Abstract
Fast disintegrating tablets (FDTs) have received ever-increasing demand during the last decade, and the field has become a rapidly growing area in the pharmaceutical industry. Oral drug delivery remains the preferred route for administration of various drugs. Recent developments in the technology have prompted scientists to develop FDTs with improved patient compliance and convenience. Upon introduction into the mouth, these tablets dissolve or disintegrate in the mouth in the absence of additional water for easy administration of active pharmaceutical ingredients. The popularity and usefulness of the formulation resulted in development of several FDT technologies. FDTs are solid unit dosage forms, which disintegrate or dissolve rapidly in the mouth without chewing and water. FDTs or orally disintegrating tablets provide an advantage particularly for pediatric and geriatric populations who have difficulty in swallowing conventional tablets and capsules. This review describes various formulations and technologies developed to achieve fast dissolution/dispersion of tablets in the oral cavity. In particular, this review describes in detail FDT technologies based on lyophilization, molding, sublimation, and compaction, as well as approaches to enhancing the FDT properties, such as spray drying and use of disintegrants. In addition, taste-masking technologies, experimental measurements of disintegration times, and dissolution are also discussed.
Keywords: Fast dissolving tablets, freeze drying, spray drying, taste masking
INTRODUCTION
Dysphagia and Fast Disintegrating Tablets
The concept of Fast Dissolving Drug Delivery System emerged from the desire to provide patient with conventional means of taking their medication. Because of physiological changes associated with, especially, elderly and pediatrics are quite unable to swallow (Dysphagia); rather, this is a common problem of all age groups patients. Solid dosage forms that can be disintegrated, dissolved, or suspended by saliva in the mouth resulting in easy swallowing can provide significant benefits to the pediatric and geriatric population, as well as other patients who prefer the convenience of easily swallowable dosage forms. This tablet disintegrates instantaneously when placed on tongue, releasing the drug that dissolves or disperses in the saliva.[1]
Recently, pharmaceutical preparations used for elderly patients have been investigated to improve the treatment compliance and quality of life of such patients. A tablet which can rapidly disintegrate in saliva (rapidly disintegrating tablet) is an attractive dosage form and a patient-oriented pharmaceutical preparation.[2] The mouth-dissolving tablets have attracted the interest of many researchers. Many elderly patients have difficulty swallowing tablets, capsules, or powders. To alleviate this problem, these tablets are expected to dissolve or disintegrate in the oral cavity without drinking water. The disintegrated mass can slide down smoothly along the esophagus with the help of saliva, so even people who have swallowing or chewing difficulties can take it with ease.[3] There are two different types of dispersible tablets which have to be distinguished: One dosage form disintegrates instantaneously in the mouth, to be swallowed without the need for drinking water, while the other tablet formulation can readily be dispersed in water, to form dispersion, easy to ingest by the patient.[4]
Advantages of Fast Disintegrating Tablets
Fast disintegrating tablets (FDTs) are meant for administration to the patients who cannot swallow, such as the elderly, stroke victims, bedridden patients, patients affected by renal failure, and patients who refuse to swallow, such as pediatric, geriatric, and psychiatric patients. By the use of FDTs, rapid drug therapy intervention can be achieved, achieve increased bioavailability/rapid absorption through pregastric absorption of drugs from mouth, pharynx, and esophagus as saliva passes down. FDTs are convenient for administration and patient compliant for disabled, bedridden patients, and for travelers and busy people who do not always have access to water. Their good mouth feel property helps to change the perception of medication as bitter pill, particularly in pediatric patients. The risk of chocking or suffocation during oral administration of conventional formulations due to physical obstruction is avoided, thus providing improved safety. The new business opportunity like product differentiation, product promotion, patent extension, and life cycle management become easy after the intervention of FDTs. The FDTs are often formulated for existing drugs with an intention to extend the patent life of the drug through product differentiation.
Recently, the European Pharmacopoeia[5] adopted the term orodispersible tablet as a tablet to be placed in the mouth where it disperses rapidly before swallowing and which disintegrates in less than 3 minutes. There was no specification concerning either the hardness or the friability of this kind of tablets. That is why we find certain Rapidly Disintegrating Tablets (RDT) in the market that disintegrate in less than 1 minute or maybe 30 seconds, but are brittle and require specified peelable blister packaging and thus higher costs.[6]
DESIRED CHARACTERISTICS AND DEVELOPMENT CHALLENGES OF FAST DISINTEGRATING TABLETS
Fast Disintegration
A fast-dissolving drug delivery system, in most cases, is a tablet that dissolves or disintegrates quickly in the oral cavity upon the contact with saliva, resulting in solution or suspension of the administered medicine.[7] FDT dosage forms, also commonly known as fast melt, quick melt, orally disintegrating tablets, and orodispersible systems, have the unique property of disintegrating the tablet in the mouth in seconds.[8]
Taste of Active Ingredients
Taste is an important parameter in administering drugs orally. Undesirable taste is one of the important formulation problems that are encountered with many drugs. Administration of bitter drugs orally with acceptable level of palatability is a key issue for healthcare providers. Proven methods for bitterness reduction and inhibition have resulted in improved palatability of oral pharmaceuticals.[9] Taste masking of the drug may be achieved with preventing the exposure of drug to the tongue through processing or adding competing taste-masking agents. Exposure of solubilized drug to the oral cavity can be prevented by encapsulation in polymer system or complexation.[10] Taste-masking technologies are increasingly focused on aggressively bitter-tasting drugs like the macrolide antibiotics, non-steroidal anti-inflammatory drugs, and penicillins. Taste masking of water-soluble bitter drugs, especially those with a high dose, is difficult to achieve by using sweeteners alone. As a consequence, more efficient techniques such as coating, microencapsulation, and granulation have been used in combination with the sweeteners.[11]
In 1953, Freudenberg et al.[12] got a patent for taste masking of bad taste of bromoisovaleryl urea by CD complexation. In that research, they described that only the dissolved substances elicit taste sensation and the substances which are completely insoluble in water are tasteless. In many cases, however, the drugs are so intensely bitter that they even at ppm levels are hardly tolerable.[13]
Drug Properties
For the ideal FDT technology, the drug properties should not significantly affect the tablet property. Many drug properties could potentially affect the performance of FDTs. For example, the solubility, crystal morphology, particle size, hygroscopicity, compressibility, and bulk density of a drug can significantly affect the final tablet's characteristics, such as tablet strength and disintegration. The FDT technology should be versatile enough to accommodate unique properties of each drug.[14] The drugs belonging to Biopharmaceutical Classification System Class II, i.e., the drugs with poor solubility and high permeability are best suitable moieties for FDTs in a dose of 125 and 250 mg.[15,16] Tizanidine HCl,[1] Oxybutynin HCl,[2] Rofecoxib,[3] Ibuprofen,[4] Promethazine Theoclate,[17] prednisone,[18] Indomethacin,[19] Glyburide,[20] Fentanyl citrate,[21] Griseofulvin,[22] Hydrochlorothiazide,[23] Crystallized Paracetamol,[24] and Nimesulide[25] are few examples of drugs that has been formulated as fast-dissolving drug delivery system.
Tablet Strength and Porosity
Many attempts for fast-disintegrating behavior have been reported by lyophilizing or molding, and compressing wet powders to construct highly porous structure.[26] When the FDT is orally applied, the drug substance has to be dissolved so that it can be absorbed. Dissolution process consists of various processes, e.g., wetting, disintegration, and dissolution. FDTs which generally contains several excipients are involved in a complex series of dissolution process that begins when the solvent contacts the solid and penetrates the tablet matrix. Effect of excipients is assumed to be related to the surface properties of the particles and solid matrix structure.[27]
The fabrication of lyophilized FDTs is based on creating a porous matrix by subliming the water from pre-frozen aqueous formulation of the drug containing matrix-forming agents and other excipients such as lyoprotectants, preservatives, and flavors. The FDTs comprise of two component frameworks of lyophilized matrix system that work together to ensure the development of a successful formulation. The first component is water-soluble polymers such as gelatin, dextran, alginate,[28] and maltodextrin.[29] This component maintains the shape and provides mechanical strength to the tablets (binder). The second constituent is matrix-supporting/disintegration-enhancing agents such as sucrose and mannitol, which acts by cementing the porous frame work, provided by the water-soluble polymer and accelerates the disintegration of the FDT.[30] Although there is wide availability of literature describing the preparation of RDTs by lyophilization, the number of matrix-supporting/disintegration-enhancing agents used has been limited to saccharides and polyols, with majority of the work dedicated to the inclusion of mannitol.[28,30] This is primarily because the incorporation of these matrix-forming agents requires fulfillment of stringent characteristics such as reasonable drying time, stability during freeze-drying process, as well as formation of elegant tablets with short disintegration time and adequate mechanical properties.
Moisture Sensitivity
Hygroscopicity is, of course, an important characteristic of a powder. It can be shown, roughly, for a fairly soluble compound that the hygroscopicity is related to its solubility.[31,32] FDTs should have low sensitivity to humidity. This problem can be especially challenging because many highly water-soluble excipients are used in formulation to enhance fast-dissolving properties as well as to create good mouth feel. Those highly water-soluble excipients are susceptible to moisture; some will even deliquesce at high humidity. A good package design or other strategy should be created to protect FDTs from various environmental conditions.[33]
FORMULATION PROCESSES FOR MAKING FAST-DISSOLVING TABLETS
Freeze Drying
Freeze-drying technique is used in order to improve the dissolution rate and oral bioavailability of drugs with poor solubility and high permeability (biopharmaceutic classification system Class II drugs). Freeze drying (Lyophilization) is a process in which water is sublimated from the product after freezing. This process can be performed in many ways to achieve the same end product, i.e., I) drug is physically trapped in a water-soluble matrix (water-soluble mixture of saccharide and polymer, formulated to provide rapid dispersion, and physical strength), which is freeze dried to produce a product that dissolves rapidly when placed in mouth. For such types of formulations, a chemically stable and water-insoluble drug with particle size less than 50 μm are required;[8,34] II) Formation of porous solid form obtained by freezing an aqueous dispersion or solution of the active containing matrix by removing the water using an excess of alcohol (solvent extraction) and for that, the active should be insoluble in the extraction of solvent with the advantage that the thermolabile drugs can be formulated at non-elevated temperature, thereby eliminating adverse thermal effects, and stored in a dry state with few shelf-life stability problems;[35] III) solid form lyophilization of an oil-in-water emulsion (porous solid galenic form) is placed directly in the blister alveolus.[36] The main disadvantage of these dosage forms are, in addition to the cost-intensive production process, the lack of physical resistance in standard blister packs and their limited ability to incorporate higher concentrations of active drug.[37]
Gelatin was first dissolved in distilled water at about 40°C to obtain the required concentration. Sorbitol (or mannitol) and glycine were then added to the gelatin solution in the predetermined concentration. An accurately weighed amount of Nemusulide (NM) powder was dispersed in the prepared aqueous solution using a magnetic stirrer to result in a dose of 50 mg NM per 1 ml. One milliliter of the suspension was then poured in each pocket of a Polyvinyl Chloride (PVC) blister pack with a diameter of 13 mm and a depth of 3 mm resulting in a dose of 50 mg per tablet. The tablet blister packs were then transferred to a freezer at –22°C and kept in the freezer for 24 hours. The frozen tablets were placed in a lyophilizer for 24 hours using a Novalyphe-NL 500 Freeze Dryer with a condenser temperature of –45°C and a pressure of 7 × 10-2 mbar. The best of these formulations (based on tablet properties) was taken forward to the next stage which involved the addition of a water-soluble surface-active agent or polymer in order to improve disintegration time and/or friability. These disintegration accelerators were sodium lauryl sulfate (SLS); three grades of PEG, namely PEG 400, PEG 4000, and PEG 6000; three grades of PVP, namely PVP K25, PVP K30, and PVP K90; and two grades of Tweens, namely Tween 20 and Tween 80. All of these were added at a concentration of 1% w/v except SLS, which was added at 0.05% w/v.[25]
This technique is used in some patented technologies like Zydis, Quicksolv, and Lyoc technologies, which are used to manufacture RDTs as given below:
Zydis technology
Zydis technology (ZT) is a patented technique.[26] ZT utilizes a unique freeze-drying process to manufacture finished dosage units which significantly differ from conventional oral systems. In this technology, the solution or suspension of drug in water is poured in preformed blisters (providing shape to the tablet) and passing them to a specially designed cryogenic freezing process to control the size of ice crystals which ensures that the tablet is having porous matrix for rapid disintegration. Then, these frozen units are then transferred to large-scale freeze dryers for the sublimation process, where the majority of the remaining moisture is removed from the tablets and open blisters are packed using a heat seal process (steps involved in freeze-drying process [Figure 1]).
Figure 1.
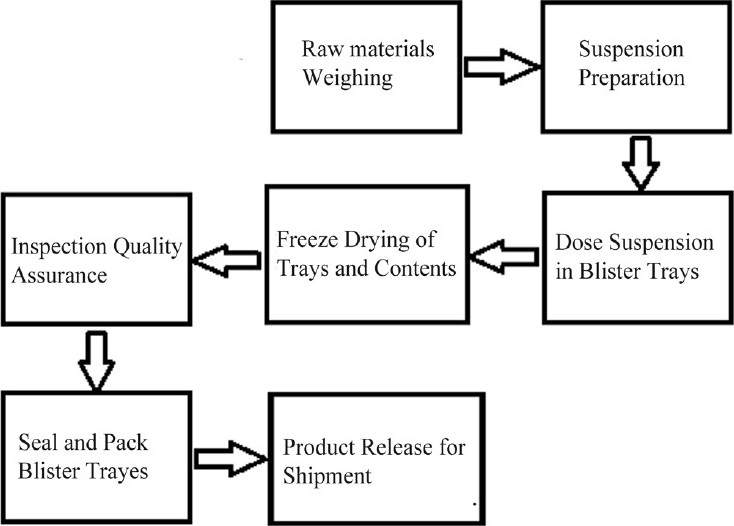
Zydis® tablet manufacturing process.[38]
Lyoc
Lyoc is a porous and solid galenic form obtained by lyophilization of an oil-in-water emulsion placed directly in the blister alveolus.[39] The method of preparation involves freezing a thickened (paste-like) emulsion containing the active as bulk or as coated microparticles. This product is capable of accommodating high dose and disintegrates rapidly but possesses poor mechanical strength.[40]
Quicksolv
In Quicksolv porous solid dosage forms are obtained by freezing an aqueous dispersion/solution of the drug-containing matrix and then drying it by removing the water using excess of alcohol by solvent extraction.[41] The final form disintegrates very rapidly, but is limited to low drug content and can be used only for those drugs that are insoluble in the extraction solvent. The ideal drug characteristics required for this technology are relative low aqueous solubility, fine particle size <50 μm,[42] and good aqueous stability in the suspension.
Advantages
The tablets produced by this technology have very low disintegration time and
Tablets having great mouth feel due to fast melting effect.
Molding
Compression molding is a process in which tablets are prepared from soluble ingredients such as sugars by compressing a powder mixture previously moistened with solvent (usually ethanol or water) into mold plates to form a wetted mass.[43] The advantages of molded tablets is that these tablets disintegrate more rapidly and offer improved taste as these tablets are made from water-soluble sugars. dispersion matrix is made from water-soluble sugars, molded tablets disintegrate more rapidly and offer improved taste. These properties are enhanced when tablets with porous structures are produced or when components that are physically modified by the molding process are used. In comparison with lyophilization process, tablets produced by molding technique are easier to adapt to the industrial scale. The lyophilization and molding techniques produce RDT which disintegrate within about 30 seconds, but that have low physical resistance and high friability. On the other hand, tablets obtained by direct compression are less friable but disintegrate in a longer time.[44]
The molded tablets formed by compression molding are air-dried. As the compression force employed is lower than conventional tablets, the molded tablet result in highly porous structure, which increases the disintegration and dissolution rate of the product. However, to further improve dissolution rate of the product, powder mixture should be sieved through very fine screen. Moulding process is usually employed for the soluble ingredients (saccharides) which offer improved mouth feel and disintegration of tablets. However, molded tablets have low mechanical strength, which results in erosion and breakage during handling.[45]
Compaction
Attempts were made in order to decrease the disintegration time of RDT that have sufficient hardness prepared by direct compression. Bi et al.[46] and Watanabe et al.[47] used microcrystalline cellulose (MCC) and low-substituted hydroxypropyl cellulose (L-HPC) as disintegrants to prepare RDT by direct compression. According to the authors, the ratios of these two disintegrants MCC/L-HPC in the range of 8:2-9:1 resulted in tablets with the shortest disintegration times. However, Bi et al.[48] and Sunada and Bi[49] used a wet compression method where wet granules of -lactose monohydrate were compressed and then the formed wet tablets were dried at 60°C and kept in a desiccator for 12 hours at room temperature. Formed RDT showed a disintegration time of less than 30 seconds and a hardness of 0.5 MPa.[24]
Direct compression is the simplest and most cost-effective tablet manufacturing technique for Mouth Dissolving Tablets (MDT) as they can be fabricated using conventional tablet manufacturing and packaging machinery and also due to availability of tableting excipients with improved flow, compressibility, and disintegration properties.
Flashtab
Flashtab is a patented technology, but the tablets are directly compressed. Flashtab contains coated crystals of drug and microgranules along with disintegrants.[50] In this technology, two types of disintegrants are used: A disintegrating agent that has a high swelling force and a swelling agent that has a low swelling force.[51]
Orasolv
Orasolv [Figure 2] is also a patented technology. Orasolv tablet are lightly compressed and are weaker and more brittle than the conventional tablets. CIMA LABS develops a special handling and packaging system for Orasolv.[53] An advantage of low degree of compaction is that the particle coating used for taste masking is not compromised by fracture during compression.
Figure 2.
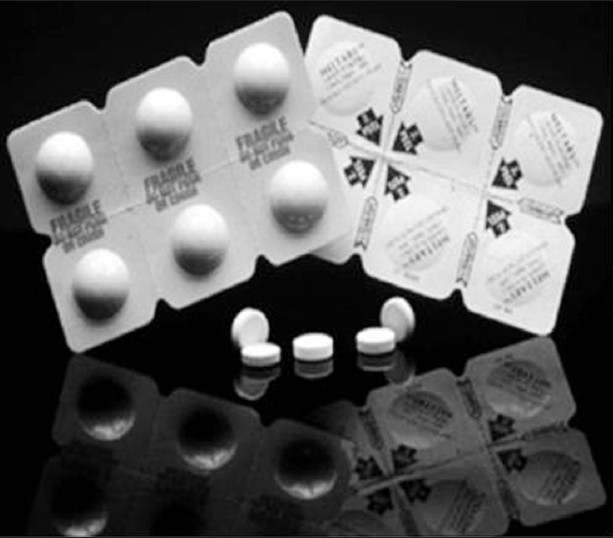
Typical OraSolv® package.[52]
Durasolv
Durasolv is a second-generation patented technology that was developed to produce robust MDTs. Durasolv has much higher mechanical strength than Orasolv due to use of higher compaction pressure during compression. Thus, it is produced in a much faster and cost-effective manner and can be packed in either traditional blister packs or vials. The limitations of Durasolv is its low drug loading capacity and high compaction pressure which are not suitable for incorporation of taste masked coated pellets.
Wowtab
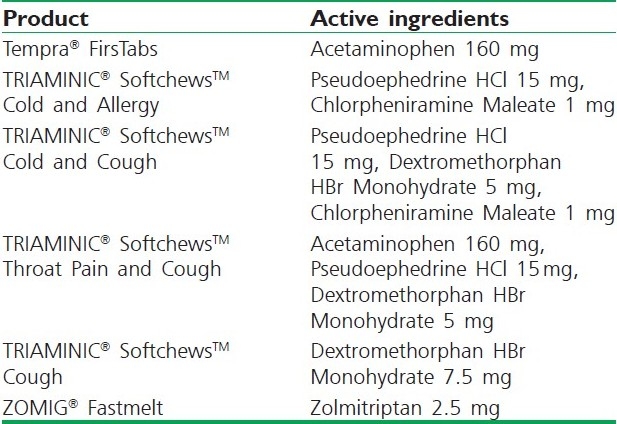
Wowtab is a patented technology developed in Japan. This technique has been used in the production of Benadryl Fast melt tablets. In this technology, two different types of saccharides are combined to obtain a tablet formulation with adequate hardness and fast dissolution rate.[54] Due to its significant hardness, the WOWTAB formulation is more stable to the environmental conditions than the Zydis or Orasolv and is suitable for both conventional bottle and blister packaging. The taste-masking technology utilized in the WOWTAB is proprietary and claims to offer superior mouth feel due to the patented smooth-melt action.[55] Table 1 depicts some patented products of CIMA Labs that are currently available in market.
Table 1.
Currently marketed intraorally disintegrating tablets manufactured by Cima Labs, Inc.[56]
Granulation Methods
Wet granulation
Wet granulation is the process in which a liquid is added to a powder in a vessel equipped with any type of agitation that will produce agglomeration or granules. Wet granulation is a commonly used method used in the manufacture of tablets, which improves the tableting process through the production of a product (granulate) that has improved flowability, uniformity, and compressibility when compared with original drug containing powder blend. There are many methods used in the pharmaceutical industry to produce granules; however, the most common is high shear. As with most pharmaceutical processes, wet granulation is a complex one, in which many factors such as binder used and processing conditions will influence the physical properties of the resultant granule.[57]
Dry granulation
When tablet ingredients are sensitive to moisture or are unable to withstand elevated temperature during drying, and when the tablet ingredients have sufficient inherent binding or cohesive properties, slugging may be used to form granules. This method is referred to as dry granulation, precompression, or double compression.
Melt granulation
Melt granulation is a mixture of active agent and a water-soluble carrier, which is heated until it is melted. The melt is solidified rapidly in an ice bath under vigorous stirring, pulverizing, and then sieving. Rapid congealing is desirable because it result in super saturation of the drug as a result of entrapment of solute molecules in the solvent matrix by instantaneous solidification. The solidification process can be achieved on stainless steel plates attached to a cooling system to favor rapid heat loss. Two advantages of the melt method are its simplicity and its economy, as no solvents are involved.[58]
Abdelbary et al. prepared FDT by incorporating a hydrophilic waxy binder (super polystate) PEG-6-stearate. Super polystate is a waxy material with an M. P. 33 to 37°C and an HLB value of 9. It not only acts as a binder and increases the physical resistance of tablets, but also helps the disintegration of tablets as it melts in the mouth and solubilizes rapidly leaving no residue. Super polystate was incorporated in the formulation of FDT by melt granulation method where granules are formed by the molten form of this material. Crystallized paracetamol was used as model drug and in addition, the formulation included mannitol as a water-soluble excipient and croscarmellose sodium as disintegrating agent.[24]
Spray drying
Spray drying was used for the preparation of the microspheres. Spray drying is widely used in pharmaceutical processing because it requires only a one-step process and can be easily controlled and scaled up.[59,60] Spray drying is widely used in pharmaceutical and biochemical fields and the final particle size is controlled by a number of factors including the size of the nozzle used in the processing.[61] Figure 3 illustrates various steps involved in spray-drying process.
Figure 3.
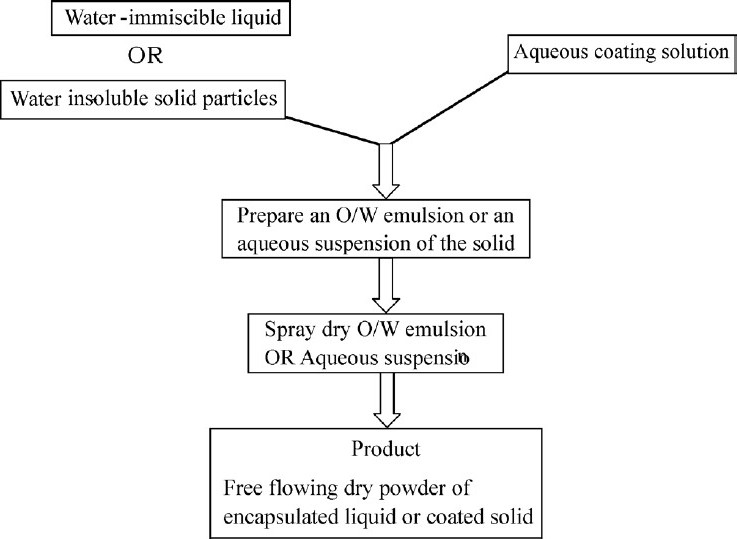
Flow chart for spray- dry process of coating liquid or solid particles.[62]
Highly porous fine powders are obtained by this method. Allen and Wang utilized this process for preparing FDT. The FDT formulations consisted of hydrolyzed/unhydrolyzed gelatin as supporting agent for matrix, mannitol as bulking agent, and sodium starch glycolate and croscarmellose sodium as disintegrating agent.[63] Disintegration and dissolution were further improved by adding effervescent components, i.e., citric acid (an acid) and sodium bicarbonate (an alkali). The formulation was spray dried to yield a porous powder. The FDT made from this method disintegrated in <20 seconds.[64,65] Jianchen et al. prepared taste-masked orally disintegrating tablets. The microspheres were produced by spray drying a mixture of the model compound (famotidine) with taste-masking material.[66]
Flash heat process
Flash flow processing can be accomplished in several ways. Flash-heat and flash-shear are two processes which are quite popular. In the flash-heat process, the feedstock material is heated sufficiently to create an internal flow condition that forces part of the feedstock to move at sub particle level with respect to the rest of the mass and exit from the openings provided in the perimeter of a spinning head. The centrifugal force created in the spinning head flings the flowing feedstock material outwardly from the head so that it reforms with a changed structure. The force necessary to separate and discharge the flowable feedstock is the centrifugal force that is produced by the spinning head. One preferred apparatus for implementing a flash heat process is a “cotton candy” fabricating type of machine.[67] Sucrose (78.25%), sorbitol (11.0%), xylitol (10.0%), and tween 80 (0.75%), Ibuprofen, cimitedine, vitamin C, calcium carbonate/vitamin D, or acetaminophen were successfully used to prepare FDT.[68]
Direct Compression
A direct compression method uses conventional equipment, commonly available excipients, and a limited number of process steps. This process may involve granulation prior to final blend. The direct compression tablet's disintegration and solubilization are based on the single or combined action of super disintegrants, water-soluble excipients, and effervescent agents.[69] Tizanidine HCl,[1] Oxybutynin HCl,[2] Rofecoxib,[3] and Ethenzamide[70] are some examples of model drugs that have been formulated as FDT by direct compression method.
The choice of superdisintegrant for a tablet formulation depends largely on the nature of the drug being used. For example, the solubility of the drug component could affect the rate and mechanism of tablet disintegration. Water-soluble materials tend to dissolve rather than disintegrate, while insoluble materials generally tend to disintegrate if an appropriate amount of disintegrant is included in the formulation.[71] Table 2 shows some disintegrants and superdisintegrants used by various researchers to formulate FDTs.
Table 2.
Disintegrants used in fast disintegrating tablets
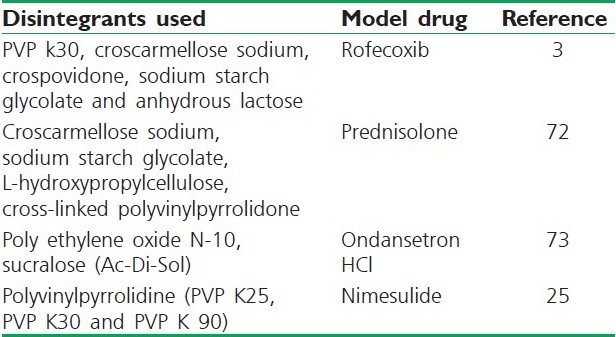
Compaction and Subsequent Treatments
Sublimation
The presence of a highly porous structure in the tablet matrix is the key factor for rapid disintegration of FDT. Even though the conventional tablets contain highly water-soluble ingredients, they often fail to disintegrate rapidly because of low porosity. To improve the porosity, volatile substances such as camphor can be used in tableting process, which sublimated from the formed tablet. Recently, it has been confirmed that a compressed tablet prepared with crystalline cellulose and L-HPC rapidly disintegrated (within 15 seconds) in saliva (or a small amount of water) in the mouth of human being.[47]
However, patients sometimes feel a rough texture in their mouth due to incomplete solubilization of this type of tablets in saliva. To eliminate the rough texture in the mouth, the researchers attempted to use a water-soluble material (mannitol) as an excipient instead of crystalline cellulose and L-HPC, in the preparation of this type of tablet. Koizumi et al. developed FDT utilizing camphor; a subliming material that is removed from compressed tablets prepared using a mixture of mannitol and camphor. Camphor was sublimated in vacuum at 80°C for 30 minutes after preparation of tablets.[74]
Taste Masking
Addition of sweeteners and flavors
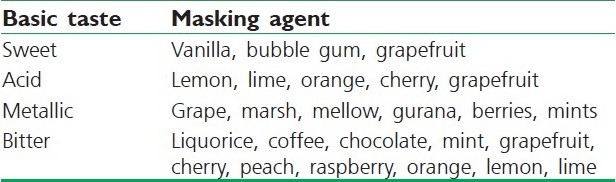
The materials for taste-masking purpose have often been classified depending upon the basic taste that is masked in Table 3.[71] Flavoring and perfuming agents can be obtained from either natural or synthetic sources. Natural products include fruit juices, aromatic oils such as peppermint and lemon oils, herbs, spices, and distilled fractions of these. They are available as concentrated extracts, alcoholic or aqueous solutions, syrups, or spirit.[76] Apart from these conventional materials, many compositions have been found to show effective taste-masking abilities with improved flavor such as alkaline earth oxide, alkaline earth hydroxide, or an alkaline hydroxide.[77] Another composition includes phosphorylated amino acid such as phosphotyrosine, phosphoserine, and phosphothreonine and mixtures thereof.[78] Anethole effectively masked bitter taste as well as the aftertaste of zinc, which is used in treating the common cold.[79] Clove oil and calcium carbonate, which has been found to be particularly useful to mask the unpalatable active in formulations which are intended to be chewed or dissolve in mouth prior to ingestion in solution.[80]
Table 3.
Flavoring agents for taste masking
Maximum patient acceptability with Orally Disintegrating Tablets (ODT) is seen if they provide pleasant taste and mouth feel. To provide this property in tablets, various sweeteners and flavors are employed. Usually, sugar-based excipients are used as they are highly water soluble and dissolve quickly in saliva and provide pleasant taste and mouth feel to the final product.[81]
Adjustment of pH values
Many drugs are less soluble at pH different from the pH value of the mouth, which are around 5.9. Drugs can be insufficiently solubilized to be available to taste if the equilibrium concentration is below the taste threshold.[82] After a solubilization inhibitor, such as sodium carbonate, sodium bicarbonate, sodium hydroxide, or calcium carbonate was added to increase the pH; when granules including a drug sildenafil dissolved in aqueous medium, the bitter taste of the drug was successfully masked by a sweetener alone.[83]
Coating or encapsulation of unpleasant drugs
Coating of drugs using a suitable polymer offers an excellent method of concealing the drug from the taste buds. The coated composition may be incorporated into much number of pharmaceutical formulations, including chewable tablet, effervescent tablets, powder, and liquid dispersion.[84–86]
Kato studied the low melting point substances for masking bitter taste of the drug. Beef tallow (a low melting point substance) was mixed with micropulverized active ingredients (e.g., antiulcer methyl benactyzuim bromide) and the mixture was nozzle sprayed to form coated spheres having homogenous particle size.[82]
Maccari et al. conducted a special study to assess the bioavailability of a Flucloxacillin preparation microencapsulated for taste abatement with 17% ethyl cellulose made up as a granular product for extemporaneous resuspension when compared with commercially available Flucloxacillin preparations. Both dosage forms were bioequivalent proving that Flucloxacillin microencapsulated for taste abatement is as available from the dosage form as the raw unprocessed antibiotics.[88]
Determination of Disintegration Time of Fast Disintegrating Tablets
In vivo determination of disintegration time
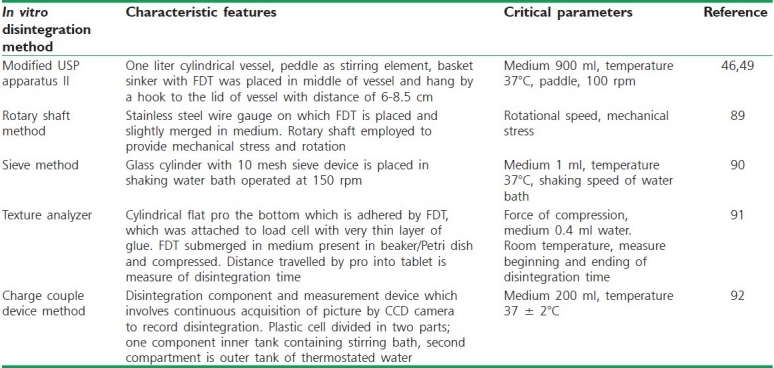
The time for disintegration of ODTs is generally <1 minute and actual disintegration time that patient can experience ranges from 5 to 30 seconds. The standard procedure of performing disintegration test for these dosage forms has several limitations and they do not suffice the measurement of very short disintegration times. The disintegration test for ODT should mimic disintegration in mouth within salivary contents. Various disintegration methods developed are discussed in Table 4.
Table 4.
In vitro disintegration methods for orally disintegrating tablets[45]
Dissolution Test
The development of dissolution methods for ODTs is comparable with the approach taken for conventional tablets and is practically identical. Dissolution conditions for drugs listed in a pharmacopoeia monograph, is a good place to start with scouting runs for a bioequivalent ODT. Other media such as 0.1N HCl and buffers (pH - 4.5 and 6.8) should be evaluated for ODT much in the same way as conventional tablets.
USP dissolution apparatus 1 and 2 can be used. USP 1 Basket apparatus may have certain applications, but sometimes, tablet fragments or disintegrated tablet masses may become trapped on the inside top of the basket at the spindle where little or no effective stirring occurs, yielding irreproducible dissolution profiles. Kancke[93] proposed USP 2 Paddle apparatus, which is the most suitable and common choice for ODTs, with a paddle speed of 50 rpm commonly used. Typically, the dissolution of ODT is very fast when using USP monograph conditions; hence, slower paddle speeds may be utilized to obtain a profile.
The USP 2 Paddle apparatus at 50 to 100 rpm is suitable for dissolution testing of taste-masked drug as well. The media used for the taste-masked drug should match that of the finished product to maximize the value of the test. High-performance liquid chromatography is often required to analyze dissolution aliquots due to presence of Ultraviolet (UV) absorbing components, specifically flavors and sweetener. Excipient to drug ratio may be higher since the formulation is designed to have good taste and mouth feel, decreasing the detection of the drug to background (excipient) in the UV spectrophotometer.[45]
Fast Disintegrating Tablet Formulation Examples
OraSolv® and DuraSolv® technology
OraSolv and DuraSolv are CIMA's core ODT tablet-based technologies. The ingredients contained in the technology include polyols as fillers, disintegrant, which may include an effervescence couple, flavor, sweetener, and lubricant. The drug may be taste masked if required, typically utilizing a fluid-bed coating process. The tabletting process includes direct compression, and can accommodate a wide range of potency from less than 1 mg to as high as 500 mg. Tablets manufactured with OraSolv technology should contain an effervescence couple along with microparticles of drug within a rupturable coat.[94]
The tablets manufactured are compressed at a low hardness that promotes fast disintegration. The dosage forms need to be packaged in foil–foil aluminum blisters with a dome shape that impact physical protection and impermeability to moisture. This constitutes the PakSolv Technology.[38] Tablets manufactured with DuraSolv technology contain a non-directly compressible filler and a lubricant. They may or may not contain effervescence, and the drug need not be taste masked.[52] DuraSolv tablets are compressed at higher hardness compared with OraSolv that allows for packaging in bottles or push through blisters. The advantages of tablet-based technology include low cost of goods, standard manufacturing technology, standard packaging format and materials, and low development costs and risks. Disadvantages include slightly longer disintegration time.
WOWTAB® technology
Yamanouchi Pharmaceutical Co. Ltd., Japan, has developed and commercialized a quick-disintegrating “Without Water Tablet” (WOWTAB®) technology. WOWTAB® is a tablet that has sufficient hardness to maintain physical and mechanical integrity of the dosage form prior to contact with saliva.[95] WOWTAB® consists of commonly used tablet excipients which are Generally Recognized As Safe (GRAS) materials. WOWTAB® when placed in the mouth rapidly becomes soft by absorption of saliva and disintegrates or dissolves within 15 to 20 seconds. WOWTAB® disintegrates or dissolves more quickly when pressure between the upper jaw and tongue or a licking movement is applied to the tablets. WOWTAB® is manufactured using conventional granulators, tablet machines, and packaging equipment, which ensure excellent drug content uniformity and batch-to-batch reproducibility.[96] WOWTAB® tablets are produced by a standard tablet compression molding process.
WOWTAB tablets are developed by Yamanouchi Pharma Technologies.[97] The main ingredients in the tablets include low- and high-moldable sugars. The low-moldable sugars promote quick dissolution and include mannitol, lactose, and glucose. High-moldable sugars promote good hardness upon compaction and include maltose, sorbitol, and maltitol. The active and other excipients are granulated with a solution containing both the sugars in a fluid-bed granulator. The granules obtained are blended with lubricants and flavors and then compressed to form tablets. The tablets are then stored in a controlled humidity and temperature system for conditioning and then packaged in blisters or bottles. Taste masking of the active may be achieved by the use of cyclodextrins.
Flashtab® technology
Flashtab tablet matrix consists of a swellable agent (modified starch or MCC) and a super disintegrant (crospovidone or croscarmellose). The system may also contain, depending on the need, a highly water-soluble polyol with binding properties such as mannitol, sorbitol, maltitol, or xylitol, instead of the swellable agent as mentioned before.[98] The active is taste masked by direct coating. Tablets manufactured using this technology produce durable tablets in which the excipients are first granulated using wet or dry granulation process, then the coated drug is mixed with the excipient granules and compressed into tablets that can be handled and packaged using conventional processing equipment. Tablets for blister packaging can withstand the pressure used to push the tablet out of the lidding foil of the blister card. Tablets containing hygroscopic material can also be blister packaged, by using high-quality polyvinyl chloride or aluminum foils, which provide a higher degree of moisture protection than ordinary polyvinyl chloride or polypropylene foils.
AdvaTab™ technology
Eurand is the owner of Advatab drug delivery system. Eurand is known for its Microcaps_ technology, which involve taste-masking drug particles using microencapsulation process based on coacervation/phase separation technique.[99] Eurand applied Microcaps technology to design ODTs (Advatab), which contains taste-masked active ingredients. The primary ingredients in the dosage form include sugar alcohols and saccharide with particle size less than 30 mm along with disintegrant and lubricant. The lubricant used in the formulation is added as an external lubricant compared with conventional formulations, which contain an internal lubricant. The company claims that this allows the tablets to be stronger compared with conventional tablets as internal lubricants are hypothesized to decrease binding of the drug particles. The dosage forms are manufactured using conventional tabletting and packaging equipments. The tablets, which can handle high drug loading and coated particles, can be packed in both bottles and pushed through blisters.
Frosta® technology
Akina owns Frosta technology. The technology incorporates manufacture of highly plastic granules using a plastic material, a material enhancing water penetration, and a wet binder.[100] These granules can then be compressed into tablets at low pressure, thus enabling fast disintegration upon administration.
Lyoc
Lyoc technology is owned by Cephalon Corporation. CIMA is a subsidiary of Cephalon, and currently manages the Lyoc RandD efforts. This was the first freeze-drying-based technology introduced for ODTs. The process involves preparation of a liquid solution or suspension of the drug-containing fillers, thickening agents, surfactants, non-volatile flavoring agents, and sweeteners.[101] This homogenous liquid is then deposited in blister cavities and subjected to freeze drying. Advantages of Lyoc compared with other freeze-dried dosage forms include absence of preservatives. Tables 5 and 6 shows various patented technologies and drugs formulated as FDT with their Brand names available in market.
Table 5.
ODT technologies and corresponding commercial products[8]
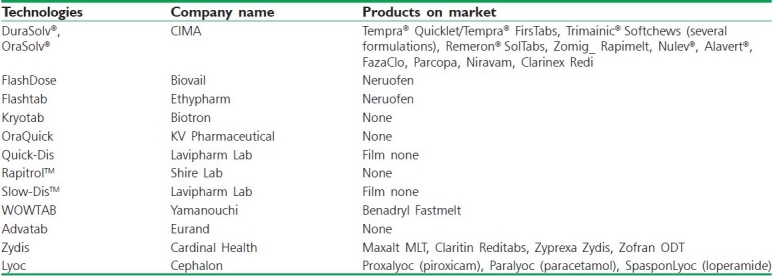
Table 6.
Orally disintegrating tablet products available in Indian market[45]
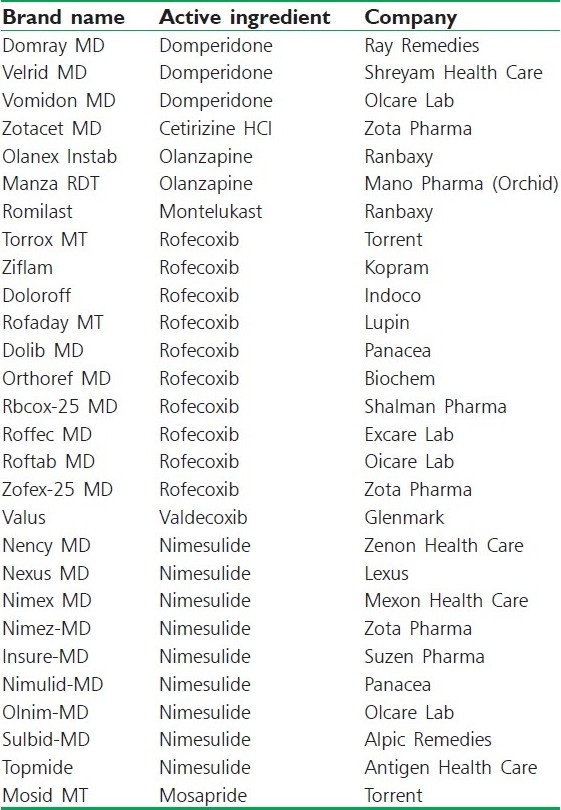
Dispersible tablet technology
Lek, Yugoslavia patents this technology. It offers development of ODT with improved dissolution rate by incorporating 8 to 10% of organic acids and disintegrating agents. Disintegrating agent facilitates rapid-swelling and good-wetting capabilities to the tablets that results in quick disintegration. Disintegrants include starch, modified starches, MCC, alginic acid, cross-linked sodium carboxymethyl cellulose, and cyclodextrins. Combination of disintegrants improved disintegration of tablets usually less than 1 minute.[102]
Pharmaburst™ technology
SPI Pharma, New Castle, patents this technology. It utilizes the coprocessed excipients to develop ODT, which dissolves within 30 to 40 seconds. This technology involves dry blending of drug, flavor, and lubricant followed by compression into tablets. Tablets obtained have sufficient strength, so they can be packed in blister packs and bottles.[103]
FUTURE RESEARCH TRENDS IN FAST DISINTEGRATING TABLETS
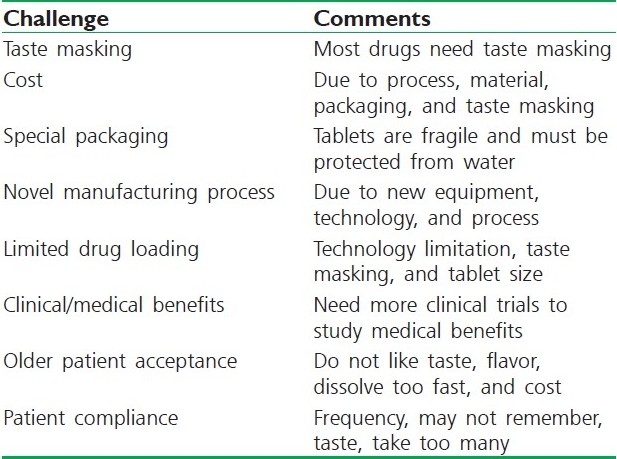
FDT products and technologies face various challenges, as listed in Table 7. These challenges are related to new technologies and products. As these technologies mature and new products are developed, some of these challenges will be addressed by various companies. Several technologies are used to achieve quick dispersion and drug delivery to the oral cavity. The four FDT technologies reviewed in this chapter include WOWTAB®, Zydis®, OraSolv®, and Shearform™. The formulation considerations including selection of drugs, excipients, packaging, and manufacturing process have been contrasted among these different FDT technologies. Each FDT technology has its own advantages and disadvantages but common to all are their rapid disintegration and convenience of dosing to patients. Special in vitro and in vivo test methods to study the performance of these products are required. Although FDT technology and products face many challenges as they are fairly new in the marketplace, these technologies are rapidly evolving and continue to undergo improvement which will address the future challenges and changing patient and healthcare needs. Overall, FDT products have enormous commercial potential, which will be realized in the next decade as more effective FDT products are being developed to address the unmet needs of the patients.
Table 7.
Challenges for quick-dissolving intraoral dosage form technology and products[104]
CONCLUSION
Quick-dispersing oral drug delivery systems are defined as oral drug delivery systems that when placed in the mouth dissolve or disintegrate within a few seconds to a few minutes and do not require water to aid swallowing. The FDT dosage forms are ideal for many groups of patients including geriatrics, pediatrics, and those people who have difficulty swallowing. An important benefit of FDT dosage forms is the ability to provide the advantages of a liquid medication in the form of a solid preparation. This feature enables the patient to take the dose as directed at any time without water and inconvenience. There is clear medical need and clinical benefits provided by these technologies and products.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Zade PS, Kawtikwar PS, Sakarkar DM. Formulation, evaluation and optimization of fast dissolving tablet containing tizanidine hydrochloride. Inter J Pharm Tech Res. 2009;1:34–42. [Google Scholar]
- 2.Ishikawa T, Watanabe Y, Utoquchi N, Matsumoto M. Preparation and evaluation of tablets rapidly disintegrating in saliva containing bitter-taste-masked granules by the compression method. Chem Pharm Bull. 1999;47:1451–4. doi: 10.1248/cpb.47.1451. [DOI] [PubMed] [Google Scholar]
- 3.Omaima SA, Mohammed HA, Nagia MA, Ahmed SZ. Formulation and optimization of mouth dissolve tablets containing rofecoxib solid dispersion. AAPS Pharm Sci Tech. 2006;7:E1–9. doi: 10.1208/pt070255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simone S, Peter CS. Fast dispersible ibuprofen tablets. Eur J Pharm Sci. 2002;15:295–305. doi: 10.1016/s0928-0987(02)00011-8. [DOI] [PubMed] [Google Scholar]
- 5.5th ed. 1.0. Strasbourg, France: 2005. European Pharmacopoeia 5.0; p. 628. [Google Scholar]
- 6.Habib W, Khankari R, Hontz J. Fast-dissolve drug delivery systems. Crit Rev Ther Drug Carrier Syst. 2000;17:61–72. [PubMed] [Google Scholar]
- 7.Li Q, Wei WX, Xiaofeng C, Tao H. Evaluation of disintegrating time of rapidly disintegrating tablets by a paddle method. Pharm Dev Tech. 2006;11:295–305. doi: 10.1080/10837450600767649. [DOI] [PubMed] [Google Scholar]
- 8.Vikas A, Bhavesh HK, Derek VM, Rajendra KK. Drug delivery: Fast dissolve systems. Encycl Phar Tech. 2007;1:1104–14. [Google Scholar]
- 9.Sohi H, Sultana Y, Khar RK. Taste masking technologies in oral pharmaceuticals: Recent developments and approaches. Drug Dev Ind Pharm. 2004;30:429–48. doi: 10.1081/ddc-120037477. [DOI] [PubMed] [Google Scholar]
- 10.Pondell R. Taste masking with coatings, Coating technology. 1996 [Google Scholar]
- 11.Zelalem A, Vibha P, Lokesh K, Arvind KB. Trends in pharmaceutical taste masking technologies: A patent review. Recent Pat Drug Deliv Formul. 2009;3:26–39. doi: 10.2174/187221109787158364. [DOI] [PubMed] [Google Scholar]
- 12.Freudenberg K, Cramer F, Plieninger H. Inclusion compounds of physiologically active organic compounds. Ger Pat. 1953:895769. [Google Scholar]
- 13.Szejtli J, Szente L. Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur J Pharm Biopharm. 2005;61:115–25. doi: 10.1016/j.ejpb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Fu Y, Yang S, Jeong SH, Kimura S, Park K. Orally fast disintegrating tablets: Developments, technologies, taste-masking and clinical studies. Crit Rev Ther Drug Carrier Syst. 2004;21:433–76. doi: 10.1615/critrevtherdrugcarriersyst.v21.i6.10. [DOI] [PubMed] [Google Scholar]
- 15.Amidon GL, Lennernas H, Shah VP, Crison JR. Theoritical basis for a biopharmaceutics drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 16.Lindenberg M, Kopp S, Dressman JR. Classification of orally administered drugs on the world health organization model list of essential medicines according to the biopharmaceutical classification system. Eur J Pharm Biopharm. 2004;58:265–278. doi: 10.1016/j.ejpb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Gupta GD. Farmulation and charracterization of fast dissolving tablet of promethazine theoclate. Asian J Pharm. 2008;2:70–72. [Google Scholar]
- 18.Dario L, Maria GB, Maria CL, Claudio JS. Development of prednisone: Polyethylene glycol 6000 fast release tablets from solid dispersion: Solid state characterization, dissolution behaviour, and formulation parameters. AAPS Pharm Sci Tech. 2007;8:221–228. doi: 10.1208/pt0804108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh J, Philip AK, Pathak K. Optimization studies on design and evaluation of orodispersible pediatric formulation of indomethacin. AAPS Pharm Sci Tech. 2008;9:60–6. doi: 10.1208/s12249-007-9018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzia C, Francesca M, Giovanna C, Paola M. Fast dissolving tablets of Glyburide based on ternary solid dispersions with PEG 6000 and surfactants. Drug Deliv. 2007;14:247–55. doi: 10.1080/10717540601067802. [DOI] [PubMed] [Google Scholar]
- 21.Sussanne B, Margareta D, Bo L, Hans L, Anders P, Marie W, et al. In vitro and In vivo evaluation of new sublingual tablet system for rapid oromucosal absorption using fentanyl citrate as the active substance. Eur J Pharm Sci. 2003;20:327–34. doi: 10.1016/j.ejps.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Iman SA, Mona HA. in vitro and in vivo evaluation of a fast disintegrating lyophilized dry emulsion tablet containing Griseofulvin. Eur J Pharm Sci. 2007;32:58–68. doi: 10.1016/j.ejps.2007.05.114. [DOI] [PubMed] [Google Scholar]
- 23.Sam C, Jean PR. Formulation and Production of Rapidly Disintegrating tablets By Lyophilization Using Hydrocholorothiazide as a Model Drug. Int J Pharm. 1997;152:215–25. [Google Scholar]
- 24.Abdelbary G, Prinderre P, Eouani C, Joachim J, Reynier JP, Piccerelle PH. The Preparation of Orally Disintegrating Tablets Using A Hydrophilic Waxy Binder. Int J Pharm. 2004;278:423–33. doi: 10.1016/j.ijpharm.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Raguia AS, Iman SA, Rehab NS. In vitro and In vivo evaluation of nimesulide lyophilized orally disintegrating tablets. Eur J Pharm Biopharm. 2009;73:162–71. doi: 10.1016/j.ejpb.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Verley P, Yarwood R. Zydis–a novel fast dissolving dosage form. Manuf Chem. 1990;61:36–7. [Google Scholar]
- 27.Jinichi F, Etsuo Y, Yasuo Y, Katsuhide T. Evaluation of rapidly disintegrating tablets containing glycine and carboxymethylcellulose. Inter J Pharm. 2006;310:101–109. doi: 10.1016/j.ijpharm.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 28.Segar H. Drug delivery products and the Zydis fast dissolving dosage. J Pharm Pharmacol. 1998;50:375–83. doi: 10.1111/j.2042-7158.1998.tb06876.x. [DOI] [PubMed] [Google Scholar]
- 29.Corveleyn S, Remon J. Formulation of a lyophilised dry emulsion tablet for the delivery of poorly soluble drugs. Int J Pharm. 1998;166:65–74. [Google Scholar]
- 30.Chandrasekhar R, Hassan Z, AlHusban F, Smith A, Mohammed A. The role of formulation excipients in the development of lyophilized fast-disintegrating tablets. Eur J Pharm Biopharm. 2009;72:119–29. doi: 10.1016/j.ejpb.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Carstensen JT. New York: Wiley Interscience; 1977. Pharmaceutics of Solids and Solid Dosage Forms; pp. 11–5. [Google Scholar]
- 32.Van Campen L, Zografi G, Carstensen JT. An approach to the evaluation of hygroscopicity for pharmaceutical solids. Int J Pharm. 1980;5:1–18. [Google Scholar]
- 33.Chang RK, Guo X, Burnside BA, Couch RA. Fast-dissolving tablets. Pharm Technol N Am. 2000;24:52–8. [Google Scholar]
- 34.Blank RG, Mody DS, Kenny RJ, Aveson MC. Fast dissolving dosage form. US Patent 4,946,684. 1990 Aug 7; [Google Scholar]
- 35.Green R, Kearney P. Process for preparing fast dispersing solid dosage form. US Patent 5,976,577. 1999 Nov 2; [Google Scholar]
- 36.Lawrence J, Posage G. Bicovex rapidly disintegrating dosage form. US Patent 6,224,905. 1998 Dec 3; [Google Scholar]
- 37.Simone S, Peter CS. Fast dispersing ibuprofen tablets. Eur J Pharm Sci. 2002;15:295–305. doi: 10.1016/s0928-0987(02)00011-8. [DOI] [PubMed] [Google Scholar]
- 38.Katzner L, Jones B, Khattar J, Kosewick J. Blister package and packaged tablet. US Patent 6,155,423. 2000 Dec 5; [Google Scholar]
- 39.Jaccard TT, Leyder J. A new galenic form: lyoc. Ann Pharm Fr. 1985;43:123–31. [PubMed] [Google Scholar]
- 40.Lafon L. Galenic form for oral administration and its method of preparation by lyophilization of an oil-in-water emulsion. US Patent 4616047. 1986 Oct 7; [Google Scholar]
- 41.Gole D, Savall T, fu GL, Dale W, Paul K, Davies JD. Tastemasked resinate and preparation thereof. US Patent application 20050036977. 2005 Feb 17; [Google Scholar]
- 42.Iles MC, Atherton AD, Copping NM. Freeze-dried dosage forms and methods for preparing the same. US Patent 5,188,825. 1993 Feb 23; [Google Scholar]
- 43.Ford J. The current status of solid dispersion. Pharm Acta Helv. 1986;61:69–88. [PubMed] [Google Scholar]
- 44.Dobetti L. Fast melting tablets: Developments and technologies. Pharm Technol Drug Deliv. 2001;(Suppl):44–50. [Google Scholar]
- 45.Suresh B, Rajendar KM, Ramesh G, Yamsani MR. Orodispersible tablets: An overview. Asian J Pharm. 2008;2:2–11. [Google Scholar]
- 46.Bi Y, Sunada H, Yorinobu Y, Danjo K, Otsuka A, Iida K. Preparation and evaluation of a compressed tablet rapidly disintegrating in the oral cavity. Chem Pharm Bull. 1996;44:2121–7. doi: 10.1248/cpb.44.2121. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe Y, Koizumi K, Zama Y, Kiriyama M, Matsumoto Y, Matsumoto M. New compressed tablet rapidly disintegrating in saliva in the mouth using crystalline cellulose and a disintegrant. Biol Pharm Bull. 1995;18:1308–10. doi: 10.1248/bpb.18.1308. [DOI] [PubMed] [Google Scholar]
- 48.Bi Y, Yorinobu Y, Sunada H. Rapidly disintegrating tablets prepared by wet compression method: Mechanism and optimization. J Pharm Sci. 1999;88:1004–10. doi: 10.1021/js990061z. [DOI] [PubMed] [Google Scholar]
- 49.Sunada H, Bi Y. Preparation, evaluation and optimization of rapidly disintegrating tablets. Powder Technol. 2002;122:188–98. [Google Scholar]
- 50.Di CM. Flashtab and T-Mask Technologies, Paper presented at: Proceedings of the 7th International Glatt Symposium. 1997:1–9. [Google Scholar]
- 51.Cousin G, Bruna E, Gendrot E. Rapidly disintegratable multiparticulate tablet, US Patent 5,464, 632. 1995 Nov 7; [Google Scholar]
- 52.Khankari R, Hontz J, Chastain S, Katzner L. Rapidly dissolving robust dosage form. US Patent 6,024,981. 2000 Feb 15; [Google Scholar]
- 53.CIMA LABS Inc., “Easy-to-take orally disintegrating tablets”. [Last cited on 2011 Jun 30]. available from: http://www.cimalabs.com/orallydisintegratingtablets .
- 54.Chang RK, Xiaodi B, Beth A, Couch RA. Fast-dissolving tablets. Pharm Technol. 2000;24:52–8. [Google Scholar]
- 55.Yamanouchi Pharma Technologies, Inc. WOWTAB. [Last cited on 2011 Jun 30]. available from: http://www.imagesrising.com .
- 56.Pather SI, Khankari R, Siebert J. “Quick-dissolving intraoral tablets” in Drug Delivery to the Oral Cavity: Molecules to Market. In: Ghosh TK, Pfister WR, editors. New York NY: CRC Press; 2005. pp. 291–310. [Google Scholar]
- 57.Bytul MR, Mir Imam IW, Proma K, Maruf A, Robiul I, Ranjan KB, et al. Effect of starch 1500 as a binder and disintegrant in lamivudine tablets prepared by high shear wet granulation. Pak J Pharm Sci. 2008;21:455–9. [PubMed] [Google Scholar]
- 58.Madhu KV. Coprecipitates and Melts. In: Swarbrick J, Boylen JC, editors. ‘Encyclopedia of Pharmaceutical Technology’. 3rd ed. Vol. 1. Pinehurst, North California, USA: Pharmaceutics Inc; 2007. pp. 774–81. [Google Scholar]
- 59.Giunchedi P, Conti B, Genta I, Conte U, Puglisi G. Emulsion spray drying for the preparation of albumin-loaded PLGA microspheres. Drug Dev India Pharm. 2001;27:745–50. doi: 10.1081/ddc-100107331. [DOI] [PubMed] [Google Scholar]
- 60.Alysson LR, Wanderley PO. Spray drying conditions and encapsulating composition effects on formation and properties of sodium diclofenac microparticles. Powder Technol. 2007;171:7–14. [Google Scholar]
- 61.Donald LW. 1st ed. New York: Marcel Dekker Inc; 2005. Handbook of Pharmaceutical Controlled Release Technology; pp. 112–3. [Google Scholar]
- 62.Lieberman HA, Lachman L, Schwartz JB. 2nd ed. Vol. 3. New York: Marcel Dekker Inc; 2005. Pharmaceutical Dosage Forms: Tablets; p. 187. [Google Scholar]
- 63.Allen LV, Wang B. Process for making a Particulate Support Matrix for Making a Rapidly Dissolving Dosage Form. US Patent 6,207,199. 2001 [Google Scholar]
- 64.Allen LV, Wang B. Process for making a Particulate Support Matrix for Making a Rapidly Dissolving Tablet. US Patent 5,587,180. 1996 [Google Scholar]
- 65.Allen LV, Wang B, Davis LD. Rapidly Dissolving Tablet. US Patent 5,807,576. 1998 [Google Scholar]
- 66.Jianchen X, Li LB, Kang Z. Taste masking microspheres for orally disintegrating tablets. Int J Pharm. 2008;359:63–9. doi: 10.1016/j.ijpharm.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 67.Honey G, Parshuram R, Vikas R, Ashok KT. Orally disintegrating systems: Innovations in formulation and technology. Recent Pat Drug Deliv Formul. 2008;2:258–74. doi: 10.2174/187221108786241660. [DOI] [PubMed] [Google Scholar]
- 68.Misra TK, Currington JW, Montwill BK, Satish V, Sanghvi PP, Sisak JR, et al. US20006048541. 2000 [Google Scholar]
- 69.Roser BJ, Blair J. Rapidly soluble oral dosage forms, methods of making same, and composition thereof. US Patent 5,762,961. 1998 Jun 9; [Google Scholar]
- 70.Jinichi F, Etsuo Y, Yasuo Y, Katsuhide T. Evaluation of rapidly disintegrating tablets containing glycine and carboxymethylcellulose. Int J Pharm. 2006;310:101–9. doi: 10.1016/j.ijpharm.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 71.Johnson JR, Wang LH, Gordon MS, Chowhan ZT. Effect of formulation solubility and hygroscopicity on disintegrating efficiency in tablets prepared by wet granulation. J Pharm Sci. 1991;80:469–71. doi: 10.1002/jps.2600800514. [DOI] [PubMed] [Google Scholar]
- 72.Anand V, Kandarapu R, Garg S. Preparation and evaluation of taste-masked orally disintegrating tablets of prednisolone. Asian J Pharm Sci. 2007;2:227–38. [Google Scholar]
- 73.Sumitha C, Karuna SN, Divya B, Madhavi K, Vimal KV, Charbe NN. Taste masking of ondansetron hydrochloride by polymer carrier system and formulation of rapid-disintegrating films. Int J Chem Res. 2009;1:24–7. [Google Scholar]
- 74.Koizumi K, Watanable Y, Morita K, Utoguchi N, Matsumoto M. New Method of preparing high-porosity rapidly saliva soluble compressed tablets using mannitol with camphor: A subliming material. Int J Pharm. 1997;152:127–31. [Google Scholar]
- 75.Adjei A, Doyle R, Reiland T, Swarbrick J, Boylan JC, editors. Vol. 6. New York: Marcel Dekker; 1992. Encyclopedia of Pharmaceutical Technology; p. 117. [Google Scholar]
- 76.Billany MR, Aulton ME, editors. New York: Churchill Livingstone; 1996. Pharmaceutics; The science of Dosage form Design, International Edition; p. 263. [Google Scholar]
- 77.Catania JS, Johnson AD. U. S. Patent 5633006. 1997 [Google Scholar]
- 78.Nelson SL. U.S. Patent 5766622. 1998 [Google Scholar]
- 79.Eby III, Georage A. U.S. Patent 5002970. 1991 [Google Scholar]
- 80.Pandya HB, Callan TP. U.S. Patent 5.837, 286. 1998 [Google Scholar]
- 81.Chang RK, Guo X, Burnside B, Couch R. Fast-dissolving tablets. Pharm Technol. 2000;24:52–8. [Google Scholar]
- 82.Morella AM, Pitman IH, Heinicke GW. Taste masked liquid suspensions. US Patent 6,197,348. 2001 [Google Scholar]
- 83.Tian W, Langride J. Fast dissolving and taste masked oral dosage form comprising sildenafil. Patent WO2004017976. 2004 [Google Scholar]
- 84.Corbo M, Desai J, Patell M. U.S. Patent 6.663.893. 2003 [Google Scholar]
- 85.Friend DR, Steve N, Sarabia RF, Weber TP, Geoffory J. U.S. Patent, 6, 139.865. 2000 [Google Scholar]
- 86.Augello M, Dell SM, Reier GE, Stamato HJ, DiMemmo LM. U.S. Patent, 6099.865. 2000 [Google Scholar]
- 87.Kato M. Japan Patent, 8259466. 1996 [Google Scholar]
- 88.Maccari M, Calanchi M, Kydonieus AF, editors. 1st ed. Vol. 2. Boca, Raton, Florida: CRS Press, Incl; 1980. Controlled Release Technologies, Methods, Theory and Applications; p. 113. [Google Scholar]
- 89.Narazaki R, Harada T, Takami N, Kato Y, Ohwaki T. A new method for disintegration studies for Rapid Disintegrating Tablet. Chem Pharm Bull. 2004;52:704–7. doi: 10.1248/cpb.52.704. [DOI] [PubMed] [Google Scholar]
- 90.Fu Y, Jeong SH, Park K. Preparation of Fast Dissolving Tablets Based on Mannose. Polym Mater Sci Eng Preprint. 2003;89:821–2. [Google Scholar]
- 91.Dor PJ, Fix JA. In vitro determination of disintegration time of quick- dissolving tablets using a new method. Pharm Dev Technol. 2002;7:361–71. doi: 10.1081/pdt-100102041. [DOI] [PubMed] [Google Scholar]
- 92.Mortia Y, Tsushima Y, Yasui M, Termoz R, Ajioka J, Takayama K. Evaluation of the disintegration time of rapidly disintegrating tablets via a novel method utilizing a CCD camera. Chem Pharm Bull. 2002;50:1181–6. doi: 10.1248/cpb.50.1181. [DOI] [PubMed] [Google Scholar]
- 93.Kancke J. Dissolution testing of orally disintegrating tablets. Disso Tech. 2003;10:6–8. [Google Scholar]
- 94.Wehling F, Schuehle S, Madamala N. Effervescent dosage form with microparticles. US Patent 5,178,878. 1993 Jan 12; [Google Scholar]
- 95.Yamanouchi Pharmaceutical Co. Press release: Open Yamanouchi Shaklee Pharma Research Center at Stanford Research Park. 1997 Nov 24; [Google Scholar]
- 96.Mizumoto T, Masuda Y, Fukui M. Intrabuccally dissolving compressed molding and production process thereof. U.S. Patent 5,576,014. 1996 Nov 19; [Google Scholar]
- 97.Mizumoto T, Masuda Y, Fukui M. Intrabuccally dissolving compressed moldings and production process thereof. US Patent 5,576,014. 1996 Nov 19; [Google Scholar]
- 98.Pebley WS, Jager NE, Thompson SJ. Rapidly Disintegrating Tablets. US Patent 5,298,261. 1994 Mar 29; [Google Scholar]
- 99.Cremer K. Fast dissolving technologies in detail: Orally disintegrating dosage forms. Pharma Concepts, Humberg, Germany, GmbH and Co. KG. 2001:62–97. [Google Scholar]
- 100.Jeong SH, Fu Y, Park K. Frosta: A new technology for making fast-melting tablets. Expert Opin Drug Deliv. 2005;2:1107–16. doi: 10.1517/17425247.2.6.1107. [DOI] [PubMed] [Google Scholar]
- 101.Laboratoire L. Galenic form for oral administration and its method of preparation by lyophilization of an oil-in-water emulsion. Eur Pat 0,159,237. 1985 [Google Scholar]
- 102.Kovacic M, Milovac J, Cvelbar P, Stalc A, Trost Z, Kopitar Z. Dispersible cimetidine tablets. US Patent 5,069,910. 1991 [Google Scholar]
- 103.Fu Y, Yang S, Jeong SH, Kimura S, Park K. Orally fast disintegrating tablets: Developments, technologies, taste-masking and clinical studies. Crit Rev Ther Drug Carrier Sys. 2004;21:433–76. doi: 10.1615/critrevtherdrugcarriersyst.v21.i6.10. [DOI] [PubMed] [Google Scholar]
- 104.Sharma K, Pfister WR, Ghosh TK. “Quick-dispersing oral drug delivery systems” in Drug Delivery to the Oral Cavity: Molecules to Market. In: Ghosh TK, Pfister WR, editors. New York, NY: CRC Press; 2005. pp. 261–90. [Google Scholar]


