Abstract
Summary
Background and objectives
It has been suggested that moderate reductions in estimated GFR (eGFR) among older adults may not reflect chronic kidney disease (CKD).
Design, setting, participants, & measurements
We examined age-specific (<60, 60 to 69, 70 to 79, and ≥80 years) associations between eGFR level and six concurrent CKD complications among 30,528 participants from the National Health and Nutrition Examination Survey (NHANES) 1988 to 1994 and 1999 to 2006 (n = 8242 from NHANES 2003 to 2006 for hyperparathyroidism). Complications included anemia (hemoglobin <12 g/dl women, <13.5 g/dl men), acidosis (bicarbonate <22 mEq/L), hyperphosphatemia (phosphorus ≥4.5 mg/dl), hypoalbuminemia (albumin <3.5 mg/dl), hyperparathyroidism (intact parathyroid hormone ≥70 pg/ml), and hypertension (systolic/diastolic BP ≥140/90 mmHg or antihypertensive use).
Results
Among participants ≥80 years old, compared with those with estimated GFR (eGFR) ≥60 ml/min per 1.73 m2, the multivariable adjusted prevalence ratios (95% confidence interval) associated with eGFR levels of 45 to 59 and <45 ml/min per 1.73 m2 were 1.39 (1.11 to1.73) and 2.06 (1.59 to 2.67) for anemia, 1.33 (0.89 to 1.98) and 2.47 (1.52 to 4.00) for acidosis, 1.11 (0.70 to 1.76) and 2.16 (1.36 to 3.42) for hyperphosphatemia, 2.04 (1.39 to 3.00) and 2.83 (1.76 to 4.53) for hyperparathyroidism and 1.09 (1.03 to 1.14), and 1.12 (1.05 to 1.19) for hypertension, respectively. Higher prevalence ratios for these complications at lower eGFR levels were also present at younger ages. Reduced eGFR was associated with hypoalbuminemia only for adults <70.
Conclusions
Reduced eGFR was associated with a higher prevalence of several concurrent CKD complications, regardless of age.
Introduction
The prevalence of moderate to severe chronic kidney disease (CKD), defined as an estimated GFR (eGFR) <60 ml/min per 1.73 m2, increases with age (1). In the National Health and Nutrition Examination Survey (NHANES) 1999 to 2004, the prevalence of reduced eGFR (<60 ml/min per 1.73 m2) was 37.8% among participants more than 70 years old (1). Importantly, for older adults, reduced eGFR has been shown to be associated with higher rates of mortality, cardiovascular disease, and geriatric conditions such as functional decline, frailty, and cognitive impairment (2–6).
Despite these data, some have suggested that reduced eGFR levels among older adults may not reflect the presence of kidney disease, but instead may be a normal age-related decline in GFR (7). Along these lines, age-specific cut points for staging CKD in the National Kidney Foundation Disease Outcomes Quality Initiative (NKF-KDOQI) guidelines have been proposed (8). However, the use of age-specific cut points for staging CKD remains controversial.
In the general population, eGFR levels <60 ml/min per 1.73 m2 are associated with a higher prevalence of concurrent CKD-related complications including anemia, acidosis, hyperphosphatemia, hypoalbuminemia, hyperparathyroidism, and hypertension (9–12). Determining whether an increased prevalence of these complications is present with reduced eGFR among older adults may help define the need for clinical evaluation of CKD-related complications in this population (13). Accordingly, we evaluated the age-specific association between eGFR levels with six concurrent complications of CKD among participants in the US National Health and Nutrition Examination Surveys (NHANES) conducted in 1988 to 1994 and 1999 to 2006.
Study Population and Methods
Study Participants
The NHANES was conducted by the National Center for Health Statistics and includes cross-sectional, multistage, stratified clustered probability samples of the US civilian noninstitutionalized population. The current analysis used NHANES data from the 1988 to 1994 surveys (conducted in two phases: 1988 to 1991 and 1991 to 1994) and from the 1999 to 2006 surveys (conducted in four phases: 1999 to 2000, 2001 to 2002, 2003 to 2004, and 2005 to 2006) (14). This analysis was limited to participants 20 years of age and older who completed a medical evaluation in the NHANES mobile examination center (n = 39,316). After excluding those with missing serum creatinine, hemoglobin, bicarbonate, phosphorus, albumin, urinary albumin, urinary creatinine, or BP measurements, those who had eGFR <15 ml/min per 1.73 m2 or were pregnant, 30,528 participants had complete data for the analysis of the associations between eGFR and anemia, acidosis, hyperphosphatemia, hypoalbuminemia, and hypertension. Intact parathyroid hormone (iPTH) was available in NHANES 2003 to 2004 and 2005 to 2006 only and was analyzed for 8242 participants.
Data Collection
Age, gender, and race/ethnicity were obtained via self-report. Participants who reported having smoked 100 or more cigarettes during their lifetime were classified as current smokers if they reported current smoking in NHANES III or smoking “some days” or “most days” in NHANES 1999 to 2006. Waist circumference was measured midway between the lowest rib and the iliac crest with the participant standing. Diabetes mellitus was defined by a prior diagnosis, excluding during pregnancy, use of insulin or oral hypoglycemic medication, fasting glucose ≥126 mg/dl or nonfasting glucose ≥200 mg/dl. C-reactive protein (CRP) was measured using a low-sensitivity assay in NHANES III, whereas a high-sensitivity assay was used in NHANES 1999 to 2006. CRP was categorized as <3, 3 to <10, and ≥10 mg/L.
Measures of Kidney Function
After recalibration of serum creatinine to standardized measurements obtained from the Cleveland Clinic Research Laboratory (15), eGFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation (16). eGFR was categorized as ≥60, 45 to 59, and <45 ml/min per 1.73 m2. Urine albumin-to-creatinine ratio (ACR) was calculated from spot urine albumin and creatinine samples obtained during the medical examination.
Assessment of Concurrent CKD Complications
Six concurrent complications of CKD were studied. Hemoglobin was measured by Coulter Splus Jr. in NHANES III and Beckman Coulter MAXM in NHANES 1999 to 2006. Anemia was defined using National Kidney Foundation guidelines as hemoglobin <12 g/dl for women and <13.5 g/dl for men (17). Bicarbonate, phosphate, and serum albumin were assayed using Hitachi 737 in NHANES III, Hitachi 704 in NHANES 1999 to 2006, and Beckman-Synchron LX20 in the NHANES 2001 to 2006. Acidosis was defined as serum bicarbonate <22 mEq/L. Hyperphosphatemia was defined as serum phosphate ≥4.5 mg/dl. Hypoalbuminemia was defined as serum albumin <3.5 g/dl. Serum iPTH was measured at the University of Washington, in Seattle, Washington, on an Elecsys 1010 autoanalyzer (Roche Diagnostics, Mannheim, Germany), using an electrochemiluminescent process. Hyperparathyroidism was defined as iPTH levels ≥70 pg/ml. To standardize the laboratory values across all of the phases of NHANES, the age, gender, and race/ethnicity adjusted mean level for hemoglobin, bicarbonate, phosphate, serum albumin, and iPTH for participants 20 to 39 years old without diabetes and hypertension, eGFR ≥ 60 ml/min per 1.73 m2, and ACR <10 mg/g for each survey was calculated. Differences from the value for NHANES 2005 to 2006 were then added to or subtracted from values for the other NHANES phases. Standardized laboratory values were used for the main analyses, whereas nonstandardized values were used in a sensitivity analysis. BP was measured six times in NHANES III and three times in NHANES 1999 to 2006. Using the average of all available BP measurements, hypertension was defined as a systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or self-reported use of antihypertensive medication.
Statistical Analyses
The NHANES study population characteristics and prevalence of concurrent CKD complications were calculated by age group (20 to 59, 60 to 69, 70 to 79, and ≥80 years of age). The crude prevalence of concurrent complications was calculated by eGFR category (≥60, 45 to 59, and <45 ml/min per 1.73 m2) for each age group, separately. Prevalence ratios for concurrent CKD complications were calculated, adjusting for age, gender, race/ethnicity, cigarette smoking, waist circumference, diabetes mellitus, hypertension (except for the model with hypertension as the outcome), ACR, and CRP. We used prevalence ratios to determine the associations between eGFR level and concurrent CKD complications given the cross-sectional data and high frequency of events (18,19). Prevalence ratios are interpreted similarly to odds ratios and relative risks. Formal tests for multiplicative interactions were conducted by comparing −2 [astctr] log likelihood in regression models including the full population with and without interaction terms (age group [astctr]level of eGFR). Analyses were repeated using the Modification of Diet in Renal Disease (MDRD) Study equation to calculate eGFR and using nonstandardized laboratory values.
Within age grouping, multivariable adjusted prevalence ratios for each complication were calculated by eGFR modeled as a continuous variable, using restricted quadratic splines with knots at 30, 60, 90, and 120 ml/min per 1.73 m2. Analyses were performed incorporating the NHANES sampling weights to obtain unbiased estimates using SUDAAN version 10 (Research Triangle Institute, Research Triangle Park, North Carolina) and R version 2.9.2 (R Foundation for Statistical Computing, Vienna, Austria). Sampling weights were combined across all survey phases from NHANES 1988 to 2006. The SEs for all estimates were obtained using the Taylor series (linearization) method accounting for the complex, multistage recruitment of NHANES participants.
Results
Participant Characteristics
Compared with individuals included in the present analyses, those excluded were more likely to be women (63.5% versus 50.8% of those included) and non-Hispanic black (17.1% versus 10.2% of those included) and less likely to be non-Hispanic white (66.1% versus 74.7% of those included). Differences between those excluded versus included were small with respect to age (45.7 versus 45.5 years, respectively) and Mexican-American race-ethnicity (10.1% versus 9.0%, respectively).
Of those included in the current analysis, participants 80 years and older were more likely than their counterparts in the younger age groups to be women, non-Hispanic white, and nonsmokers (Table 1). Additionally, systolic BP and ACR levels were higher and eGFR levels were lower in the older age groupings. Older age was also associated with a higher prevalence of anemia, hyperparathyroidism, hypoalbuminemia, and hypertension. The prevalence of acidosis and hyperphosphatemia was lower at older age.
Table 1.
Characteristics of National Health and Nutrition Examination Survey participants by age group
| Age Group (years) | ||||
|---|---|---|---|---|
| Participant Characteristic | 20 to 59 (n = 20,251) | 60 to 69 (n = 4611) | 70 to 79 (n = 3394) | ≥80 (n = 2272) |
| Age (years) | 38.5 (0.1) | 64.3 (0.1) | 73.9 (0.1) | 83.3 (0.1) |
| Women (%) | 49.3 | 52.9 | 57.2 | 62.7 |
| Race/ethnicity (%) | ||||
| non-Hispanic white | 72.2 | 81.1 | 85.7 | 88.1 |
| non-Hispanic black | 10.9 | 8.4 | 7.2 | 6.1 |
| Mexican-American | 10.2 | 5.3 | 4.2 | 3.5 |
| other | 6.7 | 5.3 | 3.0 | 2.3 |
| Current smoker (%) | 27.9 | 17.7 | 9.2 | 3.9 |
| Waist circumference (cm)a | 93.2 (0.2) | 99.9 (0.3) | 98.2 (0.3) | 95.5 (0.3) |
| Systolic BP (mmHg)a | 118.6 (0.2) | 134.4 (0.4) | 140.5 (0.6) | 147.3 (0.5) |
| Diastolic BP (mmHg)a | 73.6 (0.2) | 73.9 (0.2) | 70.2 (0.3) | 66.7 (0.5) |
| Antihypertensive medication (%) | 8.8 | 36.6 | 43.2 | 42.8 |
| Diabetes mellitus (%) | 4.1 | 15.1 | 15.9 | 13.1 |
| C-reactive protein (mg/L) | ||||
| <3 | 69.9 | 58.5 | 58.5 | 58.4 |
| 3 to 9 | 22.5 | 29.8 | 30.3 | 31.3 |
| ≥10 | 7.6 | 11.7 | 11.3 | 10.3 |
| ACR (mg/g)b | 5.4 (3.6 to 9.1) | 7.6 (4.6 to 16.0) | 10.6 (5.8 to 25.4) | 16.2 (7.9 to 43.7) |
| eGFR (ml/min per 1.73 m2) | ||||
| ≥60 | 99.1 | 90.3 | 73.5 | 48.8 |
| 45 to 59 | 0.8 | 7.4 | 18.2 | 32.8 |
| <45 | 0.1 | 2.3 | 8.3 | 18.3 |
| Anemia (%) | 4.3 | 6.2 | 9.2 | 16.4 |
| Acidosis (%) | 11.6 | 8.5 | 7.5 | 7.8 |
| Hyperphosphatemia (%) | 7.7 | 6.9 | 7.1 | 6.2 |
| Hypoalbuminemia (%) | 1.0 | 2.2 | 3.1 | 3.6 |
| Hyperparathyroidism (%) | 6.7 | 11.5 | 16.4 | 25.9 |
| Hypertension (%) | 16.7 | 55.4 | 68.6 | 74.5 |
ACR, urinary albumin-to-creatinine ratio; eGFR, estimated GFR; iPTH, intact parathyroid hormone. Anemia (hemoglobin <12 g/dl women, <13.5 g/dl men), hyperphosphatemia (serum phosphorus ≥4.5 mg/dl), hypoalbuminemia (serum albumin <3.6 mg/dl), acidosis (serum bicarbonate <22 mEq/L), hyperparathyroidism (iPTH ≥70 pg/ml), and hypertension (systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg or use of antihypertensive medication).
Values are mean with SD in parentheses.
Values are median with 25th and 75th percentiles in parentheses.
Age-Specific Association of eGFR Categories and CKD Complications
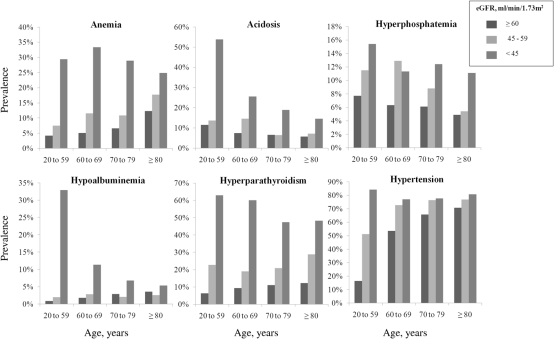
The unadjusted age-specific prevalence of concurrent CKD complications by eGFR categories is displayed in Figure 1. Among participants ≥80 years of age the prevalence of anemia, acidosis, hyperphosphatemia, hyperparathyroidism, and hypertension increased progressively at lower eGFR categories (Figure 1). The prevalence of hypoalbuminemia increased at lower eGFR categories only among participants <70 years old. For participants ≥70 years of age, the unadjusted prevalence of hypoalbuminemia was lower among individuals with an eGFR 45 to 59 ml/min per 1.73 m2 compared with ≥60 ml/min per 1.73 m2 and was highest among those with eGFR <45 ml/min per 1.73 m2.
Figure 1.
Unadjusted age-specific prevalence of concurrent complications of chronic kidney disease by estimated GFR (eGFR, ml/min per 1.73 m2).
Among participants ≥80 years of age, compared with those with eGFR ≥60 ml/min per 1.73 m2, the multivariable adjusted prevalence ratios (95% confidence interval [CI]) associated with eGFR levels of 45 to 59 ml/min per 1.73 m2 and <45 ml/min per 1.73 m2 were 1.39 (1.11 to 1.73) and 2.06 (1.59 to 2.67) for anemia, 1.33 (0.89 to 1.98) and 2.47 (1.52 to 4.00) for acidosis, 1.11 (0.70 to 1.76) and 2.16 (1.36 to 3.42) for hyperphosphatemia, 0.39 (0.16 to 0.93) and 1.15 (0.44 to 3.01) for hypoalbuminemia, 2.04 (1.39 to 3.00) and 2.83 (1.76 to 4.53) for hyperparathyroidism, and 1.09 (1.03 to 1.14) and 1.12 (1.05 to 1.19) for hypertension, respectively (Table 2). A significant interaction between age and eGFR was present for hypoalbuminemia (P-interaction <0.001) and hypertension (P-interaction = 0.04) but not the other complications studied (P-interaction >0.20). Among participants 20 to 59 and 60 to 69 years, lower eGFR levels were associated with increased multivariable adjusted prevalence ratios for hypoalbuminemia. In contrast, among individuals 70 to 79 and ≥80 years old, an eGFR of 45 to 59 ml/min per 1.73 m2 was associated with lower prevalence of hypoalbuminemia than among participants with eGFR ≥60 ml/min per 1.73 m2. Among participants ≥80 years old, the prevalence ratios for hypertension were smaller when compared with younger participants. Results were similar when analyses were repeated using the MDRD study equation and when nonstandardized laboratory values were used (data not shown).
Table 2.
Multivariable adjusted prevalence ratios for anemia, acidosis, hyperphosphatemia, hypoalbuminemia, hyperparathyroidism, and hypertension associated with reduced eGFR by age group
| Age Group (years) | ||||
|---|---|---|---|---|
| eGFR Categories (ml/min per 1.73 m2) | 20 to 59 | 60 to 69 | 70 to 79 | ≥80 |
| Prevalence Ratio (95% CI) for Anemia | ||||
| ≥60 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 45 to 59 | 1.32 (0.84, 2.10) | 2.06 (1.34, 3.18) | 1.47 (1.09, 1.99) | 1.39 (1.11, 1.73) |
| <45 | 3.73 (1.90, 7.32) | 3.88 (2.19, 6.89) | 3.29 (2.32, 4.66) | 2.06 (1.59, 2.67) |
| Prevalence Ratio (95% CI) for Acidosis | ||||
| ≥60 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 45 to 59 | 1.36 (0.80, 2.31) | 1.90 (1.43, 2.52) | 1.00 (0.67, 1.51) | 1.33 (0.89, 1.98) |
| <45 | 6.39 (4.22, 9.69) | 3.05 (1.65, 5.64) | 2.85 (1.92, 4.23) | 2.47 (1.52, 4.00) |
| Prevalence Ratio (95% CI) for Hyperphosphatemia | ||||
| ≥ 60 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 45 to 59 | 1.77 (1.10, 2.84) | 1.97 (1.23, 3.15) | 1.39 (0.91, 2.13) | 1.11 (0.70, 1.76) |
| <45 | 3.12 (1.13, 8.62) | 1.33 (0.57, 3.11) | 2.51 (1.60, 3.92) | 2.16 (1.36, 3.42) |
| Prevalence Ratio (95% CI) for Hypoalbuminemia | ||||
| ≥60 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 45 to 59 | 1.17 (0.35, 3.86) | 2.02 (0.78, 5.24) | 0.45 (0.19, 1.04) | 0.39 (0.16, 0.93) |
| <45 | 7.88 (1.67, 37.3) | 3.36 (1.53, 7.39) | 1.13 (0.48, 2.68) | 1.15 (0.44, 3.01) |
| Prevalence Ratio (95% CI) for Hyperparathyroidism | ||||
| ≥60 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 45 to 59 | 2.78 (1.31, 5.89) | 1.86 (1.08, 3.18) | 1.90 (1.32, 2.73) | 2.04 (1.39, 3.00) |
| <45 | 3.49 (1.71, 7.11) | 4.77 (2.68, 8.49) | 4.13 (3.11, 5.48) | 2.83 (1.76, 4.53) |
| Prevalence Ratio (95% CI) for Hypertension | ||||
| ≥60 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 45 to 59 | 1.29 (0.99, 1.69) | 1.23 (1.11, 1.35) | 1.12 (1.03, 1.20) | 1.09 (1.03, 1.14) |
| <45 | 1.31 (0.97, 1.76) | 1.04 (0.89, 1.22) | 1.09 (0.99, 1.20) | 1.12 (1.05, 1.19) |
eGFR, estimated GFR; CI, confidence interval; iPTH, intact parathyroid hormone. Prevalence ratios are adjusted for age, race/ethnicity, gender, cigarette smoking, waist circumference, diabetes, albumin-to-creatinine ratio, C-reactive protein, and hypertension (except for the model with hypertension as the outcome). Anemia (hemoglobin <12 g/dl women, <13.5 g/dl men), hyperphosphatemia (serum phosphorus ≥4.5 mg/dl), hypoalbuminemia (serum albumin <3.6 mg/dl), acidosis (serum bicarbonate <22 mEq/L), hyperparathyroidism (iPTH ≥ 70 pg/ml), and hypertension (systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg or use of antihypertensive medication).
Age-Specific Association of eGFR Modeled as a Continuous Variable and CKD Complications
With the exception of hypertension for adults 60 to 69 years of age, within each age group, increased multivariable adjusted prevalence ratios for each complication were present at lower eGFR modeled as a continuous variable (Supplemental Figures 1 to 6). A trend toward higher prevalence ratios for anemia and hypoalbuminemia were seen at higher eGFR. However, the confidence intervals for these estimates were wide in the older age groupings.
Discussion
In the current analysis of a large US national cross-sectional sample, reduced eGFR was associated with a higher prevalence of several concurrent CKD complications regardless of age. When compared with individuals with an eGFR ≥60 ml/min per 1.73 m2, a higher prevalence of concurrent CKD complications at eGFR <45 ml/min per 1.73 m2 was present for anemia, acidosis, hyperphosphatemia, hyperparathyroidism, and hypertension. Also, a higher prevalence of these complications was present at an eGFR of 45 to 59 ml/min per 1.73 m2 even among adults ≥80 years of age. Although previous studies have established the associations of GFR level and the concurrent CKD complications studied herein, the current study extends these findings to older adults.
Prior studies have reported a higher prevalence of anemia, higher basal PTH levels, as well as a higher prevalence of hyperparathyroidism and hypertension among older adults irrespective of kidney function (20–23). The prevalence of hyperphosphatemia has been reported to be less common at older ages (24,25). Similar to these reports, in the current study a higher prevalence of anemia, hyperparathyroidism, and hypertension and a lower prevalence of hyperphosphatemia was present in the older age groups. Population-based data on chronic metabolic acidosis are limited. In contrast to one previous report (26), in the current study, the prevalence of acidosis decreased with age. Although this could potentially be explained by the more common use of thiazide-type diuretics among older adults, further research into the age-specific prevalence of chronic metabolic acidosis may be warranted.
Despite overall age-related changes in the prevalence of these complications, at eGFR levels <45 ml/min per 1.73 m2, a higher prevalence of anemia, hyperphosphatemia, acidosis, hyperparathyroidism, and hypertension was consistently present for all age groups. These findings were robust even after age-stratification and multivariable adjustment and thus are unlikely to be explained by the effect of age alone. Furthermore, among participants ≥80 years of age, eGFR 45 to 59 ml/min per 1.73 m2 was associated with a higher prevalence of anemia, hyperparathyroidism, and hypertension. Although not statistically significant, similar trends were seen for acidosis and hyperphosphatemia among participants ≥80 years. When eGFR was modeled as a continuous variable, with the exception of hypertension among participants 60 to 69 years, similar patterns of increased prevalence ratios for each complication were present at lower eGFR within each age group. For participants <70 years of age there appeared to be a stable prevalence of hyperphosphatemia at eGFR levels below 45 ml/min per 1.73 m2.
In the current study, the overall prevalence of hypoalbuminemia was higher in the older age groups, but the association between reduced eGFR and hypoalbuminemia was only seen among participants <70 years. Although serum albumin is an indicator of visceral protein, serum concentrations are affected by other factors including rate of synthesis, catabolism, and inflammation and may vary by etiology of kidney disease and severity of proteinuria (27). Thus, among older participants other factors such as subclinical inflammation may be more strongly associated with hypoalbuminemia than reduced eGFR (28). In addition, individuals with lower levels of creatinine production, and thus higher eGFR, may also have lower serum albumin. This may also explain the high prevalence of hypoalbuminemia and anemia at higher eGFR levels when modeled as a continuous variable (29). Prior studies have shown adverse outcomes associated with high eGFR levels and may reflect low muscle mass (30).
The importance of reduced eGFR, especially in the 45 to 59 ml/min per 1.73 m2 range, among older adults is controversial (7). Prior studies have shown weaker associations between eGFR and adverse events and a lower correlation between kidney function and evidence of nephrosclerosis on renal biopsy among older adults (31,32). However, more recent studies clearly demonstrate that moderate reductions in eGFR are associated with adverse outcomes and a disproportionately higher prevalence of frailty, cognitive impairment, and functional decline (3,5,6). In meta-analyses conducted by the Chronic Kidney Disease Prognosis Consortium of general population and high-risk cohorts, eGFR <60 ml/min per 1.73 m2 was associated with increased all-cause and cardiovascular mortality among adults <65 years of age as well as their counterparts ≥65 years of age (33,34). Similarly, in the population-based Reasons for Geographic and Racial Differences in Stroke study, the association between reduced eGFR and all-cause mortality was evaluated across narrow age groups (i.e., 45 to 59, 60 to 69, 70 to 79, and ≥80 years) (4). Higher rates of all-cause mortality at eGFR levels of 45 to 59 and <45 ml/min per 1.73 m2, compared with ≥60 ml/min per 1.73 m2, were present in all age groups including individuals ≥80 years.
Findings from the current study add to the growing evidence of the clinical importance of even moderate reductions in eGFR among older adults. First, our findings, together with previous reports of increased mortality and other adverse outcomes, do not support using age-specific cut points for CKD diagnosis and staging. Second, the current findings suggest that clinical evaluation of concurrent CKD complications should be considered for all patients with reduced eGFR, regardless of their age. Recognition of concurrent complications may be especially important in older adults. Although CKD management in general prioritizes slowing the progression of kidney disease to prevent ESRD, many older adults are unlikely to have progressive disease (35). Among older adults with reduced eGFR, the risk of ESRD may be less of a concern than the risk of a hip fracture with subsequent functional decline and nursing home placement. In addition to the increased risk of fracture associated with reduced eGFR, renal osteodystrophy, and acidosis-related bone turnover (36), several concurrent CKD complications have been shown to be associated with fall risk (37). Although an association between acidosis and falls has not been directly reported, uremic acidosis has also been shown to cause muscle wasting, and muscle weakness predicts falls (38). Whether or not treatment of these complications reduces falls remains unknown. Further research is needed to better determine how to prioritize outcomes among older adults with reduced eGFR. Subsequent clinical trials that include outcomes other than mortality and ESRD may be necessary.
The findings from the present analyses should be interpreted within the context of known and potential limitations. First, the cross-sectional design does not allow for evaluation of temporal associations between reduced eGFR and CKD complications. Among older adults, it is possible that the complications studied may have preceded kidney dysfunction and a causal relationship between reduced eGFR and CKD complications cannot be inferred (39,40). Data on kidney function were available from a one-time blood draw and misclassification is possible. However, misclassification of those with normal kidney function into the reduced GFR group would likely have biased the results toward the null. There is also a possibility of survivor bias as reduced eGFR has been shown to be associated with increased mortality (30). The current analysis was limited to community-dwelling adults and may not reflect the experience of older adults residing in nursing homes. Associations of eGFR and some concurrent complications, as well as interactions may not have been statistically significant because of the low numbers of cases in certain age strata, rather than the absence of true associations. Despite this limitation, the patterns between lower eGFR and a higher prevalence of CKD complications were similar in magnitude within each age strata. Furthermore, the low numbers did not allow us to use a more restricted reference group, which may be more appropriate when examining associations with estimated GFR from creatinine, especially in the elderly.
Conclusions
In conclusion, in a large, nationally representative sample of US adults, reduced eGFR was associated with a higher prevalence of anemia, acidosis, hyperphosphatemia, hyperparathyroidism, and hypertension at all ages, even among participants ≥80 years of age. These findings suggest that clinical evaluation of concurrent CKD complications should be considered for all patients with reduced eGFR, regardless of age.
Disclosures
None.
Supplementary Material
Acknowledgments
Support was provided through the Birmingham/Atlanta GRECC Special Fellowship in Advanced Geriatrics and John A. Hartford Foundation/Southeast Center of Excellence in Geriatric Medicine to C.B.B. Additional support was provided in part by the Deep South Resource Center for Minority Aging Research, from the National Institute on Aging (R.M.A.: Grant P30AG031054), and from the National Institute of Diabetes and Digestive and Kidney Diseases (L.A.I.: Grant K23 DK081017)
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate online is available for additional clinical information at www.cjasn.org.
See related editorial, “Age, eGFR, and CKD Complications,” on pages 2729–2731.
References
- 1. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB: Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol 41: 1364–1372, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, Allman RM, Warnock DG, McClellan W: Kidney function and cognitive impairment in US adults: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 52: 227–234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muntner P, Bowling CB, Gao L, Rizk D, Judd S, Tanner RM, McClellan W, Warnock DG: Age-specific association of reduced estimated glomerular filtration rate and albuminuria with all-cause mortality. Clin J Am Soc Nephrol 6, 2200–2207, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilhelm-Leen ER, Hall YN, K Tamura M, Chertow GM: Frailty and chronic kidney disease: The Third National Health and Nutrition Evaluation Survey. Am J Med 122: 664–671, e2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bowling CB, Sawyer P, Campbell RC, Ahmed A, Allman RM: Impact of chronic kidney disease on activities of daily living in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 66: 689–694, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glassock RJ, Winearls C: CKD—Fiction not fact. Nephrol Dial Transplant 23: 2695–2696 [Author reply 2696–2699], 2008 [DOI] [PubMed] [Google Scholar]
- 8. Glassock RJ, Winearls C: Screening for CKD with eGFR: Doubts and dangers. Clin J Am Soc Nephrol 3: 1563–1568, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Astor BC, Muntner P, Levin A, Eustace JA, Coresh J: Association of kidney function with anemia: The Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 162: 1401–1408, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Eustace JA, Astor B, Muntner PM, Ikizler TA, Coresh J: Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int 65: 1031–1040, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Hsu CY, Chertow GM: Elevations of serum phosphorus and potassium in mild to moderate chronic renal insufficiency. Nephrol Dial Transplant 17: 1419–1425, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Muntner P, Jones TM, Hyre AD, Melamed ML, Alper A, Raggi P, Leonard MB: Association of serum intact parathyroid hormone with lower estimated glomerular filtration rate. Clin J Am Soc Nephrol 4: 186–194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 14. National Center for Health Statistics. Centers for Disease Control and Prevention: National Health and Nutrition Examination Survey. Available from: http://www.cdc.gov/nchs/nhanes.htm Accessed July 21, 2011
- 15. Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, Coresh J: Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis 50: 918–926, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Macdougall IC, Eckardt KU, Locatelli F: Latest US KDOQI Anaemia Guidelines update—What are the implications for Europe? Nephrol Dial Transplant 22: 2738–2742, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Barros AJ, Hirakata VN: Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 3: 21, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeger SL, Liang KY: Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42: 121–130, 1986 [PubMed] [Google Scholar]
- 20. Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ: Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension 52: 818–827, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC: Prevalence of anemia in persons 65 years and older in the United States: Evidence for a high rate of unexplained anemia. Blood 104: 2263–2268, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Haden ST, Brown EM, Hurwitz S, Scott J, El-Hajj Fuleihan G: The effects of age and gender on parathyroid hormone dynamics. Clin Endocrinol (Oxf) 52: 329–338, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Portale AA, Lonergan ET, Tanney DM, Halloran BP: Aging alters calcium regulation of serum concentration of parathyroid hormone in healthy men. Am J Physiol 272: E139–E146, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Cirillo M, Botta G, Chiricone D, De Santo NG: Glomerular filtration rate and serum phosphate: An inverse relationship diluted by age. Nephrol Dial Transplant 24: 2123–2131, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Moranne O, Froissart M, Rossert J, Gauci C, Boffa JJ, Haymann JP, M'Rad MB, Jacquot C, Houillier P, Stengel B, Fouqueray B: Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol 20: 164–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alpern RJ, Sakhaee K: The clinical spectrum of chronic metabolic acidosis: Homeostatic mechanisms produce significant morbidity. Am J Kidney Dis 29: 291–302, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Kopple JD: The National Kidney Foundation K/DOQI clinical practice guidelines for dietary protein intake for chronic dialysis patients. Am J Kidney Dis 38: S68–S73, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ: Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 47: 639–646, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Stevens LA, Levey AS: Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 20: 2305–2313, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Warnock DG, Muntner P, McCullough PA, Zhang X, McClure LA, Zakai N, Cushman M, Newsome BB, Kewalramani R, Steffes MW, Howard G, McClellan WM: Kidney function, albuminuria, and all-cause mortality in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. Am J Kidney Dis 56: 861–871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rule AD, Am H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, Textor SC, Stegall MD: The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 152: 561–567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van der Velde M, Bakker SJ, de Jong PE, Gansevoort RT: Influence of age and measure of eGFR on the association between renal function and cardiovascular events. Clin J Am Soc Nephrol 5: 2053–2059, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT: Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T: Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 35. O'Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, Landefeld CS: Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 18: 2758–2765, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Dooley AC, Weiss NS, Kestenbaum B: Increased risk of hip fracture among men with CKD. Am J Kidney Dis 51: 38–44, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E: Risk factors for falls in community-dwelling older people: A systematic review and meta-analysis. Epidemiology 21: 658–668, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Abramowitz MK, Hostetter TH, Melamed M: Association of serum bicarbonate levels with gait speed and quadriceps strength in older adults. Am J Kidney Dis 58: 29–38, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kestenbaum B, Rudser KD, de Boer IH, Peralta CA, Fried LF, Shlipak MG, Palmas W, Stehman-Breen C, Siscovick DS: Differences in kidney function and incident hypertension: The multi-ethnic study of atherosclerosis. Ann Intern Med 148: 501–508, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Seaghdha CM, Hwang SJ, Muntner P, Melamed ML, Fox CS: Serum phosphorus predicts incident chronic kidney disease and end-stage renal disease. Nephrol Dial Transplant 26: 2885–2890, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.