Abstract
Summary
Background and objectives
Studies have evaluated acute kidney injury (AKI) using biomarkers in various settings, but their prognostic utility within current practice is unclear. Thus, we sought to determine the prognostic utility of newer biomarkers or traditional markers (fractional excretion of sodium [FeNa] and urea [FeUrea] and microscopy) over clinical assessment alone.
Design, setting, participants, & measurements
This is a prospective cohort study of adults on the first day of meeting AKI criteria. We measured urine concentrations of neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and IL-18 and determined FeNa, FeUrea, and microscopy score for casts and tubular cells. Primary outcome was worsened AKI stage from enrollment to peak serum creatinine or in-hospital death.
Results
In 249 recipients, 57% were ≥65 years old, 48% were from intensive care, and mean baseline GFR was 69 ± 30 ml/min per 1.73 m2. AKI was considered prerenal in 164 (66%), acute tubular necrosis (ATN) in 51 (20%), and “other” in 34 (14%). All mean protein biomarker concentrations, FeNa, FeUrea, and microscopy scores were statistically different between prerenal and ATN. Seventy-two patients (29%) developed the primary outcome. There was an approximate three-fold increase in adjusted risk for the outcome for upper versus lower values of NGAL, KIM-1, IL-18, and microscopy score (P values <0.05). Net reclassification improved after adding these to baseline clinical assessment. FeNa and FeUrea were not useful.
Conclusions
On the first day of AKI, urine protein biomarkers and microscopy significantly improve upon clinical determination of prognosis, indicating their potential utility in current practice.
Introduction
The incidence of acute kidney injury (AKI) in hospitalized patients, which is associated with short- and long-term mortality (1), ranges between 2 and 7% (2). Clinicians rely on three traditional markers to evaluate AKI: serum creatinine (SCr), urine output, and microscopic urine sediment examination. The peak change in SCr during hospitalization associates with mortality by the Risk, Injury, Failure, Loss and End-stage renal disease (RIFLE) (3) and Acute Kidney Injury Network (AKIN) (4) classification systems (5,6); however, these staging methods have limited predictive power for outcomes at the initial moment of AKI diagnosis. Apart from reflex laboratory evaluation of abnormal urinalyses for AKI, urine microscopy is typically only performed by consulting nephrologists days later. Other frequently utilized markers to characterize hospital-associated AKI include fractional excretion of sodium (FeNa) and urea (FeUrea), although their utility in hospitalized patients is often questionable (7–9).
Considering the caveats and limitations of these AKI markers (10–13), in addition to their relatively unknown prognostic utility at the time of AKI diagnosis, it is necessary to improve AKI risk stratification as early as possible given current practice constraints. In fact, early AKI diagnosis and identification of those at high risk for worsening may ultimately improve outcomes (14,15). Advances in urinary protein biomarker research may lead to better AKI prognostication and earlier detection, both of which are necessary to develop effective treatments and prevention.
Animal experiments have demonstrated the utility of many novel urinary proteins of kidney injury, and human studies support their potential role in clinical practice (16). Three frequently cited proteins are neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and IL-18. We hypothesized that these newer markers would provide additional prognostic information when added to clinical assessment at the time of AKI diagnosis in hospitalized patients. We conducted a prospective cohort study to test this hypothesis in a tertiary care setting compared with traditional markers at the time of AKI (FeNa, FeUrea, and urine microscopy).
Materials and Methods
We adhered to the ethical standards of the Declaration of Helsinki. The Yale Human Investigation Committee approved this study, and its reporting here follows Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies (17). Although de-identification of patient information and samples (freshly collected urine that would have otherwise been discarded) permitted waiver of written consent, we sought verbal consent from all patients and/or surrogates.
Study Design and Participants
Patients 18 years of age and older at Yale-New Haven Hospital were prospectively screened for AKI by AKIN criteria between July 2008 and September 2009. Patients were eligible if they were admitted with at least stage 1 AKI or if they developed at least stage 1 AKI during their hospitalization. We excluded patients with end-stage kidney disease or kidney transplant, those discharged within 24 hours of enrollment, and the few with stage 3 AKI at enrollment (given little room for further worsening kidney function). Electronic medical records were used to screen all patients on medical and surgical floors and in intensive care units (ICUs) on a daily basis. Real-time SCr graphs were utilized to detect increases by at least 0.3 mg/dl or by 50% over the previous 2 days.
Data Collection
Serum values for creatinine, sodium, and urea, available as part of routine patient care from the day of admission to discharge, were recorded for study patients. For patients with AKI within 48 hours of admission, we obtained baseline SCr from available out-patient records within the prior year. For those who developed hospital-acquired AKI, baseline SCr was defined as the admission SCr. A small proportion of patients may have had AKI on admission, subsequently improved, and then developed recurrent AKI that led to study enrollment. For these patients, who were either missed on admission or had no available out-patient SCr values, baseline SCr was defined as the lowest SCr within 2 days before the AKI episode at enrollment. Baseline GFR was estimated from baseline SCr using the Modification of Diet in Renal Disease study equation (18). We recorded laboratory, demographic, and other clinical variables on standardized collection forms from available records. Patient characteristics (including comorbidities) were recorded from the medical histories obtained by admitting/consulting physicians.
Outcomes
AKI stage on the mornings of diagnosis and peak SCr were determined relative to baseline SCr according to AKIN criteria (urine output not used): stage 1, increase in SCr by ≥0.3 mg/dl or 0.5- to <2-fold increase and stage 2, 2- to <3-fold increase; and stage 3, ≥3-fold increase, or SCr ≥4.0 mg/dl after a rise of at least 0.5 mg/dl or acute dialysis requirement.
The primary outcome was a composite of worsened AKIN stage (i.e., progression to higher stage after AKI diagnosis as determined by peak SCr or dialysis requirement) or in-hospital mortality. We further analyzed variables significantly associated with the primary outcome for potential associations with the secondary outcomes of in-hospital death, worsened AKIN stage, dialysis, and nephrology consult. We also determined duration of AKI (number of days from the initial rise in SCr to its return to below the cutoff for stage 1 AKI) and length of hospital stay.
For descriptive purposes, we classified AKI as prerenal azotemia, acute tubular necrosis (ATN), or “other” according to the nephrology consultation diagnosis when available. For those without nephrology consults, all available records were used by study physicians to retrospectively classify AKI with blinding to biomarker values (including microscopy). The following key variables were considered: volume status and response to resuscitation (if administered), SCr kinetics (slope of rise, duration of elevation and fall), exposure to nephrotoxins, and indications for dialysis. An independent nephrologist (M.A.P.) abstracted a random sample of 30 charts to determine reliability of our AKI classification and adjudicator agreement.
Specimen Handling and Microscopy
One 10-ml urine sample was collected from the catheter tubing, or a clean catch was requested directly from the patient within a few hours of the routine morning blood draw that indicated the patient had AKI. The samples were immediately refrigerated and then centrifuged at 5000 × g for 10 minutes at 4°C within 4 hours of collection. Aliquots of 1 ml were promptly stored in labeled microvials at −80°C for subsequent NGAL, KIM-1, IL-18, FeNa, and FeUrea measurements.
Bright-field urine microscopy was performed, and urine sediment scores were calculated, as described previously on the subset of samples available within 1 hour of collection (19,20). Microscopy score was calculated by adding the points given for the number of granular casts per low-power field (magnification, ×100) and the points given for the number of renal tubular epithelial cells (RTEs) per high-power field (×400): 0 casts or 0 RTEs, 0 points; 1 to 5 casts or 1 to 5 RTEs, 1 point each; and ≥6 casts or ≥6 RTEs, 2 points each. Urine sediments were verified by two physicians utilizing representative and specific digital photomicrographs of microscopy samples after screening the entire slide, with debatable sediments agreed upon by consensus with an independent nephrologist (C.R.P.). All of the microscopists, who were extensively trained and assessed for quality control as described previously (19,20), were blinded to clinical and patient characteristics at the time of examination.
Biomarker Measurements
ELISA methods were performed as described previously for NGAL (21), KIM-1 (22), and IL-18 (23), with intra- and interassay variability for all biomarkers documented at <10%. Standard hospital laboratory systems were used to measure urine sodium and urea. Both serum and urine creatinine were measured by a modified Jaffé method standardized against isotope dilution mass spectrometry, with intra- and interassay variability documented at <2.3%. All of the laboratory measurements were performed by personnel blinded to patient information.
Statistical Analyses
Analyses were assessed at a two-tailed alpha = 0.05. We compared dichotomous variables using chi-squared or exact tests, categorical outcomes with the Jonckheere-Terpstra trend test, and continuous variables with the two-sample t test. The kappa statistic was calculated between adjudicators for retrospective AKI classification. We stratified the cohort into quartiles of urine NGAL, KIM-1, and IL-18, respectively, and examined the occurrence of primary and secondary outcomes among these groups. We also examined outcome occurrences in the cohort after stratifying by FeNa (<1%, 1 to 2%, and >2%), FeUrea (<35%, 35 to 50%, and >50%), and microscopy score (0, 1, 2, and ≥3). Multivariable logistic and log-binomial regression was used to determine the crude and adjusted relationship between the composite primary outcome and appropriate predictor levels given additional clinical variables that were consistently available at the time of AKI. The first set of covariates (limited adjustment model) was age ≥65 years, body mass index, male gender, and non-Caucasian race. We defined the baseline clinical model as all of these covariates plus baseline GFR, surgery before AKI, diabetes, and hypertension. Given only three primary outcome occurrences for the lowest microscopy score, we only report results from the limited adjustment model for microscopy. Each predictor/biomarker was assessed individually in all models without combining biomarkers.
For biomarkers that appeared useful for the primary outcome by regression analysis, we calculated net reclassification improvement (NRI) and integrated discrimination improvement (IDI) indices to determine the prognostic benefit of adding those biomarkers individually to the baseline clinical model (24). These statistics have been increasingly utilized to evaluate the prognostic benefit of adding biomarkers to clinical prediction models. NRI describes the reclassification of risk in the desired direction, whereas IDI describes the increased division of events and nonevents, after adding the biomarker to the baseline model. These measures may be more informative than changes in traditional areas under the curve (AUCs), but they depend on levels of risk set by the investigator, as well as the quality of calibration (i.e., goodness-of-fit) of the baseline model (25). We set three levels of risk, with intermediate risk defined as a predicted outcome occurrence approximately equal to the overall occurrence for the cohort (approximately 30%) and low and high risk as <20 and >40%, respectively. We also performed receiver operating characteristic curve analysis to compare individual predictors and multivariable models using the method suggested by DeLong et al. (26). All of the analyses were performed with SAS version 9.2 statistical software for Windows (SAS Institute, Cary, NC).
Subgroup Analyses
We performed subgroup analyses for the primary outcome with the biomarkers that appeared useful by regression analysis according to baseline SCr source (admission, outpatient, or lowest stable SCr 2 days before enrollment), enrollment location (ICU or floor), and days from admission to AKI (<3, 3 to 7, or >7 days).
Results
Cohort Description
A total of 284 patients were enrolled. After prespecified exclusions, 249 were available for analysis (Figure 1). Table 1 describes the characteristics of the entire cohort. At enrollment, 83% of the entire cohort had AKI stage 1, and almost 25% had pre-existing chronic kidney disease.
Figure 1.
Study enrollment and exclusions.
Table 1.
Cohort characteristics
| Characteristic | n = 249 |
|---|---|
| Demographics | |
| age ≥65 years | 143 (57) |
| male gender | 143 (57) |
| non-Caucasian race | 59 (24) |
| body mass index | 29.9 ± 8 |
| Clinical characteristics | |
| pre-existing CKD | 62 (25) |
| hypertension | 171 (69) |
| diabetes | 102 (41) |
| CHF | 92 (37) |
| CAD | 102 (41) |
| COPD | 49 (20) |
| stroke | 34 (14) |
| dementia | 20 (8) |
| liver failure/cirrhosis | 27 (11) |
| active cancer | 60 (24) |
| OSA | 21 (8) |
| Number of comorbidities | |
| none | 17 (7) |
| 1 | 34 (14) |
| ≥2 | 198 (79) |
| Tobacco use | |
| never | 130 (52) |
| prior | 60 (24) |
| current | 42 (17) |
| Enrollment location | |
| ICU | 120 (48) |
| floor | 129 (52) |
| Kidney function | |
| baseline SCr (mg/dL) | 1.2 ± 0.5 |
| baseline GFRa | 68.5 ± 30 |
| Stage of AKI at enrollment | |
| stage 1 | 207 (83) |
| stage 2 | 42 (17) |
| Stage of AKI at peak SCr | |
| stage 1 | 176 (71) |
| stage 2 | 41 (16) |
| stage 3 | 18 (7) |
| stage 3-dialysisb | 14 (6) |
| Oliguria/anuria | 39 (16) |
| Day 0 SCr (mg/dl) | 1.8 ± 0.60 |
| Peak SCr (mg/dl) | 2.2 ± 1.1 |
| Discharge SCr (mg/dl) | 1.4 ± 0.7 |
| Length of AKI (days) | 6.9 ± 11 |
| Length of stay (days) | 20 ± 26 |
| AKI etiology by chart review | |
| ATN | 51 (20) |
| prerenal azotemia | 164 (66) |
| other | 34 (14) |
The values presented as n (%) or mean ± SD. AKI, acute kidney injury; ATN, acute tubular necrosis; CKD, chronic kidney disease (if specifically listed in patient medical history); CHF, congestive heart failure; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnea; ICU, intensive care unit; SCr, serum creatinine.
GFR (in ml/min per 1.73 m2) was estimated by the Modification of Diet in Renal Disease equation.
Those acutely dialyzed for AKI; excludes those with stage 3 AKI who were not dialyzed.
AKI Prognosis
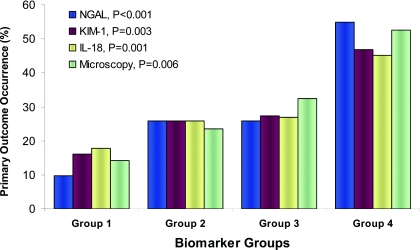
Seventy-two patients (29%) developed the primary outcome. Mean and median values for all three protein biomarkers were significantly higher in those who experienced the outcome compared with those who did not (Table 2). Irrespective of AKI etiology, quartiles of all three protein biomarkers had a graded relationship with the risk for the outcome (Table 3). NGAL provided the strongest association by regression analysis. Compared with the first quartiles, protein biomarker levels in the fourth quartiles had over two-fold higher adjusted relative risks for the primary outcome. The traditional cutoffs for FeNa and FeUrea were not associated with the primary outcome, but patients with microscopy scores of ≥3 had 3.5-fold higher risk compared with those with scores of 0 after limited adjustment. Figure 2 shows the incidence of the primary outcome by protein biomarker quartiles and microscopy scores.
Table 2.
Urine biomarkers and microscopy by primary outcome
| All | No Primary Outcome | Primary Outcome | Pa | |
|---|---|---|---|---|
| Urine biomarkers | ||||
| n | 249 | 177 | 72 | |
| NGAL (ng/ml) | 62 (5.8 to 1110) | 43 (4.9 to 388) | 203 (21 to 2709) | <0.001 |
| KIM-1 (ng/ml) | 2.8 (0.6 to 9.8) | 2.5 (0.5 to 7.3) | 4.1 (1.2 to 13.3) | <0.001 |
| IL-18 (pg/ml) | 70 (0 to 437) | 61 (0 to 348) | 103 (0 to 573) | 0.002 |
| FeNa (%) | 0.9 (0.1 to 4.8) | 0.8 (0.1 to 5.0) | 1.0 (0.1 to 4.3) | 0.95 |
| FeUrea (%) | 13.5 (4.9 to 27.9) | 14.0 (5.0 to 27.9) | 10.1 (3.9 to 28.7) | 0.03 |
| Urine microscopy | ||||
| n | 165 | 116 | 49 | Pb |
| score 0 | 21 (13) | 18 (16) | 3 (6) | 0.006 |
| score 1 | 51 (31) | 39 (34) | 12 (24) | |
| score 2 | 74 (45) | 50 (43) | 24 (49) | |
| score ≥3 | 19 (12) | 9 (8) | 10 (20) |
The values are presented as medians (10th to 90th percentile) or n (%). Primary outcome was a composite of worsened AKIN stage (progression to higher stage following AKI diagnosis based on peak SCr or dialysis requirement) or in-hospital mortality. AKI, acute kidney injury; SCr, serum creatinine; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule-1; FeNa, fractional excretion of sodium; FeUrea, fractional excretion of urea.
Value for Wilcoxon test.
P value for Jonckheere-Terpstra trend test.
Table 3.
Association of newer and traditional biomarker levels at time of AKI diagnosis with the primary outcome
| Biomarker Cutoffs | Percent with the Outcomea | Unadjusted RR (95% CI) | AUC (95% CI) | Limited Adjustment RR (95% CI)b | AUC (95% CI) | Full Adjustment RR (95% CI)c | AUC (95% CI) |
|---|---|---|---|---|---|---|---|
| Urine NGAL (ng/ml) | |||||||
| Q1 (<18.3) | 10% | Ref | 0.71 (0.64 to 0.77) | Ref | 0.74 (0.67 to 0.81) | Ref | 0.75 (0.68 to 0.81) |
| Q2 (18.3 to 62.3) | 26% | 2.7 (1.1 to 6.4) | 1.9 (1.0 to 3.8) | 1.6 (0.9 to 2.80) | |||
| Q3 (62.3 to 234.9) | 26% | 2.7 (1.1 to 6.4) | 1.9 (1.0 to 3.8) | 1.6 (0.9 to 2.8) | |||
| Q4 (>234.9) | 55% | 5.7 (2.6 to 12.6) | 3.6 (1.9 to 6.7) | 2.6 (1.6 to 4.3) | |||
| Urine KIM-1 (ng/ml) | |||||||
| Q1 (<1.5) | 16% | Ref | 0.64 (0.57 to 0.72) | Ref | 0.68 (0.60 to 0.75) | Ref | 0.69 (0.61 to 0.76) |
| Q2 (1.5 to 2.8) | 26% | 1.6 (0.8 to 3.2) | 1.6 (0.8 to 3.2) | 1.5 (0.8 to 3.1) | |||
| Q3 (2.8 to 4.9) | 27% | 1.7 (0.8 to 3.4) | 1.7 (0.9 to 3.5) | 1.6 (0.8 to 3.2) | |||
| Q4 (>4.9) | 47% | 2.9 (1.5 to 5.4) | 3.0 (1.6 to 5.5) | 2.8 (1.5 to 5.3) | |||
| Urine IL-18 (pg/ml) | |||||||
| Q1 (<25.7) | 18% | Ref | 0.63 (0.55 to 0.70) | Ref | 0.66 (0.59 to 0.74) | Ref | 0.68 (0.60 to 0.76) |
| Q2 (25.7 to 69.8) | 26% | 1.5 (0.7 to 2.9) | 1.4 (0.7 to 2.7) | 1.3 (0.7 to 2.7) | |||
| Q3 (69.8 to 178.1) | 27% | 1.5 (0.8 to 3.0) | 1.4 (0.7 to 2.7) | 1.3 (0.7 to 2.7) | |||
| Q4 (>178.1) | 45% | 2.5 (1.4 to 4.6) | 2.5 (1.4 to 4.6) | 2.7 (1.4 to 5.0) | |||
| FeNa | |||||||
| <1% | 27% | Ref | 0.50 (0.42 to 0.57) | Ref | 0.58 (0.50 to 0.67) | Ref | 0.62 (0.54 to 0.70) |
| 1 to 2% | 35% | 1.3 (0.8 to 2.2) | 1.3 (0.8 to 2.1) | 1.2 (0.7 to 2.1) | |||
| ≥2% | 25% | 1.0 (0.6 to 1.6) | 1.0 (0.6 to 1.7) | 1.0 (0.6 to 1.7) | |||
| FeUrea | |||||||
| <35% | 29% | Ref | 0.51 (0.48 to 0.55) | Ref | 0.6 (0.52 to 0.67) | Ref | 0.64 (0.56 to 0.72) |
| 35 to 50% | 11% | 0.4 (0.1 to 2.5) | 0.4 (0.1 to 2.7) | 0.4 (0.1 to 2.6) | |||
| ≥50% | 29% | 1.0 (0.3 to 3.3) | 1.1 (0.3 to 3.7) | 1.1 (0.3 to 3.6) | |||
| Microscopy score | |||||||
| 0 | 14% | Ref | 0.63 (0.54 to 0.71) | Ref | 0.66 (0.57 to 0.75) | ||
| 1 | 24% | 1.6 (0.5 to 5.2) | 1.6 (0.5 to 5.2) | N/Ad | |||
| 2 | 32% | 2.3 (0.8 to 6.8) | 2.3 (0.8 to 6.8) | ||||
| 3 | 53% | 3.7 (1.2 to 11) | 3.5 (1.1 to 11) |
Each biomarker listed was assessed individually in all models without combining biomarkers. All regression models shown were sufficiently calibrated with “goodness-of-fit” P values >0.1 by the Hosmer-Lemeshow method. AKI, acute kidney injury; RR, relative risk from log-binomial regression; CI, confidence interval; AUC, area under the receiver operating characteristic curve; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule-1; FeNa, fractional excretion of sodium; FeUrea, fractional excretion of urea; Ref, reference value.
Percentage with the outcome within each quartile or group.
Adjusted for age ≥65 years, body mass index, male gender, and non-Caucasian race. The AUC for this model alone was 0.58 (0.50 to 0.66).
Adjusted for all covariates above plus baseline glomerular filtration rate, surgery before AKI, diabetes, and hypertension. The AUC for this model alone was 0.62 (0.54 to 0.70). By the DeLong test, AUCs were only significantly different between this model and the model with the addition of urine NGAL quartiles (P = 0.003).
Full adjustment was not applicable for the urine microscopy score given only three actual occurrences for the primary outcome for the lowest (reference) score.
Figure 2.
Urine protein biomarker quartiles and microscopy score by primary outcome of worsened AKIN stage or death. NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule-1. For urine protein levels, group 1 is quartile 1 (NGAL: <18.3 ng/ml, n = 62; KIM-1: <1.5 ng/ml, n = 62; IL-18: <25.7 pg/ml, n = 63), group 2 is quartile 2 (NGAL: 18.3 to 62.3 ng/ml, n = 62; KIM-1: 1.5 to 2.8 ng/ml, n = 62; IL-18: 25.7 to 69.8 pg/ml, n = 63), group 3 is quartile 3 (NGAL: 62.3 to 239.4 ng/ml, n = 62; KIM-1: 2.8 to 4.9 ng/ml, n = 62; IL-18: 69.8 to 178.1 pg/ml, n = 63), and group 4 is quartile 4 (NGAL: >239.4 ng/ml, n = 62; KIM-1: >4.9 ng/ml, n = 62; IL-18: >178.1 pg/ml, n = 63). For urine microscopy, group 1 represents scores of 0 (n = 21), group 2 is scores of 1 (n = 51), group 3 is scores of 2 (n = 74), and group 4 is scores of ≥3 (n = 19). P values are for the Jonckheere-Terpstra trend test for each marker.
The AUC (95% confidence interval) for the primary outcome improved from a baseline of 0.62 (0.54 to 0.70) for the full clinical model to 0.75 (0.68 to 0.81), 0.69 (0.61 to 0.76), and 0.68 (0.60 to 0.76), respectively, with the individual additions of NGAL, KIM-1, and IL-18 quartiles from the first day of AKI (DeLong P value only significant for NGAL, P = 0.003). Adding microscopy improved the limited adjustment model from an AUC of 0.58 (0.50 to 0.66) to 0.66 (0.57 to 0.75) (P = 0.12). We compared predicted risk categories and actual primary outcome occurrences between the baseline clinical model and the same model plus NGAL, KIM-1, IL-18, or microscopy via NRI and IDI (Table 4). NRI was highly significant and of substantial magnitude for each marker (NGAL: 46.4%, P < 0.0001; KIM-1: 21.6%, P = 0.03; IL-18: 26.1%, P = 0.007; microscopy: 24.3%, P = 0.002). IDI was also highly significant for each marker. As an example of improved reclassification, among the 50 patients with the primary outcome that were initially categorized as intermediate risk by the clinical model alone, adding NGAL appropriately reclassified 22 as high risk (eight as low risk).
Table 4.
Reclassification of primary outcome risk after adding urine biomarker quartiles or urine microscopy score at AKI diagnosis to the baseline clinical model
| Risk with Clinical Model Alone | Risk with Clinical Model + Biomarker Shown |
|||
|---|---|---|---|---|
| Low | Intermediate | High | Row Total | |
| NGAL | ||||
| patients without the outcome | ||||
| low, frequency (row %) | 20 (71%) | 2 (7%) | 6 (21%) | 28 |
| intermediate, frequency (row %) | 59 (44%) | 57 (42%) | 19 (14%) | 135 |
| high, frequency (row %) | 2 (15%) | 4 (31%) | 7 (54%) | 13 |
| column total | 81 | 63 | 32 | 176 |
| patients with the outcome | ||||
| low, frequency (row %) | 3 (38%) | 4 (50%) | 1 (13%) | 8 |
| intermediate, frequency (row %) | 8 (16%) | 20 (40%) | 22 (44%) | 50 |
| high, frequency (row %) | 0 (0%) | 1 (7%) | 13 (93%) | 14 |
| column total | 11 | 25 | 36 | 72 |
| NRI and IDI with NGAL (95% CI, P value): 46.4% (26.9 to 66.1, P < 0.0001) and 11.9% (7.8 to 16, P < 0.0001) | ||||
| KIM-1 | ||||
| patients without the outcome | ||||
| low, frequency (row %) | 19 (68%) | 9 (32%) | 0 (0%) | 28 |
| intermediate, frequency (row %) | 48 (36%) | 68 (50%) | 19 (14%) | 135 |
| high, frequency (row %) | 0 (0%) | 6 (46%) | 7 (54%) | 13 |
| column total | 67 | 83 | 26 | 176 |
| patients with the outcome | ||||
| low, frequency (row %) | 3 (38%) | 5 (63%) | 0 (0%) | 8 |
| intermediate, frequency (row %) | 12 (24%) | 23 (46%) | 15 (30%) | 50 |
| high, frequency (row %) | 0 (0%) | 3 (21%) | 11 (79%) | 14 |
| column total | 15 | 31 | 26 | 72 |
| NRI and IDI with KIM-1 (95% CI, P value): 21.6% (2.7 to 40.6, P = 0.03) and 6.0% (2.8 to 9.3, P < 0.0001) | ||||
| IL-18 | ||||
| patients without the outcome | ||||
| low, frequency (row %) | 18 (62%) | 11 (38%) | 0 (0%) | 29 |
| intermediate, frequency (row %) | 46 (34%) | 74 (55%) | 15 (11%) | 135 |
| high, frequency (row %) | 0 (0%) | 4 (31%) | 9 (69%) | 13 |
| column total | 64 | 89 | 24 | 177 |
| patients with the outcome | ||||
| low, frequency (row %) | 6 (75%) | 2 (25%) | 0 (0%) | 8 |
| intermediate, frequency (row %) | 8 (16%) | 22 (44%) | 20 (40%) | 50 |
| high, frequency (row %) | 0 (0%) | 5 (36%) | 9 (64%) | 14 |
| column total | 14 | 29 | 29 | 72 |
| NRI and IDI with IL-18 (95% CI, P value): 26.1% (7.3 to 44.8, P = 0.007) and 5.7% (2.6 to 8.8, P < 0.0001) | ||||
| Microscopy score | ||||
| patients without the outcome | ||||
| low, frequency (row %) | 15 (71%) | 4 (19%) | 2 (10%) | 21 |
| intermediate, frequency (row %) | 25 (29%) | 52 (60%) | 10 (11%) | 87 |
| high, frequency (row %) | 1 (13%) | 1 (13%) | 6 (75%) | 8 |
| column total | 41 | 57 | 18 | 116 |
| patients with the outcome | ||||
| low, frequency (row %) | 2 (40%) | 3 (60%) | 0 (0%) | 5 |
| intermediate, frequency (row %) | 4 (11%) | 18 (47%) | 16 (42%) | 38 |
| high, frequency (row %) | 0 (0%) | 2 (33%) | 4 (67%) | 6 |
| column total | 6 | 23 | 20 | 49 |
| NRI and IDI with microscopy score (95% CI, P value): 24.3% (8.8 to 39.7, P = 0.002) and 6.7% (2.7 to 10.7, P = 0.001) | ||||
For risk classification, predicted primary outcome occurrence (increased AKI stage from enrollment to peak creatinine, need for dialysis or in-hospital death) of <20%, 20 to 40%, and >40% was considered low, intermediate, and high risk, respectively. The number within each cell indicates the number of patients (within each row) reclassified for the multivariable risk of the primary outcome by changing from the clinical model alone to the clinical model with the addition of each biomarker shown. The number in parentheses is the row percentage for each cell. The clinical model consisted of age ≥65 years, body mass index, male gender, non-Caucasian race, baseline glomerular filtration rate, surgery prior to AKI, diabetes, and hypertension. AKI, acute kidney injury; NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule-1; NRI, net reclassification improvement; IDI, integrated discrimination improvement.
Secondary Outcomes and AKI Classification
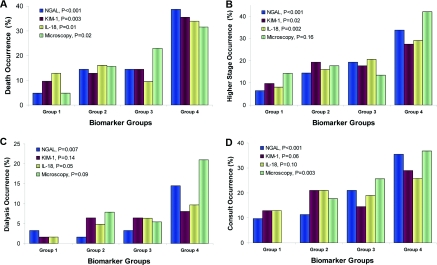
Patients with elevated protein biomarkers or higher urine microscopy scores experienced higher secondary outcome occurrence rates (Figure 3). The trend test was significant for death for all three proteins and microscopy. Mortality for the first quartile versus the fourth quartile of NGAL, KIM-1, and IL-18 was 5% versus 39% (P < 0.0001), 10% versus 35% (P = 0.0003), and 13% versus 34% (P = 0.01), respectively. Mortality for the lowest versus highest microscopy score was 5% versus 32% (P = 0.02). All three protein biomarkers were significantly associated with progression to higher AKI stage, whereas only NGAL and IL-18 were associated with the need for acute dialysis. Only NGAL and microscopy scores were significant for nephrology consult.
Figure 3.
Urine protein biomarker quartiles and microscopy score by secondary outcomes. NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule-1. For urine protein levels, group 1 is quartile 1 (NGAL: <18.3 ng/ml, n = 62; KIM-1: <1.5 ng/ml, n = 62; IL-18: <25.7 pg/ml, n = 63), group 2 is quartile 2 (NGAL: 18.3 to 62.3 ng/ml, n = 62; KIM-1: 1.5 to 2.8 ng/ml, n = 62; IL-18: 25.7 to 69.8 pg/ml, n = 63), group 3 is quartile 3 (NGAL: 62.3 to 239.4 ng/ml, n = 62; KIM-1: 2.8 to 4.9 ng/ml, n = 62; IL-18: 69.8 to 178.1 pg/ml, n = 63), and group 4 is quartile 4 (NGAL: >239.4 ng/ml, n = 62; KIM-1: >4.9 ng/ml, n = 62; IL-18: >178.1 pg/ml, n = 63). For urine microscopy, group 1 represents scores of 0 (n = 21), group 2 is scores of 1 (n = 51), group 3 is scores of 2 (n = 74), and group 4 is scores of ≥3 (n = 19). (A) Death alone as the outcome. (B) Progression in AKI stage from enrollment to peak serum creatinine as the outcome. (C) Dialysis alone as the outcome. (D) Nephrology consultation as the outcome. P values are for the Jonckheere-Terpstra trend test for each marker.
By retrospective chart review, 164 (66%) were classified as prerenal, 51 (20%) were classified as ATN, and 34 (14%) were classified as “other,” which included diagnoses such as acute interstitial nephritis, hepatorenal syndrome, lupus nephritis, and urinary obstruction (supplemental Table 1). The kappa between adjudicators for AKI classification was 0.76. Mean and median levels of all three protein biomarkers were significantly higher for ATN compared with prerenal. Mean values for FeNa and FeUrea were actually slightly lower in the ATN group. For microscopy, the trend test suggested overall scores were higher for ATN and lower for prerenal.
Subgroup Analyses
Baseline SCr was defined by admission SCr in 180 patients (72%), by outpatient SCr in 52 patients (21%), and by the lowest stable SCr within 2 days before enrollment in 17 patients (7%). Unadjusted risk for the primary outcome for all protein biomarkers and microscopy was essentially no different for the admission SCr subgroup than for the entire cohort (supplemental Table 2). The other baseline SCr subgroups lacked outcome occurrences in multiple groups, making them too small to characterize. Unadjusted risks were also similar for protein biomarkers and microscopy irrespective of the number of days from admission to enrollment (<3, 3 to 7, or >7 days). Although point estimates were attenuated in patients enrolled from the ICU compared with the floor, likelihood ratio tests for interaction between enrollment location and each biomarker were not statistically significant (all P values were >0.4).
Discussion
In this cohort of general hospitalized patients, a single protein biomarker measurement or microscopy score at the first rise in SCr was able to risk stratify patients for progression to higher AKIN stage or in-hospital death irrespective of AKI etiology. After adjusting for multiple demographic and clinical variables, patients with protein biomarker levels in the fourth quartile versus first quartile or highest versus lowest microscopy scores had over two- to three-fold increased risk of the primary end point. On the basis of outcome reclassification, NGAL, KIM-1, IL-18, and microscopy all significantly improved upon baseline clinical assessment for predicting the outcome. FeNa and FeUrea were not useful for AKI prognosis in this cohort.
To our knowledge, this study is unique in the collection of urine samples for both novel and traditional biomarker assessment to predict worsened clinical status on the first day of meeting current criteria for AKI. Because AKI is now defined by very small changes in SCr (4), its overall incidence is higher than with prior criteria. Thus, it is imperative to distinguish between “low-risk” and “high-risk” AKI with regard to important clinical outcomes. This underlies the typical clinical approach of discriminating between prerenal azotemia and ATN, which is considered high risk. It is critical to remember that “other” causes of AKI (interstitial nephritis, obstruction, etc.) also occur in hospitalized patients and are quite variably associated with clinical outcomes. For this reason, we chose not to exclude patients with “other” AKI causes, improving the generalizability of our findings to commonly encountered hospital-associated AKI.
Using SCr to detect AKI simply identifies diminished GFR without providing information on the structural integrity of the kidney and the propensity to improve or worsen. In this study, we see the potential advantages of three urinary protein biomarkers and a urinary microscopy score, all of which provide prognostic information that can be useful in caring for hospitalized patients. Although the current consensus guidelines have helped reveal AKI's association with in-hospital mortality (5), the recognition that peak SCr has occurred is required to assign AKI stage (potentially days after the peak). Using newer urine biomarkers and/or manual microscopy on the first day of AKI allows for risk stratification without delay to make informed decisions regarding more aggressive care (e.g., fluid management, renal replacement therapy) or early nephrologist involvement, which may improve outcomes (27).
These findings build upon data from other investigators demonstrating that urinary protein biomarkers of AKI obtained at the time of ICU and emergency room admission predict development and severity of AKI. A meta-analysis by Haase et al. (28) noted that NGAL from several different time points predicted the need for dialysis and death. Koyner et al. (29) found that urine NGAL soon after arrival into the ICU after cardiac surgery accurately predicted the development of stage 3 AKI and that preoperative urine KIM-1 levels were moderately predictive. Parikh et al. (30) demonstrated that urine IL-18 levels significantly predicted time to death in a subgroup of ICU patients within the ARDS (Acute Respiratory Distress Syndrome) Network trial.
In a more recent report of urine biomarkers obtained during the EARLARF trial, in which no differences were seen (i.e., development of AKI in 48 hours, need for dialysis, or death in 7 days) between erythropoietin and placebo on admission to the ICU (31), five of the six biomarkers studied predicted death within 7 days with AUCs from 0.61 to 0.68 (32). Urine NGAL, IL-18, and cystatin C also predicted the need for dialysis with AUCs from 0.71 to 0.79. Although the negative predictive values for dialysis were excellent (≥0.97) for all biomarkers studied, positive predictive values were uniformly poor (all <0.10). This indicates that current urine biomarkers may not adequately predict outcomes on their own but may have utility when considered in context of the overall clinical picture.
Our results reinforce this more effective approach to biomarker assessment by demonstrating improved reclassification of risk over baseline clinical appraisal and by more selective urine biomarker measurement at the first sign of AKI by SCr, as opposed to nonspecific biomarker screening. We view the use of protein biomarkers within the context of current clinical practice as an important strength of this study. Because samples were collected on the first day of meeting AKI criteria, this represents the shortest time from injury onset to injury biomarker expression that is possible with usual clinical assessment. Additional strengths include our fairly large sample size, a substantial proportion experiencing the primary outcome, and reasonable case-mix distribution (mild-to-severe AKI, ICU, and non-ICU patients, medical and postsurgical patients) compared with previous AKI biomarker studies.
As for limitations, this was a single-center study, and we have only measured three of the most well described protein biomarkers. Urine albumin, other proteins, and even discovery proteomics could be planned for remaining samples. Accurate urine output was not consistently available in this cohort, especially for non-ICU medical patients, which may have limited the discriminatory ability of FeNa or FeUrea, given that these tests have only been validated in limited settings. Urine microscopy was performed by two physicians well trained in this technique. Thus, its utility may be infeasible in current clinical practice, although improved training could potentially reverse this trend (33). Although automated urine sediment evaluation systems are evolving, they are not widely available and are inferior to microscopists in identifying cells and casts (34–36). We retrospectively classified AKI as prerenal, ATN, or “other” with blinding to biomarker values; however, some degree of clinical subjectivity was undoubtedly present. Although additional nephrologist chart abstraction indicated reliable agreement, we reported biomarker discrimination between AKI classifications in this study for descriptive purposes only in favor of the “harder” end point of worsened AKIN stage or in-hospital death.
In summary, urinary protein biomarkers (NGAL, KIM-1, and IL-18) and urine microscopy have the potential to risk-stratify patients for meaningful outcomes early in the course of hospital-associated AKI. Ultimately, we should consider supplementing SCr with accurate markers of kidney tissue injury in the context of clinical practice to evaluate new therapies for AKI in hospitalized patients.
Disclosures
Dr. Parikh is coinventor on the IL-18 patent issued to the University of Colorado.
Supplementary Material
Acknowledgments
We thank Dr. Prasad Devarajan and Dr. Michael Bennett from the University of Cincinnati College of Medicine and Christine Simpson from the Yale Center for Clinical Investigation for their assistance in biomarker measurement. We also thank Dr. Madiha Koraishy and Dr. Christine Vigneault, for their assistance with sample collection and data abstraction.
Satellite Healthcare (via a Norman S. Coplon Grant awarded to Dr. Parikh) and the National Institutes of Health Grant RO1HL-085757 supported this research, but neither had any role in study design, implementation, analysis, interpretation, or manuscript submission. Dr. Hall and Dr. Parikh had full access to all data and take responsibility for its integrity and the accuracy of all analyses.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. James MT, Hemmelgarn BR, Wiebe N, Pannu N, Manns BJ, Klarenbach SW, Tonelli M: Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: A cohort study. Lancet 376: 2096–2103, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Nash K, Hafeez A, Hou S: Hospital-acquired renal insufficiency. Am J Kidney Dis 39: 930–936, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup: Acute renal failure: Definition, outcome measures, animal models, fluid therapy and information technology needs: The second international Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical Care 8: R205–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A: Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang CH, Lin CY, Tian YC, Jenq CC, Chang MY, Chen YC, Fang JT, Yang CW: Acute kidney injury classification: Comparison of AKIN and RIFLE criteria. Shock 33: 247–252, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Akram AR, Singanayagam A, Choudhury G, Mandal P, Chalmers JD, Hill AT: Incidence and prognostic implications of acute kidney injury on admission in patients with community-acquired pneumonia. Chest 138: 825–832, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Langenberg C, Wan L, Bagshaw SM, Egi M, May CN, Bellomo R: Urinary biochemistry in experimental septic acute renal failure. Nephrol Dial Transplant 21: 3389–3397, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Schonermarck U, Kehl K, Samtleben W: Diagnostic performance of fractional excretion of urea and sodium in acute kidney injury. Am J Kidney Dis 51: 870–871; author reply 871, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Zarich S, Fang LS, Diamond JR: Fractional excretion of sodium: Exceptions to its diagnostic value. Arch Intern Med 145: 108–112, 1985 [PubMed] [Google Scholar]
- 10. Bagshaw SM, Langenberg C, Bellomo R: Urinary biochemistry and microscopy in septic acute renal failure: A systematic review. Am J Kidney Dis 48: 695–705, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Doi K, Yuen PS, Eisner C, Hu X, Leelahavanichkul A, Schnermann J, Star RA: Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol 20: 1217–1221, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller TR, Anderson RJ, Linas SL, Henrich WL, Berns AS, Gabow PA, Schrier RW: Urinary diagnostic indices in acute renal failure: A prospective study. Ann Intern Med 89: 47–50, 1978 [DOI] [PubMed] [Google Scholar]
- 13. Waikar SS, Sabbisetti VS, Bonventre JV: Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int 78: 486–494, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee JB, Forni LG: Recently published papers: Acute kidney injury: Diagnosis and treatment. Crit Care 13: 140, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soni SS, Ronco C, Katz N, Cruz DN: Early diagnosis of acute kidney injury: The promise of novel biomarkers. Blood Purif 28: 165–174, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Coca SG, Yalavarthy R, Concato J, Parikh CR: Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systematic review. Kidney Int 73: 1008–1016, 2008 [DOI] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP: The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med 4: e296, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levey AS, Greene T, Kusek J, Beck G: A simplified equation to predict glomerular filtration rate from serum creatinine (abstract). J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]
- 19. Perazella MA, Coca SG, Hall IE, Iyanam U, Koraishy M, Parikh CR: Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 5: 402–408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR: Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 3: 1615–1619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Han WK, Wagener G, Zhu Y, Wang S, Lee HT: Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 4: 873–882, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shibata M, Hirota M, Nozawa F, Okabe A, Kurimoto M, Ogawa M: Increased concentrations of plasma IL-18 in patients with hepatic dysfunction after hepatectomy. Cytokine 12: 1526–1530, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr, Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172; discussion 207–112, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Pepe MS, Janes H: Commentary: Reporting standards are needed for evaluations of risk reclassification. Int J Epidemiol 40: 1106–1108, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44: 837–845, 1988 [PubMed] [Google Scholar]
- 27. Balasubramanian G, Al-Aly Z, Moiz A, Rauchman M, Zhang Z, Gopalakrishnan R, Balasubramanian S, El-Achkar TM: Early nephrologist involvement in hospital-acquired acute kidney injury: A pilot study. Am J Kidney Dis 57: 228–234, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A: Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 54: 1012–1024, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, Raman J, Jeevanandam V, O'Connor MF, Devarajan P, Bonventre JV, Murray PT: Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol 5: 2154–2165, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL: Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol 16: 3046–3052, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Endre ZH, Walker RJ, Pickering JW, Shaw GM, Frampton CM, Henderson SJ, Hutchison R, Mehrtens JE, Robinson JM, Schollum JB, Westhuyzen J, Celi LA, McGinley RJ, Campbell IJ, George PM: Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial). Kidney Int 77: 1020–1030, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Endre ZH, Pickering JW, Walker RJ, Devarajan P, Edelstein CL, Bonventre JV, Frampton CM, Bennett MR, Ma Q, Sabbisetti VS, Vaidya VS, Walcher AM, Shaw GM, Henderson SJ, Nejat M, Schollum JB, George PM: Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int 79: 1119–1130, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Claure-Del Granado R, Macedo E, Mehta RL: Urine microscopy in acute kidney injury: Time for a change. Am J Kidney Dis 57, 657–660, Epub 2011 Jan 22 2011 [DOI] [PubMed] [Google Scholar]
- 34. Chien TI, Kao JT, Liu HL, Lin PC, Hong JS, Hsieh HP, Chien MJ: Urine sediment examination: A comparison of automated urinalysis systems and manual microscopy. Clin Chim Acta 384: 28–34, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Chien TI, Lu JY, Kao JT, Lee TF, Ho SY, Chang CY, Liu CW, Lee CH, Yan SN, Yang JY, Lin PC, Wang MJ: Comparison of three automated urinalysis systems: Bayer Clinitek Atlas, Roche Urisys 2400 and Arkray Aution Max for testing urine chemistry and detection of bacteriuria. Clin Chim Acta 377: 98–102, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Shayanfar N, Tobler U, von Eckardstein A, Bestmann L: Automated urinalysis: First experiences and a comparison between the Iris iQ200 urine microscopy system, the Sysmex UF-100 flow cytometer and manual microscopic particle counting. Clin Chem Lab Med 45: 1251–1256, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.