Abstract
It is well known that the over production of reactive oxygen species is harmful for living organisms and it damages major cellular constituents such as DNA, protein, and lipid. At present, searching of new plant sources having free radical scavenging activity is an important field of research in phytomedicine as natural products are safe and relatively low cost. In this respect, attention has been focused to evaluate the antioxidant potential of hydro-methanolic extract of seed of Caesalpinia bonduc (Caesalpenacae) using different in vitro models. To evaluate the antioxidant activity, extract was examined on 2, 2-diphenyl-1-picrylhydrazyl radical scavenging effect, scavenging of hydrogen peroxide, hydroxyl radical scavenging potential, and anti-lipid peroxidation activity by biochemical methods. Total phenol and flavonoids contents in the said extract were measured biochemically as per standard methods. Results were compared with butylated hydroxyl toluene and α-tocopherol. Results indicated that hydro-methanolic extract has strong scavenging activity on 2, 2-diphenyl-1-picrylhydrazyl radical with IC50 value 157.4 μg/ml, hydroxyl radical with IC50 value 61.9 μg/ml and hydrogen peroxide with IC50 value 64.32 μg/ml. Hydro-methanolic extract also showed notable inhibition in lipid peroxidation having IC50 value 58.87 μg/ml. Phytochemical study focused that the extract is rich in phenolic compounds (24.66 mg gallic acid equivalent/g dried extract) and flavonoids (136.65 mg quercetin equivalent/g dried extract). Findings of the experiment indicated that the hydro-methanolic extract of seed of Caesalpinia bonduc is a source of natural antioxidants.
Keywords: Antioxidant, Caesalpinia bonduc, free radicals, lipid peroxidation
INTRODUCTION
Majority of the diseases or disorders including diabetes mellitus, arthritis, cancer, ageing processes are often connected with reactive oxygen species (ROS) and lipid peroxidation.[1,2] The main contributor of oxidative stress is uncontrolled generation of free radical together with reduced levels of antioxidative vitamins and enzymes.[3] These free radicals interfere with biochemical processes and represent an essential part of aerobic life and metabolism.[4] Therefore, antioxidant with free radical scavenging activities may have great relevance for the management and therapeutics of free radical inducing diseases. Polyphenolic compounds like flavonoids and phenolic acid commonly found in plant have multiples biological effects including antioxidant activity, free radical scavenging abilities, anti inflammatory, and anti carcinogenic activities.[5] Currently, available synthetic antioxidants like butylated hydroxytoluene (BHT), tertiary butylated hydroquinone, and gallic acid esters have suspected to cause or prompt negative health effects.[6] Hence, strong restrictions have been placed on their application and there is a trend to substitute them with phyto-antioxidants. Neutraceuticals having antioxidant properties are non toxic or may have minimum side effects than synthetic compounds. In this concern, our attempt is to search out the neutraceuticals for substitute of synthetic antioxidant drug. Therefore, the aim of the present study is to investigate the antioxidant activity of hydro-methanolic extract of seed of Caesalpinia bonduc (C. bonduc) on different in vitro models such as 2, 2-diphenyl-1-picrylhydrazyl (DPPH), hydrogen peroxide, hydroxyl radical, and lipid peroxidation inhibition activity. The antioxidant activities of the said plant part have been expressed in term of percentage of inhibition on free radical generation on the different in-vitro experimental models.
C. bonduc is a medicinal plant belonging to the family of Caesalpenacae. In Indian traditional plant medicine, it has been considered as an important remedy for the treatment of filarial infection, tumor, asthma, and diabetes.[7] This plant part also has a remedial effect on hyperglycemic and hyperlipidemic state in diabetic rats which was noted in our previous report.[8]
MATERIALS AND METHODS
Chemicals
Chemicals like 2, 2-diphenyl-1-picrylhydrazyl (DPPH), trichloroacetic Acid (TCA), thiobarbituric Acid (TBA), gallic acid, ascorbic acid, α-tocopherol, butylated hydroxytoluene (BHT), and folin-ciocalteu (FC) reagent were used in this experiment and these were purchased from Himedia Laboratories Pvt. Ltd, Mumbai, India. Ethylene diamine tetra acetic acid (EDTA), hydrogen peroxide, sodium hydroxide (NaOH), sodium carbonate (Na2CO3), ferric chloride (FeCl2), and sodium nitrite (NaNO2) were purchased from Sisco Research Laboratories (SRL) Pvt. Ltd. Mumbai, India. Aluminium chloride (AlCl3) was obtained from Sd Fine Chemicals Ltd, Mumbai, India.
Collection of Plant Material
The dried seeds of C. bonduc were collected from village area of Paschim Medinipur district, West Bengal, India, in the months of July-September. The plant was identified by the taxonomist, Prof. R. K. Bhakat, Department of Botany and Forestry, Vidyasagar University, Midnapore, West Bengal, and the voucher specimen was deposited having the Reference No. Bio-Med/V.U/C.B/24/10.
Preparation of Hydro-Methanolic Extract of Seed of C. bonduc
Hydro-methanolic extract of seeds of C. bonduc was prepared as per the standard method.[9] In brief, fresh seeds of C. bonduc were dried in an incubator for 2 days at 40°C, then crushed in an electric grinder and pulverized. From this powder, 50 g was suspended in the mixture solvent consisting of 80 ml of water and 120 ml methanol in a container for 48 hrs at room temperature and then the supernatant was filtered through No. 3 Whatman filter paper. The filtrate was concentrated and the collected residue was preserved in a refrigerator at 2-8°C for use in the experiments.
Phytochemical Screening
Qualitative tests for phytochemicals of the seeds of C. bonduc were performed as per the standard methods.[10]
Determination of Total Flavonoid Content
The total flavonoid content was determined with the AlCl3 method[11] using quercetin as a standard. The seed extract (0.25 ml) was added to 1.25 ml of distilled water followed by addition of 75 μl of 5% NaNO2. The preparation was allowed to incubate at room temperature for 5 minutes, and then AlCl3 (0.15 ml, 10%) was added. After a further incubation for 6 min at room temperature, the reaction mixture was treated with 0.5 ml of 1mM NaOH. Finally, the reaction mixture was diluted with 275 μl of distilled water. Further incubation for 20 min at room temperature was performed and the absorbance was measured at 510 nm. All tests were performed in triplet. The flavonoid content was expressed as mg of quercertin equivalents (QE) per gram (g) of dried extract.
Determination of Total Phenolic Content
Total phenolic content was determined using the Folin-Ciocalteu (FC) reagent method[12] with slight modification. Briefly, the seed extract (0.5 ml) was mixed with 0.5 ml of FC reagent (previously diluted with 1:1 with distilled water) and incubated for 5 min at room temperature, and then 1 ml of 2% Na2CO3 solution was added. After incubation at room temperature for 10 min, the absorbance was noted. Gallic acid monohydrate was used as the standard. The phenolic content was expressed as mg of gallic acid equivalents (GAE) per g of dried extract.
Assessment of in-vitro Antioxidant Activity of Hydro-Methanolic Extract
DPPH radical scavenging assay
The radical scavenging activity of C. bonduc against DPPH was determined spectrophotometrically.[13] DPPH reacts with an antioxidant compound that can donate hydrogen and thereby DPPH is reduced. Change in color of the solution, (from deep violet to light yellow) was measured. The intensity of the yellow color depends on the amount and nature of radical scavenger present in the sample and standard compounds. The reaction mixture containing 1 ml of 0.1 mM 1, 1-diphenyl-2-picryl-hydrazyl (DPPH) and various concentrations of extract (50, 100, 150, 200 and 250 μg) were made up to 3 ml with water. Then the tubes were incubated for 10 minutes. Once the blue color chromophore was formed, the absorbance of this solution was measured at 517 nm, against reagent blank containing water in place of extract. BHT was used as the standard for the comparison. The ability to scavenge the DPPH radical in terms of percentage of inhibition was calculated according to the following equation: % inhibition = {(A0–A 1)/A0 × 100} where A0 is the absorbance of the control (without extract) and A1 is the absorbance in the presence of the extract.
Hydrogen peroxide scavenging assay
A solution of hydrogen peroxide (20 mM) was prepared in phosphate buffer saline (PBS) (pH-7.4). Various concentrations (20, 40, 60, 80 and 100 μg) of extract or standard in hydro-methanol (1 ml) were added to 2 ml of hydrogen peroxide solution in PBS. After 10 min of incubation, the absorbance was measured at 230 nm against a blank solution containing phosphate buffer without hydrogen peroxide.[14] The result was compared with alpha tocopherol as a standard. The percentage of inhibition was calculated using the formula given before.
Hydroxyl radical scavenging assay
Hydroxyl radical inhibitory activity was performed as per deoxyribose method.[15] To the reaction mixture containing deoxyribose (3 mM, 0.2 ml), FeCl2 (0.1 mM, 0.2 ml), EDTA, disodium salt (0.1 mM, 0.2 ml), ascorbic acid (0.1 mM, 0.2 ml), and hydrogen peroxide (2 mM, 0.2 ml) in PBS (pH, 7.4, 20 mM), various concentrations (20, 40, 60, 80 and 100 μg) of 0.2 ml of the extract or standard in DMSO were added to give a total volume of 1.1 ml. The solutions were then incubated for 30 min at 37°C. After incubation, ice-cold TCA (0.2 ml, 15% w/v) and TBA (0.2 ml, 1% w/v) in 0.25 N hydrochloric acid (HCl) were added. The reaction mixture was kept in a boiling water bath for 30 min, cooled in room temperature, and the absorbance was measured at 532 nm with reagent blank containing water in the place of extract. Alpha tocopherol was used as the standard for the comparison. The percentage of inhibition was calculated using the formula given before.
Lipid Peroxidation Inhibitory Activity
The lipid peroxidation inhibitory activity of hydro-methanolic extract was studied in-vitro following the modified method.[16,17] Rats were killed by cervical dislocation (NIH, 1985), the liver tissue was excised, rinsed in ice-cold saline solution, and blotted dry. Then, 0.5 gm of the liver was sliced and homogenized with 10 ml of 150 mM KCL-Tris-HCl buffer (pH-7.2). The reaction mixture was composed of 0.25 ml of liver homogenate, Tris-HCl buffer (pH-7.2), 0.1 mM ascorbic acid (AA), 4 mM FeCl2, and 0.05 ml of various concentration of extract (25, 50, 75, 100 and 150 mg). The mixture was incubated at 37°C for 1 hr in capped tubes. Then, 0.5 ml of 0.1 N HCl, 0.2 ml of 9.8% SDS, 0.9 ml of distilled water, and 2 ml of 0.6% TBA were added to each tube and the tubes were vigorously shaken. All the tubes were then placed in boiling water bath at 100°C for 30 minutes. The tubes were allowed to keep at room temperature and centrifuged at 3000 rpm for 20 minutes. The absorbance of the supernatant was measured at 532 nm against reagent blank containing water in place of extract. The result was compared with BHT as a standard. The percentage inhibition of lipid peroxidation was calculated by comparing the results of test with those of controls not treated with the extract as per the formula:
% inhibition = {(A0–A1)/A0 × 100} where A0 is the absorbance of the control (without extract) and A1 is the absorbance in the presence of the extract.
Statistical Analysis
Statistical analysis was performed by software (Origin-8.1). Data was expressed as means ± SD of three measurements. Data was analyzed using analysis of variance (ANOVA) followed by multiple comparison two tail ‘t-test’. The results obtained were considered statistically significant if the P-value was <0.05. The amount of extract needed to inhibit free radicals concentrations by 50%, IC50 was performed by software (STATISTICA) based on the percentage of inhibition in different doses or concentration.
RESULTS AND DISCUSSION
Oxidative stress has been implicated in the pathology of many diseases and conditions including diabetes, cardiovascular disease, inflammatory condition, cancer, ageing etc.[18,19] Antioxidants may offer resistance against the oxidative stress by scavenging the free radicals, inhibiting the lipid peroxidation, and by many other mechanisms and thus prevent diseases.[20] Consequently, we studied the antioxidant activities of hydro-methanolic extract by a series of in-vitro protocols using some biologically relevant models.
The findings of the phytochemical screening indicated that the seeds of C. bonduc are rich in flavonoids, phenols, and saponins which may be responsible for the antioxidative efficacy as these phytochemicals act as antioxidants.[21–23]
Phenolic compounds may contribute directly to the antioxidative action. The total phenolic content was 24.66 mg GAE/g dried extract. The total flavonoids content of the hydro-methanolic extract was 136.65 mg QE/g dried extract [Table 1]. Due to redox properties, phenolic compounds play an important role in adsorbing and neutralizing free radicals, quenching singlet and triplet oxygen or decomposing peroxides.[24,25] It has been also recognized that flavonoids show antioxidant activity through scavenging or chelating process and their effects on human nutrition and health are considerable.[26]
Table 1.
Total phenolic and flavonoids contents of hydro-methanolic extract of seed of C. bonduc

The scavenging ability of hydro-methanolic extract on DPPH radical is represented by line diagram [Figure 1] and compared with BHT. The scavenging activity of the investigated extract varied widely from 23.32% to 75.92% (IC50 value 157.4 mg/ml) and in standard 26.78% to 79.88% (IC50 value 145.89 mg/ml). From the result, we say that DPPH antioxidant assay is based on the ability of DPPH, a stable free radical, to decolorize in the presence of antioxidants. The DPPH radical contains an odd electron which is responsible for the absorbance at 517 nm and also for a visible deep purple color. When DPPH accepts an electron donated by an antioxidant compound, the DPPH is decolorized which can be quantitatively measured from the changes in absorbance.[27] It was observed that the radical scavenging activity is increasing with the increase of phenolic compound content.[28] The two separate studies were also reported a high concentration between DPPH radical scavenging potential and total phenolic content.[29,30]
Figure 1.
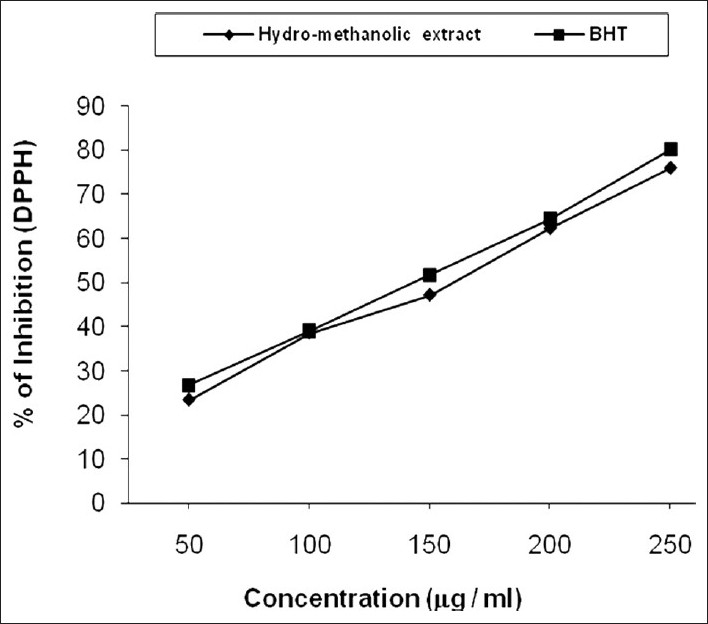
Inhibition in DPPH radical by hydro-methanolic extract of seed of C. bonduc and standard BHT. The IC50 value of the extract was 157.4 μg/ml
Hydrogen peroxide neutralization ability of the extract [Figure 2] varied from 11.22% to 80.53% (IC50 value 64.32 mg/ ml) and in standard 18.26% to 85.16% (IC50 value 57.06 mg/ml). The ability of the said extract to neutralize hydrogen peroxide was dose dependent. Hydrogen peroxide is important because of its ability to penetrate biological membranes. Hydrogen peroxide itself is not very reactive, but it can sometimes be toxic to cell because it may give rise to hydroxyl radical in the cells.[31] Scavenging of hydrogen peroxide by C. bonduc may be attributed to their phenolic compound which could donate electron to hydrogen peroxide, thus it is neutralizing to water.
Figure 2.
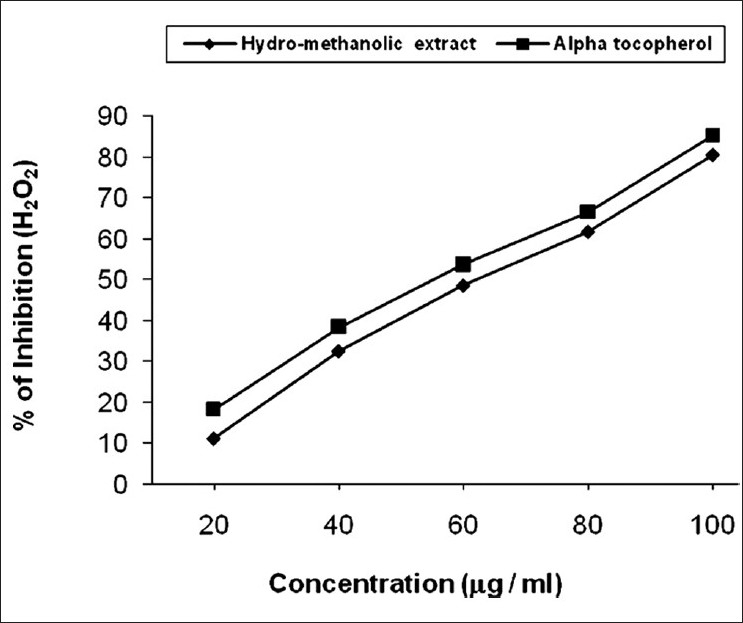
Scavenging of hydrogen peroxide by hydro-methanolic extract of seed of C. bonduc and standard α-tocopherol. The IC50 value of the extract was 64.32 μg/ml
Hydroxyl radical scavenging ability of hydro-methanolic seed extract was shown in [Figure 3] and was compared with a-tocopherol. The extract inhibited the degradation of deoxyribose in dose dependent manner. Thereby, hydroxyl radical neutralization values ranges from 14.21% to 87.82% (IC50 value 61.9 mg/ml) and in standard from 17.98% to 92.66% (IC50 value 54.97 mg/ml). Hydroxyl radical is an extremely reactive free radical formed in biological systems and has the capacity to cause DNA strand breakage which contributes to carcinogenesis, mutagenesis, and cytotoxicity.[32] Like many free radicals, hydroxyl radical can be neutralized if it is provided with hydrogen atoms. Oxygen radical may attack DNA either in sugar or in base giving rise to a large number of products. Phytochemical study of seed extract revealed the presence of phenolic compounds which may responsible for the hydroxyl radical scavenging activity.[33,34]
Figure 3.
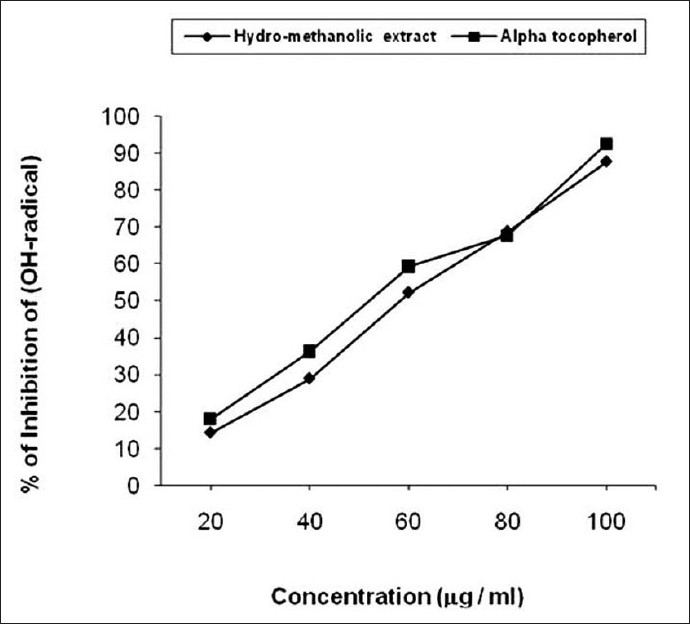
Hydroxyl radical scavenging ability of hydro-methanolic extract of seed of C. bonduc and a-tocopherol as a standard. The IC50 value of hydro-methanolic extract was 61.9 μg/ml
Inhibition percentage in lipid peroxidation varies widely in different doses ranges from 27.23% to 91.26% (IC50 value 58.87 mg/ ml) in hydro-methanolic extract and 32.18% to 94.54% (IC50 value 51.34 mg/ml) in case of standard [Figure 4]. Lipid peroxidation has been broadly defined as the oxidative deterioration of polyunsaturated fatty acids and involves formation of lipid radicals leading to membrane damage. Free radicals induced lipid peroxidation mainly occurs in brain and liver due to presence of polyunsaturated lipid.[35] Increased lipid peroxidation is a salient characteristic of chronic diabetes which impairs membrane function by reducing the activity of enzymes as well as receptors.[36] Results focused that the hydro-methanolic extract of the seeds of C. bonduc inhibit lipid peroxidation under in vitro conditions indicating the anti-lipid peroxidant effect of the seed of C. bonduc.
Figure 4.
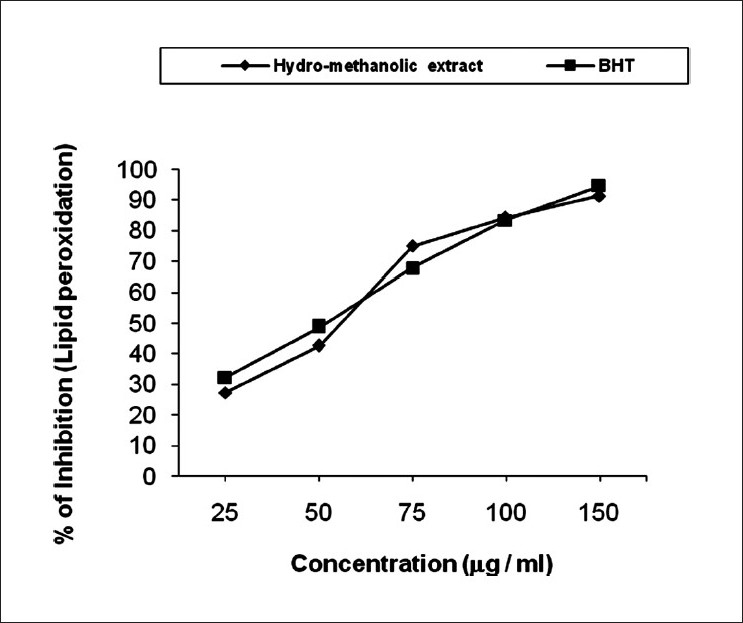
Inhibition in lipid peroxidation by hydro-methanolic extract of seed of C. bonduc and standard BHT. The IC50 value of the extract was 58.87 μg/ml
CONCLUSION
The results of the study clearly indicate that hydro-methanolic extract of seed of C. bonduc possess in vitro antioxidant activity. The encouraging results of this extract in various in vitro tests proved that the plant seeds act as a reducing agent, its hydrogen donating ability and effectiveness as scavengers of hydrogen peroxide and hydroxyl radical. Hence, it is worthwhile to isolate and elucidate the bioactive principle(s) responsible for the antioxidant activity of the extract which is underway in our laboratory.
ACKNOWLEDGMENTS
The authors are thankful to the Ayurvedic division, Southern Health Improvement Samity (SHIS), Bhangar, South 24-Paraganas, West Bengal, India, for providing the necessary help, support and information.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Van de Vijver LP, Kardinaal AF, Grobbee DE, Princen HM, Van Poppel G. Lipoprotein oxidation, antioxidants and cardiovascular risk: Epidemiologic evidence. Prostagland Leukot Essent Fatty Acids. 1997;57:479–87. doi: 10.1016/s0952-3278(97)90432-4. [DOI] [PubMed] [Google Scholar]
- 2.Smith MA, Perry G, Pryor WA. Causes and consequences of oxidative stress in Alzheimer's disease. Free Radic Biol Med. 2002;32:1049. doi: 10.1016/s0891-5849(02)00793-1. [DOI] [PubMed] [Google Scholar]
- 3.Ellnain-Wojtaszek M, Kruczynski Z, Kasprzak J. Investigation of the free radical scavenging activity of Ginkgo biloba leaves. Fitoterapia. 2003;74:1–6. doi: 10.1016/s0367-326x(02)00306-4. [DOI] [PubMed] [Google Scholar]
- 4.Cheeseman KH, Slater TF. An introduction to free radical biochemistry. British Med Bull. 2010;49:481–93. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 5.Yildirim A, Oktay M, Bulaloulu V. The antioxidant activity of the leaves of Cydonia vulgaris. Turkesh J Med Sci. 2001;31:23–7. [Google Scholar]
- 6.Anagnostopoulou MA, Kefalas P, Papageorgiou VP, Assimopoulou AN, Boskou D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis) Food Chem. 2006;94:19–25. [Google Scholar]
- 7.Gaur RL, Sahoo MK, Dixit S, Fatma N, Rastogi S, Kulshreshtha DK, et al. Antifilarial activity of Caesalpinia bonducella against experimental filarial infections. Indian J Med Res. 2008;128:65–70. [PubMed] [Google Scholar]
- 8.Jana K, Chatterjee K, Bera TK, Maiti S, De D, Ali KM, et al. Antihyperglycemic and antihyperlipidemic effect of seed of Caesalpinia bonduc in streptozotocin induced diabetic male albino rat. Int J Pharm Tech Res. 2010;2:2234–42. [Google Scholar]
- 9.Ali KM, Chatterjee K, De D, Jana K, Bera TK, Ghosh D. Inhibitory effect of hydro-methanolic extract of seed of Holarrhena antidysenterica on alpha-glucosidase activity and postprandial blood glucose level in normoglycemic rat. J Ethnopharmcol. 2011;135:194–6. doi: 10.1016/j.jep.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Trease GE, Evans WC. 15th ed. Uttar Pradesh, India: Elsevier India Pvt. Ltd; 2008. Pharmacognosy. [Google Scholar]
- 11.Zhishen J, Mengchen T, Jianming W. The determination of flavonoids content in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–9. [Google Scholar]
- 12.Singleton VL, Rossi JA. Colometry of total phenolics with phosphomolybodic-phosphotungtic acid reagents. Am J Enol Vitic. 1965;6:144–58. [Google Scholar]
- 13.Williams BW, Cuverlier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm Wiss Technol Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 14.Vaijanathappa J, Badami S, Bhojraj S. In-vitro antioxidant activity of Enicostemma axillare. J Hel Sci. 2008;54:524–8. [Google Scholar]
- 15.Badami S, Rai SR, Suresh B. Antioxidant activity of Aporosa lindleyana root. J Ethnopharmacol. 2005;37:1–5. doi: 10.1016/j.jep.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Masao H, Yang XW, Miyashrio H, Nabma T. Inhibitory effects of monomeric and dimeric phenyl propanoids from mice on lipid peroxidation in vivo and in vitro. Phytother Res. 1993;7:395–401. [Google Scholar]
- 17.Sunil C, Latha PG, Suja SR, Shine V, Shyamal SJ, Anuja GI, et al. Effect of ethanolic extract of Pisonia alba Span.leaves on blood glucose levels and histological changes in tissues of alloxan-induced diabetic rats. Int J Appl Res Nat Prod. 2009;2:4–11. [Google Scholar]
- 18.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: Food sources and bioavailability. J Am Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 19.Marx JL. Oxygen free radicals linked too many diseases. Science. 1987;235:529. doi: 10.1126/science.3810154. [DOI] [PubMed] [Google Scholar]
- 20.Bravgghler JM, Duncan CA, Chase LR. The involvement of iron in lipid peroxidation.Importance of ferrous to ferric ratio in initiation. J Biol Chem. 1986;261:282–9. [PubMed] [Google Scholar]
- 21.Loew D, Kaszkin M. Approaching the problem of bioequivalence of herbal medicinal products. Phytother Res. 2002;16:705–11. doi: 10.1002/ptr.1248. [DOI] [PubMed] [Google Scholar]
- 22.Sharma US, Kumar A. In vitro antioxidants activity of Rubus ellipticus fruits. J Adv Pharm Tech Res. 2011;2:47–50. doi: 10.4103/2231-4040.79805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gautam A, Jhade D, Ahirwar D, Sujane M, Sharma GN. Phamacognostic evaluation of Toona ciliate Bark. J Adv Pharm Tech Res. 2010;1:216–20. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhehg W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49:5165–70. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- 25.Paliwal P, Pancholi SS, Patel RK. Pharmacognostic parameters for evaluation of the rhizomes of Curcuma caesia. J Adv Pharm Tech Res. 2011;2:56–61. doi: 10.4103/2231-4040.79811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kessler M, Ubeaud G, Jung L. Anti and pro-oxidant activity of rutin and quercetin derivatives. J Pharm Pharmacol. 2003;55:131–42. doi: 10.1211/002235702559. [DOI] [PubMed] [Google Scholar]
- 27.Pal D, Mitra S. A preliminary study on the in vitro antioxidant activity of the stem of Opuntia vulgaris. J Adv Pharm Tech Res. 2010;1:268–72. [PMC free article] [PubMed] [Google Scholar]
- 28.Oki T, Masuda M, Furuta S, Nishibia Y, Terahara N, Suda I. Involvement of anthocyanins and other phenolic compounds in radical-scavenging activity of purple-fleshed sweet potato cultivars. Food Chem Toxicol. 2002;67:1752–6. [Google Scholar]
- 29.Lu Y, Foo YL. Antioxidant and free radical scavenging activities of selected medicinal herbs. J Life Sci. 2000;66:725–35. doi: 10.1016/s0024-3205(99)00643-8. [DOI] [PubMed] [Google Scholar]
- 30.Siriwardhana N, Lee KW, Kim SH, Ha JW, Jeon YJ. Antioxidant activity of Hizikia fusiformis on reactive oxygen species scavenging and lipid peroxidation inhibition. Food Sci Tech Int. 2003;9:339–46. [Google Scholar]
- 31.Flora SJ. Role of free radicals and antioxidants in health and disease. Cell Mol Biol (Noisy-le-grand) 2007;53:1–2. [PubMed] [Google Scholar]
- 32.Chatgilialoglu C, O’Neill P. Free radicals associated with DNA damage. Exp Gerontol. 2001;36:1459–71. doi: 10.1016/s0531-5565(01)00132-2. [DOI] [PubMed] [Google Scholar]
- 33.Sinha BN, Sasmal D, Basu SP. Pharmacological studies on Melothria maderaspatana. Fitoterapia. 1997;1:75–8. [Google Scholar]
- 34.Oyaizu M. Studies on product of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 35.Kappus H. Lipid peroxidation-mechanism and biological relevance. In: Aruoma O I, Halliwell B, editors. Free radicals and food additives. London, UK: Taylor and Francis; 1991. pp. 59–75. [Google Scholar]
- 36.Ali KM, Chatterjee K, De D, Bera TK, Mallick C, Ghosh D. Hypoglycemic, antioxidant and antihyperlipidemic effects of the aqueous sepal extracts of Salmalia malabarica in streptozotocin-induced diabetic rat. Ethiop Pharm J. 2009;27:1–15. [Google Scholar]


