Abstract
Summary
Background and objectives
Approximately 70% of illicit cocaine consumed in the United States is contaminated with levamisole. Most commonly used as a veterinary antihelminthic agent, levamisole is a known immunomodulating agent. Prolonged use in humans has been associated with cutaneous vasculitis and agranulocytosis. We describe the development of a systemic autoimmune disease associated with antineutrophil cytoplasmic antibodies (ANCA) in cocaine users. This complication appears to be linked to combined cocaine and levamisole exposure.
Design, setting, participants, & measurements
Cases were identified between March 2009 and November 2010 at Massachusetts General Hospital's ANCA laboratory. Cocaine exposure was identified from patient history in all cases. Medical records were reviewed for clinical presentation and for laboratory and diagnostic evaluation.
Results
Thirty cases of ANCA positivity associated with cocaine ingestion were identified. All had antimyeloperoxidase antibodies and 50% also had antiproteinase 3 antibodies. Complete clinical and laboratory data were available for 18 patients. Arthralgia (83%) and skin lesions (61%) were the most frequent complaints at presentation. Seventy-two percent of patients reported constitutional symptoms, including fever, night sweats, weight loss, or malaise. Four patients had biopsy-proven vasculitis. Two cases of acute kidney injury and three cases of pulmonary hemorrhage occurred. From the entire cohort of 30, two cases were identified during the first 3 months of our study period and nine cases presented during the last 3 months.
Conclusions
We describe an association between the ingestion of levamisole-contaminated cocaine and ANCA-associated systemic autoimmune disease. Our data suggest that this is a potentially life-threatening complication of cocaine use.
Introduction
Over 38 million Americans have used cocaine at some point in their lives (1). In 2009, approximately 6.2 million people in the United States used cocaine, equivalent to an annual prevalence rate of 2.8% in the population aged 15 to 64 years (2). Levamisole, a veterinary antihelminthic agent, is a common contaminant in cocaine. The extent of this contamination has increased dramatically in recent years, from less than 5% in 2006 to 30% in 2008 (3,4). Currently, it is estimated that over 70% of cocaine is affected (4).
Levamisole has been used clinically as an immunomodulatory agent for various indications, including treatment of rheumatoid arthritis and pediatric nephrotic syndrome, and as adjuvant therapy for colon cancer (5,6). It was voluntarily withdrawn from the US market in 2000 due to its side-effect profile, which includes idiosyncratic agranulocytosis and the development of vasculitic lesions with prolonged exposure (7–9). Agranulocytosis was observed at rates of 2.5 to 13% in patients treated with moderate to high doses for protracted periods (10). Although the exact mechanism remains unclear, anti-neutrophil antibodies have been described in some patients (11,12).
ANCA-associated vasculitis (AAV) has been associated with a variety of drugs, including hydralazine, propylthiouracil, and minocycline (13). Cases can present with very high titers of antimyeloperoxidase (MPO) antibodies, often greater than 10 times that seen in idiopathic cases (14). Combined positivity of both anti-MPO and antiproteinase 3 (PR3) antibodies is occasionally seen in drug-induced AAV, but is extremely uncommon outside this setting (15). Patients with propylthiouracil and minocycline-induced AAV commonly present with arthralgia and skin rashes, but hydralazine has frequently been associated with rapidly progressive glomerulonephritis (14,16).
Massachusetts General Hospital (MGH) ANCA laboratory has been performing ANCA testing continuously since 1989. In recent months, we noted a marked increase in the frequency of samples with very high anti-MPO antibody titers, and in samples positive for both anti-MPO and anti-PR3 antibodies. In addition, a disproportionate number of these patients presented with leukopenia, an uncommon feature in idiopathic AAV (17). On careful review of the clinical details with referring physicians, cocaine was identified as the common drug exposure among these cases. Over the same period, there were a series of reports in the literature linking levamisole with agranulocytosis and vasculitic skin lesions in cocaine users (4,11,12,18–23). Many clinical and laboratory features of these cases are in keeping with previously described levamisole-related autoimmune disease (12,18,23). However, ANCA has not been commonly associated with autoimmune phenomena related to cocaine or levamisole ingestion (23). Here we report the identification of 30 cases of ANCA-positive systemic disease associated with cocaine use. We hypothesize that combined exposure to both agents has led to the observed clinical picture.
Materials and Methods
Patients included in this analysis had a new diagnosis of ANCA between March 2009 and November 2010 at the MGH ANCA laboratory, which is a referral center for ANCA testing for the northeast region of the United States. This study was approved by the institutional review board at our institution as a retrospective analysis and, therefore, informed consent was not required.
ANCA testing at MGH includes direct immunofluorescence and capture ELISA for anti-PR3 antibodies and direct ELISA for anti-MPO antibodies (24). Per protocol at our laboratory, when a new positive ANCA is identified, the referring physician is contacted. The result and its clinical significance are discussed in the context of the patient's history, clinical presentation, and laboratory data. Drug exposures are discussed in detail. Where the clinical picture and antibody titer are suggestive of a drug-induced etiology (high titer antibodies, combined positivity for anti-MPO and anti-PR3 antibodies), an attempt is made to identify a precipitating agent. Information discussed is recorded in the ANCA laboratory correspondence logbook.
During the study period, 327 new positive ANCA patients were identified. Those with readily identifiable drug exposures known to be associated with AAV were excluded (propylthiouracil, n = 9; hydralazine, n = 9; minocycline, n = 5). Active cocaine use was identified in 30 of the remaining cases. Details regarding cocaine use were personally provided by patients to their individual physicians and recorded in the patient's medical record. Urine toxicology screening for cocaine and its metabolites were reviewed (positive in 16 of 18 cases, where tested).
Demographic data and details of ANCA serology are presented for the entire cohort of 30 patients. Eighteen of the 30 cases were treated within the Partners Healthcare system, and complete clinical and laboratory data were available. Medical records were not available for review in the remaining 12 cases.
Results
Patient Characteristics
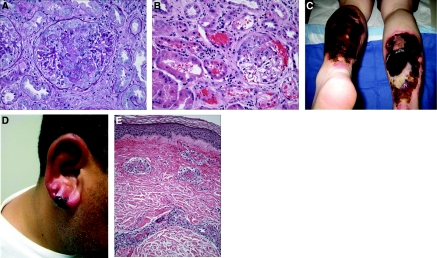
In the entire group of 30 patients, the mean age was 47.3 (± 8.0) years and 66% were female. Detailed clinical features at presentation are presented in Table 1. The most common clinical manifestations were arthralgias (83%), which generally involved the large joints, and skin lesions (61%). Cutaneous manifestations were heterogeneous, including necrotic lesions (n = 3), purpura (n = 6), digital abscesses (n = 1), and ecchymotic bullous skin lesions (n = 1). In one patient, the necrotic lesions were very extensive, measuring 15 cm × 20 cm over both calves, and ultimately required skin grafting. Furthermore, five patients developed purpuric lesions over their ear lobes, a classically described manifestation of levamisole-induced vasculitis (7,9) (Figure 1).
Table 1.
Clinical features of patients at presentation
| Number | Sex | Age, Years | Duration of Symptoms | Anti-MPO | Anti-PR3 | Arthralgia | Skin Lesions | Fever/Night Sweats | Weight Loss | Lethargy | Myalgia | ENT | Leukopenia | Ear Lesions | Pulmonary | Oral Ulceration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 44 | 3 wk | 1050 | + | + | + | – | + | + | – | – | + | – | – | |
| 2 | M | 55 | n/a | 1553 | 172 | – | – | – | + | – | – | – | – | – | – | – |
| 3 | M | 43 | 28 wk | 10,103 | + | – | + | + | – | + | + | – | – | + | – | |
| 4 | F | 46 | 4 wk | 132 | + | + | + | + | + | – | – | – | – | +a | – | |
| 5 | M | 46 | >12 wk | 7655 | + | + | – | – | – | – | – | – | + | – | – | |
| 6 | F | 57 | 28 wk | 1127 | 8601 | + | + | – | – | – | + | + | + | + | – | – |
| 7 | F | 50 | 6 wk | 24,986 | + | – | + | – | + | + | + | + | – | – | + | |
| 8 | F | 53 | 12 wk | 19,797 | 230 | + | + | + | + | + | + | – | + | – | – | – |
| 9 | F | 50 | 8 wk | 1826 | + | + | – | – | – | + | – | – | – | – | – | |
| 10 | M | 41 | 6 wk | 12,698 | + | + | – | – | + | + | + | – | + | – | + | |
| 11 | M | 42 | 32 wk | 1715 | 118 | + | – | + | + | – | + | + | – | – | +a | – |
| 12 | F | 49 | 7 wk | 4711 | + | – | – | + | + | – | + | – | – | – | + | |
| 13 | F | 36 | 8 wk | 18 | + | + | – | – | + | + | – | – | – | + | – | |
| 14 | F | 29 | 12 wk | 1152 | 153 | – | + | + | + | + | – | + | +b | + | – | + |
| 15 | F | 52 | n/a | 1741 | 342 | – | + | + | – | – | – | – | + | – | – | – |
| 16 | F | 51 | 12 wk | 1075 | 201 | + | + | – | – | – | – | + | – | – | – | – |
| 17 | M | 47 | 8 wk | 12,415 | 522 | + | + | – | – | – | + | – | – | – | – | – |
| 18 | M | 43 | 24 wk | 2406 | 49 | + | – | – | – | + | – | – | – | – | – | – |
n/a, not available; ENT, Ear, Nose, Throat.
Pulmonary hemorrhage.
Treatment of neutropenia with granulocyte macrophage colony-stimulating factor prior to diagnosis.
Figure 1.
(A) Kidney biopsy. The glomerulus reveals fibrinoid necrosis of the central part of the tuft associated with focal leukocytoclasia and a small cellular crescent filling the upper portion of Bowman's space; there is no significant hypercellularity of the uninvolved segments of the tuft. (Periodic acid-Schiff). (B) Kidney biopsy. The cortex shows an active glomerulitis with a small cellular crescent that has resulted in prominent bleeding into Bowman's space and the tubules. Mild interstitial inflammation is also present. (Hematoxylin & eosin). (C) Necrotic skin lesions. Large areas of full thickness, necrotic ulceration, near circumferential over both lower extremities. (D) Earlobe vasculitis. Purpuric violaceous lesion is seen with surrounding erythema. Biopsy revealed leukocytoclastic vasculitis. (E) Skin biopsy. The small vessel in the dermis shows active inflammation, with associated fibrinoid necrosis of the wall, leukocytoclasia, and thrombosis. The perivascular space reveals edema and extravasation of red blood cells. (Hematoxylin & eosin).
A total of 72% of patients reported at least one constitutional symptom at the time of diagnosis, with fever, night sweats, weight loss, malaise, or myalgia being particularly common (Table 1).
ENT involvement was seen in 44% of cases, of which the most common symptoms were persistent rhinorrhea or recurrent sinusitis. Four patients had an absent nasal septum; one had a saddle nose deformity. However, the route of cocaine ingestion is an important factor in this context, as nasal inhalation of cocaine may have contributed to these symptoms. Details of the route of cocaine ingestion were not available.
Significant end-organ damage occurred in a subset of individuals. Abnormal urinalysis (defined as dipstick proteinuria, hematuria, or the presence of cellular/RBC casts on microscopy) was present in eight patients at diagnosis (Table 2). Two developed severe acute kidney injury, presenting with serum creatinine of 7.7 mg/dl and 5.6 mg/dl (patients 2 and 8, respectively). In both cases, dipstick urinalysis was positive for blood and protein, and casts were observed on urine microscopy. Patient 8 underwent renal biopsy, revealing pauci-immune focal necrotizing and crescentic glomerulonephritis (GN) (Figure 1). Neither required renal replacement therapy. Despite improved renal function with immunosuppressive treatment, both were left with significant chronic kidney disease (i.e., estimated GFR (eGFR) <30 ml/min per 1.73 m2). Six other individuals had abnormalities on urine dipstick, without casts or dysmorphic RBCs on urine microscopy. However, all of these individuals had eGFR ≥ 60 ml/min per 1.73 m2 and were without documented increase in serum creatinine (Table 2).
Table 2.
Relationship between ANCA serology and renal involvement
| Number | Anti-MPO | Anti-PR3 | Protein | Blood | Casts | Creatinine (mg/dl) |
|---|---|---|---|---|---|---|
| 1 | 1050 | – | – | – | 0.62 | |
| 2 | 1553 | 172 | 2+ | 2+ | + | 7.7 |
| 3 | 10,103 | – | – | – | 1.2 | |
| 4 | 132 | + | – | + (Granular) | 0.52 | |
| 5 | 7655 | + | – | – | 0.85 | |
| 6 | 1127 | 8601 | + | 3+ | – | 0.79 |
| 7 | 24,986 | – | – | – | 1.19 | |
| 8 | 19,797 | 230 | 3+ | 2+ | + (Cellular) | 5.57 |
| 9 | 1826 | + | + | – | 0.83 | |
| 10 | 12,698 | 3+ | + | – | 0.77 | |
| 11 | 1715 | 118 | – | – | – | 1.16 |
| 12 | 4711 | – | + | – | 0.7 | |
| 13 | 18 | – | – | – | 0.6 | |
| 14 | 1152 | 153 | – | – | – | 0.93 |
| 15 | 1741 | 342 | – | – | – | 0.78 |
| 16 | 1075 | 201 | – | – | – | n/a |
| 17 | 12,415 | 522 | n/a | 0.96 | ||
| 18 | 2406 | 49 | – | – | – | 0.8 |
In total, three patients developed pulmonary hemorrhage, none requiring intubation. Two of these patients had normal serum creatinine values (patient 4 = 0.52 mg/dl; patient 11 = 1.0 mg/dl). Urinalysis was negative and microscopy revealed granular casts in patient 4. Patient 11 had a normal urine dipstick and microscopy. The third patient, from an outside institution, was reported to have normal serum creatinine but details of urinalysis were not available. Overall, there was little definitive evidence of combined pulmonary-renal disease in this cohort.
Pathologic features
Seven patients underwent skin biopsy, with histologic findings including leukocytoclastic vasculitis (n = 3), thrombotic microangiopathy (n = 2), panniculitis (n = 1), and necrotic lesions (n = 1). Pauci-immune GN was seen on renal biopsy, as described previously. Representative histology from kidney and skin biopsies is shown in Figure 1.
Serologic Characteristics
All cases had positive anti-MPO antibodies and, strikingly, 15 (50%) also showed positive anti-PR3 antibodies (Table 3). By immunofluorescence, all cases were P-ANCA-positive. Samples with coexistent anti-PR3 antibodies were also C-ANCA-positive.
Table 3.
Characteristics of ANCA serology
| % | n | Median Titer | Interquartile Range | |
|---|---|---|---|---|
| Antimyeloperoxidase | 100% | 30/30 | 1702 | 1075 to 7988 |
| Antiproteinase 3 | 50% | 15/30 | 172 | 70 to 252 |
On review of the ANCA serology from MGH during the 21-month period, antibodies against both PR3 and MPO were identified in 21 new cases. Fifteen of these were cocaine-associated and two were in patients treated with propylthiouracil. Of the remaining four, two patients also were neutropenic on presentation, but no information regarding cocaine or other exposures could be ascertained.
Importantly, patients with cocaine-associated ANCA had anti-MPO antibody titers that were up to 15-fold higher than patients diagnosed with idiopathic AAV over the same time period (median 1702, interquartile range [IQR] 1075 to 7988 versus median 112, IQR 23 to 768, respectively; P < 0.01).
Other Laboratory Findings
A range of other autoantibodies were identified in these patients. Fourteen were positive for antinuclear antibodies (14 of 17 tested), seven had decreased complement C3 and/or C4 (7 of 11), and three had positive anti-double-stranded DNA antibodies (3 of 9). Six patients had a positive lupus anticoagulant (6 of 9) and three had positive anticardiolipin antibodies. Two patients had both antibodies.
In terms of correlating antibody profile with biopsy findings, we observed the following: In cases of thrombotic microangiopathy (n = 2), decreased circulating C4 with normal C3 were seen. One had an anticardiolipin antibody and the other had positive testing for the lupus anticoagulant. In biopsy-proven leukocytoclastic vasculitis (n = 3), complements were normal and lupus anticoagulant was present in two of three cases. While renal biopsy in one case confirmed pauci-immune GN, this patient also had decreased C3, anti-dsDNA antibodies and positive lupus anticoagulant.
Twenty-eight percent of patients had leukopenia at presentation, with the median white cell count (WCC) 4.9 × 103/μL. Eight patients had a WCC below 3.0 × 103/μL; four of these had an absolute neutrophil count (ANC) of <1000. One of these patients had been treated at an outside institution with granulocyte-macrophage colony-stimulating factor (GM-CSF) for profound neutropenia. In keeping with prior reports of levamisole exposure, leukopenia was the main hematologic abnormality observed (Table 4).
Table 4.
Hematological/biochemical characteristics
| Median | Interquartile Range | |
|---|---|---|
| Hemoglobin (g/dl; n = 17) | 12.0 | 11.0 to 13.6 |
| WCC (× 103/μl; n = 17) | 4.9 | 2.7 to 6.6 |
| Absolute neutrophil counta (n = 17) | 1925 | 1456 to 5168 |
| Platelet count (/μl; n = 17) | 299 | 260 to 366 |
| Erythrocyte sedimentation rate (n = 15) | 49 | 22 to 59 |
| C-reactive protein (mg/L; n = 6) | 36.9 | 25 to 72 |
One patient treated with granulocyte macrophage colony-stimulating factor and initial absolute neutrophil count was not available.
As described, two patients had acute kidney injury at the time of presentation. One additional patient had elevated liver function tests at presentation, secondary to hepatitis B. Synthetic function was normal. No other patients had liver or kidney disease of sufficient severity to potentially alter metabolism of cocaine or its contaminants.
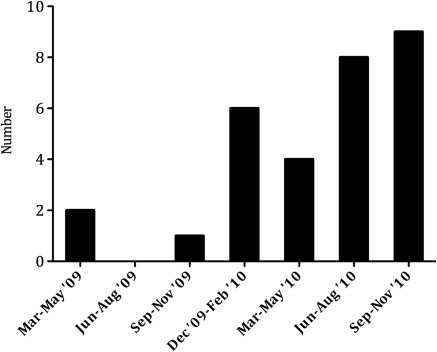
One of the most striking features noted is the increase in incidence of these cases toward the end of the study period (Figure 2). During the first year of this study, we identified less than one case of cocaine-associated ANCA per month. However, over the last 3 months, from September to November 2010, we identified approximately three cases per month.
Figure 2.
New cases of cocaine-associated antineutrophil cytoplasmic antibodies diagnosed per quarter, March 2009 to November 2010.
Discussion
Despite widespread use of cocaine for several decades, there has been a relative paucity of reports linking it with autoimmunity and vasculitis. A small number of case reports describe patients with systemic vasculitis and cocaine exposure (25,26). However, in contrast to the group we describe, these cases have been associated with anti-PR3 antibodies alone, without anti-MPO antibodies (25–27).
Rashes have been reported in association with cocaine use, but these have typically been ANCA-negative and without biopsy findings diagnostic for vasculitis (28,29). Cerebral vasculitis has occasionally been associated with cocaine use but, again, many of these cases were ANCA-negative (30–32). Merkel et al. reviewed eight cases of cocaine associated cerebral vasculitis (31). Brain biopsy revealed small vessel angiitis in 75%. Seven patients underwent cerebral angiography, of whom four had findings that were consistent with cerebral vasculitis. Testing for ANCA was carried out in two cases and was negative (31).
Cocaine-induced destructive midline lesion (CIMDL) arises due to locally destructive effects of cocaine on the upper respiratory tract (33–35). These individuals are frequently ANCA-positive, and it has been challenging to differentiate these cases from granulomatosis with polyangiitis (GPA; known formerly as Wegener granulomatosis). However, recent studies indicate that these patients differ clinically. Patients with GPA demonstrate much higher rates of constitutional symptoms and biochemical evidence of chronic disease than those with CIMDL, despite high rates of ANCA positivity (33). It has been proposed that the presence of antineutrophil elastase antibodies may be more predictive of a cocaine-induced etiology in this setting (27,33,36). In our patient population, testing for antineutrophil elastase antibodies was not available. However, the systemic nature of illness and characteristics of ANCA serology identify this as a drug-induced process, with features quite distinct from CIDML or idiopathic GPA.
Taken together, the existing body of literature regarding the link between cocaine and vasculitis differs from our cohort of patients in several important respects. These features point to an additional toxic exposure, which we hypothesize to be levamisole.
Levamisole has been used therapeutically as an immunomodulatory agent and has been shown to promote neutrophil mobility and chemotaxis, enhance dendritic cell maturation, and promote T cell proliferation (37–39). It is known to induce circulating autoantibodies (8). These effects on innate and adaptive immune responses may explain its propensity to induce autoimmunity and vasculitis.
The reason why levamisole is used so widely as an adulterant in cocaine is unknown. Its ubiquity in the cocaine supply suggests it is added at source, most likely in Colombia (40). Given the relatively low percentage present, varying between 2 and 10%, it has been postulated that levamisole is added for expected pharmacologic effects (20). Levamisole acts as a nicotinic acetylcholine receptor agonist and stimulates the sympathetic nervous system (41). In animal models, it has been shown to alter central metabolism of norepinephrine, dopamine, and serotonin, and attenuate symptoms of opiate withdrawal (42). Therefore, it has been proposed that levamisole may potentiate the impact of increased dopamine release induced by cocaine. Furthermore, these peripheral and central actions could combine synergistically to increase the perceived effects from cocaine use and, possibly, its addictive potential (41). Finally, others have suggested that due to similar physical properties to cocaine, levamisole may be more difficult to detect as an adulterant than other more commonly used agents.
Due to its pharmacokinetic profile, levamisole is difficult to detect in biologic samples. It has a plasma elimination half-life of around 5.5 hours, and only 2 to 5% is excreted in urine, where it can be detected for up to 3 days (38,43,44). Interestingly, a recent study demonstrated that over two thirds of urine samples positive for cocaine had detectable levels of levamisole, using gas chromatography/mass spectroscopy (45). This study, carried out in an inner-city emergency department, emphasizes the need for a very high degree of clinical suspicion, early in disease presentation, to definitively confirm exposure.
It is extremely difficult to accurately quantify the amount of levamisole our patients were exposed to, both due to underreporting of cocaine use and inconsistent levels present in the product consumed (20). Eight patients gave a detailed history of cocaine use, and all of these had been abusers for more than 10 years. Most patients used cocaine several times per week and some used daily. Therefore, it appears that many of these affected individuals were chronic, habitual cocaine users, suggesting a large cumulative exposure to cocaine and, by association, levamisole, possibly over an extended period of time.
The emergence of these cases parallels the increase in levamisole contamination of the US cocaine supply (3). Before the development of any drug-induced autoantibody, patients typically have prolonged exposure to the causative agent. In prior cases of levamisole-induced vasculitis, patients were treated for longer than 24 months before the appearance of cutaneous vasculitis (9). Contamination of the cocaine supply has become an increasing issue, reaching a level of 70% in 2009. It is therefore biologically plausible that, after prolonged cumulative exposure to levamisole, cocaine users are now presenting with drug-induced autoantibodies. The frequency of these presentations appears, from our raw data, to be increasing. Obviously, given the illicit nature of cocaine abuse, it is almost impossible to get accurate data on the number of at-risk individuals, making estimates of incidence challenging.
Lack of documentation of levamisole in patient samples is clearly a limitation of our study. However, recent studies have confirmed high rates of levamisole exposure in cocaine users (45). We feel that features of the cases presented, including high rates of leukopenia, rashes observed (including highly suspicious ear lobe vasculitis), and ANCA serology typical of drug-induced antibodies, provide highly compelling circumstantial evidence to link these cases with levamisole contamination of ingested cocaine.
Many of our patients had other autoantibodies identified. Prior studies have similarly reported a high frequency of antinuclear antibodies in patients with drug-induced ANCA (14). The development of anticardiolipin antibodies and decreased C3 has previously been reported with levamisole treatment (7,9,46). In this patient cohort, no firm correlation was observed between either decreased circulating complement components or the presence of other autoantibodies and symptom complex at presentation. However, the presence of hypocomplementemia with antinuclear antibodies and anti-dsDNA antibodies raised the question of drug-induced lupus in a subset of these patients. While the finding of thrombotic microangiopathy on skin biopsies of two hypocomplementemic patients emphasizes the importance of histologic evaluation of each case, it remains challenging to strictly define the syndrome observed. Indeed, our patient with pauci-immune GN on renal biopsy had several other antibodies at the time of presentation. Histologic diagnosis confirmed that, in her case, the pathogenic antibody was ANCA. On this basis, we feel these cases are most accurately described as ANCA-associated autoimmune disease, a subset of which includes biopsy-proven vasculitis.
In conclusion, illicit cocaine sold in the United States is now widely adulterated with levamisole. We report 30 cases of ANCA and systemic illness in cocaine users. Four of these individuals had biopsy-proven vasculitis, including one case of pauci-immune glomerulonephritis. These cases are notable for the high titer anti-MPO antibodies seen in all patients, and the presence of antibodies against both MPO and PR3 in 50%, features highly suggestive of a drug-induced etiology. These cases differ from earlier descriptions of cocaine-induced ANCAs, where anti-MPO antibodies were absent (25–27,47). We propose that this clinical syndrome has arisen due to exposure to both agents.
This issue is of pressing public health significance due to the widespread use of cocaine. Based on experience from other causes of drug-induced autoantibodies, the first line in management should include the complete avoidance of cocaine consumption. However, issues regarding compliance with therapy and ongoing cocaine use make this a challenging patient group to manage. In particular, a significant number have coexistent leukopenia at presentation, and this raises concerns regarding the safety of intensive immunosuppressive therapy. Longer-term follow-up data are needed to determine the optimal management and the ultimate prognosis of individuals affected.
Disclosures
None.
Acknowledgments
Part of this work was presented as a poster at the annual meeting of the American Society of Nephrology, Denver, Colorado, November 16 to 21, 2010, and as an oral presentation at The International Vasculitis & ANCA Workshop, Chapel Hill, North Carolina, May 15 to 18, 2011.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Substance Abuse and Mental Health Services Administration: Results from the 2008 National Survey on Drug Use and Health: National Findings. Office of Applied Studies, NSDUH Series H-36, HHS Publication No SMA 09–4434, 2009
- 2. United Nations Office on Drugs and Crime: World drug report, New York, United Nations, 2010 [Google Scholar]
- 3. Casale JF, Corbel EM, Hays PA: Identification of levamisole impurities found in illicit cocaine exhibits. Microgram Journal 6: 134–137, 2008 [Google Scholar]
- 4. Agranulocytosis associated with cocaine use–four states, March 2008-November 2009. MMWR Morb Mortal Wkly Rep 58: 1381–1385, 2009 [PubMed] [Google Scholar]
- 5. Levamisole in rheumatoid arthritis. Final report on a randomised double-blind study comparing a single weekly dose of levamisole with placebo. Multicentre Study Group Ann Rheum Dis 41: 159–163, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA, Tormey DC, Glick JH, et al. : Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 322: 352–358, 1990 [DOI] [PubMed] [Google Scholar]
- 7. Menni S, Pistritto G, Gianotti R, Ghio L, Edefonti A: Ear lobe bilateral necrosis by levamisole-induced occlusive vasculitis in a pediatric patient. Pediatr Dermatol 14: 477–479, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Laux-End R, Inaebnit D, Gerber HA, Bianchetti MG: Vasculitis associated with levamisole and circulating autoantibodies. Arch Dis Child 75: 355–356, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rongioletti F, Ghio L, Ginevri F, Bleidl D, Rinaldi S, Edefonti A, Gambini C, Rizzoni G, Rebora A: Purpura of the ears: A distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol 140: 948–951, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Thompson JS, Herbick JM, Klassen LW, Severson CD, Overlin VL, Blaschke JW, Silverman MA, Vogel CL: Studies on levamisole-induced agranulocytosis. Blood 56: 388–396, 1980 [PubMed] [Google Scholar]
- 11. Czuchlewski DR, Brackney M, Ewers C, Manna J, Fekrazad MH, Martinez A, Nolte KB, Hjelle B, Rabinowitz I, Curtis BR, McFarland JG, Baumbach J, Foucar K: Clinicopathologic features of agranulocytosis in the setting of levamisole-tainted cocaine. Am J Clin Pathol 133: 466–472, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Buchanan JA, Vogel JA, Eberhardt AM: Levamisole-induced occlusive necrotizing vasculitis of the ears after use of cocaine contaminated with levamisole. J Med Toxicol 7: 83–84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao Y, Zhao MH: Review article: Drug-induced anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrology (Carlton) 14: 33–41, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Choi HK, Merkel PA, Walker AM, Niles JL: Drug-associated antineutrophil cytoplasmic antibody-positive vasculitis: Prevalence among patients with high titers of antimyeloperoxidase antibodies. Arthritis Rheum 43: 405–413, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Wiik A: Drug-induced vasculitis. Curr Opin Rheumatol 20: 35–39, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Bonaci-Nikolic B, Nikolic MM, Andrejevic S, Zoric S, Bukilica M: Antineutrophil cytoplasmic antibody (ANCA)-associated autoimmune diseases induced by antithyroid drugs: Comparison with idiopathic ANCA vasculitides. Arthritis Res Ther 7: R1072–1081, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akhtari M, Curtis B, Waller EK: Autoimmune neutropenia in adults. Autoimmun Rev 9: 62–66, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Bradford M, Rosenberg B, Moreno J, Dumyati G: Bilateral necrosis of earlobes and cheeks: Another complication of cocaine contaminated with levamisole. Ann Intern Med 152: 758–759, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Buchanan JA, Oyer RJ, Patel NR, Jacquet GA, Bornikova L, Thienelt C, Shriver DA, Shockley LW, Wilson ML, Hurlbut KM, Lavonas EJ: A confirmed case of agranulocytosis after use of cocaine contaminated with levamisole. J Med Toxicol 6: 160–164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang A, Osterloh J, Thomas J: Levamisole: A dangerous new cocaine adulterant. Clin Pharmacol Ther 88: 408–411, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Knowles L, Buxton JA, Skuridina N, Achebe I, Legatt D, Fan S, Yan Zhu N, Talbot J: Levamisole tainted cocaine causing severe neutropenia in Alberta and British Columbia. Harm Reduct J 6: 30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wiens MO, Son WK, Ross C, Hayden M, Carleton B: Cases: Cocaine adulterant linked to neutropenia. CMAJ 182: 57–59, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walsh NM, Green PJ, Burlingame RW, Pasternak S, Hanly JG: Cocaine-related retiform purpura: Evidence to incriminate the adulterant, levamisole. J Cutan Pathol 37: 1212–1219, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Merkel PA, Polisson RP, Chang Y, Skates SJ, Niles JL: Prevalence of antineutrophil cytoplasmic antibodies in a large inception cohort of patients with connective tissue disease. Ann Intern Med 126: 866–873, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Neynaber S, Mistry-Burchardi N, Rust C, Samtleben W, Burgdorf WH, Seitz MA, Messer G, Wollenberg A: PR3-ANCA-positive necrotizing multi-organ vasculitis following cocaine abuse. Acta Derm Venereol 88: 594–596, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Tervaert JW, Stegeman CA: A difficult diagnosis. Lancet 364: 1313–1314, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Wiesner O, Russell KA, Lee AS, Jenne DE, Trimarchi M, Gregorini G, Specks U: Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum 50: 2954–2965, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Friedman DR, Wolfsthal SD: Cocaine-induced pseudovasculitis. Mayo Clin Proc 80: 671–673, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Bhinder SK, Majithia V: Cocaine use and its rheumatic manifestations: A case report and discussion. Clin Rheumatol 26: 1192–1194, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Kaye BR, Fainstat M: Cerebral vasculitis associated with cocaine abuse. JAMA 258: 2104–2106, 1987 [PubMed] [Google Scholar]
- 31. Merkel PA, Koroshetz WJ, Irizarry MC, Cudkowicz ME: Cocaine-associated cerebral vasculitis. Semin Arthritis Rheum 25: 172–183, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Fredericks RK, Lefkowitz DS, Challa VR, Troost BT: Cerebral vasculitis associated with cocaine abuse. Stroke 22: 1437–1439, 1991 [DOI] [PubMed] [Google Scholar]
- 33. Trimarchi M, Gregorini G, Facchetti F, Morassi ML, Manfredini C, Maroldi R, Nicolai P, Russell KA, McDonald TJ, Specks U: Cocaine-induced midline destructive lesions: Clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine (Baltimore) 80: 391–404, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Plaza G, Espinosa A, Ferrando J, Pinedo F: [Wegener granulomatosis and cocaine-induced midline destructive lesion: differential diagnosis.]. Med Clin (Barc) 133: 237–238, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Angit C, Dabrowski MT, Owen CM: Cocaine-induced midline destructive lesion. Clin Exp Dermatol 34: e469–470, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Scheenstra RJ, van Buren M, Koopman JP: [A patient with both cocaine-induced nasal septum destruction and antibodies against anti-neutrophil cytoplasmic antibodies (ANCA); potential confusion with Wegener's disease]. Ned Tijdschr Geneeskd 151: 2395–2399, 2007 [PubMed] [Google Scholar]
- 37. Chen LY, Lin YL, Chiang BL: Levamisole enhances immune response by affecting the activation and maturation of human monocyte-derived dendritic cells. Clin Exp Immunol 151: 174–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levamisole Physician's Desk Reference Generics. Montvale, NJ: Medical Economics 1670–1672, 1995 [Google Scholar]
- 39. Szeto C, Gillespie KM, Mathieson PW: Levamisole induces interleukin-18 and shifts type 1/type 2 cytokine balance. Immunology 100: 217–224, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. National Drug Intelligence Centre UDoJ: National Drug Threat Assessment 2010. http://www.justice.gov/ndic/pubs38/38661/index.htm
- 41. Raymon LP, Isenschmid DS: Letter to the editor: The possible role of levamisole in illicit cocaine preparations. J Anal Toxicol 33: 620–622, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Spector S, Munjal I, Schmidt DE: Effects of the immunostimulant, levamisole, on opiate withdrawal and levels of endogenous opiate alkaloids and monoamine neurotransmitters in rat brain. Neuropsychopharmacology 19: 417–427, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Kouassi E, Caille G, Lery L, Lariviere L, Vezina M: Novel assay and pharmacokinetics of levamisole and p-hydroxylevamisole in human plasma and urine. Biopharm Drug Dispos 7: 71–89, 1986 [DOI] [PubMed] [Google Scholar]
- 44. Reid JM, Kovach JS, O'Connell MJ, Bagniewski PG, Moertel CG: Clinical and pharmacokinetic studies of high-dose levamisole in combination with 5-fluorouracil in patients with advanced cancer. Cancer Chemother Pharmacol 41: 477–484, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Buchanan JA, Heard K, Burbach C, Wilson ML, Dart R: Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA 305: 1657–1658, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Barbano G, Ginevri F, Ghiggeri GM, Gusmano R: Disseminated autoimmune disease during levamisole treatment of nephrotic syndrome. Pediatr Nephrol 13: 602–603, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Rachapalli SM, Kiely PD: Cocaine-induced midline destructive lesions mimicking ENT-limited Wegener's granulomatosis. Scand J Rheumatol 37: 477–480, 2008 [DOI] [PubMed] [Google Scholar]