Abstract
Microencapsulation is an accepted process used to achieve controlled release and drug targeting for many years. Mucoadhesion has been a topic of interest in the design of drug delivery systems to prolong its intestinal residence time. Mucoadhesion facilitates the intimate contact of the dosage form with the underlying absorption surface for improved bioavailability of drugs. Aceclofenac is a newer nonsteroidal anti-inflammatory drug (NSAID) having short biological half-life of 4–4.3 h, and therefore a sustained release medication is required to get prolonged effect and to reduce fluctuations in drug plasma concentration levels. Aceclofenac microcapsules were prepared employing sodium alginate as the coat material in combination with some mucoadhesive polymers such as (hydroxypropyl methyl cellulose) HPMC, (sodium carboxymethyl cellulose) Sod. CMC, Carbopol and methyl cellulose (MC) (drug:SA:polymer at ratios 2:2:1, 2:3:1 and 2:4:1), following orifice-ionic gelation technique. Infrared (IR) spectroscopy, differential scanning calorimetry and X-ray diffraction studies proved the compositions were compatible, without any interaction between the drug and excipients. The prepared microcapsules were evaluated for various physical and release parameters. The resulted microcapsules were found to be discrete and spherical in scanning electron microscopy studies and free flowing in rheological studies. The size of microcapsules was found to be around 757.44 ± 5.201 μm to 814.46 ± 6.586 μm. The microencapsulation efficiency was found to be higher in HPMC than in Carbopol > MC > Sod. CMC containing formulations, but the swelling index was found to be higher in Sod. CMC formulations. The microcapsules with HPMC exhibited good mucoadhesive property in the in vitro wash-off test. In vitro drug release studies of aceclofenac microcapsules were carried out up to 24 h and they followed zero-order release kinetics with Super Case II mechanism. The drug release from the microcapsules was sustained over a prolonged period with greater retardation in drug:SA:HPMC (2:4:1) containing microcapsules and this proved to be the best formulation.
Keywords: Aceclofenac, controlled release, ionic-orifice gelation, microencapsulation, mucoadhesion
INTRODUCTION
Microcapsules can be defined as solid, approximately spherical particles made of polymeric, waxy or other protective materials ranging in size from 1 to 1000 μm. Microencapsulation is a process used to achieve controlled release and drug targeting. Mucoadhesion has been a topic of interest in the design of drug delivery systems to prolong the residence time of the dosage form in gastrointestinal tract (GIT), which facilitates the intimate contact with the absorption surface to enhance the bioavailability of drugs.[1]
Rheumatism is an older term used to describe a number of painful conditions of muscles, tendons, joints, and bones connective tissue. Rheumatic conditions have been classified as localized, regional, or generalized. Localized rheumatism includes bursitis and tendinitis. Regional rheumatic conditions include chest wall pain, temporomandibular joint pain, and myofascial pain syndromes. Generalized rheumatic conditions include fibromyalgia. Another category of rheumatism is psychogenic rheumatism. With this term, it is understood that the patient is reporting inconsistent pains of muscles and joints that do not correspond to true anatomy and physiology. The patient feels to have underlying psychological causes for the symptoms. Although these disorders probably have little in common in terms of their epidemiology, they do share two characteristics like acute and chronic pain which is difficult to treat. Among the ill, elderly people, majority suffer from rheumatism in which medication should be used for a prolonged period, which may extend up to some months also.[2]
Aceclofenac is a newer nonsteroidal anti-inflammatory drug (NSAID). It is a phenylacetic acid derivative showing anti-inflammatory and analgesic properties with low and rare side effects, and is mainly used in the treatment of osteoarthritis, rheumatoid arthritis, post-traumatic pain, ankylosing spondylitis, etc. Aceclofenac is rapidly and efficiently absorbed after oral administration, but has short biological half-life of 4–4.3 h. Therefore, sustained release medication is required to reduce the dosing frequency, which gives prolonged effect with improved bioavailability and also improves the safety and efficacy of the medication. While the therapy it is given twice or thrice in a day, if the medicine is given as conventional dosage form, this leads to lot of inconvenience and fluctuations in therapy with some adverse effects of aceclofenac like gastrointestinal disturbances, peptic ulceration and gastrointestinal bleeding also. To overcome the demerits of a conventional dosage form, a suitable controlled drug delivery system should be developed.[3–5]
Several studies have reported on controlled drug delivery systems in the form of tablets, films, patches, and gels for oral, buccal, nasal, ocular, and topical routes. Aceclofenac is made available as many forms in the market like conventional tables and sustained release tablets, but microencapsulation is a technique used to deliver the medicament at controlled rate by targeting. Microcapsules have more advantages over conventional and simple sustained release tablet formulations, such as targeting, less dosing frequency, zero-order release and high margin of safety, which are not possible with the existing formulations. Amongst the polymers used for microencapsulation, alginate has gained much attention since it is nontoxic, biodegradable and can be prepared by a safe technique avoiding organic solvents. Hence, orifice-ionic gelation technique was developed as an alternative approach, even though so many other techniques are available like single and double emulsification techniques, normal and interfacial polymerization, coacervation phase separation, spray drying, spray congealing, etc.[6]
Mucoadhesion is the process by which a natural or a synthetic polymer can be adhered to a (biological substrate) mucosal layer. The substrate possessing mucoadhesive property can help in devising a delivery system capable of delivering a drug for a prolonged period of time at a specific delivery site and offers several advantages over other oral controlled systems by virtue of prolongation of residence of the drug in GIT. Mucoadhesive microcapsules provide the needed continuous therapy in the management of rheumatism with high margin of safety by evaluating pre- and post-formulation parameters.[7,8]
There are numerous drugs for treating rheumatism and inflammatory problems. The objective of the present work was to develop, characterize (pre- and post-formulation parameters) and evaluate aceclofenac mucoadhesive microcapsules by following orifice-ionic gelation technique using sodium alginate (SA) as the release rate retarding polymer with sodium carboxymethyl cellulose (Sod. CMC), hydroxypropyl methyl cellulose (HPMC), Carbopol and methyl cellulose (MC) as mucoadhesive polymers. Sod. CMC, HPMC and MC are economic and easily available synthetic hydrophilic polymers, and these can be extensively used for designing mucoadhesive delivery systems due to their ability to exhibit strong hydrogen bonding with the mucin present in the mucosal layer as compared to thiolated polymers, lectin-based polymers and other natural polymers. Basically, polymers of natural source containing polysaccharides, carbohydrates and cystine are less stable as compared to those containing synthetic polymers as these are highly prone for microbial degradation.[9]
MATERIALS AND METHODS
Aceclofenac pure drug was obtained as a gift sample from M/s Halmark Pharmaceuticals Pvt. Ltd., Hyderabad, India. HPMC and MC were procured from M/s Central Drug House (P) Ltd., New Delhi, India. SA (having a viscosity of 5.5 cps in a 1% w/v aqueous solution at 25°C), calcium chloride and petroleum ether were procured from M/s S. D. Fine Chemicals Pvt. Ltd., Mumbai, India.
Preparation of Aceclofenac Mucoadhesive Microcapsules
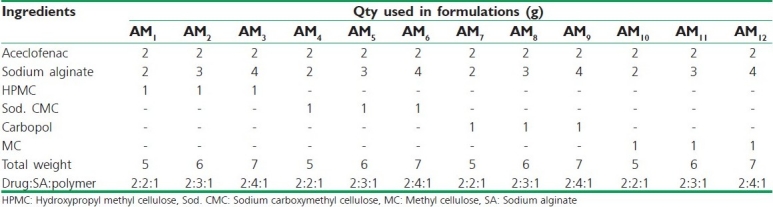
Aceclofenac mucoadhesive microcapsules were prepared by employing SA as the coat material in combination with four mucoadhesive polymers such as HPMC, Sod. CMC, Carbopol and MC (drug:SA:polymer at ratios 2:2:1, 2:3:1 and 2:4:1) by following orifice-ionic gelation process. SA (2.0 g, 3.0 g and 4.0 g) and the mucoadhesive polymer (1.0 g) were dissolved in purified water (25 ml) to form a homogenous polymer solution to which core material, aceclofenac (2.0 g), was added and mixed thoroughly to get smooth viscous dispersion [Table 1]. The resulting dispersion was then added drop wise to 100 ml calcium chloride (10% w/v) solution through a syringe with a needle of No. 22 size. The added droplets were retained in the calcium chloride solution for 15 min to complete the curing reaction and to produce spherical rigid microcapsules. The microcapsules were separated by decantation and the product was washed with water and petroleum ether and dried at 45°C for 12 h.[10–12] The stated ratios were fixed as per the results obtained in manual optimization of SA and mucoadhesive polymer. When drug:SA:polymer was less than 2:2:1, the formulation was found to disintegrate within a short time, and when the ratio was more than 2:4:1, the dosage form weight increased to more than 1100 mg, making it difficult to fill in a capsule and the release was also retarded for more than 24 h. When the ratio of mucoadhesive polymer was decreased less than the fixed ratio, formulations became non-adhesive, and when it was increased more than the fixed ratio, all the microcapsules became sticky and this also led to drying problem.
Table 1.
Composition of various batches of aceclofenac mucoadhesive microcapsules
Evaluation of Prepared Microcapsules
Particle size analysis
All the batches prepared were analyzed for particle size where the microcapsules were placed on a set of standard sieves ranging from sieve No. 16# to 60#, using an electromagnetic sieve shaker (Electro Lab, EMS-8). The sieves were arranged in such a way that they were in a descending order with the mesh size 16# on the top and 60# mesh in the bottom. The microcapsules passed through the set of sieves and the amount retained on each sieve was weighed and the average mean diameter was determined and considered as mean particle size:[13]

Bulk density
Accurately weighed microcapsules (M) were transferred to a 100 ml graduated cylinder to measure the apparent volumes or bulk volume (Vb). The measuring cylinder was tapped for a fixed period of time and tapped volume (Vt) occupied in the cylinder was measured. The bulk density and tapped/true density were calculated in gram per milliliter by the following formula:
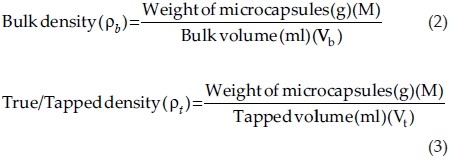
where M = mass of the powder, Vb = bulk volume of the powder and Vt = tapped volume of the powder.
Carr's index and Hausner's ratio
The static angle of repose was measured according to the fixed funnel and free standing cone method. The bulk density of the mixed microcapsules was calculated for determining the Hausner's ratio and Carr's index from the poured and tapped bulk densities of a known weight of sample using a measuring cylinder.[14,15] The following equations were used for the calculations:

Angle of repose
A funnel was fixed in a stand in such a way that the top of the funnel was at a height of 6 cm from the surface. The microcapsules were passed from the funnel so that they formed a pile. The height and the radius of the heap were measured and the angle of repose was calculated using the equation:[16]
![]()
Scanning electron microscopy
The surface, morphology, microcapsule size, microcapsule shape, etc., were determined using Scanning Electron Microscopy (BIOMETRICS: SEM-CS491Q/790Q). Dry microcapsules were placed on an electron microscope brass stub that was coated with gold (thickness 200 nm) in an ion sputter. Pictures of microparticles were taken by random scanning of the stub under reduced pressure (0.001 torr).
% Drug content evaluation
Aceclofenac content in the microcapsules was estimated by UV-spectrophotometric method at a wavelength of 275 nm in phosphate buffer, pH. 7.4, with 10% methanol (Elico, SL-158). The method obeyed Beer's law in the concentration range 5–25 mg/ml. Microcapsules containing equivalent to 100 mg of aceclofenac were crushed as fine powder, extracted with 10 ml of methanol, and made up to 100 ml with pH. 7.4. One milliliter of the sample solution was taken and made up to 10 ml with phosphate buffer, pH. 7.4, and the absorbance was measured at wavelength 275 nm. The procedure was repeated with pure aceclofenac. The absorbance values from the pure drug aceclofenac and microcapsules were measuredand the % drug content was calculated. The method was validated for linearity, accuracy and precision.
Microencapsulation efficiency
Microencapsulation efficiency was calculated using the following formula:[17]

Determination of wall thickness
Wall thickness of microcapsules was determined using the equation:[18]
![]()
where h = wall thickness, Γ = arithmetic mean radius of microcapsules, d1 and d2 are densities of core and coat material, respectively, and P is the proportion of medicament in microcapsules. All the experimental units were studied in triplicate (n=3).
Swelling index
Pre-weighed aceclofenac microcapsules (W0) formulated with mucoadhesive polymers by employing different coat:core ratios were placed in pH. 7.4 phosphate buffer maintained at 37°C. After the 3rd h, the microcapsules were collected and blotted to remove excess water and weighed (Wt). The swelling index was calculated with the following formula:[19]
![]()
where Wt = weight of microcapsules observed at the 3rd h and W0 = the initial weight of microcapsules.
Permeability studies
The permeability constant Pm of the microcapsules was calculated using the equation:[20]
![]()
where V is the volume of the dissolution medium (cm3), H the wall thickness of the microcapsules (mm), A the surface area of the microcapsules (cm2), Cs the solubility of the core material (mg) in the dissolution medium and K is the release rate constant (mg/h or h–1).
For a given microcapsule and under standard testing conditions, the values of V, A and Cs remain constant and hence the equation can be written as:
Pm = K × H (11)
where K is the release rate constant and H is the wall thickness of the microcapsule.
Fourier transform infrared studies
Fourier Transform Infrared (FT-IR) analysis measurements of pure drug, carrier and drug-loaded microcapsule formulations were obtained using a Perkin-Elmer system 200FT-IR spectrophotometer. The pellets were prepared on KBr-press under a hydraulic pressure of 150 kg/cm2; the spectra were scanned over the wave number range of 4000–400 cm–1 at the ambient temperature.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) was performed on aceclofenac drug loaded microcapsules using Seiko (Japan) DSC model 220C. Samples were sealed in aluminum pans and the DSC thermograms were reported at a heating rate of 10°C/min from 20 to 260°C.
X-ray diffraction studies
Different samples were evaluated by X-ray powder diffraction. Diffraction patterns were obtained using X-ray diffractometer with a radius of 240 mm. The Cu Ka radiation was Ni filtered. A system of diverging and receiving slits of 1 and 0.1 mm, respectively, was used. The pattern was collected with 40 kV of tube voltage and 30 mA of tube current and scanned over the 2q range of 10°–80°.
In vitro wash-off test for mucoadhesive microcapsules
The mucoadhesive property of the microcapsules was evaluated by an in vitro adhesion testing method known as wash-off method. A piece of goat intestinal mucus (2 × 2 cm) was mounted onto glass slides of 3 × 1 inch with elastic bands. Glass slide was connected with a suitable support. About 50 microcapsules were spread onto each wet tissue specimen, and thereafter the support was hung onto the arm of a USP tablet disintegrating test machine (Electro Lab, ED 2AL). The disintegration machine containing tissue specimen was adjusted for a slow, regular up and down movement in a test fluid at 37°C taken in a beaker. At the end of 1 h and later at hourly intervals up to 8 h, the machine was stopped and the number of microcapsules still adhering onto the tissue was counted. The test was performed in phosphate buffer of pH. 6.8.[21]
In vitro drug release studies of microcapsules
In vitro drug release studies of microcapsules were carried out using USP XXIII Eight-station dissolution rate test apparatus Type-I with a basket stirrer (Electro Lab, EDT 08 LX) at 100 rpm in 900 ml 0.1 N HCl for the 1st 2 h, then in phosphate buffer of pH. 7.4 at 50 rpm and temperature 37 ± 0.5°C. Microcapsules equivalent to 100 mg of aceclofenac were tied in a muslin bag and kept in the basket. Five milliliter samples of the dissolution fluid were withdrawn at regular intervals and replaced with fresh quantity of dissolution fluid. The samples were filtered, diluted and analyzed using Elico, SL-158 Double-beam UV-Visible Spectrophotometer at wavelength 275 nm. For all the formulations, the dissolution was carried out in triplicates and statistically analyzed using InStat3®. The obtained data were used to calculate the % drug release and to determine the order and mechanism of the release.[22] The formulation showed that best release was prepared six times and six formulations form each batch were evaluated for drug release and the results were statistically analyzed by analysis of variance (ANOVA).[23]
Curve Fitting Analysis[24–27]
Zero-order release rate kinetics
To study the zero-order release kinetics, the release rate data are fitted to the following equation:
Q=K0t (12)
where “Q” is the fraction of drug released, “K” the release rate constant and “t” is the release time.
First-order kinetics
A first-order release would be predicated by the following equation:
![]()
where C = amount of drug remaining at time “t”, Co = initial amount of the drug and K = first-order rate constant (h–1).
When the data are plotted as cumulative percent drug remaining versus time, it yields a straight line, indicating that the release follows first-order kinetics. The constant “K” can be obtained by multiplying 2.303 with slope.
Higuchi release model
To study the Higuchi release kinetics, the release rate data were fitted to the following equation:
Q=K.t1/2 (14)
where “Q” is the amount of drug released, “K” the release rate constant, and “t” is the release time.
When the data are plotted as accumulative drug released versus square root of time, it yields a straight line, indicating that the drug was released by diffusion mechanism. The slope is equal to “K”.
Korsmeyer-Peppas release model
The release rate data were fitted into the following equation:
Mt/M∞(Q)=K.tn (15)
where Mt/M∞ is the fraction of drug released, “K” the release constant, “t” the release time, and “n” is the diffusion exponent for the drug released that is dependent on the shape of the matrix dosage form.
When the data are plotted as log of drug released versus log time, it yields a straight line with a slope equal to “n” and the “K” value can be obtained from Y intercept:
Q=Ktn (16)
When n approximates 0.45, a Fickian/diffusion control release is implied; where 0.45>n<0.89, it implies non-Fickian transport; and n≥0.89 for zero-order release.
RESULTS AND DISCUSSION
The SEM and sieve analysis results showed the microcapsules to be discrete, spherical and free flowing. The particle size of microcapsules was found to be between 757.44 ± 5.201 μm and 814.46 ± 6.586 μm, with an average size of 757.44 ± 5.894 μm [Figure 1a and b]. Angle of repose, bulk density, Carr's index and Hausner's ratio were found to be between 24.41 ± 0.802 and 27.74 ± 0.515, 0.491 ± 0.102 and 0.598 ± 0.729, 10.526 ± 2.785 and 15.904 ± 4.527 and 1.1176 ± 0.0176 and 1.1891 ± 0.0425, respectively [Table 2].
Figure 1.
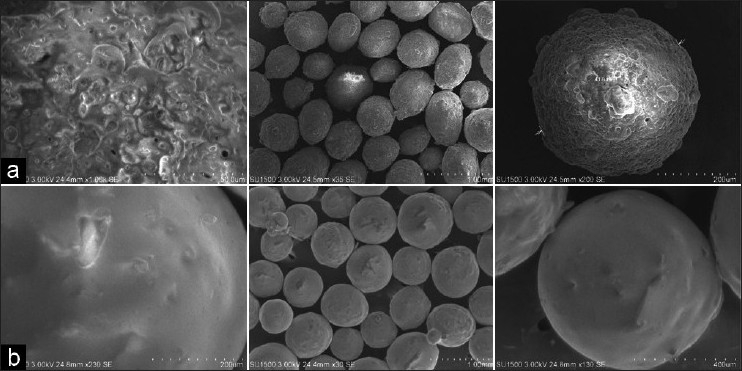
(a) SEM pictogram of aceclofenac mucoadhesive microcapsules prepared with HPMC; (b) SEM pictogram of aceclofenac mucoadhesive microcapsules prepared with Sod. CMC
Table 2.
Physical parameters’ data of aceclofenac mucoadhesive microcapsules AM1-AM12

Drug excipient compatibility was proved by FT-IR spectroscopy, DSC and X-ray diffraction (XRD) studies. In the IR spectra of aceclofenac, the pure drug formed a number of peaks prominently at different wave numbers, indicating the presence of functional groups and substituents, for example, peaks at 1683 cm-1 wave number were due to C-C stretching in aliphatic chain and prominent peaks at 1716 cm-1, 1282 cm-1, 1417 cm-1 and 1247-1255 cm-1 were due to C=O stretching, C–O stretching in acidic group, C–O–H stretching, and C–O–C stretching in aliphatic chain, respectively, indicating the presence of carboxylic group and keto group in the structure. Broad peaks appeared between 3000 cm-1 and 2850 cm-1 wave numbers due to C=C stretching in the aromatic structure. Peaks appearing at 2962 cm-1 and 1452 cm-1 were because of C–H stretching aromatic and in CH2 aliphatic respectively. A number of noise peaks between 600 cm-1 and 800 cm-1 wave numbers were found because of C–Cl asymmetric and symmetric vibrations and it indicates the presence of halogen group. A more intense peak was found at 3390 cm-1 because of N-H stretching, indicating the presence of amino group in the structure, and peak at 1438 cm-1 and 1452 cm-1 wave numbers also indicates C–N stretching. All these peaks appeared unchanged in the IR spectra of combinations like aceclofenac + SA + HPMC, aceclofenac + SA + Sod. CMC, aceclofenac + SA + Carbopol and aceclofenac + SA + MC; the above interpretational data clearly state that there was no interaction between the pure drug aceclofenac and other excipients. Therefore, it can be said that the drug and excipients are compatible [Figure 2].
Figure 2.

FT-IR spectra of aceclofenac pure drug, aceclofenac + SA + HPMC, aceclofenac + SA + Sod. CMC, aceclofenac + SA + Carbopol and aceclofenac + SA + MC
The melting point of pure aceclofenac was found to be 149.40°C and followed endothermic type of reaction for which the onset was at 140.89°C and ended at 152.36°C. The glass transition lag was found around 11.47°C and the same exothermic type of reaction was found in all combinations like aceclofenac + SA + HPMC, aceclofenac + SA + Sod. CMC, aceclofenac + SA + Carbopol, and aceclofenac + SA + MC. No change was found in the melting point as well as glass transition lag, but special peaks were found indicating melting point of SA as 219.93°C, HPMC as 109.48°C, Sod. CMC as 109.71°C, Carbopol as 93.98°C and MC as 101.97°C, and the influence of excipients was found to be only in changing on's and end's sets of melting point peaks of aceclofenac by absorbing heat but not by interactions. The above interpretational data clearly indicate that the crystalline nature of the drug had not been changed and it did not undergo any polymorphism because there was no interaction, which has been proved by its unchanged melting point in all the combinational spectra. X-ray diffractogram of aceclofenac proves its crystalline nature as evidenced from the number of sharp and intense peaks. The diffractogram of aceclofenac with polymers showed diffuse peaks indicating amorphous nature of the polymers and sharp, intense peaks indicating the crystalline nature of drug. Diffraction pattern of drug with polymer mixture showed simply the sum of the characteristic peaks of polymer, indicating the presence of drug in a crystalline form. Diffraction patterns of sample spectra represent the availability of crystalline peaks of drug situated at 11.56, 15.35, 32.26, and 44.11 (2θ) similar to the pure drug with corresponding intensities and linear counts respectively. The obtained 2θ values as characteristic peaks were found at the same position in combinations like aceclofenac + SA + HPMC, aceclofenac + SA + Sod. CMC, aceclofenac + SA + Carbopol and aceclofenac + SA + MC, but the intensities got reduced because of diffused peaks and more orientation in the case of polymers. Finally, the DSC and XRD data indicate that the crystallinity of pure drug was unchanged and stable, and indirectly show that the compositions are compatible [Figures 3 and 4].
Figure 3.
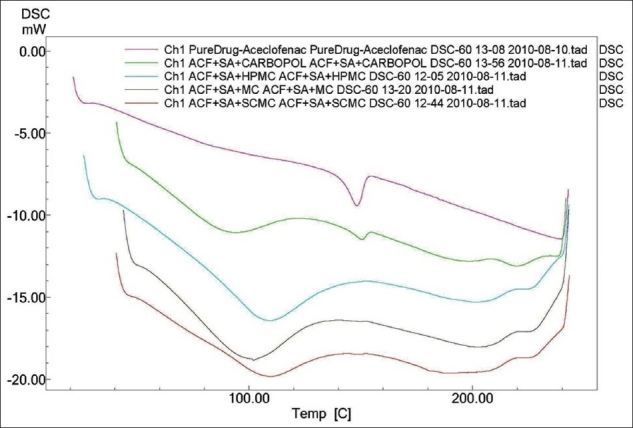
DSC spectra of aceclofenac pure drug, aceclofenac + SA + HPMC, aceclofenac + SA + Sod. CMC, aceclofenac + SA + Carbopol and aceclofenac + SA + MC
Figure 4.
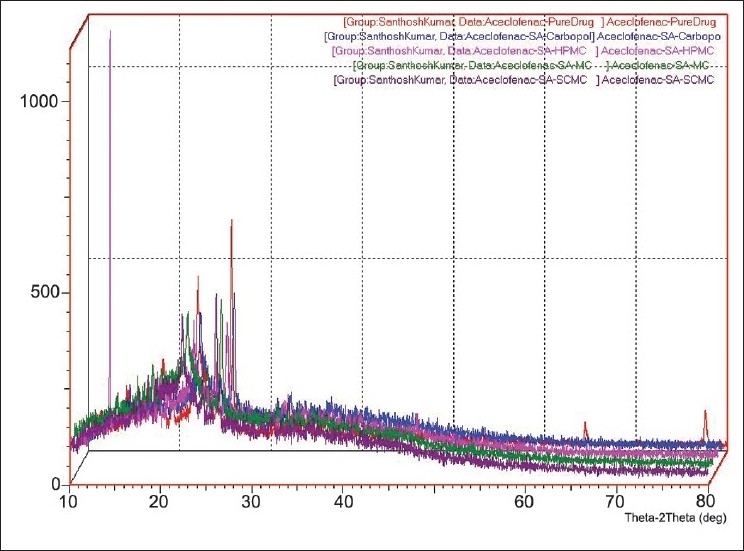
X-ray diffraction spectra of aceclofenac pure drug, aceclofenac + SA + HPMC, aceclofenac + SA + Sod. CMC, aceclofenac + SA + Carbopol and aceclofenac + SA + MC
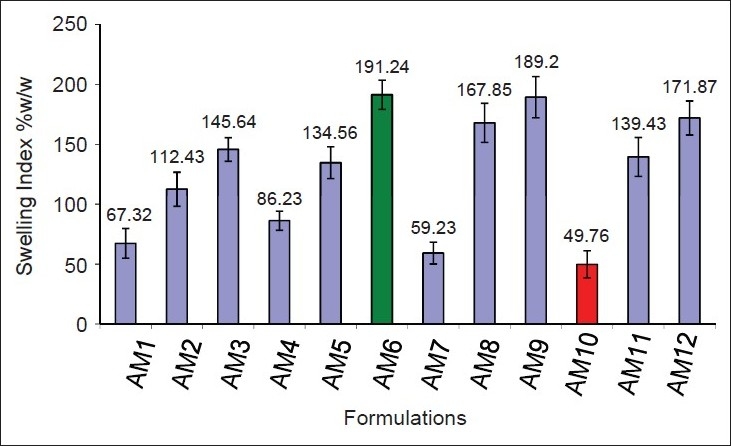
The microencapsulation efficiency was from 83.250 ± 1.687 to 99.939 ± 0.736%, with practical % drug content values around 23.31 ± 0.745 to 37.33 ± 1.198% [Table 3]. Wall thickness and permeability coefficient were found to be around 88.615 ± 2.345 to 107.6281 ± 5.234 μm and 452.361-537.170 μg/h, respectively. Swelling index was the highest in formulation AM8 (around 191.24 ± 12.194% w/w) and the least in AM10 (around 49.76 ± 11.232% w/w) [Figure 5]. All microcapsules exhibited good mucoadhesive property in the in vitro wash-off test [Figure 6] and microcapsules with HPMC AM3 showed better mucoadhesion where 38% of microcapsules were found adhered to the mucosal layer after 8 h [Table 4]. In the in vitro drug release studies, the highest release retardation was found to be around 99.716 ± 2.745% in the formulation AM3 up to 24 h, whereas the least retardation was observed to be around 99.635 ± 3.654% in the formulation AM4 after 17 h [Figure 7]. When that the best formulation was prepared 6 times from which 6 samples from each batch were evaluated for drug release (n=6) and statistically analyzed by (ANOVA), the data showed Df1 (5) and Df2 (30) with an F-value of 0.33724. The obtained F-value found less than f-table value, around 2.53, indicating less difference in between the groups and within the groups. P-value was found to be significant around 0.8862, proving maximum closeness between the results. All formulations followed zero-order non-Fickian release kinetics with Super Case II Transport mechanism [Table 5].
Table 3.
Drug content/encapsulation efficiency of formulations AM1–AM12
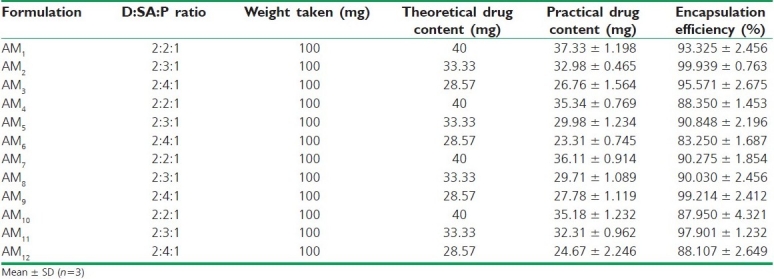
Figure 5.
Swelling index histogram of aceclofenac mucoadhesive microcapsules AM1–AM12
Figure 6.
In vitromucoadhesive wash-off test histogram results of aceclofenac mucoadhesive microcapsules AM1–AM12 after 8 h
Table 4.
In vitro wash-off test data of formulations AM1–AM12

Figure 7.
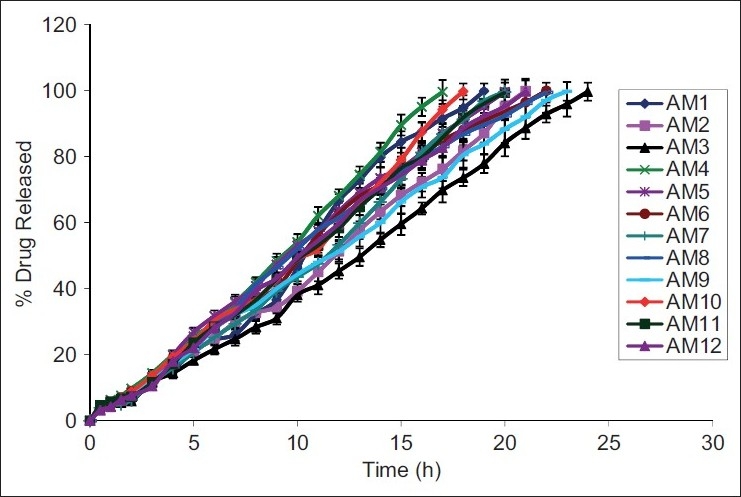
In vitro drug release plots of aceclofenac mucoadhesive microcapsules AM1–AM12
Table 5.
Release kinetic data of formulations AM1–AM12

All physical parameters were found in the acceptable range. The microencapsulation efficiency and mucoadhesive efficiency were found to be greater with HPMC than in other formulations, whereas swelling index was higher in formulations with Sod. CMC. All compositions were found compatible in IR, DSC and XRD studies and thus are suitable for extending the scope of work in this research area. The drug release from the microcapsules was sustained over an extended period of time. The study states that release depended on the core:coat ratio, which got retarded as the coat material percentage got increased. Microcapsules prepared using HPMC showed better sustained action, and formulation containing drug:SA:HPMC in the ratio 2:4:1 was found to be the best formulation as it released the maximum drug up to 24 h.
CONCLUSION
The mucoadhesive microencapsulation by following orifice-ionic gelation technique could be adopted in the laboratory as well as in the industry, as it is simple and reproducible. In conclusion, HPMC and Carbopol microcapsules could be used for better mucoadhesive action and SA could be used for better sustained action over an extended period of time. However, further in vivo studies are needed to optimize the drug for sustained action in human beings for better bioavailability and efficacy, and thus safety.
ACKNOWLEDGMENTS
Authors wish to thank M/s Halmark Pharmaceuticals Pvt. Ltd, Hyderabad, (Andhra Pradesh India) for providing gift sample of Aceclofenac and authors will be thankful to management of Vaageswari College of Pharmacy, Karimnagar, (Andhra Pradesh, India) for supporting us to finish up this study successfully.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Robinson RJ, Lee VH. Revised and expanded. 2nd ed. Vol. 29. New York: Marcel Dekker Inc; 2005. Controlled Drug Delivery: Fundamentals and Applications; pp. 9–19. [Google Scholar]
- 2.Salvador G, Gomez A, Vinas O, Ercilla G, Canete JD, Munoz-Gomez J, et al. Prevalence and clinical significance of anti-cyclic citrullinated peptide and antikeratin antibodies in palindromic rheumatism. Rheumatology (Oxford) 2003;42:972–5. doi: 10.1093/rheumatology/keg268. [DOI] [PubMed] [Google Scholar]
- 3.Vol. 1. Ghaziabad: Indian Pharmacopoeia Commission; 2007. The Indian Pharmacopoeia; pp. 267–8. [Google Scholar]
- 4.1 and 2. London: British pharmacopoeia Commission; 2009. British Pharmacopoeia; pp. 78–82. [Google Scholar]
- 5.Solanki SS, Dahima R. Formulation and evaluation of aceclofenac mouth-dissolving tablet. J Adv Pharm Tech Res. 2011;2:128–31. doi: 10.4103/2231-4040.82951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solmaz D, Reza A, Mohammadreza A, Ramin K. Formulation optimization of nifedipine containing microspheres using factorial design. African J Pharm Pharmacology. 2010;4:346–54. [Google Scholar]
- 7.Boddupalli BM, Mohammed ZN, Ravinder Nath A, Banji D. Mucoadhesive drug delivery system: An overview. J Adv Pharm Tech Res. 2010;1:381–7. doi: 10.4103/0110-5558.76436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pranshu TS, Sathish MN. Mucoadhesive drug delivery: Mechanism and methods of evaluation. Int J Pharma Bio Sci. 2011;2:458–67. [Google Scholar]
- 9.Carvalho FC, Bruschi ML, Evangelista RC, Gremião MP. Mucoadhesive drug delivery systems. Braz J Pharm Sci. 2010;46:1–17. [Google Scholar]
- 10.Chowdary KP, Srinivas L. Mucoadhesive drug delivery system: Status of Current review. Indian Drugs. 2000;37:400–10. [Google Scholar]
- 11.Liu XD, Yu WY, Zhang Y, Xue WM, Yu WT, Xiong Y, et al. Characterization of structure and diffusion behavior of Ca-alginate beads prepared with external or internal calcium sources. J Microencapsul. 2002;18:775–82. doi: 10.1080/0265204021000022743. [DOI] [PubMed] [Google Scholar]
- 12.Bahadur S, Chanda R, Roy A. Preparation and evaluation of mucoadhesive microcapsules of captopril for oral controlled release. Res J Pharm Tech. 2008;1:100–5. [Google Scholar]
- 13.Lachman L, Lieberman HA, Kanig JL. 3rd ed. Bombay: Varghese Publishing House; 1987. The theory and practice of industrial pharmacy; pp. 22–8. [Google Scholar]
- 14.Hausner HH. Friction conditions in a mass of metal powder. Int J Metall. 1967;3:7–13. [Google Scholar]
- 15.Carr RL. Evaluating flow properties of solids. Chem Eng. 1965;72:163–8. [Google Scholar]
- 16.Aulton ME. 3rd ed. New York: Churchill Livingstone; 1988. Pharmaceutics: The science of dosage form design; pp. 605–13. [Google Scholar]
- 17.Zinutti C, Hoffman M. Preparation and characterization of ethyl cellulose microspheres containing 5-fluoro-uracil. J Microencapsul. 1994;11:555–63. doi: 10.3109/02652049409034994. [DOI] [PubMed] [Google Scholar]
- 18.Si-Nang L, Carlier PF, Delort P, Gazzola J, Labont D. Determination of coating thickness of microcapsules and influence upon diffusion. J Pharm Sci. 1973;62:452–5. doi: 10.1002/jps.2600620320. [DOI] [PubMed] [Google Scholar]
- 19.Ma XJ, Xie YB, Zhou L, Yu XJ, Yuan Q, Li CC, et al. Relationship between preparation conditions and membrane strength of APA biomicrocapsules. Chinese J Organ Transplant. 1995;16:156–7. [Google Scholar]
- 20.Koida Y, Kobayashi M, Samejima M. Studies on microcapsules.IV: Influence of properties of drugs on microencapsulation and dissolution behavior. Chem Pharma Bull (Tokyo) 1986;34:3354–61. doi: 10.1248/cpb.34.3354. [DOI] [PubMed] [Google Scholar]
- 21.Lehr CM, Bowstra JA, Tukker JJ, Junginer HE. Intestinal transit of bioadhesive microspheres in an in situ loop in the rat. J Control Rel. 1990;13:51–62. [Google Scholar]
- 22.Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–33. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 23.Yadav A, Jain DK. Gastroretentive microbaloons of metformin: Formulations development and characterization. J Adv Pharm Tech Res. 2011;2:51–5. doi: 10.4103/2231-4040.79806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vol. 2. Ghaziabad: Indian Pharmacopoeia Commission; 2007. The Indian Pharmacopoeia; pp. 740–42. [Google Scholar]
- 25.Higuchi T. Mechanism of sustained action medication: Theoretical analysis of rate of release of solid drug dispersed in solid matrices. J Pharm Sci. 1963;52:1145–9. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 26.Ritger PL, Peppas NA. The simplest equation for description of solute release II.Fickian and anomalous release from swellable devices. J Controlled Rel. 1987;52:37–42. [Google Scholar]
- 27.Sipemann J, Peppas NA. Modeling of drug release for delivery systems based on hydroxypropyl methyl cellulose (HPMC) Adv Drug Del Rev. 2001;48:139–57. doi: 10.1016/s0169-409x(01)00112-0. [DOI] [PubMed] [Google Scholar]


