Abstract
Cardiac resynchronization therapy (CRT) represents the major new advance for the treatment of heart failure since the new millennium. With this therapy, failing hearts with discoordinate contraction due to conduction delay are subjected to bi-ventricular stimulation to “resynchronize” contraction and improve chamber function. Remarkably, CRT was mostly developed and tested in patients first, and the speed at which the concept became translated to an approved clinical therapy was unusually quick. To date, CRT is the only treatment for heart failure that can both acutely and chronically improve the systolic pump performance of the human failing heart, yet also enhances long term survival. This raises the importance of understanding how CRT works at the molecular and cellular level, as these insights might shed light on new approaches to treating heart failure more generally. Over the past 7 years, my laboratory and others at Johns Hopkins developed novel animal models to start to address this question, and new results are revealing intriguing insights into the mechanisms of CRT. This review, presented on the occasion of the 4th Annual Douglas Zipes Lecture at the 2009 Scientific Sessions of the Heart Rhythm Society, highlights these advances and new directions in CRT research.
Introduction
Congestive heart failure (CHF) is the leading cause of morbidity and mortality in adults throughout Westernized societies1–3. At least half of these patients have dilated cardiomyopathy (reduced ejection fraction), and among these, many develop left ventricular dyssynchrony associated with conduction delay. The presence of dyssynchrony markedly worsens CHF morbidity and mortality independent of traditional risk factors4–7. Dyssynchrony generates marked regional heterogeneity of both function and loading, effectively dividing the heart into early and late contracting zones. The early-activated territory is relatively unloaded, its work being wasted first by pre-stretching a still quiescent opposing wall and then by being stretched itself in late systole as the delayed territory contracts. Stress in the latter region is correspondingly greater8, and chamber function inefficient9. Depending upon the metric used, current estimates of the prevalence of dyssynchrony vary from 25–30% of CHF patients (based on QRS widening), to >60% based on tissue Doppler or MRI measures of dyssynchrony 10,11. To treat this, cardiac resynchronization therapy was developed in the mid 1990’s, after investigators found that bi-ventricular (or LV only) pre-excitation could restore mechanical synchrony and improve acute LV mechanics12, energetic efficiency13, and regional metabolism14. Subsequent large-scale clinical trials demonstrated benefits for selected patients for improving symptoms, and lowering rehospitalization rates and mortality15–17.
Despite its overall success, problems remain with CRT as nearly a third of seemingly appropriate recipients do not benefit clinically from the therapy, and our ability to identify non-responders before the fact remains poor. We have presumed that dyssynchrony – first measured electrophysiologically and more recently using various imaging metrics to examine wall motion timing - is the key property to be identified, but is this correct? Mechanical dyssynchrony seems important, yet our measures have not predicted response well18, and even improvement in dyssynchrony after CRT is initiated weakly predicts chronic response19. We all recognize that having a low EF does not predict whether the patient will be able to play golf, conduct normal daily activities, or be virtually bed ridden – there is a lot more to the disease than is apparent from the wall motion. Regional dyssynchrony may act the same way – we need to look under the hood.
The ability of CRT to both acutely and chronically improve systolic pump function yet also prolong survival is unique among heart failure treatments to date. There are no existing drugs that can achieve this; positive inotropes not only fail to prolong survival but have often worsened arrhythmia and mortality, whereas agents such as beta blockers work chronically, but do not acutely enhance pump function. How is this achieved? Is this all due to improving chamber level mechanics and efficiency or is there more going on? Is dyssynchronous CHF (dys-HF) simply a worse form of disease, or does it have unique aspects that play a role in when CRT is effective? How does CRT influence basic cellular and molecular properties, and is this purely regional in nature or can global changes occur? Despite more than a decade of clinical experience with CRT, such basic mechanistic questions have been largely unanswered until recently. CRT in this regard was somewhat unusual as a heart failure therapy, as it moved from bedside to bench rather than the other way around. Only recently have new animal models been developed that are beginning to reveal how potent CRT is at the molecular and cellular level, and define unique features of dys-HF.
Developing an animal model
To initiate analysis of how dyssynchrony affects the failing heart and what CRT does about it, we developed a large animal model. Leveraging decades of research in the canine rapid-pacing model of dilated HF, we modified the model in two ways. First, we doubled the time course to 6 weeks, allowing for a 3-wk period in which dyssynchronous rapid pacing (atrial pacing superimposed on a left bundle branch block) was implemented that could be followed by 3-wks of the same (dys-HF) or of bi-ventricular rapid pacing (CRT). Both models involved 6 wks of rapid tachypacing, and indeed this generated similar heart failure with reduced function, dilation, and elevated filling pressures. However, as in humans, dogs treated with bi-V rapid pacing during weeks 4-6 had a slight improvement in EF and stroke volume, while in those with continued dys-HF, both declined. The difference after 6 weeks was significant (Fig 1a). Furthermore, we showed evidence for resynchronization based on standard tissue Doppler parameters (Fig 1b). Thus, we have two conditions, both involving the same underlying rapid pacing model, but with the pattern of contraction altered mid-way through in one instance. It is worth keeping this in mind, as the changes observed with the CRT model are all the more remarkable given how little was altered. This is not a regionally ischemic model, nor a common form of human heart failure, but it does provide a platform to selectively alter dyssynchrony vs resynchronization of contraction in the setting of depressed dilated HF.
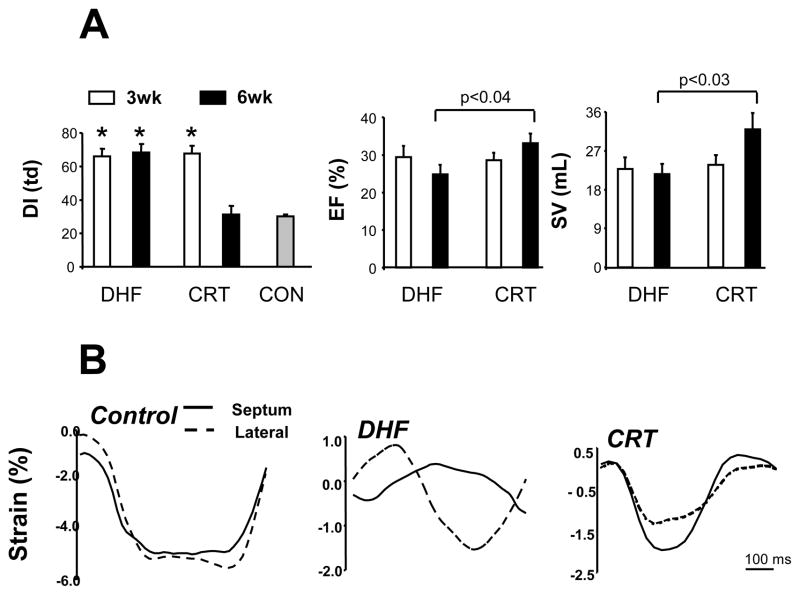
Figure 1.
Development of a canine model of cardiac dyssynchrony and resynchronization. In this model, both dysynchronous heart failure (DHF) and resynchronized heart failure (CRT) groups are exposed first to tachypacing for 3-wks (in the presence of a pre-established LBBB). This is continued for an additional 3-wks in DHF hearts, but switched to rapid bi-ventricular pacing in CRT. A) LV dysynchrony (DI) is assessed by the variance in timing delay in systolic motion using tissue Doppler. This is similar in both groups at 3 wks, and declines to control levels (synchrony) only in the CRT group. Middle and right panels show slight improvement in ejection fraction (EF) and stroke volume (SV) in CRT groups that are greater than the DHF group at 6 weeks. B) Myocardial strain patterns obtained in the anterior and lateral wall (tissue Doppler) for a normal control, DHF, and CRT heart. With DHF, there are major disparities in the timing of shortening and reciprocal shortening/stretch in each region, and this is amelioratedby CRT. (adapted from Chakir et al.29).
Molecular Polarization and the impact of CRT
Initial molecular insights into dys-HF came in a brief report by Spragg et al.20 who examined the effects at 3 wks of dyssynchronous HF revealing selective downregulation (calcium handling proteins, connexin 43) and upregulation (mitogen activated protein kinases) of key proteins involved with stress remodeling, contraction, and conduction occurred in the lateral wall only. This regional molecular change was not observed in synchronous CHF. More recently, Chakir et al.21 used the 6-wk model and showed dys-HF showed regional amplification of lateral stress kinase and cytokine stimulation, whereas in the CRT ventricle these changes were reversed and expression/activity was again homogeneous (Fig 2). This is just the tip of the iceberg. Using genome-wide analysis, we recently reported >3000 genes were differentially altered between anterior and lateral walls, with most changes occurring in the anterior wall that broadly recapitulated a fetal gene pattern22. Importantly, CRT reverses this geographically disparate gene expression – rendering patters that are similar to normal controls, though absolute expression levels remain characteristic of CHF. Thus, the dys-HF ventricle is strikingly heterogeneous at the molecular and protein expression/activation level, and is not just worse heart failure. This molecular substrate may be important if not essential for CRT to really benefit the heart.
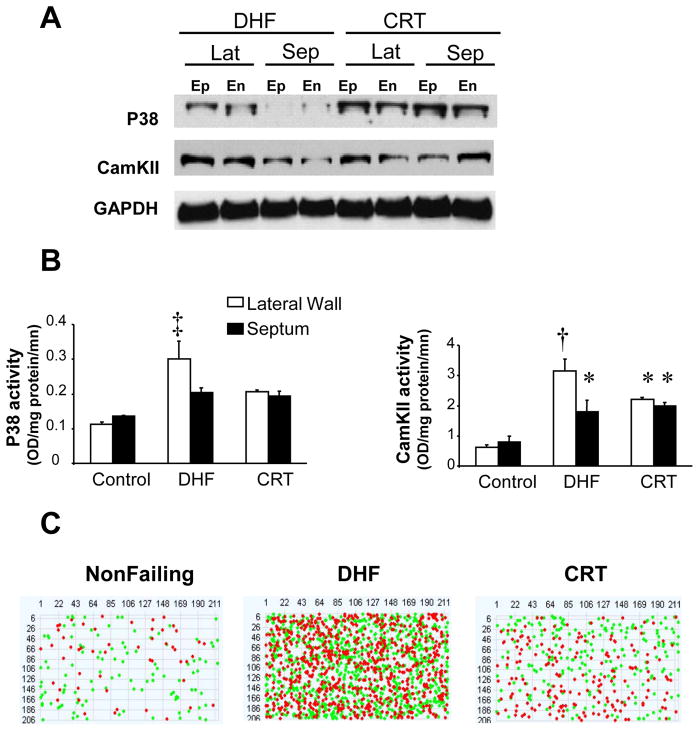
Figure 2.
A) Regional differences in stress response kinases (p38 mitogen activated kinase and calcium-calmodulin kinase II are shown) in DHF hearts (higher in the lateral (lat) versus septal (sep) wall in both endocardial (En) and epicardial (Ep) layers) are rendered homogeneous by CRT. Example protein expression (Western blot) is shown, with GAPDH expression shown as a loading control. B) Summarydata showing kinase activity for each respective enzyme, confirming regional enhancement in the lateral versus septal wall with DHF that is re- homogenized by CRT. C) Heterogeneity of gene expression revealed by sample pseudoarray plots of microarrays (each dot is a different gene, row and columns reflect full 43.4K array). Genes that are up (green) versus down (red) regulated between anterior/septal versus lateral wall from an individual heart are depicted. There is little heterogeneity normally, profound heterogeneity of gene expression in DHF, and amelioration though not complete restoration of this in CRT hearts. From Barth et al.22.
Human gene expression data are scant, but some results have been reported. The inability to biopsy from different targeted regions has prevented heterogeneity from being examined, evidence that reduced expression of calcium handling proteins, beta-1 receptor, and elevated TNFα occur in dys-HF and are reversed by CRT has been obtained 23,24. Some limited studies have suggested that the ability of CRT to reverse these molecular changes may be related to its capacity to benefit the patient clinically25, though this work remains preliminary.
Electrophysiologic influences of dys-HF and CRT
The dys-HF heart is characterized by several marked electrophysiologic abnormalities. The Carnes laboratory first reported prolongation of the action potential, a reduction in the L-type Ca2+ current, and reduced whole cell Ca2+ transients, all of which were reversed by CRT in their chronic canine model26. Our group recently reported more detailed analyses27, revealing reductions in the inward and delayed rectifier current (Fig 3a) with dys-HF that were improved by CRT. In contrast, the transient outward current was markedly depressed in dys-HF and unaltered by CRT, confirming the persistence of a HF phenotype. We also found reduced L-type Ca2+ current but only in the late activated (lateral) wall, and this corresponded to reduced expression of the beta-2 sub-unit of the channel. The resulting prolongation of the action potential in dys-HF was coupled to more easily induced after-depolarizations, and this too was markedly blunted in the CRT model showing physiologic relevance to these changes (Fig 3b).
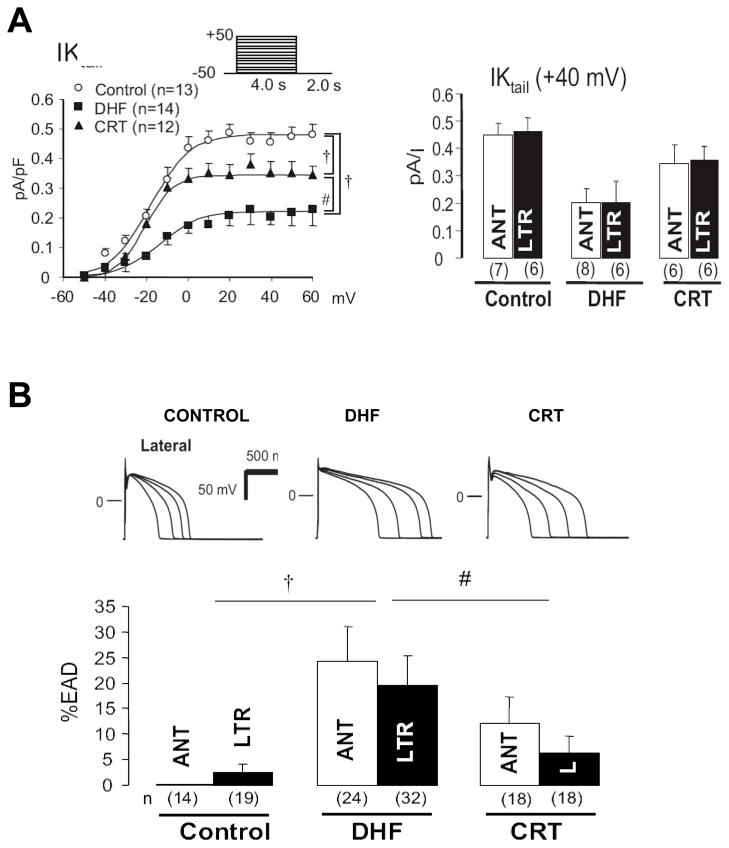
Figure 3.
Electrophysiologic effects of DHF and CRT. A) Summary data are shown for the delayed rectifier (IK tail current), a potassium current. The left panel displays current-voltage dependence and the left summary data at +40 mV. The tail current is globally and substantially reduced by DHF and partially and significantly restored by CRT. B) DHF displays prolongation of action potential duration (APD) notably in the lateral wall. APD tracings in upper panels are generated at varying stimulation frequency (longer APD at slower rates), and at each rate, APD prolongs with DHF, but is improved by CRT. The lower bar graph shows the frequency of early afterdepolarizations (%EAD) in each condition. DHF displayed increased EADs, and this was reduced (particularly in the lateral wall) by CRT. Adapted from Aiba et al.27.
Global changes in cell survival signaling
As noted, though some changes in molecular signaling (e.g. stress kinases) had been found to be vary strikingly between early (antero-septum) versus late (lateral wall) activated regions, other changes (e.g. IK1, IK) were more globally altered. This observation that global molecular signaling could also be generated by CRT was first revealed by Chakir et al21 with respect to cell survival signaling. Apoptosis was enhanced chamber-wide in dys-CRT, and this was found coupled with a global decline in the phosphorylation and activation of the cell survival signaling kinase, Akt. Apoptosis is modulated downstream of Akt by several potential proteins, including BAD, which when bound to Bcl-2 in the mitochondria promotes cell death, but when phosphorylated, binds to 14-3-3 and is exported out of the mitochondria where it enhances survival. This signaling cascade appears to play a role globally with dys-HF, and is favorably influenced by CRT (Fig 4).
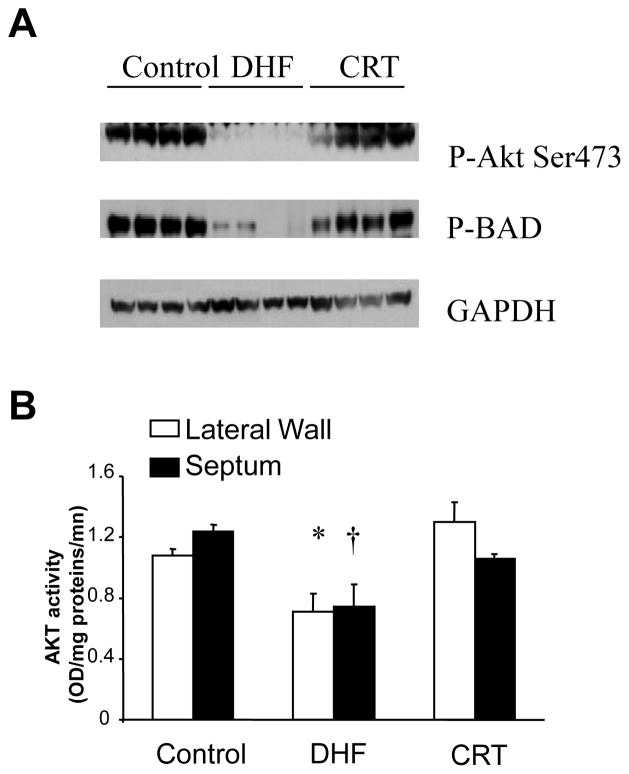
Figure 4.
CRT improves cell survival signaling associated with enhanced phosphorylation (activation) of the serine threonine kinase Akt, and the mitochondrial-apoptosis regulating protein BAD. A) Example phosphor-protein blot showing marked reduction of both Akt and BAD phosphorylation in DHF that are improved by CRT. The four columns (lanes) for each condition (control, DHF, CRT) reflect anterior and lateral walls, and epicardial and endocardial tissue from each region. Thus, these changes are also global in nature. GAPDH is shown for protein loading control. B) Akt activity assay confirms differential regulation that is similar in septal and laterals with DHF and improved by CRT. Adapted from Chakir et al.21.
Improved survival signaling raised an interesting question of whether mitochondrial function was favorably altered by CRT. This was also examined by our investigative group. In a new study, Agnetti et al.28 systematically studied the mitochondrial sub-proteome and revealed many changes at the expression and post-translational level induced by CRT that support improved oxidative phosphorylation, mitochondrial synthesis and stabilization, and anti-oxidant effects. Thus, CRT may improve energy balance not only by improving chamber level efficiency, but by targeted improvement of the mitochondrial function. Just how this is being achieved and its implications for CRT and/or heart failure generally is under exploration.
Rest and adrenergic-stimulated myocyte function
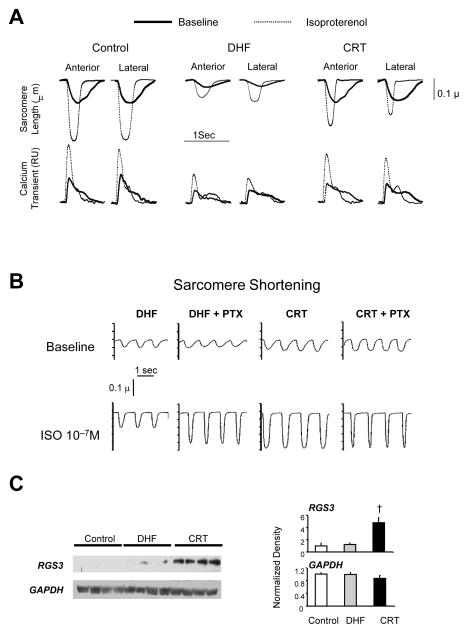
Congestive heart failure is typically a disease of exertion, therefore understanding mechanisms for cardiac reserve capacity are paramount. In new studies, Chakir et al.29 isolated myocytes from dys-HF and CRT hearts, and compared their rest sarcomere shortening and Ca2+ transient behavior to each other and to normal control myocytes. Dys-HF myocytes had very reduced rest and isoproterenol (ISO) stimulated shortening and Ca2+-transients compared to normal, consistent with what we have come to understand with heart failure. Yet, both were markedly improved with CRT (Fig 5a). This is indeed striking, since recall that our CRT model still involves 6-wks of tachypacing, and indeed CRT and dys-HF hearts have substantial and similar dilation, elevated EDP. Improved cellular function was accompanied in vivo by a decline in myocardial catecholamines, so the balance of neuro-stimulation and myocyte responsiveness was restored towards normal by CRT. Intriguingly, these cellular changes were also global, not regional. We further tested for mechanisms underlying depressed β-AR signaling in dys-HF that were ameliorated by CRT. Both β1 and β2 receptor gene expression and number (radioligand binding assay) were reduced by dys-HF, and CRT enhanced β1 but not β2 receptor number. Using forskolin to directly activate adenylate cyclase activity, we found this too was depressed in dys-HF and improved by CRT. Among the most striking changes, however, was in inhibitory G-protein (Gi) signaling. As shown in figure 5b, myocytes from dys-HF hearts showed marked potentiation of the ISO response if they were first incubated with pertussis toxin, which inhibits G i. In contrast, CRT myocytes displayed enhanced responses at baseline, and PTX had no effect on changing this – as if Gi was already inhibited by CRT. Giα was upregulated in dys-HF, as has been seen in human HF, but this remained high in CRT tissue, so could not itself explain the change. However, we found selective upregulation of proteins known as RGS (regulators of g-coupled protein signaling). RGS proteins negatively regulate G-coupled signaling by acting as GTPase accelerators, removing GTP from the activated α-subunit so the trimeric G-protein complex re-forms and coupled signaling is suppressed. RGS3, a protein known to suppress Gi, was selectively upregulated in CRT hearts (Fig 5c), and this may be quite important to improved functional reserve with this therapy. Ongoing genetic studies are testing this hypothesis more directly.
Figure 5.
CRT improves rest and beta-adrenergic responsiveness in isolated myocytes from both early and late activated regions. A) Example time tracings of sarcomere length (upper) and whole cell calcium transients (Fura2-AM; lower) for myocytes isolated from anterior versus lateral walls from control, DHF, and CRT left ventricles. Baseline and results with isoproterenol (ISO) stimulation are shown. There is marked depression of resting function and calcium transients, and the ISO response in both, and this is strikingly improved by CRT. B) DHF results in enhanced inhibitory G-protein (Gi) coupling in DHF myocytes that is suppressed by CRT. In DHF, pretreatment with the Gi inhibitor – pertussis toxin (PTX) enhanced the ISO stimulated contraction, whereas CRT myocytes had enhanced shortening without ISO, and there was no further increase by adding PTX. C) Upregulation of regulator of G-protein signaling 3, a negative modulator of Gi coupled signaling, by CRT. Left panels show example blots and right panels show summary densitometry. The four lanes are from four different hearts in each group, data from the lateral wall. Similar changes were observed in the anterior wall as well. Adapted from Chakir et al.29.
Mechanisms for Molecular/Cellular Changes and Possible Role in Non-Responders
The root causes for the many changes observed in both global and regional cellular/molecular signaling with DHF and CRT remain uncertain, but we can speculate. As noted, CRT both alters regional stresses and strains and improves global mechanoenergetics. While many of the alterations we’ve observed appear global in nature, I suspect this is not simply related to the modest improvement in chamber function our model generated. This is because both DHF and CRT models induce heart failure from 6-wks of rapid pacing, and so functional differences at the whole heart level while present are not dramatic. Yet, the amelioration of cell survival, potassium currents, and myocyte function and β-AR responsiveness are quite striking. Rather, we hypothesize this reflects a general yet still cardiac targeted effect from CRT resulting from the restoration of normal homogeneity of excitation-contraction, perhaps involving local (myocardial) changes in neuron-humoral activation. Both early and late activated regions experience altered timing and magnitude of local stretch and stress, and perhaps for some cascades (e.g stress kinases) this difference is quite important and signaling specific to each area. For other changes such as Akt and IK, the specifics of regional electromechanical heterogeneity may be less important than simply that they exist throughout the heart, and may contribute to altered local regulation. Ongoing studies are attempting to better sort out this interesting issue.
Secondly, it is worth considering how these results may relate to the non-responder problem. As noted, having heart failure and a depressed ejection fraction does not necessarily predict the response to specific pharmacologic therapies, and variability in underlying genetics, as well as molecular and cellular biology are increasingly thought to be key determining factors30,31. We do not yet know whether underlying cellular and/or molecular signaling responses to dysynchrony vary among individuals with DHF who are typically targeted for resynchronization therapy, but it would be surprising if this were not the case. Differences in etiology, patient’s genetics, and myocardial responses to discoordination may well dictate how well a patient will respond to CRT. One could imagine that lack of depressed Akt signaling, IK, or up-regulated Gi coupling in a given DHF patient might diminish the impact of CRT if amelioration of these changes are indeed important primary targets. The level of molecular heterogeneity as depicted in Figure 2C may itself prove a useful marker that the dysynchrony has adversely impacted the ventricle. Others have suggested that non-responders do not display the same extent of improvement in some gene expression abnormalities as responders24, though this does not address whether a molecular signature may predict a future lack of response independent of apparent mechanical dysynchrony. It is worth repeating that while studies have claimed improvement in mechanical dysynchrony is mandatory for a clinical CRT response, the correlation between response (reverse remodeling based on reduction in end-systolic volume) and the extent of resynchronization (tissue Doppler) is poor to non-existent 19,32. While this could reflect our metrics, differences in underlying myocardial pathobiology may indeed play an important role in explaining the variance.
Conclusion
In a relatively short period, we and others have revealed profound basic cellular and molecular changes in the dys-HF heart – many of which appear characteristic of this form of failure and not observed in synchronous HF – as well as how CRT can substantially target these changes and reverse them. Reverse remodeling the likes of what we have observed has been previously reported with LV assist devices, but it is all the more remarkable with CRT given that these hearts remain loaded and contract. It is intriguing to speculate that responders to CRT indeed have a molecular signature that could prove an important adjunct to visible wall motion changes that we have solely focused on to date. Future studies to test this possibility are needed.
Acknowledgments
This work reflects the summary efforts of a terrific team of investigators at Johns Hopkins that I have worked closely with for many years. I would particularly recognize my faculty colleagues, Gordon Tomaselli, Jennifer VanEyk, Albert Lardo, Ronald Berger, Henry Halperin, and David Spragg, and an extremely talented group of post doctoral fellows whose efforts I highlighted in this review: Khalid Chakir, Takeshi Aiba, Andreas Barth, and Giulio Agnetti. I also want to thank Dr. Christophe Leclercq, who back in the early 2000’s decided to take a sabbatical from his Cardiology life in Rennes, come to Baltimore, and who ended up playing a central role in getting our CRT model established and first studied. The work highlighted in this review was funded by National Heart Lung and Blood Institute Program Project Grant (HL-077180) and Fellow training grant: HL07227-34.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rodeheffer RJ. The new epidemiology of heart failure. Curr Cardiol Rep. 2003;5:181–186. doi: 10.1007/s11886-003-0046-8. [DOI] [PubMed] [Google Scholar]
- 2.Tsuyuki RT, Shibata MC, Nilsson C, Hervas-Malo M. Contemporary burden of illness of congestive heart failure in Canada. Can J Cardiol. 2003;19:436–438. [PubMed] [Google Scholar]
- 3.Masoudi FA, Havranek EP, Krumholz HM. The burden of chronic congestive heart failure in older persons: magnitude and implications for policy and research. Heart Fail Rev. 2002;7:9–16. doi: 10.1023/a:1013793621248. [DOI] [PubMed] [Google Scholar]
- 4.Iuliano S, Fisher SG, Karasik PE, Fletcher RD, Singh SN. QRS duration and mortality in patients with congestive heart failure. Am Heart J. 2002;143:1085–1091. doi: 10.1067/mhj.2002.122516. [DOI] [PubMed] [Google Scholar]
- 5.Kashani A, Barold SS. Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol. 2005;46:2183–2192. doi: 10.1016/j.jacc.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 6.Bader H, Garrigue S, Lafitte S, et al. Intra-left ventricular electromechanical asynchrony. A new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol. 2004;43:248–256. doi: 10.1016/j.jacc.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 7.Cho GY, Song JK, Park WJ, et al. Mechanical dyssynchrony assessed by tissue Doppler imaging is a powerful predictor of mortality in congestive heart failure with normal QRS duration. J Am Coll Cardiol. 2005;46:2237–2243. doi: 10.1016/j.jacc.2004.11.074. [DOI] [PubMed] [Google Scholar]
- 8.Prinzen FW, Hunter WC, Wyman BT, McVeigh ER. Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol. 1999;33:1735–1742. doi: 10.1016/s0735-1097(99)00068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owen CH, Esposito DJ, Davis JW, Glower DD. The effects of ventricular pacing on left ventricular geometry, function, myocardial oxygen consumption, and efficiency of contraction in conscious dogs. Pacing Clin Electrophysiol. 1998;21:1417–1429. doi: 10.1111/j.1540-8159.1998.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 10.Chalil S, Stegemann B, Muhyaldeen S, et al. Intraventricular dyssynchrony predicts mortality and morbidity after cardiac resynchronization therapy: a study using cardiovascular magnetic resonance tissue synchronization imaging. J Am Coll Cardiol. 2007;50:243–252. doi: 10.1016/j.jacc.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 11.Yu CM, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart. 2003;89:54–60. doi: 10.1136/heart.89.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kass DA, Chen CH, Curry C, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:1567–1573. doi: 10.1161/01.cir.99.12.1567. [DOI] [PubMed] [Google Scholar]
- 13.Nelson GS, Berger RD, Fetics BJ, et al. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation. 2000;102:3053–3059. doi: 10.1161/01.cir.102.25.3053. [DOI] [PubMed] [Google Scholar]
- 14.Nowak B, Sinha AM, Schaefer WM, et al. Cardiac resynchronization therapy homogenizes myocardial glucose metabolism and perfusion in dilated cardiomyopathy and left bundle branch block. J Am Coll Cardiol. 2003;41:1523–1528. doi: 10.1016/s0735-1097(03)00257-2. [DOI] [PubMed] [Google Scholar]
- 15.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 16.Cleland JG, Daubert JC, Erdmann E, et al. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase] Eur Heart J. 2006;27:1928–1932. doi: 10.1093/eurheartj/ehl099. [DOI] [PubMed] [Google Scholar]
- 17.Abraham WT. Cardiac resynchronization therapy. Prog Cardiovasc Dis. 2006;48:232–238. doi: 10.1016/j.pcad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 19.Bleeker GB, Mollema SA, olman ER, et al. Left ventricular resynchronization is mandatory for response to cardiac resynchronization therapy. Circulation. 2007;116:1140–1148. doi: 10.1161/CIRCULATIONAHA.106.677005. [DOI] [PubMed] [Google Scholar]
- 20.Spragg DD, Leclercq C, Loghmani M, et al. Regional alterations in protein expression inthe dyssynchronous failing heart. Circulation. 2003;108:929–932. doi: 10.1161/01.CIR.0000088782.99568.CA. [DOI] [PubMed] [Google Scholar]
- 21.Chakir K, Daya SK, Tunin RS, et al. Reversal of global apoptosis and regional stress kinase activation by cardiac resynchronization. Circulation. 2008;117:1369–1377. doi: 10.1161/CIRCULATIONAHA.107.706291. [DOI] [PubMed] [Google Scholar]
- 22.Barth AS, Aiba T, Halperin V, et al. Cardiac resynchronization therapy corrects dyssynchrony-induced regional gene expression changes on a genomic level. Circ Cardiovasc Gen. 2009 doi: 10.1161/CIRCGENETICS.108.832345. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanderheyden M, Mullens W, Delrue L, et al. Endomyocardial upregulation of beta1 adrenoreceptor gene expression and myocardial contractile reserve following cardiac resynchronization therapy. J Card Fail. 2008;14:172–178. doi: 10.1016/j.cardfail.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Vanderheyden M, Mullens W, Delrue L, et al. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders. J Am Coll Cardiol. 2008;51:129–136. doi: 10.1016/j.jacc.2007.07.087. [DOI] [PubMed] [Google Scholar]
- 25.Mullens W, Bartunek J, Wilson Tang WH, et al. Early and late effects of cardiac resynchronization therapy on force-frequency relation and contractility regulating gene expression in heart failure patients. Heart Rhythm. 2008;5:52–59. doi: 10.1016/j.hrthm.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Nishijima Y, Sridhar A, Viatchenko-Karpinski S, et al. Chronic cardiac resynchronization therapy and reverse ventricular remodeling in a model of nonischemic cardiomyopathy. Life Sci. 2007;81:1152–1159. doi: 10.1016/j.lfs.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aiba T, Hasketh GG, Barth AS, et al. Electrophysioloigcal consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation. 2009;119:1220–1230. doi: 10.1161/CIRCULATIONAHA.108.794834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agnetti G, Kaludercic N, Kane LA, et al. Modulation of mitochondrial proteome and improved mitochondrial function by bi-ventricular pacing of dyssynchronous failing hearts. Circ Cardiovasc Gen. 2009 doi: 10.1161/CIRCGENETICS.109.871236. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakir K, Daya SK, Aiba T, et al. Mechanisms of enhanced beta-adrenergic reserve from cardiac resynchronization therapy. Circulation. 2009;119:1231–1240. doi: 10.1161/CIRCULATIONAHA.108.774752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liggett SB, Cresci S, Kelly RJ, et al. A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14:510–517. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mialet PJ, Rathz DA, Petrashevskaya NN, et al. Beta 1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat Med. 2003;9:1300–1305. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- 32.Kass DA. Highlighting the R in CRT. Circulation. 2007;116:1434–1436. doi: 10.1161/CIRCULATIONAHA.107.728748. [DOI] [PubMed] [Google Scholar]