Abstract
Mikania scandens (L.) Willd. (Asteraceae), known as climbing hemp weed in English, is a herbaceous climbing vine grown as a weed throughout the plains of the Indian subcontinent. The present study evaluated some neuropharmacological properties of hydroalcoholic extract of aerial parts from M. scandens (HAMS) in experimental animal models. HAMS (at 250 and 500 mg/kg body weight, i.p.) was evaluated for central antinociceptive activity by tail flick method. Locomotor depressant activity was measured by means of an actophotometer. Skeletal muscle relaxant effect was evaluated by using rotarod apparatus and sedative potentiating property by phenobarbitone-induced sleep potentiation study. The results of the present study revealed significant (P<0.001) and dose-dependent central antinociceptive, locomotor depressant, muscle relaxant, and sedative potentiating effects of HAMS, demonstrating its depressant action on the central nervous system (CNS). From the present study, it can be concluded that the aerial parts of M. scandens possessed prominent depressant action on the CNS, as manifested by the important neuropharmacological properties in mice.
Keywords: Central nervous system depressant, locomotor, muscle relaxant, phenobarbitone
INTRODUCTION
Natural products have contributed significantly towards the development of modern medicine. Recently traditional medicine worldwide is being re-evaluated by extensive research on different plant species and their active therapeutic principles. The rich wealth of plant kingdom can represent a novel source of newer compounds with significant therapeutic activities. The major merits of herbal medicine seem to be their perceived efficacy, low incidence of serious adverse effects, and low cost.
Mikania scandens (L.) Willd. (Asteraceae), known as climbing hemp weed in English, is a twining herbaceous climbing vine with long-petioled, opposite leaves and small homogamous flower-heads grown as a common weed throughout the plains of India and Bangladesh. Traditionally, the plant has been used for some medicinal purposes in the Indian subcontinent. Aqueous leaf extracts of this plant have been used in folk medicine to treat stomach ulcers. The plant is thought to be efficacious in the treatment of gastric problems. Traditionally, its leaf juice is applied to the affected area of body in treatment of wounds and bruises. The plant is regarded as a rich source of vitamin A and C and also contains vitamin B, mikanin, friedelin, efifriedinol, and some sesquiterpene dilactones including mikanolide, dihydromikanolide, deoxymikanolide, and scandenolide. Three deterpenic acids known as kaurenic acid, butyryloxykaurenic acid, and benzoyloxykaurenic acid, stigmasterol, and betasitosterin have also been isolated from this plant.[1–3]
It has come to the author's notice that the rural people of Hooghly, Bardhaman, and Medinipur districts of West Bengal state of India use the young leaves of this plant in management of insect bites and stings. Previous workers reported analgesic and in vitro antioxidant activities of M. scandens leaf.[4] As very limited pharmacological work has been reported on this plant, the present study was aimed to assess some neuropharmacological activities of M. scandens aerial parts in experimental rodent models.
MATERIALS AND METHODS
Plant Material and its Authentication
The aerial parts of M. scandens were collected during June-July, 2011 from Gotan region of Bardhaman district of West Bengal, India. The species was authenticated by Dr. P. Lakshminarasimhan, Scientist D, at the Central National Herbarium, Botanical Survey of India, Howrah, West Bengal, India, and a voucher specimen (CNH/44/2011/Tech.II/476) was deposited at the Pharmacognosy Research Laboratory, Bengal School of Technology, Delhi Road, Sugandha, Hooghly 712102, India. Just after collection, the plant material was washed thoroughly with running tap water and shade dried at room temperature (24-26°C) and ground mechanically into a coarse powder.
Drugs and Chemicals
Morphine sulfate, diazepam, chlorpromazine hydrochloride, and phenobarbitone sodium were from Sigma-Aldrich Corporation, St. Louis, MO, USA. All the other chemicals were of analytical grade obtained commercially. Doubled distilled water from all-glass still was employed throughout the study.
Preparation of Extract
The powdered plant material was first defatted by using petroleum ether (60-80°C). The defatted plant material (45 g) was extracted with 50% aqueous ethanol (400 ml) by boiling under reflux for 90 minutes. The extract was filtered and evaporated to dryness to yield the dry extract (HAMS, yield: 11.78%). The dry extract was kept in a refrigerator until use. Preliminary phytochemical studies were performed on HAMS as per reported method.[5]
Experimental Animals
Adult Swiss albino mice of either sex weighing 20 ± 2 g were used in the present study. The mice were grouped and housed in polyacrylic cages (38 × 23 × 10 cm) with not more than three animals per cage and maintained under standard laboratory conditions (temperature 25 ± 2°C, relative humidity 48%, with dark/light cycle 12/12 h). They were allowed free access to standard diet and water ad libitum. The mice were acclimatized to laboratory conditions for 7 days before commencement of the experiments. All experimental procedures were reviewed and approved by the Institutional Animal Ethics Committee, Bengal School of Technology (No. BST/11/SB/001).
Acute Toxicity
The acute oral toxicity of HAMS in Swiss albino mice was studied as per reported method.[6]
Assessment of Neuropharmacological Properties
Evaluation of antinociceptive activity (tail flick test)
Before treatment, the basal reaction time for each mouse to radiant heat (Analgesiometer, Techno) was determined by placing the tip of the tail on the radiant heat source (nichrome wire). The strength of the current passing through the naked nichrome wire was kept constant at 6 Amps. The tail withdrawal time in seconds from the heat (flicking response) was considered as nociceptive end point. Any mouse failing to withdraw its tail within 2 to 4 seconds was excluded from the study.
The prescreened animals (reaction time: 2-4 seconds) were divided into three groups (n=6). The first two groups received the HAMS at the doses of 250 and 500 mg/kg body weight i.p., respectively. The last group (which served as reference) received morphine sulfate at a dose of 5 mg/kg body weight s.c. The reactions of all groups of mice were determined after 5, 15, 30, 60, and 90 minutes of treatment by tail flick method, similarly as mentioned above, and the latency times (in seconds) were recorded. Each animal served as its own control. The mean reaction times for each group were calculated.[7]
Evaluation of locomotor activity
The central nervous system (CNS) depressant activity of HAMS was evaluated by studying locomotor activity of mice using an actophotometer (Techno, India). The mice were divided into three groups (n=6). The equipment was turned on and animals of each group were placed individually in the activity cage for 10 minutes and the basal activity score of all the animals were monitored and recorded. Then, the first group (which served as reference) received chlorpromazine hydrochloride at the dose of 3 mg/kg body weight i.p. The rest two groups received the HAMS at the doses of 250 and 500 mg/kg body weight i.p., respectively. The animals were again tested for activity scores similarly 30 and 60 minutes after these treatments. Each animal served as its own control.[8] Percent reduction in motor activity was calculated for each animal by using the following formula:-
% reduction in motor activity = (Wa –Wb/Wa) × 100%.
Where, Wa and Wb are the mean activity scores before and after treatment, respectively.
Evaluation of muscle relaxant activity
The muscle relaxant activity of HAMS was evaluated by studying neurological deficit of mice using rotarod apparatus (Techno, India). The mice were divided into three groups (n=6). The equipment was turned on and adjusted to appropriate speed (20 rpm). Animals of each group were placed individually on the rotating rod and the fall off time of all the animals were recorded when the mouse falls from the rotating rod. Then, the first two groups received the HAMS at the doses of 250 and 500 mg/kg body weight i.p., respectively. The last group (which served as reference) received diazepam at the dose of 4 mg/kg body weight i.p. The animals were again tested for determination of fall off time similarly 30 and 60 minutes after these treatments. Each animal served as its own control.[9] Percent reduction in fall off time was calculated for each animal by using the following formula:-
% reduction in fall off time = (Wa–Wb/Wa) × 100%.
Where, Wa and Wb are the mean fall off times before and after treatment, respectively.
Evaluation of phenobarbitone-induced sleep potentiation
The animals were divided into four groups (n=6). The first group (which served as control) received normal saline 5 ml/ kg body weight i.p. The second and third groups received the HAMS at the doses of 250 and 500 mg/kg body weight i.p., respectively. The last group (which served as reference) received chlorpromazine hydrochloride at the dose of 3 mg/kg body weight i.p. Thirty minutes after these treatments, all the animals received phenobarbitone sodium at the dose of 20 mg/kg i.p. Immediately after phenobarbitone administration, each animal was kept in an individual cage under observation. The latency to the loss of righting reflex (i.e., onset of action or induction time in minutes) and the time required to recover righting reflex or awakening (i.e., duration of action or sleeping time in minutes) for each group were recorded.[10]
Statistical Analysis
All results were expressed as the mean ± standard error of mean (SEM). The results were analyzed for statistical significance by Student's ‘t’ test. P<0.001 was considered as statistically significant.
RESULTS
Preliminary phytochemical analysis revealed the presence of flavonoids, steroids, alkaloids, tannins, saponins, and sugars in HAMS. The HAMS was found to be safe in Swiss albino mice up to the dose of 2 000 mg/kg body weight p.o. LD50 could not be determined because physical factors were limiting for administration of more amount of HAMS.
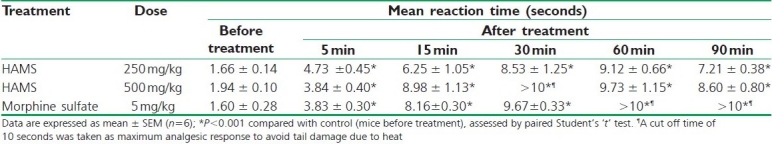
The results of antinociceptive activity, i.e., tail flick test are shown in Table 1. HAMS at the both doses exhibited significant (P<0.001, after 5 to 90 minutes) and dose-dependent steady increase in reaction time of mice up to 60 minutes of administration followed by minor decrease in activities, observed at both doses. Peak analgesic effect was observed at 30 to 60 minutes indicating maximum increase in reaction times.
Table 1.
Effect of HAMS on tail flick test in mice
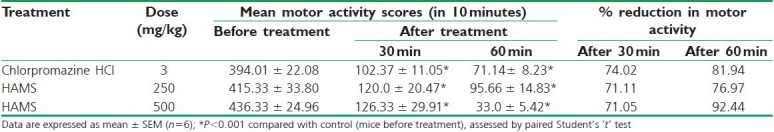
In locomotor activity study, it was found that HAMS significantly (P<0.001) depressed the locomotor activity in mice in a dose and time-dependent fashion. The activities increased as time approached to 60 minutes. The results are summarized in Table 2.
Table 2.
Effect of HAMS on locomotor activity in mice
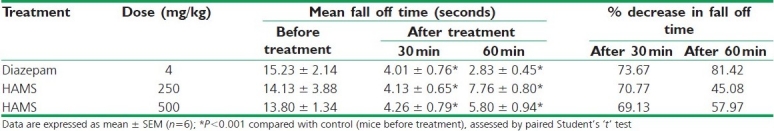
In muscle relaxant study, the HAMS at both doses significantly (P<0.001) and dose dependently decreased the fall off time in mice demonstrating its skeletal muscle relaxant property. The effect was most prominent after 60 minutes of administration [Table 3].
Table 3.
Effect of HAMS on muscle relaxant activity in mice
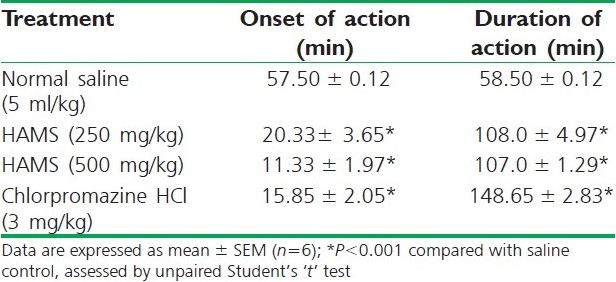
The results of phenobarbitone sodium-induced sleeping time are presented in Table 4. Here, HAMS at all doses pretreatment exhibited significant (P<0.001) and potentiation of phenobarbitone-induced sleeping time in mice by hastening the onset of sleep (dose dependent) and delaying recovering the animals from sleep (non-dose dependent) as compared with the vehicle control group.
Table 4.
Effect of HAMS on phenobarbitone induced sleeping time in mice
DISCUSSION
The present study examined some important neuropharmacological activities of hydroalcoholic extract from aerial parts of M. scandens (HAMS) in Swiss albino mice. The acute toxicity results revealed that this plant might be considered as non-toxic.
The antinociceptive activity of M. scandens extract (HAMS) was evaluated by tail flick method in mice to assess central (narcotic) analgesic activity.[11] The results of tail flick study clearly indicated that the HAMS had significant central antinociceptive action revealing the involvement of the CNS in antinociception. This implies that the HAMS exerted analgesic activity interfering the central mechanisms for the transmission of painful messages in mice.
The tail flick test is thermally induced nociception model where radiant heat is used as a source of pain. Here, radiant heat (through a hot nichrome wire) is applied to the tail of mice and the withdrawal of tail from the radiant heat source (hot nichrome wire) is considered as flicking response to thermally induced pain. The flicking reaction which is the end point of this test may be mediated as a spinal reflex. Analgesics of only narcotic (central) type, e.g., morphine, pethidine, pentazocine, etc., can increase the tail flick latency period indicating antinociception.[11,12]
Most of the centrally acting analgesics have certain CNS depressant effects. The locomotor activity was evaluated to assess the CNS-depressant property of HAMS on the motor activity in mice. Most of the centrally active analgesic agents influence the locomotor activities in human beings and rodents mainly by reducing the motor activity because of their CNS depressant property.[13] Locomotor activity is considered as an index of wakefulness or alertness of mental activity and a decrease may lead to calming and sedation as a result of reduced excitability of the CNS.[14] The results of the present study showed significant influence in locomotor activity of mice by HAMS treatment demonstrating decrease in locomotor activity and hence indicating its CNS depressant property in mice.
In muscle relaxant evaluation, the HAMS-induced decrease in fall off tine was due to the loss of muscle grip implying skeletal muscle relaxation.[11] Demonstration of marked muscle relaxant effect by the rotarod study indicated that HAMS induced neurological deficit accompanied with taming or calming effect in mice, thereby further supporting its CNS-depressant effect.
Barbiturates are putative sedatives inducing sleep in human beings and animals by depressing the CNS.[15] Phenobarbitone, although a long-acting barbiturate, at lower doses, it can serve as short to intermediate acting barbiturate.[10] In the present study, in the saline control group of mice, phenobarbitone (20 mg/kg) produced intermediate onset and duration of sleep as indicated by the loss of righting reflex (inability to maintain posture) and awakening or regaining righting reflex subsequently.[16] HAMS pretreatment remarkably reduced the sleep induction time in mice in a dose-dependent manner. It markedly and significantly prolonged the duration of sleep in phenobarbitone-induced mice; however, here the observed effect was not found dose dependent. Potentiation of phenobarbitone induced sleeping time by HAMS indicated the anxiolytic or sedative property of HAMS, thereby confirming its CNS depressant role in mice.
Polyphenolic compounds, like flavonoids, tannins, and phenolic acids, commonly found in higher plants have been reported to possess multiple biological effects.[17] Previous research showed that plants containing flavonoids, saponins, and tannins are useful in many CNS disorders.[18] Many flavonoids and neuroactive steroids were found to be ligands for the GABAA receptors in the CNS, which led to the assumption that they may act as benzodiazepine-like molecules (which act through GABAA receptor).[19] In the present study, phytochemical investigations revealed the presence of alkaloids, flavonoids, saponins, and tannins in the HAMS; therefore, presence of these phytoconstituents may be responsible for its CNS depressant properties.
From the present preliminary study, it can be concluded that the aerial parts of M. scandens possessed promising centrally mediated antinociceptive, locomotor depressant, skeletal muscle relaxant, and sedative potentiating effects in the experimental rodent models demonstrating its prominent depressant action on the CNS, as manifested by these important neuropharmacological properties in Swiss mice. Purification of the plant extract and further studies may reveal the exact mechanisms and constituents behind the observed neuropharmacological activities of M. scandens.
ACKNOWLEDGMENTS
The authors are grateful to the authority of the Bengal School of Technology (A College of Pharmacy), Sugandha, Hooghly, 712102, West Bengal, India, for providing necessary facilities for the present study.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Ghani A. Dhaka: The Asiatic Society of Bangladesh; 2003. Medicinal plants of Bangladesh. [Google Scholar]
- 2.Mahabub Nawaz AH, Hossain M, Karim M, Khan M, Jahan R, Rahmatullah M. An ethnobotanical survey of Jessore district in khulna division, Bangladesh. Am Euras J Sustain Agric. 2009;3:238–43. [Google Scholar]
- 3.Herz W, Subramaniam PS, Santhanam PS, Aota K, Hall AL. Structure elucidation of sesquiterpene dilactones from Mikania scandens. J Org Chem. 1970;35:1453–64. doi: 10.1021/jo00830a043. [DOI] [PubMed] [Google Scholar]
- 4.Hasan SM, Jamila M, Majumder MM, Akter R, Hossain MM, Mazumder ME, et al. Analgesic and antioxidant activity of the hydromethanolic extract of Mikania scandens (L.) Willd. leaves. Am J Pharm Toxicol. 2009;4:1–7. [Google Scholar]
- 5.Harborne JB. New Delhi: Springer (India) Pvt. Ltd; 1998. Phytochemical methods, a guide to modern techniques of plant analysis. [Google Scholar]
- 6.Lorke DA. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–87. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya S, Nagaich U. Assessment of anti-nociceptive efficacy of Costus speciosus rhizome in Swiss albino mice. J Adv Pharm Tech Res. 2010;1:34–40. [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharya S, Haldar PK, Zaman MK. Anti-nociceptive and locomotor activity of Zanthoxylum nitidum stem bark extracts in experimental animal models. J Comp Integr Med. 2010;7:1–8. [Google Scholar]
- 9.Turner RA. New York: Academic Press; 1965. Screening methods in pharmacology. [Google Scholar]
- 10.Awe SO, Olajide OA, Adeboye JO, Makinde JM. Some pharmacological studies on Morlnda luclda. Indian J Pharmacol. 1998;30:38–42. [Google Scholar]
- 11.Vogel HG. Berlin, Heidelberg: Springer Verlag; 2002. Drug discovery and evaluation, pharmacological assays. [Google Scholar]
- 12.Seth UK, Dadkar NK, Kamt UG. Bombay: Mohanlal B. Kothari Book Depot; 1972. Drugs acting on CNS: Selected topics in experimental pharmacology. [Google Scholar]
- 13.Muthal AV, Chopde CT. Effect of neuropeptide FMR Famide on morphine and amphetamine stimulated locomotor activity. Indian J Pharmacol. 1993;25:167–9. [Google Scholar]
- 14.Singh N, Kaur S, Bedi PM, Kaur D. Anxiolytic effects of Equisetum arvense Linn.extracts in mice. Indian J Exp Biol. 2011;49:352–6. [PubMed] [Google Scholar]
- 15.Tripathi KD. New Delhi: Jaypee Brothers Medical Publishers; 1999. Essentials of medical pharmacology. [Google Scholar]
- 16.Kulkarni SK. New Delhi: Vallabh Prakashan; 1999. Hand book of experimental pharmacology. [Google Scholar]
- 17.Bhattacharya S. Are we in the polyphenols era? Pharmacognosy Res. 2011;3:147. doi: 10.4103/0974-8490.81966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatacharya SK, Satyan KS. Experimental methods for evaluation of psychotropic agents in rodents: I–Anti-anxiety agents. Indian J Exp Biol. 1997;35:565–75. [PubMed] [Google Scholar]
- 19.Hossain MM, Biva IJ, Jahangir R, Vhuiyan MM. Central nervous system depressant and analgesic activity of Aphanamixis polystachya (Wall.) parker leaf extract in mice. Afr J Pharm Pharmacol. 2009;3:282–6. [Google Scholar]


