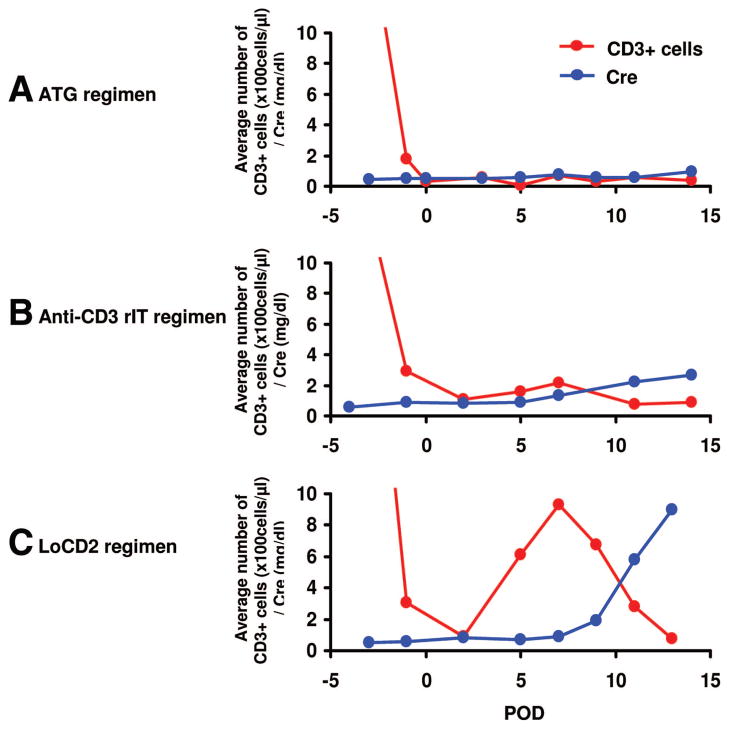
Successful transient depletion of peripheral T cells in the perioperative period (days 0–3) was achieved in all animals. The rATG-based model led to persistent TCD for the first 2 weeks. In that study, T-cell levels were below 200/μL during induction (Fig. 4A) and increased (>400) approximately 5 weeks after transplantation, without concomitantly increased serum creatinine. This correlated with in vitro donor-specific hyporesponsiveness in CML at day 49 (5). In contrast, the two animals treated with the LoCD2 (group 3) regimen had only transient TCD (Fig. 4C). T-cells returned to 928/μL by day 7. Approximately 48 hr after T-cell levels began to rise, serum creatinine increased sharply, and subsequent rejection ensued. In the CD3-rIT regimen (group 2), TCD was less-complete when compared with our rATG model. T-cell levels were slightly higher in group 2, 150 to 250 cells/μL, at day 7 (Fig. 4B). Although serum creatinine fluctuated, graft rejection was not seen histologically, suggesting that the absolute T-cell count at day 7 may be a good predictor of thymokidney transplant outcome.
FIGURE 4.
Average absolute number of CD3+ cells in peripheral blood and serum creatinine levels (mg/dL) after thymokidney transplantation with the ATG (A), anti-CD3 rIT (B), and LoCD2 (C) regimens.
On further phenotypic analysis, we observed that T cells in group 3 returned faster than T cells in group 2 (Fig. 5). As shown in Figure 5(B), the returning cells were predominantly CD8. We observed that these cells rapidly increased starting on day 3 and peaked at day 7 after transplantation in group 3.
FIGURE 5.
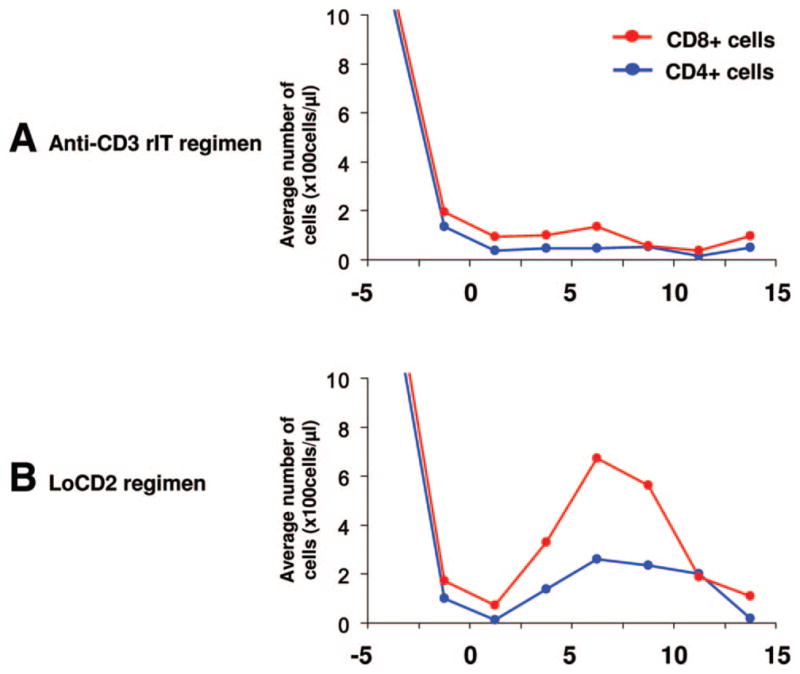
Phenotypic analysis of returning T cells after TCD in groups 2 (Fig. 5A) and 3 (Fig. 5B). In group 3, both CD4 and CD8 T cells increased after TCD; however, these cells were predominantly CD8. This increase began around day 3 and peaked at day 7. In contrast, neither CD4 nor CD8 cells markedly increased in following TCD in group 2.
DISCUSSION
We have previously published that graft-survival of xenogeneic renal grafts co-transplanted with vascularized donor thymus is prolonged (>80 days) in comparison with grafts transplanted without thymic tissue (34 days) (4). By eliminating steroids and WBI, we decreased early postoperative infection and prolonged mean graft survival to 51 days (5). In contrast, without a tolerance strategy, other groups have reported maximum survivals of 16 (11) and 21 days (12). Despite intensive immunosuppression, GalT-KO kidney grafts demonstrated cellular infiltrates and glomerular and peritubular IgG deposition, suggesting T-cell sensitization and intact host T-cell dependent B-cell responses. These findings may be consistent with reports by Korsgren and coworkers (13, 14) indicating that small numbers of T cells are capable of rejecting porcine islets in T-cell-deficient rodents. Because xenogeneic T-cell responses are as-strong-as, or stronger than, allogeneic T-cell responses (15), and because the development of anti-non-Gal antibodies is T-cell dependent (4, 5, 16, 17), it is clear that strategies aimed at T-cell tolerance induction will be essential for successful xenogeneic kidney transplantation.
Our colleagues have reported that TCD is essential for the development of in vitro donor-specific unresponsiveness using porcine fetal-thymic tissue transplantation in a pig-to-mouse model. Mice recipients in this study also accepted skin grafts from the porcine thymic donors (6, 7). Prolonged and complete TCD, which has been shown to be essential for thymic tissue reconstitution and function (6, 18), was consistently achieved with this regimen using a T-cell-specific mAb. Because few T-cell-specific mAbs exist for baboons, we originally used WBI and polyclonal antibodies for TCD in thymokidney recipients (4, 5). Our results indicate that rATG may eliminate mature T cells in the induction period, avoiding rejection, and allowing the recipient to become unresponsive to donor at the in vitro level during the maintenance period (5). However, T-cell-specific depletion may have an advantage over the broad-based polyclonal antibody regimens, which may lead to infection or systemic toxicity.
To assess the specific role of TCD with regard to xenogeneic cellular responses, we attempted to limit the contribution of humoral responses by preformed antibody against Gal and non-Gal. Therefore, we intentionally chose baboons with low levels of preformed non-Gal Nab and kidneys from GalT-KO pigs. In our experience, and as presented in this study, these two factors are essential to maintain stable renal function in the first week after xenogeneic transplantation (4, 5). Our findings suggest that maintenance of an “optimal number” of circulating T cells during the induction period is crucial to thymokidney xenograft survival. Comparison of T-cell levels for the 14 days after induction among animals that received the anti-CD3 rIT-based induction regimen (group 2) or LoCD2-based induction regimen (group 3) with the five baboons who received the ATG-based regimen and achieved graft-survival of greater than 40 days (5), suggests a correlation between the level of TCD at day 7 and graft survival. Animals that were treated with the ATG-based regimen demonstrated persistent TCD for the first 14 days, maintaining T-cell levels below 200/μL and stable renal function in the first month (Fig. 4A). The T-cell levels of these animals recovered (>400 cells/μL) approximately 5 weeks later, without concomitantly increased serum creatinine (5). Anti-CD3 rIT-treated animals displayed similar graft function and TCD levels (Fig. 4B) when compared with animals that received ATG. In contrast, LoCD2 led to only transient TCD (Fig. 4C), resulting in T-cell and B-cell sensitization, and subsequent rejection. However, extensive immunosuppression may lead to lethal infection. Thus, there is a balance between TCD and immunocompetence. In the ATG-based regimen (5), one baboon had prolonged TCD (<50 cells/μL) for the first 14 days. It developed lethal baboon CMV and died at day 28, likely as a result of extensive TCD. The data suggest that an “optimal level of TCD” is required for xenogeneic thymokidney graft survival and avoidance of lethal infection. We propose that T-cell levels should be maintained between 50 and 150 cells/μL when TCD is followed by other immunosuppressive drugs such as co-stimulatory blockade, MMF and FK506.
After TCD, the returning T cells in group 3 were predominantly CD8 T cells. These cells which returned in the early post-TCD period likely played an important role in xenograft rejection. We have previously observed, however, that donor-specific unresponsiveness can be induced in the maintenance period of thymokidney recipients, despite the later return CD8 T cells (4, 5). To characterize the cells returning after TCD, further phenotypic analysis (i.e., effector/central memory versus naïve) of returning T cells in our thymokidney xenogeneic model is currently in progress.
The results of anti-CD3 rIT in this study are encouraging, but further investigation is required to optimize the efficacy and side-effect profile. Major concerns associated with our current regimen of anti-CD3 rIT include elevation in transaminases and incidence of infection after induction. With regard to the first concern, we observed that doubling the dose of anti-CD3 rIT correlated with increased liver enzymes. Although these enzyme levels spontaneously decreased, the optimal timing for transplantation (based on T-cell levels) was missed, while we awaited resolution of the elevated levels. Both baboons in group 2, treated with anti-CD3 rIT died with functioning grafts. The cause of pneumonia and cardiac failure in these animals is not clear. We plan to further optimize our anti-CD3 rIT regimen in an attempt to develop an induction regimen that results in a desirable level of T-cell specific depletion. To increase the efficacy of TCD without worsening side-effects, we are currently attempting to increase the half-life in vivo.
MATERIALS AND METHODS
Animals
MGH IACUC guidelines were followed. Baboons, Papio hamadryus (n = 6) were purchased from Mannheimer Foundation, Homestead, FL. To minimize the contributions of humoral responses related to non-Gal natural antibodies, baboons were screened for preformed non-Gal natural antibodies and only baboons with levels less than 30% cytotoxicity at 1:4 ratio were selected to be recipients of thymokidneys. Thymokidneys were prepared in α1, 3– galactosyltransferase gene-knockout (GalT-KO) swine (n = 4), as published (3, 19, 20).
Experimental Groups
Group 1 (n = 2) was used to determine the effect of the anti-CD3 rIT on TCD. In group 2 (n = 2), we evaluated whether anti-CD3 rIT would lead to long-term acceptance of GalT-KO porcine thymokidneys (see details below). Group 3 (n = 2) animals received a LoCD2-based regimen using the standard GalT-KO porcine thymokidney protocol.
Surgery
Preparation of Thymokidney
Thymokidneys were prepared 2-to-3 months before transplantation as previously described (20). Through a median neck incision, thymic tissue was harvested, minced, and implanted beneath the autologous renal-capsule.
Recipient Preparation
Under general anesthesia, baboons were thymectomized 2-to-4 weeks before thymokidney transplantation. Arterial and venous catheters (Saint Gobain Performance Plastics, Reading, PA) were inserted 7 days before transplantation and maintained using a flexible tether/jacket system (Lomir Biomedical, Malone, NY). Recipients underwent bilateral native nephrectomy and splenectomy immediately before transplantation, which have been described elsewhere (5).
Graft Biopsy for Pathologic Examination
Formaldehyde-fixed tissues were stained using both hematoxylin-eosin (H&E) and analyzed according to a standardized grading system (21). Immunohistochemistry was performed using polyclonal antibodies reactive to primate CD3 (polyclonal rabbit anti-human CD3, A0452; Dako, Denmark), primate CD4 (mouse anti-human CD4 mAb, 1F6; Zymed, CA), C4d (polyclonal rabbit anti-human C4d; American Research Products), IgM (polyclonal rabbit anti-human IgM; Dako), IgG (polyclonal rabbit anti-human IgG; Dako), and C5b-9 (mouse anti-human C5b-9mAb, aE11; Dako).
LN Biopsy to Assess TCD
Inguinal and axillary LNs were analyzed for depletion of lymphocytes before (day −14, −1) and after (day 5, 10, 15, 20, 30) administration of anti-CD3 rIT.
Flow Cytometry
Assessments of TCD on Peripheral Lymphocytes
Baboon CD3+, CD4+, CD8+, and CD20+ lymphocytes were identified by direct flow-cytometry using a Becton-Dickinson microfluorimeter (Sunnyvale, CA) (5, 8) using: CD3 (polyclonal rabbit anti-human CD3, A0452; DAKO), CD4 (mouse anti-human CD4 mAb, 1F6; Zymed), CD8 (mouse anti-human CD8; BD Pharmingen), and CD20 (mouse anti-human CD20; BD Pharmingen).
Assessments of Anti Non-Gal IgM and IgG Antibodies
Anti-non Gal IgM and IgG antibody were evaluated by indirect flow-cytometry using a Becton-Dickinson FACScan (Sunnyvale, CA). FITC-conjugated goat anti-human IgG and IgM (Invitrogen) were secondary reagents (22). Data were expressed as median fluorescence intensity.
Acknowledgments
This work was supported by NIH Program Project 5PO1-A145897 (Project 1), RPrEP Core, and DF/HCC, and Grant-in-aid for 19209043 A.
The authors thank Dr. Curt Cetrulo, Dr. Isabel Hanekamp, and Dr. Masayuki Nakagawa for their helpful review of this manuscript; and Rebecca Wark for editorial assistance. They also thank Dr. David Neville for his guidance and sharing his knowledge of recombinant immunotoxin.
Footnotes
H.N. is the primary research fellow and participated in data collection, data interpretation, and manuscript preparation. J.S. is the secondary research fellow and participated in data collection, data interpretation, and manuscript preparation. Z.W. participated in the development of the recombinant immunotoxin. A.S. participated in data collection, pathology preparation, and interpretation. S.M. participated in expert animal care and data collection. B.G. participated in expert animal care and data collection. D.H.S. participated in data interpretation, and project design. K.Y. is the principal investigator and participated in project design, data interpretation, and manuscript preparation.
The authors declare no conflicts of interest.
References
- 1.Yamada K, Griesemer A, Okumi M. Pigs as xenogenic donors. Transplant Rev. 2008;19:164. [Google Scholar]
- 2.Lai L, Kolber-Simonds D, Park K, et al. Production of α-1, 3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 3.Kolber-Simonds D, Lai L, Watt SR, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibro-blasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004;101:7335. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α 1, 3-galactosyltransferase gene-knockout donors and the contrasplantation of vascularized thymic tissue. Nat Med. 2005;11:32. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 5.Griesemer AD, Hirakata A, Shimizu A, et al. Results of Gal-Knockout porcine thymokidney xenografts. Am J transplant. 2009;9:1. doi: 10.1111/j.1600-6143.2009.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y, Swenson K, Sergio JJ, et al. Skin graft tolerance across a discordant xenogeneic barrier. Nat Med. 1996;2:1211. doi: 10.1038/nm1196-1211. [DOI] [PubMed] [Google Scholar]
- 7.Lee LA, Gritsch HA, Sergio JJ, et al. Specific tolerance across a discordant xenogeneic transplantation barrier. Proc Natl Acad Sci U S A. 1994;91:10864. doi: 10.1073/pnas.91.23.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu A, Yamada K, Neville DM, et al. Xenogeneic thymus transplantation in a pig-to-baboon model. Transplantation. 2003;75:282. doi: 10.1097/01.TP.0000044137.97841.99. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto S, Lavelle JM, Vagefi PA, et al. Vascularized thymic lobe transplantation in a pig-to-baboon model: a novel strategy for xenogenic tolerance induction and T-cell reconstitution. Transplantation. 2005;80:1783. doi: 10.1097/01.tp.0000184445.70285.4b. [DOI] [PubMed] [Google Scholar]
- 10.Kim GB, Wang Z, Liu YY, et al. A fold-back single-chain diabody format enhances the bioactivity of an anti-monkey CD3 recombinant diphtheria toxin-based immunotoxin. Protein Eng Des Sel. 2007;20:425. doi: 10.1093/protein/gzm040. [DOI] [PubMed] [Google Scholar]
- 11.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboon using Gal-knockout pig kidney. Nat Med. 2005;11:1295. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cozzi E, Simioni P, Nottle MB, et al. Preliminary study in a life supporting pig to primate xenotransplantation model using Gal KO pigs transgenic for human CD39, CD55, CD59 and fucosyltransferase. Xenotransplantation. 2010;16:544. [Google Scholar]
- 13.Wallgren AC, karlsson-Parra A, Korsgren O. The main infiltrating cell in xenograft rejection is a CD4+ macrophage and not a T lymphocyte. Transplantation. 1995;60:594. doi: 10.1097/00007890-199509270-00013. [DOI] [PubMed] [Google Scholar]
- 14.Wu G, Korsgren O, Zhang J, et al. Pig islet xenograft rejection is markedly delayed in macropharge-depleted mice: a study in streptozotocin diabetic animals. Xenotransplantation. 2000;7:214. doi: 10.1034/j.1399-3089.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogenic T-cell response. Evidence for allelic specificity of MLR and for both direct and indirect pathways of recognition. J Immunol. 1995;155:5249. [PubMed] [Google Scholar]
- 16.Hisashi Y, Yamada K, Kuwaki K, et al. Rejection of cardiac xenografts transplanted from α 1, 3-galactosyltransferase gene-knockout (GalT-KO) pig to baboons. Am J transplant. 2008;8:2516. doi: 10.1111/j.1600-6143.2008.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu A, Yamada K. Histopathology of xenografts in pig to non-human primate discordant xenotransplantation. Clin Transplant. 2010;24:11. doi: 10.1111/j.1399-0012.2010.01270.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamada K, Vagefi PA, Utsugi R, et al. Thymic transplantation in miniature swine: III. Induction of tolerance by transplantation of composite thymokidneys across fully major histocompatibility complex-mismatched barriers. Transplantation. 2003;76:530. doi: 10.1097/01.TP.0000080608.42480.E8. [DOI] [PubMed] [Google Scholar]
- 19.Sachs DH. The pig as a potential xenograft donor. Path Biol. 1994;42:217. [PubMed] [Google Scholar]
- 20.Yamada K, Shimizu A, Ierino FL, et al. Thymic transplantation in miniature swine. I. Development and function of the “thymokidney. Transplantation. 1999;68:1684. doi: 10.1097/00007890-199912150-00011. [DOI] [PubMed] [Google Scholar]
- 21.Colvin RB. The renal allograft biopsy. Kidney Int. 1996;50:1069. doi: 10.1038/ki.1996.410. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu A, Yamada K, Sachs DH, Colvin RB. Intragraft events preceding chronic renal allograft rejection in a modified tolerance protocol. Kidney Int. 2000;58:2546. doi: 10.1046/j.1523-1755.2000.00440.x. [DOI] [PubMed] [Google Scholar]