Abstract
Summary
Background
Accurate prediction of prognosis in idiopathic membranous nephropathy (iMN) allows restriction of immunosuppressive therapy to patients at high risk for ESRD. Here we re-evaluate urinary low-molecular-weight proteins as prognostic markers and explore causes of misclassification.
Design, setting, participants, & measurements
In a cohort of 129 patients with serum creatinine concentration <135 μmol/L and proteinuria ≥3.0 g/10 mmol, urinary α1- (uα1m) and β2-microglobulin (uβ2m) excretion rate was determined. Urinary α1m and uβ2m-creatinine ratio was also obtained. We defined progression as a rise in serum creatinine ≥50% or ≥25% and an absolute level ≥135 μmol/L.
Results
Median survival time was 25 months, and 47% of patients showed progression. The area under the receiver operating characteristic curve for uβ2m was 0.81 (95% CI: 0.73 to 0.89). Using a threshold value of 1.0 μg/min, sensitivity and specificity were 73% and 75%, respectively. Similar accuracy was observed for the uβ2m-creatinine ratio with sensitivity and specificity of 75% and 73%, respectively, at a threshold of 1.0 μg/10 mmol creatinine. Similar accuracy was found for uα1m and uα1m-creatinine ratio. Blood Pressure and cholesterol contributed to misclassification. Repeated measurements improved accuracy in patients with persistent proteinuria: the positive predictive value of uβ2m increased from 72% to 89% and the negative predictive value from 76% to 100%.
Conclusions
Urinary excretion of uα2m and uβ2m predict prognosis in iMN. A spot urine sample can be used instead of a timed sample. A repeated measurement after 6 to 12 months increases prognostic accuracy.
Introduction
Idiopathic membranous nephropathy (iMN) is an important cause of nephrotic syndrome in adults (1). Spontaneous remission of proteinuria occurs in 30% to 50% of patients (2,3). Despite treatment with angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARB) and statins, between 25% and 50% of patients show progressive loss of renal function (4,5). Although alkylating drugs improve outcome in patients with iMN (6–8), these agents often have adverse effects such as bone marrow depression, infections, and increased risk of cancer (8). Therefore, one should restrict their use to patients at highest risk of progression to ESRD.
There has been an extensive search for tools that differentiate between patients with a favorable and poor prognosis (9). Histologic markers appeared to be of limited value, whereas the severity of proteinuria is a better marker for outcome (2–4,10). Remission of proteinuria or increased serum creatinine concentration during follow-up are the most powerful predictors of outcome; however, these are late events (11,12). In past decades, several specific urinary proteins were evaluated as early prognostic markers. Candidates such as TGF-β, βNAG (N-acetyl-beta-glucosaminidase), IgG, complement factors, urinary α1- and β2-microglobulin (uα1m and uβ2m) have been proposed (13–19). In a previous study of 57 patients we showed that uIgG and uβ2m can accurately predict prognosis (20). Because conservative treatment and prognosis may have changed in recent years, we re-evaluated the data.
Here we report the value of uα1m, uβ2m, and uIgG as predictors of outcome in a cohort of 129 patients with iMN. In addition we evaluate the role of these markers in clinical practice using low-molecular-weight protein-creatinine ratios. We also analyzed possible causes of misclassification and the value of repeated measurements.
Study Population and Methods
Population
Patients with biopsy-proven iMN who attended our clinic for urinary analysis between January 1995 and June 2009 were assessed for this study. Inclusion criteria were normal renal function, defined as serum creatinine <135 μmol/L (≈1.5 mg/dl), proteinuria ≥3.0 g/10 mmol creatinine, and an interval between biopsy and urinary analysis <3 years. Exclusion criteria were participation in the intervention arm of a immunosuppressive therapy trial (21), follow-up duration <1 year, or treatment with immunosuppressive drugs before urinary analysis. Follow-up was completed until an end point was reached or until June 2010. Patients were followed at our hospital or by nephrologists in referring centers. Patients were treated with diuretics and were given dietary sodium restriction, ACE inhibitors and/or ARBs and statins according to existing guidelines. Immunosuppressive therapy was advised only in patients with deteriorating kidney function or severe untreatable nephrotic syndrome. Patients with persistent proteinuria were invited for a repeated evaluation after 6 to 12 months.
Data Collection
Details of our protocol for the evaluation of patients with iMN are described elsewhere (20). Patients were instructed to fast overnight and take sodium bicarbonate to alkalinize urine on the evening before urinary analysis, because β2m disintegrates in acidic urine. They did not take diuretics on the morning of urinary analysis. Timed urine samples were collected, and blood samples were taken. IgG and uα1m were measured using a BNII nephelometer (Behring, Marburg, Germany), and uβ2m was measured using ELISA (22). The excretion of total protein and low-molecular-weight proteins was standardized against urinary creatinine concentration, to obtain a urine protein-creatinine ratio. Data on serum creatinine concentration, urinary protein, and creatinine excretion during follow-up and use of immunosuppressive therapy, ACE inhibitors, ARBs, and lipid-lowering drugs were gathered from medical records.
Definition of End Points
We defined progression as (1) a rise in serum creatinine >50%, (2) a rise in serum creatinine >25% and an absolute level ≥135 μmol/L, or (3) the need for immunosuppressive therapy because of severe nephrotic syndrome as judged by the treating physician (23). Partial remission of proteinuria was defined by urinary protein excretion <2.0 g/10 mmol creatinine with stable serum creatinine. We also applied the definition of partial remission as suggested by Troyanov et al. (proteinuria <3.5 g/d and a reduction of >50% with a stable kidney function) (2). Remission was considered complete when protein excretion was <0.2 g/10 mmol creatinine. Spontaneous remission means it occurred without immunosuppressive therapy.
Statistical Analyses
All analyses were performed with Stata 10 (StataCorp LP, College Station, Texas). Median values and interquartile ranges were calculated. Incidence of patient outcomes was plotted using the competing risks method. The area under the receiver operating characteristic curve (ROC-AUC) was calculated to compare prognostic value of urinary markers. We determined cutoff values so that false-positive and false-negative rates would be minimal and the proportion of correctly classified patients was maximized, and we calculated sensitivity, specificity, and positive and negative predictive values (PPV and NPV, respectively). Finally, we created a logistic model using a backward stepwise algorithm with exclusion at P > 0.10 and reinclusion at P < 0.05. The model's ROC-AUC was compared with the AUC for either uα1m or uβ2m to evaluate if it added to prognostic power. Sources of misclassification were explored by tabulation of baseline characteristics by classification and outcome. One-way ANOVA or chi-squared tests were used to compare the four groups. Classification according to repeated measurements was cross-tabulated by outcome to explore the value of repeated measurements.
Results
Population Characteristics
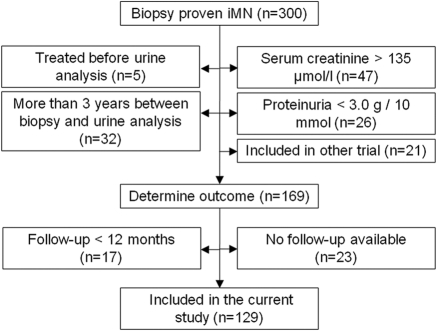
Between January 1995 and June 2009 we evaluated 300 patients with biopsy-proven iMN. One hundred sixty-nine patients met criteria for enrollment (Figure 1). In 17 patients follow-up was less than 12 months. No follow-up data were available for 23 patients. Thus, 129 patients were available for analysis. Baseline characteristics are presented in Table 1. The majority of patients was male and middle aged. Median serum creatinine concentration was 88 μmol/L (interquartile range [IQR] 76 to 103), and median proteinuria was 8.0 g/10 mmol creatinine (IQR 5.6 to 10.7). Urinary excretion of low-molecular-weight proteins was increased, with median uα1m and uβ2m excretion of 41 (reference <10) and 0.6 (reference <0.2) μg/min, respectively. Virtually all patients (99%) received ACE inhibitors and/or ARBs during follow-up, and the majority (90%) were treated with lipid-lowering medication.
Figure 1.
Flowchart of the inclusion of patients.
Table 1.
Baseline characteristics of patients with idiopathic membranous nephropathy
| Number of subjects (% male) | 129 (68%) |
| Age at time of biopsy (years)a | 51 (43 to 61) |
| Time between biopsy and urine analysis (months)a | 2 (1 to 4) |
| Survival time (months)a | 25 (13 to 51) |
| MAP (mmHg)a | 97 (86 to 106) |
| Laboratorya | |
| serum creatinine (μmol/L) | 88 (76 to 103) |
| serum albumin (g/L) | 23 (19 to 28) |
| serum cholesterol (mmol/L) | 7.3 (5.7 to 9.2) |
| eGFRMDRD4 (ml/min per 1.73 m2) | 75 (60 to 87) |
| Urine samples | |
| proteinuria (g/10 mmol creatinine)a | 8.0 (5.6 to 10.7) |
| proteinuria <4.0 g/10 mmol (%) | 9 |
| proteinuria ≥4.0 and <8.0 g/10 mmol (%) | 41 |
| proteinuria ≥8.0 and <12 g/10 mmol (%) | 35 |
| proteinuria ≥12 g/10 mmol (%) | 15 |
| β2-microglobulin (μg/min)a | 0.6 (0.2 to 4.8) |
| α1-microglobulin (μg/min)a | 41 (23 to 72) |
| IgG (mg/24 h)a | 257 (116 to 490) |
| β2-microglobulin (mg/10 mmol creatinine)a | 0.9 (0.3 to 7.0) |
| α1-microglobulin (mg/10 mmol creatinine)a | 36 (57 to 113) |
| IgG (mg/10 mmol creatinine)a | 262 (110 to 485) |
| Selectivity indexb | 0.19 ± 0.09 |
| Medication (%) | |
| ACEi/ARB use at time of biopsy | 22 |
| ACEi/ARB use during follow-up | 99 |
| statin use at time of biopsy | 13 |
| statin use during follow-up | 90 |
| Outcomes | |
| progression (%) | 47 |
| 50% rise in serum creatinine (n) | 30 |
| 25% rise and serum creatinine ≥135 μmol/L (n) | 24 |
| clinical progression (n) | 6 |
| spontaneous remission (%) | 47 |
| partial remission [<2 g/10 mmol] (n) | 61 |
| partial remission [<3.5 g/10 mmol and 50% reduction] (n) | 63 |
| complete remission (%) (n) | 26 |
MAP, mean arterial pressure; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; eGFRMDRD4, estimated GFR calculated with the Modification of Diet in Renal Disease formula.
Values are median with interquartile range in parentheses.
Values are means ± SD.
Outcomes
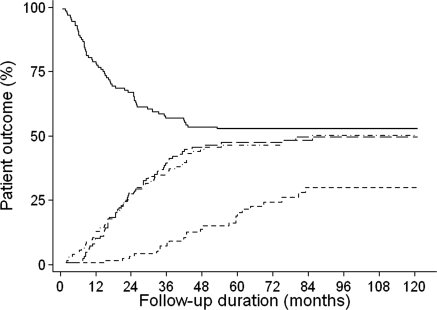
Clinical outcome is reported in Table 1 and illustrated in Figure 2. Sixty patients (47%) showed progression. In 30 patients serum creatinine concentration increased by >50%, in 24 patients serum creatinine concentration increased >25% and reached values ≥135 μmol/L, and six patients started immunosuppressive therapy because of severe nephrotic syndrome. Of the patients showing progression, 47% did so within 12 months, 72% within 24 months, and all within 5 years. In 63 patients proteinuria spontaneously decreased by >50% and reached values <3.5 g/d. With the exception of two cases, proteinuria in these patients decreased to concentrations <2.0 g/10 mmol creatinine. Twenty-three percent of patients who developed spontaneous remission (<2.0 g/10 mmol creatinine) did so within 12 months, 59% within 24 months, and 97% within 5 years. Forty-three percent of the patients who went into partial remission eventually had a complete remission of proteinuria.
Figure 2.
Patient outcomes. The solid line represents renal survival without progression. The dot and dashed line represents partial remission defined as proteinuria <3.5 g/d and <50% since baseline, the long dashed line partial remission (proteinuria <2.0 g/d), and the short dashed line complete remission (proteinuria <0.2 g/d).
Prognostic Value of uα1m and uβ2m
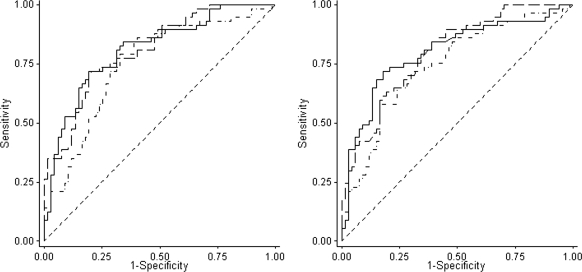
We plotted an ROC curve for the prognostic accuracy of uα1m, uβ2m, and uIgG excretion (Figure 3, left). ROC-AUC was 0.81 (95% confidence interval: 0.73 to 0.88) for uα1m, 0.81 (0.73 to 0.89) for uβ2m, and 0.75 (0.66 to 0.84) for uIgG. ROC curves for uα1m and uβ2m and uIgG-creatinine ratios are presented in Figure 3 (right). The ratios yielded similar ROC-AUCs: 0.80 (0.72 to 0.87), 0.80 (0.72 to 0.88), and 0.74 (0.66 to 0.83) for uα1m, uβ2m, and uIgG, respectively. The optimal cutoff value for the excretion of uβ2m based on our current data is 1.0 μg/min (Table 2). At this threshold, the PPV and NPV were 72% and 76%, respectively. For uα1m, a threshold value was determined at 50 μg/min, with a PPV of 76% and NPV of 73%. When excretion was standardized for urinary creatinine concentration, threshold values were 1.0 μg/10 mmol creatinine and 75 mg/10 mmol creatinine for uβ2m and uα1m, respectively (Supplementary Table S1).
Figure 3.
Left: ROC curves for prognostic accuracy of urinary excretion rate of α1- (dashed line) and β2-microglobulin (solid line) and IgG (dot and dashed line). Both α1- and β2-microglobulin excretions rates are expressed in μg/min and IgG in mg/24 h. Areas under the curve were as follows: uα1m: 0.81 (95% confidence interval: 0.73 to 0.88), uβ2m: 0.81 (0.73 to 0.89), and IgG: 0.75 (0.66 to 0.84). Right: ROC curves for the prognostic accuracy of α1- (dashed line) and β2-microglobulin (solid line) and IgG (dot and dashed line). When expressed as mg/10 mmol creatinine. Areas under the ROC curve were as follows: uα1m/creat: 0.80 (0.72 to 0.87), uβ2m/creat: 0.80 (0.72 to 0.88), and uIgG/creat: 0.74 (0.66 to 0.83).
Table 2.
Test characteristics for urinary low-molecular-weight protein excretion to predict progression in 129 iMN patients
| Threshold Value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | False Positives (n) | False Negatives (n) | Test Positives (n) |
|---|---|---|---|---|---|---|---|
| uβ2m | |||||||
| ≥0.5 μg/min | 80 | 67 | 68 | 79 | 23 | 12 | 71 |
| ≥1.0 μg/min | 73 | 75 | 72 | 76 | 17 | 16 | 61 |
| ≥1.5 μg/min | 65 | 83 | 76 | 73 | 12 | 21 | 51 |
| ≥2.0 μg/min | 58 | 83 | 76 | 70 | 12 | 24 | 47 |
| ≥2.5 μg/min | 55 | 84 | 75 | 68 | 11 | 27 | 44 |
| uα1m | |||||||
| ≥40 μg/min | 77 | 71 | 70 | 78 | 20 | 14 | 66 |
| ≥50 μg/min | 65 | 83 | 76 | 73 | 12 | 21 | 51 |
| ≥60 μg/min | 57 | 86 | 77 | 69 | 10 | 26 | 44 |
| ≥70 μg/min | 45 | 88 | 77 | 65 | 8 | 33 | 35 |
| ≥80 μg/min | 42 | 90 | 78 | 64 | 11 | 27 | 44 |
iMN, idiopathic membranous nephropathy; PPV, positive predictive value; NPV, negative predictive value; uβ2m, urinary β2-microglobulin; uα1m, urinary α1-microglobulin. Test positives are the number of patients with a urinary α1- and β2-microglobulin excretion greater than the threshold value.
Sources of Misclassification
To evaluate potential sources of misclassification, we tabulated baseline characteristics by classification based on uβ2m excretion rate (Table 3). In general, progressors showed higher median serum creatinine (110 and 90 versus 80 and 86 μmol/L) and cholesterol concentrations (8.4 and 8.5 versus 6.5 and 6.1 mmol/L) than nonprogressors. Mean arterial pressure (MAP) (94 versus 93 mmHg) and proteinuria (5.5 versus 6.2 g/10 mmol creatinine) were remarkably similar between misclassified progressors and correctly classified low-risk patients, whereas serum albumin levels were markedly higher in nonprogressing patients whose uβ2m was <1.0 μg/min than in progressors (27 versus 23 g/L). To further improve prognostic accuracy, we created two models, one based on uβ2m and the other on uα1m. We included baseline MAP, serum cholesterol, serum creatinine, serum albumin, and proteinuria. All predictors were log-transformed, and a stepwise backward selection algorithm was used. The model including uβ2m also retained serum cholesterol and creatinine as independent predictors, and its ROC-AUC was 0.85 (0.79 to 0.92). A similar model including uα1m had an ROC-AUC of 0.86 (0.80 to 0.93). The final models are presented in Supplementary Table S2.
Table 3.
Baseline characteristics for progressors and nonprogressors classified according to initial excretion of β2-microglobulin ≥1.0 μg/min
| Progressors |
Nonprogressorsa |
Pb | |||
|---|---|---|---|---|---|
| β2m ≥ 1.0 | β2m < 1.0 | β2m < 1.0 | β2m ≥ 1.0 | ||
| Number of subjects (% male subjects) | 44 (75) | 16 (62) | 52 (65) | 17 (65) | 0.69 |
| Age at time of biopsy (years)c | 57 (47 to 64) | 49 (44 to 58) | 49 (38 to 60) | 56 (51 to 64) | 0.02 |
| Time between biopsy and urine analysis (months) | 2 (1 to 4) | 2 (0 to 2) | 1 (1 to 4) | 2 (1 to 4) | 0.88 |
| Survival time (months) | 11 (6 to 25) | 16 (7 to 25) | 53 (28 to 84) | 41 (24 to 54) | |
| MAP (mmHg) | 100 (89 to 112) | 94 (81 to 105) | 93 (86 to 104) | 99 (92 to 104) | 0.16 |
| Laboratory | |||||
| serum creatinine (μmol/L) | 110 (97 to 119) | 90 (68 to 95) | 80 (70 to 87) | 86 (82 to 91) | <0.001 |
| serum albumin (g/L) | 20 (17 to 24) | 23 (18 to 26) | 27 (23 to 31) | 22 (17 to 25) | <0.001 |
| serum cholesterol (mmol/L) | 8.4 (7.0 to 9.8) | 8.5 (5.7 to 9.3) | 6.5 (5.5 to 7.7) | 6.1 (5.3 to 7.3) | 0.004 |
| eGFRMDRD4 (ml/min per 1.73 m2) | 58 (53 to 67) | 75 (65 to 97) | 85 (78 to 93) | 75 (67 to 80) | <0.001 |
| Urine samples | |||||
| proteinuria (g/10 mmol creatinine) | 10.7 (9.3 to 12.7) | 5.5 (4.8 to 8.7) | 6.2 (4.7 to 8.5) | 9.1 (5.9 to 11.0) | <0.001 |
| β2-microglobulin (μg/min) | 7.8 (2.3 to 13.8) | 0.3 (0.1 to 0.5) | 0.1 (0.2 to 0.4) | 2.6 (1.3 to 7.7) | |
| α1-microglobulin (μg/min) | 106 (61 to 131) | 31 (20 to 44) | 22 (12 to 37) | 50 (39 to 83) | |
| IgG (mg/24 h) | 511 (356 to 776) | 157 (74 to 217) | 119 (62 to 219) | 351 (158 to 607) | |
| Selectivity index>d | 0.27 ± 0.08 | 0.11 ± 0.05 | 0.15 ± 0.07 | 0.21 ± 0.08 | <0.001 |
| Medication (%) | |||||
| ACEi/ARB use at time of biopsy | 36 | 20 | 16 | 6 | 0.03 |
| ACEi/ARB use during follow-up | 100 | 100 | 98 | 100 | 0.68 |
| statin use at time of biopsy | 20 | 7 | 10 | 6 | 0.26 |
| statin use during follow-up | 93 | 93 | 84 | 94 | 0.43 |
| Outcomes | |||||
| progression (%) | 100 | 100 | 0 | 0 | |
| 50% rise in serum creatinine (n) | 18 | 12 | |||
| 25% rise and serum creatinine >135 μmol/L (n) | 24 | 0 | |||
| clinical progression (n) | 2 | 4 | |||
| Spontaneous remission (%) | |||||
| partial remission: < 2.0 g/10 mmol | 0 | 0 | 90 | 82 | |
| partial remission: <3.5 g/10 mmol and ≥50% reduction | 0 | 0 | 94 | 82 | |
| complete remission | 0 | 0 | 40 | 29 | |
β2-microglobulin, β2m; MAP, mean arterial pressure; eGFRMDRD4, estimated GFR calculated with the Modification of Diet in Renal Disease formula; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Misclassified progressors are those patients who did show progression but had uβ2m < 1.0 μg/min.
ANOVA was used to compare continuous data between the four groups and chi-squared tests to compare medication use and gender.
Values are medians with interquartile range in parentheses.
Values are mean ± SD.
We questioned if tubulointerstitial damage could be of value. In 95 patients the interval between kidney biopsy and urine analysis was <3 months. Forty-seven biopsies were available for review. Tubulointerstitial injury (scored 0 to 3) correlated with uβ2m (r = 0.58). However, the tubulointerstitial injury score did not improve the predictive accuracy in individual patients and did not explain discordances (Supplementary Table S3).
Repeated Measurements
We analyzed data of 44 patients with persistent proteinuria who underwent repeated urinary measurements. Baseline characteristics did not differ from total study population characteristics (Supplementary Table S4). At the time of repeated measurements, patients generally had lower BP (MAP 95 versus 89 mmHg) and serum cholesterol values (7.8 versus 6.0 mmol/L) compared with baseline, likely due to intensified conservative treatment. Median serum creatinine concentrations (85 versus 97 μmol/L) and uβ2m (0.5 versus 1.1 μg/min) were higher. We tabulated uβ2m at baseline and repeated measurement by outcome in Table 4. Patients with a uβ2m above 1.0 μg/min at both measurements invariably showed progression (n = 11). In contrast, none of the 17 patients with uβ2m <1.0 μg/min at two measurements showed progression. Fifteen (88%) of them went into spontaneous remission. Four patients with uβ2m > 1.0 at baseline had uβ2m below the threshold at the repeated measurement. Three of them did show progression. In all three patients BP was greatly reduced at the time of the repeated measurement, with a decrease in MAP of 7, 16, and 22 mmHg, respectively, leading to very low MAP of 69 and 81 mmHg in two of them. In summary, when uβ2m was ≥1.0 μg/min in at least one of two measurements, PPV for progression was 89%, and when uβ2m was <1.0 μg/min at both occasions, the NPV was 100%.
Table 4.
Classification according to uβ2m excretion at baseline and repeated measurement versus patient outcome in 44 patients with repeated measurements
| Measurement |
Outcome (n) |
||
|---|---|---|---|
| Baseline | Repeated | Progression | No Progression |
| uβ2m ≥ 1.0 μg/min | uβ2m ≥ 1.0 μg/min | 11 | 0 |
| uβ2m ≥ 1.0 μg/min | uβ2m < 1.0 μg/min | 3 | 1 |
| uβ2m < 1.0 μg/min | uβ2m ≥ 1.0 μg/min | 10 | 2 |
| uβ2m < 1.0 μg/min | uβ2m < 1.0 μg/min | 0 | 17 |
The positive predictive value for patients with a least one measurement ≥1.0 μg/min was 89%. The negative predictive value for patients with both measurements <1.0 μg/min was 100%. Of the 11 patients who were classified as progressors and had uβ2m > 1.0 μg/min, three had a 50% rise in serum creatinine, seven had a 25% rise and serum creatinine >135 μmol/L, and one patient had severe nephrotic syndrome. uβ2m, urinary β2-microglobulin.
Discussion
We evaluated urinary excretion of uα1m and uβ2m as prognostic markers in a cohort of 129 iMN patients with nephrotic range proteinuria and normal serum creatinine concentration. Approximately half of the patients showed progression, and the other half went into spontaneous remission. This illustrates that the “rule of thirds” does not apply to iMN patients who present with the nephrotic syndrome and normal kidney function (24). The majority of patients (61%) reached either disease progression or partial remission within 24 months and 92% within 5 years of follow-up.
Our data indicate agreement between two commonly used definitions of partial remission, i.e. proteinuria <2 versus 3.5 g/d and a decrease >50% from baseline (2). In our population, concordance between the two definitions was almost perfect, and only time to remission varied slightly. Patients with high baseline proteinuria tend to achieve remission sooner when the latter definition is used, whereas patients with limited baseline proteinuria have proteinuria <2 g/d before a reduction of 50% is achieved. Thus, our data support the use of the definition proposed by Troyanov et al. (2).
We confirmed the prognostic value of uβ2m. However, the AUC was lower than reported in our previous study: 0.81 (95% confidence interval: 0.73 to 0.89) versus 0.94 (0.87 to 1.00) (20). This difference may be caused by a distinction in the definition of end points. In our previous study, renal death was defined as a rise in serum creatinine ≥50% or an absolute level more than 135 μmol/L. In the current study, the second criterion also included a 25% rise in serum creatinine, because an absolute value could lead to biased results (19). Second, we used stricter inclusion criteria in the current study, excluding patients with limited proteinuria. Furthermore, when we inspected baseline characteristics of patients in our current cohort by year of referral, we noted a decline in baseline serum creatinine, albumin, and cholesterol, a lower MAP over time, and shortened time between biopsy and urine analysis (Supplementary Table S5). Higher referral rates and lower baseline ACE inhibitor and ARB use in recent years point toward earlier referrals by participating nephrologists.
Timed urine samples are not routinely taken in all hospitals, and uβ2m should be measured after alkalinization of urine by overnight bicarbonate intake. Our current data suggest that a timed measurement of low-molecular-weight protein excretion may not be necessary. Both α1m and β2m related to urinary creatinine concentration had the same prognostic power as the timed excretion. Contrary to uβ2m, uα1m measurement does not require alkalinization, and it can be measured using a nephelometric assay; thus, a spot urine taken at the out-patient clinic for measurement of uα1m-creatinine ratio may be sufficient to predict prognosis.
We attempted to find explanations for the discordance between predicted and actual progressive disease by comparing patient characteristics stratified for prediction and outcome. We observed notable differences in serum cholesterol, creatinine, and the ratio between serum albumin and proteinuria. A model that included these variables slightly improved prognostic power. We hypothesize that the higher cholesterol values reflect increased hepatic synthesis and are indicative of higher unmeasured protein losses due to tubular hypermetabolism. Alternatively, the high cholesterol levels may contribute to progressive renal injury. Although based on a limited number of biopsies, our data suggest evaluation of tubulointerstitial damage is of no added value.
We evaluated if repeated measurements of uα1m and uβ2m would improve prognostic accuracy. Repeated measurements were done in patients with persistent proteinuria. When one of the measurements was above the uβ2m threshold value of 1.0 μg/min, 89% of patients showed progression. Conversely, when both measurements were <1.0 μg/min, none of the patients showed progression (NPV = 100%). Noteworthy, the data show that changes in BP can influence the results. Low levels of uβ2m and uα1m in the face of very low BP cannot be used with confidence. Alternatively the opposite may also hold true, although we do not have hard data to confirm this.
Our study has several limitations. Our end point to define renal failure can be criticized. However, we feel that it is not justified to delay start of immunosuppressive therapy until doubling of serum creatinine. If we calculate estimated GFR (eGFR) using the abbreviated Modification of Diet in Renal Disease formula, 88% of the patients who fulfilled our definition of renal failure had an eGFR value below 60 ml/min per 1.73 m2. We performed additional analyses with occurrence of eGFR <60 ml/min per 1.73 m2 as the end point. ROC-AUCs for uβ2m and uα1m remained similar and were 0.84 (0.77 to 0.92) and 0.84 (0.76 to 0.92) for uβ2m in μg/min and μg/10 mmol creatinine, respectively. For uα1m, ROC-AUCs were 0.82 (0.74 to 0.89) and 0.82 (0.75 to 0.90) for μg/min and μg/10 mmol, respectively. Many patients were referred to our center for urinary analysis, but were followed and treated elsewhere, and we were unable to collect follow-up data for all patients. Also, the data we presented on repeated measurements have to be interpreted with some caution because these were performed on a subset of patients with persistent proteinuria. Finally, we were not able to calculate a proteinuria risk score for the cohort, which requires multiple measurements of serum and urine creatinine and proteinuria during each 6-month period during follow-up (4). These data were not available.
Conclusions
We have advocated that treatment decisions in the individual patient with iMN must be based on an individualized assessment of risks and benefits (21). The risks of prolonged nephrotic syndrome should be balanced against those of progression and treatment-related complications. Urinary α1m or uβ2m measurement can be of value in this balanced decision because both allow an early prediction of prognosis in iMN. A spot urine sample can be used instead of a timed sample. BP may affect excretion rates. A repeated measurement after 6 to 12 months increases prognostic accuracy.
Disclosures
None.
Acknowledgments
J.F.M.W., J.M.H., and J.A.J.G.vdB. are supported by a grant of the Dutch Kidney Foundation (NSN: OW08). We thank the staff at participating centers: Meander Medical Centre, Sint Lucas Andreas, Gelre Hospital, Alysis Medical Centre, Lievensberg Hospital, Red Cross Hospital, Bosch Medical Centre, Amphia Hospital, Maxima Medical Centre, Catharina Hospital, Gelderse Vallei Hospital, Sint Anna Hospital, Canisius-Wilhemina Hospital, Laurentius Hospital, Franciscus Hospital, Maasland Hospital, Rivierenland Hospital, Twee Steden Hospital, Sint Elizabeth Hospital, Isala Clinics. Part of the data has been presented at the ASN Renal Week 2010.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at www.cjasn.org.
References
- 1. Cattran DC. Membranous nephropathy. In: Primer on Kidney Diseases, 5th ed., edited by Greenberg A, Cheung AK, Coffman TM, Falk RJ, Jennette JC, Philadelphia, Saunders Elsevier, 2009, pp 170–178 [Google Scholar]
- 2. Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC: Idiopathic membranous nephropathy: Definition and relevance of a partial remission. Kidney Int 66: 1199–1205, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Polanco N, Gutierrez E, Covarsi A, Ariza F, Carreno A, Vigil A, Baltar J, Fernández-Fresnedo G, Martín C, Pons S, Lorenzo D, Bernis C, Arrizabalaga P, Fernández-Juárez G, Barrio V, Sierra M, Castellanos I, Espinosa M, Rivera F, Oliet A, Fernández-Vega F, Praga M: Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Española de Nefrología.: Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol 21: 697–704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cattran DC, Pei Y, Greenwood CM, Ponticelli C, Passerini P, Honkanen E: Validation of a predictive model of idiopathic membranous nephropathy: Its clinical and research implications. Kidney Int 51: 901–907, 1997 [DOI] [PubMed] [Google Scholar]
- 5. du Buf-Vereijken PW, Branten AJ, Wetzels JF: Idiopathic membranous nephropathy: Outline and rationale of a treatment strategy. Am J Kidney Dis 46: 1012–1029, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Ponticelli C, Zucchelli P, Passerini P, Cesana B, Locatelli F, Pasquali S, Sasdelli M, Redaelli B, Grassi C, Pozzi C, Bizarri D, Banfi G: A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int 48: 1600–1604, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Jha V, Ganguli A, Saha TK, Kohli HS, Sud K, Gupta KL, Joshi K, Sakhuja V: A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol 18: 1899–1904, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Hofstra JM, Wetzels JF: Alkylating agents in membranous nephropathy: Efficacy proven beyond doubt. Nephrol Dial Transplant 25: 1760–1766, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Reichert LJ, Koene RA, Wetzels JF: Prognostic factors in idiopathic membranous nephropathy. Am J Kidney Dis 31: 1–11, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Pei Y, Cattran D, Greenwood C: Predicting chronic renal insufficiency in idiopathic membranous glomerulonephritis. Kidney Int 42: 960–966, 1992 [DOI] [PubMed] [Google Scholar]
- 11. Ponticelli C, Passerini P, Altieri P, Locatelli F, Pappalettera M: Remissions and relapses in idiopathic membranous nephropathy. Nephrol Dial Transplant 7 Suppl 1: 85–90, 1992 [PubMed] [Google Scholar]
- 12. Ponticelli C, Passerini P: Can prognostic factors assist therapeutic decisions in idiopathic membranous nephropathy? J Nephrol 23: 156–163, 2010 [PubMed] [Google Scholar]
- 13. Honkanen E, Teppo AM, Meri S, Lehto T, Gronhagen-Riska C: Urinary excretion of cytokines and complement SC5b-9 in idiopathic membranous glomerulonephritis. Nephrol Dial Transplant 9: 1553–1559, 1994 [PubMed] [Google Scholar]
- 14. Kon SP, Coupes B, Short CD, Solomon LR, Raftery MJ, Mallick NP, Brenchley PE: Urinary C5b-9 excretion and clinical course in idiopathic human membranous nephropathy. Kidney Int 48: 1953–1958, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Reichert LJ, Koene RA, Wetzels JF: Urinary excretion of beta 2-microglobulin predicts renal outcome in patients with idiopathic membranous nephropathy. J Am Soc Nephrol 6: 1666–1669, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Honkanen E, Teppo AM, Tornroth T, Groop PH, Gronhagen-Riska C: Urinary transforming growth factor-beta 1 in membranous glomerulonephritis. Nephrol Dial Transplant 12: 2562–2568, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Reichert LJ, Koene RA, Wetzels JF: Urinary IgG excretion as a prognostic factor in idiopathic membranous nephropathy. Clin Nephrol 48: 79–84, 1997 [PubMed] [Google Scholar]
- 18. Bazzi C, Petrini C, Rizza V, Arrigo G, Napodano P, Paparella M, Di Amico G: Urinary N-acetyl-beta-glucosaminidase excretion is a marker of tubular cell dysfunction and a predictor of outcome in primary glomerulonephritis. Nephrol Dial Transplant 17: 1890–1896, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Hofstra JM, Deegens JK, Willems HL, Wetzels JF: Beta-2-microglobulin is superior to N-acetyl-beta-glucosaminidase in predicting prognosis in idiopathic membranous nephropathy. Nephrol Dial Transplant 23: 2546–2551, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Branten AJ, du Buf-Vereijken PW, Klasen IS, Bosch FH, Feith GW, Hollander DA, Wetzels JF: Urinary excretion of beta2-microglobulin and IgG predict prognosis in idiopathic membranous nephropathy: A validation study. J Am Soc Nephrol 16: 169–174, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Hofstra JM, Branten AJ, Wirtz JJ, Noordzij TC, du Buf-Vereijken PW, Wetzels JF: Early versus late start of immunosuppressive therapy in idiopathic membranous nephropathy: A randomized controlled trial. Nephrol Dial Transplant 25: 129–136, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Jacobs EM, Vervoort G, Branten AJ, Klasen I, Smits P, Wetzels JF: Atrial natriuretic peptide increases albuminuria in type I diabetic patients: Evidence for blockade of tubular protein reabsorption. Eur J Clin Invest 29: 109–115, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Hofstra JM, Deegens JK, Steenbergen EJ, Wetzels JF: Urinary excretion of fatty acid-binding proteins in idiopathic membranous nephropathy. Nephrol Dial Transplant 23: 3160–3165, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Cattran DC: Membranous nephropathy: Quo vadis? Kidney Int 61: 349–350, 2002 [DOI] [PubMed] [Google Scholar]