Abstract
Summary
Background and objectives
IgG commonly co-exists with IgA in the glomerular mesangium of patients with IgA nephropathy (IgAN) with unclear clinical relevance. Autoantibody (autoAb) biomarkers to detect and track progression of IgAN are an unmet clinical need. The objective of the study was to identify IgA-specific autoAbs specific to IgAN.
Design, setting, participants, & measurements
High-density protein microarrays were evaluated IgG autoAbs in the serum of IgAN patients (n = 22) and controls (n = 10). Clinical parameters, including annual GFR and urine protein measurements, were collected on all patients over 5 years. Bioinformatic data analysis was performed to select targets for further validation by immunohistochemistry (IHC).
Results
One hundred seventeen (1.4%) specific antibodies were increased in IgAN. Among the most significant were the autoAb to the Ig family of proteins. IgAN-specific autoAbs (approximately 50%) were mounted against proteins predominantly expressed in glomeruli and tubules, and selected candidates were verified by IHC. Receiver operating characteristic analysis of our study demonstrated that IgG autoAb levels (matriline 2, ubiquitin-conjugating enzyme E2W, DEAD box protein, and protein kinase D1) might be used in combination with 24-hour proteinuria to improve prediction of the progression of IgAN (area under the curve = 0.86, P = 0.02).
Conclusions
IgAN is associated with elevated IgG autoAbs to multiple proteins in the kidney. This first analysis of the repertoire of autoAbs in IgAN identifies novel, immunogenic protein targets that are highly expressed in the kidney glomerulus and tubules that may bear relevance in the pathogenesis and progression of IgAN.
Introduction
IgA nephropathy (IgAN) is diagnosed by evidence of mesangial IgA deposits along with proliferation of mesangial cells on renal biopsy. Although named for the deposition of IgA in the kidney, other types of immunoglobulins may also be involved (1). In fact, Berger described IgAN as “Les depots intercapillaires d'IgA-IgG” (2). IgG and IgM deposits accompany IgA in most cases, with IgA deposits alone seen in approximately 15% of biopsies (3). Suzuki et al. have highlighted the potential importance of IgG in IgAN when they found that specific IgG antibodies recognize aberrantly glycosylated IgA, and these antibody levels correlated with disease activity in terms of proteinuria (1). The specificities of other IgG antibodies are currently unknown in this disease.
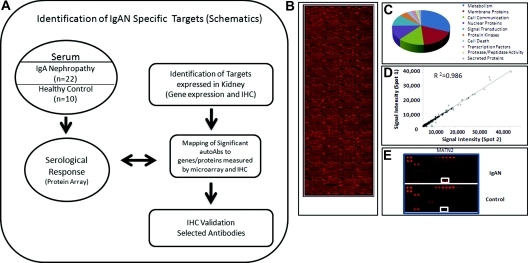
High-density protein microarrays have been successfully used to identify surrogate biomarkers for kidney and other diseases (4–6). For this report, we used protein arrays to characterize the profile of IgG autoantibodies (autoAbs) in patients with IgAN. We used an integrative genomics approach to map the significant antibodies to protein targets. The overall approach is summarized in Figure 1.
Figure 1.
(A) Study flow diagram used to identify IgA nephropathy (IgAN)-specific autoantibodies (autoAbs) by immune response biomarker profiling, bioinformatics to map targets of significant autoAbs with genes and proteins expressed in kidney by microarray and immunohistochemistry (IHC), and IHC validation. (B) A representative protein array from an IgAN patient in this study, probing approximately 8200 proteins. (C) The biologic functional classes of the proteins on the protoarray probed. (D) Quality control results from duplicate spots printed on the protoarray demonstrating very stringent correlation (R2 = 0.986). (E) A representative close-up of the protoarray showing visible Alexa fluorophore signal intensity differences in IgAN and healthy controls.
Materials and Methods
Patients
Thirty-two subjects participated in this study, including 22 patients with biopsy-confirmed IgAN and 10 age- and gender-matched healthy controls (HCs). Subjects were divided into two groups on the basis of their rate of decline of measured GFR over the 5-year follow-up. Patients were labeled as progressors (IgANp; n = 7) if their rate of measured GFR decline was ≥5 ml/min per 1.73 m2 per year. IgAN patients with a ΔGFR of <5 ml/min per 1.73 m2 per year were labeled nonprogressors (IgANnp). The demographics of all 22 IgAN patients are provided in Tables 1 and 2. The IgAN patients underwent annual clearance studies over 5 years, with the exception of those who had progressed to end-stage renal failure. GFR was examined using the urinary clearance of inulin, as described previously (7). Serum and urine samples were collected annually over 4 to 5 years. Seventeen patients with non-IgAN glomerular disease (nine focal segmental glomerulosclerosis [FSGS] and eight membranous nephropathy) were chosen for a comparison with IgAN autoAb profiling. Demographics of patients with non-IgA glomerular diseases include age (49 ± 13 years), gender (male = 10, female = 7), and serum creatinine (1.6 ± 0.83 mg/dl). Two patients with membranous glomerulonephritis were on immunosuppressant agents (one on prednisone and cyclosporine, one on cyclosporine and mycophenolate), and one patient with FSGS was on prednisone. AutoAbs elevated in non-IgAN glomerular disease were based on the comparison with autoAb levels from 12 separate HCs.
Table 1.
Patient demographics: IgAN versus healthy controls
| Demographic | IgANa | Healthy Controlsa | Pb |
|---|---|---|---|
| Age (years) | 38.0 ± 10.2 | 29.9 ± 10.4 | 0.05 |
| Gender (male/female) | 12/10 | 5/5 | 0.82 |
| Height (cm) | 170.0 ± 11.3 | 169.4 ± 10.2 | 0.85 |
| Mean systolic BP (mmHg) | 131.5 ± 9.8 | 117.1 ± 1 | 0.03 |
| Mean diastolic BP (mmHg) | 79.0 ± 13.2 | 73.6 ± 5.9 | 0.16 |
| Serum creatinine (mg/dl) | 1.0 ± 0.31 | 0.94 ± 0.18 | 0.02 |
| GFR (ml/min per 1.73 m2) | 72.0 ± 21.7 | 108.2 ± 12.6 | <0.01 |
| ΔGFR (ml/min per 1.73 m2 per year) | −5.0 ± 12.0 | NA | NA |
| Proteinuria (g/day) | 2.0 ± 2.05 | NA | NA |
IgAN, IgA nephropathy; NA, not available.
Mean values are presented as mean ± SD. Other values are presented as the number of patients.
P values are calculated to evaluate whether there is any significant difference between the two groups.
Table 2.
Patient demographics: Progressors versus nonprogressors
| Demographic | Progressora | Nonprogressora | Pb |
|---|---|---|---|
| Age (years) | 39.7 ± 7.3 | 37.6 ± 11.5 | 0.67 |
| Gender (male/female) | 5/2 | 7/8 | 0.30 |
| Height (cm) | 176.5 ± 8.9 | 168 ± 12.2 | 0.26 |
| Mean systolic BP (mmHg) | 131.5 ± 9.8 | 130.3 ± 12.4 | 0.82 |
| Mean diastolic BP (mmHg) | 82.2 ± 9.1 | 77.9 ± 14.8 | 0.49 |
| Serum creatinine (mg/dl) | 1.38 ± 0.22 | 1.05 ± 0.28 | 0.01 |
| GFR (ml/min per 1.73 m2) | 57 ± 13.7 | 79 ± 21.4 | 0.02 |
| ΔGFR (ml/min per 1.73 m2 per year) | −16.4 ± 15.3 | 0.19 ± 4.7 | <0.01 |
| Proteinuria (g/day) | 3.87 ± 2.63 | 1.28 ± 0.93 | 0.04 |
Mean values are presented as mean ± SD. Other values are presented as the number of patients.
P values are calculated to evaluate whether there is any significant difference between the two groups.
Measurement of IgG and IgA Levels in the Serum
To control for differences in levels of immunoglobulins in IgAN patients, human IgG ELISA (catalog no. E-80G) and human IgA ELISA (catalog no. E-80A) kits (Immunology Consultants Laboratory, Inc., Newberg, OR) were used to measure total IgG and IgA in the sera. After 1:80,000 dilution, ELISA analysis was done following standard protocols (8). Protein concentration was determined from the generated standard curve.
Immune Response Profiling Using Protein Microarrays
ProtoArray Human Protein Microarray version 4.0 (Invitrogen, Carlsbad, CA) was used to characterize the IgG-specific autoAb responses in IgAN. The arrays contain approximately 8000 recombinant human proteins (Figure 1B). The established protocol (http://www.invitrogen.com) (5) was followed for autoAb data acquisition. The slides were scanned using an Axon GenePix 4000B scanner (Molecular Devices, Sunnyvale, CA). Raw signal intensity data were acquired using GenePix pro 6.0 software (Molecular Devices), and initial data acquisition was done using ProtoArray Prospector 5.2 (Invitrogen). The relative fluorescence intensity was measured using GenePix pro 6.0 (Molecular Devices, Sunnyvale, CA), and initial data processing was done using ProtoArray Prospector 5.2 following the manufacturer's manual. The detailed method is described in previous publications (5,9). Statistical significance was based on the antibody titer in IgAN patients versus controls. The analysis was done on corresponding antibodies for all antigens printed on the arrays. We used the software in ProtoArray Prospector, which uses the M statistic, similar to a Fisher exact test, and uses a cutoff of the mean group signal antibody titer intensity. Differentially increased antibody signal in IgAN was analyzed after linear model normalization using the robust linear model (10). The minimal signal threshold was set at 500 relative fluorescence intensity units (5,9). Pearson correlation coefficients between selected antibodies and the rate of renal function decline (ΔGFR, ml/min per 1.73 m2 per year) were calculated with the use of base-2 logarithms. Multinomial logistic regression analysis was used to build a model for antibody targets individually and as a combination model of antibody targets and 24-hour proteinuria as a means to predict disease progression in IgAN. The analysis was done with SAS software (version 9.2, enterprise guide 4.2) and IPA (http://www.ingenuity.com).
Immunohistochemistry
Tissue-specific expression by immunohistochemical staining was performed on formalin-fixed paraffin-embedded kidney tissue to evaluate the presence of protein kinase D1 (PRKD1), ubiquitin-conjugating enzyme E2W (UBE2W), and immunoglobulin kappa constant (IGKC) using corresponding antibodies. Immunohistochemistry (IHC) of these antigens was tested on a new set of five IgAN kidney biopsy samples and five new normal kidney control samples obtained from the normal kidney region obtained from renal tumor nephrectomy samples. Protein-G-purified rabbit anti-human PRKD1 polyclonal antibody (Lifespan Biosciences, Seattle, WA; catalog no. LS-C98928), protein-G-purified mouse anti-human UBE2W monoclonal antibody, and goat anti-IGKC polyclonal antibody (Abcam Inc., Cambridge, MA; catalog no. ab55034) were used for this purpose.
Results
IgG and IgA Levels in IgAN Patients
Commercially available ELISA was used to measure total IgA level in the sera. As expected, the IgA levels were found to be significantly higher in IgAN patients (6.8 ± 3.8 mg/ml in IgAN versus 2.9 ± 1.5 mg/ml in HCs; P < 0.004). Despite greater variability in the level of IgG in subjects with IgAN, there was no statistically significant difference in IgG levels (8.8 ± 5.1 mg/ml in IgAN versus 9.4 ± 4.1 mg/ml in HCs; P > 0.75). There was also no significant difference between the IgG levels of IgANp and IgANnp (P > 0.25).
Immune Response Repertoire in IgAN
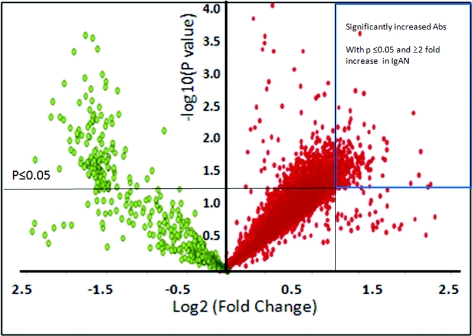
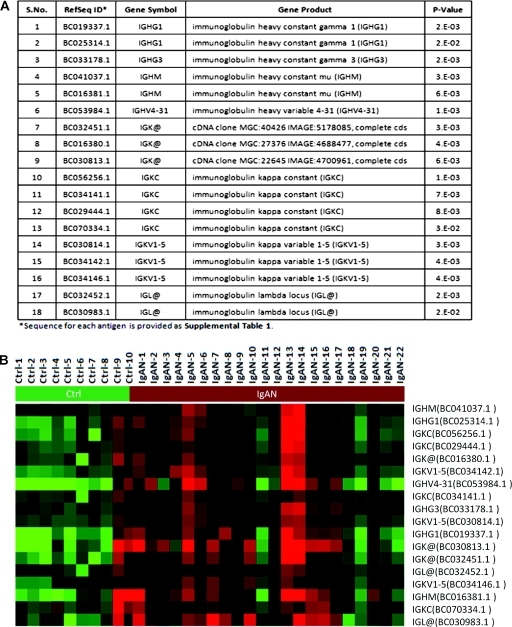
On the basis of the fluorescence intensity measured for the bound secondary antibody for each autoAb, a set of 117 autoAbs were increased in IgAN sera (P ≤ 0.05) and 28 were highly significant (P < 0.01) (Figure 2). Eighteen of these (15.4%) were related to different Ig classes (Figure 3). Cluster analysis of the samples shows clear separation of IgAN and HCs on the basis of the detection of these different Ig reactivities in IgAN patients. Of the 117 autoAbs in IgAN, 99 were mounted against non-Ig protein targets (Table 3).
Figure 2.
Increased immune response in IgA nephropathy (IgAN). The volcano plot demonstrates immune response in terms of autoantibodies (autoAbs) in IgAN when compared with normal controls. Each red spot on the plot represents an increased autoAb compared with the control, and each green spot represents a decreased autoAb against control. One hundred seventeen autoAbs with significant increase (with P ≤ 0.05 and ≥2-fold increase in IgAN) are boxed (upper right corner).
Figure 3.
An increased reactivity against several immunoglobulins was observed among IgA nephropathy (IgAN) patients. (A) The list of immunoglobulins significantly increased in the sera of IgAN patients. (B) A heat map that demonstrates separation of healthy controls from IgAN patients purely on the basis of the intensity of the different Ig responses in IgAN.
Table 3.
List of the most significant and biologically relevant proteins with increased IgG antibodies in IgAN
| Sample Number | Gene Symbol | Gene Name | Present in Glomerulusa | Present in Tubulesa | Highly Kidney Specific from Gene Expression Datab | P |
|---|---|---|---|---|---|---|
| 1 | PRKD1 | Serine/threonine-protein kinase D1 | X | X | X | 0.03 |
| 2 | MATN2 | Matrilin 2 | X | X | <0.01 | |
| 3 | DDX17 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 17 2 | X | X | <0.01 | |
| 4 | UBE2W | Ubiquitin-conjugating enzyme E2W | NA | NA | X | 0.05 |
| 5 | CDKN1B | Cyclin-dependent kinase inhibitor 1B | X | X | 0.02 | |
| 6 | SOD2 | Superoxide dismutase 2, mitochondrial | X | X | 0.05 | |
| 7 | IQCK | IQ motif containing K | X | X | X | 0.05 |
| 8 | BLZF1 | Basic leucine zipper nuclear factor 1 | X | X | 0.02 | |
| 9 | EFNA3 | Ephrin A3 | X | X | 0.01 | |
| 10 | EIF4A2 | Eukaryotic translation initiation factor 4A | X | X | 0.05 | |
| 11 | FLII | Flightless I homolog | X | X | 0.05 | |
| 12 | LIMCH1 | LIM and calponin homology domains 1 | X | X | 0.05 | |
| 13 | MAGEA4 | Melanoma antigen family A, 4 | X | X | 0.05 | |
| 14 | MEF2D | Myocyte enhancer factor 2D | X | X | 0.03 | |
| 15 | MLLT6 | Myeloid/lymphoid or mixed-lineage leukemia (trithorax homolog, Drosophila); translocated to, 6 | X | X | 0.02 | |
| 16 | CIAPIN1 | Cytokine induced apoptosis inhibitor 1 | NA | NA | X | 0.05 |
| 17 | GDI2 | GDP dissociation inhibitor 2 | NA | NA | X | 0.02 |
| 18 | HSPA8 | Heat shock 70-kD protein 8, transcript variant 1 | X | X | 0.05 | |
| 19 | SERPINA5 | Serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 5 | NA | NA | X | 0.05 |
| 20 | TGM1 | Transglutaminase 1 (K polypeptide epidermal type I, protein-glutamine-gamma-glutamyltransferase) | X | X | 0.05 |
All of the autoantibodies for the corresponding proteins were significantly increased in IgA nephropathy (IgAN) compared with healthy controls (P ≤ 0.05). X, yes. NA, not available.
No immunohistochemistry data available.
With a false discovery rate < 5%.
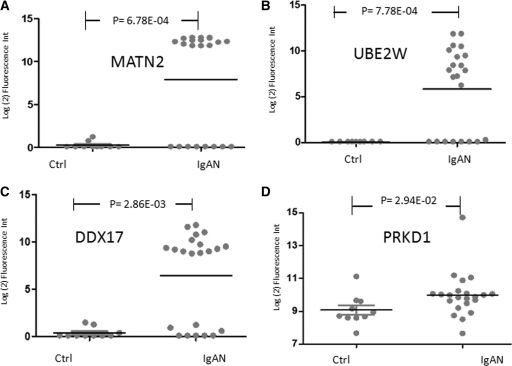
For a functional analysis of the non-Ig protein targets in IgAN, we used pathway analysis. This revealed that increased autoAb responses against cyclin-dependent kinase inhibitor 1B and superoxide dismutase 2 proteins could alter their functions, known to be involved in mesangial cell apoptosis (11,12). Furthermore, an analysis of the main functional classes by pathway analysis revealed that the most significant autoAbs were reactive against proteins involved in apoptosis (P ≤ 0.05; 21 proteins), cellular assembly and organization (P < 0.04; 20 proteins), and cellular development (P ≤ 0.05; 19 proteins). These autoAb targets are also associated with dysregulation of proteins in immunologic disease (P ≤ 0.045), connective tissue disorders (P ≤ 0.045), and dermatological conditions (P ≤ 0.045), reflecting some systems affected in IgA disease (Table 4). Other antibody targets (cAMP responsive element modulator 1, Fc fragment of IgG, low-affinity IIIa receptor for CD16, and CD79a molecule Ig-associated alpha) were reported to be dysregulated in systemic lupus erythematosus (13–15). Four of the most significantly elevated antibodies were against proteins found in or highly specific to the kidney. These were matriline2 (MATN2), UBE2W, DEAD box protein (DDX17), and PRKD1. Signal differences for these autoAbs are shown for each individual patient and HCs in Figure 4. PRKD1 antibody with IgAN remained significant after the patient with the highest signal was removed from the analysis.
Table 4.
Associated pathways and molecular functions for increased autoantibodies specific to IgA nephropathy
| Molecular Pathway/Function | Associated Antigens | P |
|---|---|---|
| Apoptosis | IGHG1, IGHG3, NEK2, TNS1, EDIL3, CDKN1B, ABCD1, KIFC3, HSPA8, RAB38, SOD2, TOM1, RASA1, AGAP1, CD7, IGHM, CKM, JAK3, SEPT5, BLZF1, and CDKN1B | ≤0.05 |
| Cellular assembly and organization | ABCD1, AGAP1, BLZF1, CD7, CDKN1B, CKM, EDIL3, HSPA8, IGHG1, IGHM, JAK3, KIFC3, NEK2, SEPT5, SOD2, TNS1, RASA1, IGHG3, TOM1, and RAB38 | ≤0.04 |
| Cellular development | CD79A, IGHG1, SART1, RASA1, EFNA3, GNAT2, MED6, CDKN1B, MEF2D, SOX5, CIAPIN1, IGHM, JAK3, NIF3L1, PRKD1, RBPJ, SOD2, TRPV4, and TGM1 | ≤0.05 |
| Immunologic disease | IGHM, IGKC, JAK3, CDKN1B, ALCAM, FCGR3A, and IGHG1 | ≤0.05 |
| Connective tissue disorders | TPRA1 | ≤0.05 |
| Dermatologic conditions | CD79A, IGHG3, IGHM, IGK@, IGL@, PLXNA1, HN1, IGHG1, IGKC, POLH, and TGM1 | ≤0.03 |
Figure 4.
Increased matriline 2 (MATN2), ubiquitin-conjugating enzyme E2W (UBE2W), DEAD box protein (DDX17), and protein kinase D1 (PRKD1) antibodies in IgA nephropathy (IgAN). Several autoAbs were identified with a significant increase in reactivities against their targets (Table 2). Scatterplots for antibodies of (A) MATN2, (B) UBE2W, (C) DDX17, and (D) PRKD1 are shown in terms of their relative fluorescence intensities in healthy controls and IgAN.
Immune Response Profiling of IgANp and IgANnp
AutoAbs against five targets, primarily protein kinases, were significantly increased in the IgANp group compared with the IgANnp group (P < 0.05). The list includes (1) the mitogen-activated protein kinase-interacting and spindle-stabilizing protein, SMITH antigen; (2) RIO kinase 3; (3) protein tyrosine phosphatase type IVA, member 1; (4) leucine-rich repeat containing 8 family, member D; and (5) and the death-associated protein kinase 3. Leucine-rich repeat containing 8 family, member D is present in glomeruli and renal tubules and is reported to be involved in cell-cycle exit in T lymphoblasts by IL-2 withdrawal (16,17). RIO kinase 3, reported to interact with caspase-10 and inhibit the nuclear factor–κB signaling pathway, is expressed in renal tubules (18).
Integrative Bioinformatics and IHC
We used publicly available tissue-specific gene expression data (19) to perform integrative genomic and antibiomic analyses to predict which, if any, corresponding proteins to the 99 autoAbs were highly expressed in the kidney (5). We next examined the kidney-specific expression of all 99 proteins by examining the publicly available IHC data from the Human Protein Atlas (http://www.proteinatlas.org) (17). There was significant enrichment for antigens expressed in the glomeruli and tubules of the kidney (n = 32; P < 1 × 106) (17), and 15 additional proteins were only expressed in the renal tubules (P < 1 × 106). A list of 20 biologically relevant antigenic targets and the information regarding their presence and location in the kidney by IHC are also provided in Table 2.
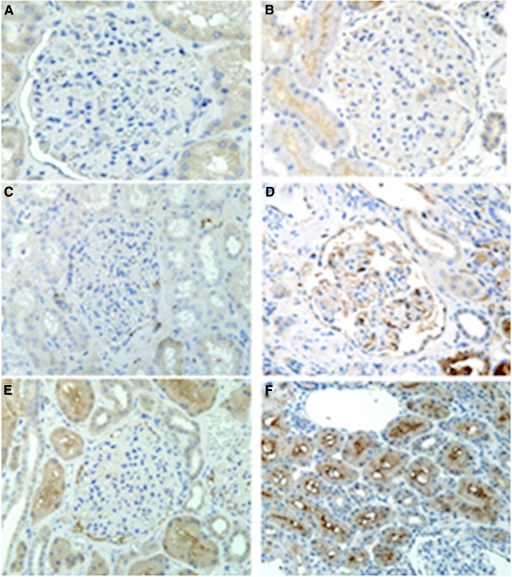
PRKD1, IGKC, and UBE2W were chosen for IHC (1) because a previously published integrative genomics study showed high expression of these proteins in the kidney and (2) on the basis of our data (5). The IHC of PRKD1 showed weak, patchy cytoplasmic staining within podocytes in a few biopsies of IgAN tissue. Mildly increased immunostaining was observed within proximal and distal tubules, especially along the apical aspects (Figure 5B); however, there was no significant staining in normal kidney tissue (Figure 5A).
Figure 5.
Immunohistochemical staining for PRKD1, immunoglobulin kappa constant (IGKC), and UBE2W in normal kidney tissue and kidney tissue with IgA nephropathy (IgAN). (A, C, E) No significant staining for PRKD1, IGKC, or UBE2W was observed in glomeruli and tubules in normal kidney. However, there was (B) a patchy staining within podocytes and increased staining within tubular cells for PRKD1, (D) increased staining for IGKC in the glomerular endothelium and proximal tubules, and (F) increased staining of the proximal tubular region for UBE2W in kidney tissue with IgAN.
In controls, no significant staining for IGKC was observed in glomeruli and tubules, and UBE2W staining revealed a moderate granular cytoplasmic pattern in proximal tubules but no staining in glomeruli (Figure 5, C and E). However, there was increased staining for IGKC in the glomerular endothelium and proximal tubules (Figure 5D) and increased staining for UBE2W in the proximal tubular cells with accentuation of the brush border in kidney tissue from IgAN patients (Figure 5F).
Prediction of Renal Function Decline
The positive predictive value (PPV) and negative predictive value of 24-hour proteinuria for the risk of ending up in the IgANp group was 42.8% and 85.7%, respectively (a cutoff of 24-hour proteinuria >1 g per day). We investigated IgG autoAbs to see if they could predict the progression of IgAN or could be used in combination with 24-hour proteinuria to improve its PPV.
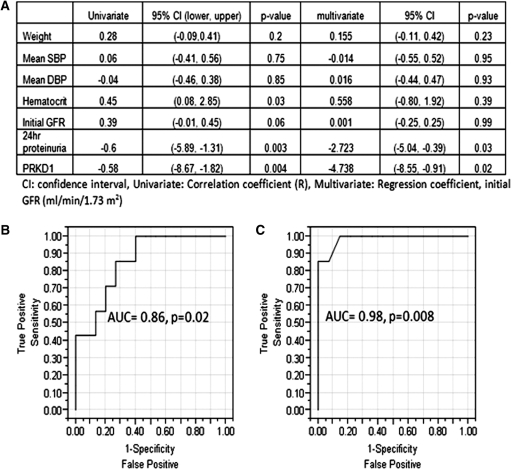
The signal intensity of IgG against PRKD1 in IgAN significantly correlated with the observed decline of kidney function during the study (Δinulin GFR; R = −0.58; P = 0.004). The results of linear regression analyses between the decline of kidney function and clinical variables are shown in Figure 6. By univariate analysis, there was no significant correlation of age at presentation, initial systolic and diastolic BP, or initial GFR with the subsequent decline in kidney function. The amount of 24-hour proteinuria at the study start correlated, as expected, with a decline in GFR (R = −0.60; P = 0.004). In multivariate analysis, after adjusting for other clinical variables including age, systolic BP, diastolic BP, hematocrit, and initial GFR, the intensity of the autoAb signal against PRKD1 correlated with a decline in kidney function better than the extent of proteinuria over 24 hours (regression coefficient: −4.73 versus −2.72). Figure 6B shows the receiver operating characteristic curve analysis when the IgG antibodies against MATN2, UBE2W, DDX17, and PRKD1 (which were selected because of the significant correlation among each other as well as significant increase in IgAN) were used in combination. The area under the curve (AUC) was 0.86 (P = 0.02) on the basis of the logistic regression model by combining the four antibodies' signal intensities when the arbitrary cutoff of −5 ml/yr of renal function decline was used to diagnose disease progression. Figure 6C shows receiver operating characteristic curve analysis when the four antibodies (MATN2, UBE2W, DDX17, and PRKD1) and 24-hour proteinuria were used in combination. The AUC was 0.98 (P = 0.0008) by performing logistic regression. The AUC of 24-hour proteinuria alone was 0.82 (P = 0.02). This finding suggests that using IgG autoAbs in addition to 24-hour proteinuria could improve the ability to predict the progression of disease.
Figure 6.
(A) Results of linear regression analysis, (B) receiver operating characteristic (ROC) analysis of four combined antibodies (MATN2, UBE2W, DDX17, PRKD1), and (C) ROC analysis of four antibodies and 24-hour proteinuria. AUC, area under the curve.
Specificity of AutoAbs to IgAN
Protoarray analysis was done in a cohort of 15 patients with other glomerular diseases (membranous and FSGS) with a similar range of GFR and urine protein as our IgAN subjects. Their autoAb profile (data not shown) was distinct with only six of the autoAbs overlapping the IgAN group as significantly increased, none of which included the autoAbs of greatest significance. This suggests that the autoAb pattern is relatively specific to IgAN, at least as compared with other proteinuric glomerular diseases.
Discussion
IgG has been identified as an important participant in the pathogenesis of IgAN. This is the first study using high-density protein protoarrays to characterize the specificity of IgG autoAb responses in patients with IgAN. In this disease, it has been demonstrated that IgG antibodies can recognize aberrant IgA and form immune complexes leading to deposition in the glomerular mesangium (20). The purpose of this study was to more fully identify specific IgG autoAbs in this disease with a view to understanding pathogenesis, to track disease by noninvasive biomarkers, and to follow disease progression. We analyzed a carefully collected and serially followed cohort of patients with biopsy-proven IgAN who had serial GFR measurements by inulin clearance on a yearly basis, such that over a 5-year period, patients could be identified as patients with or without disease progression.
We found a nonsignificant trend toward more overall IgG and a greater variability in IgG levels in IgAN patients when compared with normal controls. Mimicking the biopsy findings of different classes of Ig deposits in IgA, we found a significant and broad increase in IgG targeting multiple immunoglobulins (IgA, IgG, and IgM) in patients with IgAN. IgG targeting against total IgA was increased, but not statistically significant (P = 0.08), possibly because the assay measured the IgG against all IgA including nonaberrent IgA. This finding likely supports the prior hypotheses that increased concentrations of polymeric IgA in IgAN (21,22) activates immune complexes between IgA and IgG and could be a critical mechanism involved in immune complex formation and deposition in the mesangium in IgAN.
In addition, this study identifies a specific repertoire of autoAbs in IgAN, including antibodies to targets that may have important regulatory properties in the kidney. For example, MATN2 is expressed in various extracellular matrices that can form collagen-dependent and collagen-independent filamentous networks (23). DDX17 are putative RNA helicases involved in alteration of RNA secondary structure, leading to cellular growth and division (24). UBE2W is involved in the ubiquitination reaction that targets proteins for proteasomal degradation (25). These findings suggest that IgAN involves the development of IgG antibodies specific to the kidney and that these antibodies may play an important role in determining the manifestations and clinical course of the disease.
If these findings are further validated in other cohorts of patients, then it would suggest that the role of IgG in IgAN is not limited to forming immune complexes with IgA. It has been known that IgAN is often associated with other diseases. Examples include dermatologic diseases such as erythema nodosum, cancers such as lymphoma, infectious diseases such as HIV, and rheumatologic diseases such as systemic lupus erythematosus and inflammatory bowel disease (26–32). Our study results could help to understand common pathophysiology between IgAN and these diseases. The protein targets of the IgGs identified herein are involved in various cellular and molecular functions in cancer, cell death, cellular assembly/organization, and molecular biochemistry. The molecular networks of protein targets are associated with dermatologic diseases, infectious disease, lipid metabolism, and hair and skin development and function. There may be common pathologic pathways causing production of IgGs in these diseases that lead to forming immune complex with IgAs and developing IgAN.
On the basis of our integrative genomic analysis and an IHC database, antigen targets corresponding to approximately 50% of the increased antibodies are expressed in glomeruli and tubules of the kidney. We believe these findings support that IgG targeting of these antigens in the kidney is an important pathophysiological process in IgAN that may cause renal injury and loss of function. By IHC, separate markers were seen in glomerular endothelial cells, but we have no present explanation why the endothelium of the kidney should be disproportionately affected in this disease. A larger clinical study may be necessary to investigate these antigens and antibodies in IgAN.
We also analyzed the data to find potential biomarkers that can complement existing prognostic indicators. Traditional prognostic indicators are not able to accurately predict the progression as shown in this study by the low PPV (42%) of 24-hour proteinuria.
Analysis of our data (including MATN2, UBE2W, DDX17, and PRKD1) demonstrated that IgG autoAb levels might be useful in combination with 24-hour proteinuria to improve prediction of the progression of IgAN. Broader clinical validation will be necessary to determine if IgG autoAbs may be used as biomarkers in this clinical setting.
Potential limitations of the study include the limited size of the study, but the study needed a population that was well characterized for kidney function, structure, and outcome with adequate follow-up to classify patients into IgANp or IgANnp. Another potential drawback is the use of fusion proteins that are expressed in insect cells as antigens. The insect-expressed human proteins may not possess disease-specific characteristics associated with post-translational modification and thus might not present epitopes necessary for the identification of immune reactivity. However, the differences we see in IHC suggest that at least some of these antigens are acting differently in the kidneys of patients with IgAN. The comparisons to other glomerular diseases suggest that the response is relatively specific to IgAN. Finally, further analysis for the pathogenicity of these antibodies and validation of these autoAbs for accuracy, consistency, sensitivity, and specificity for IgAN will need to be pursued by larger, prospective studies.
In summary, this report provides new insight into the potential roles of IgGs in IgAN. These IgGs appear to be broadly involved in many molecular networks and functions not limited to forming immune complexes with IgA. The further investigation of IgG autoAbs may lead to the discovery of new biomarkers to predict the progression of IgAN and a better understanding of the pathophysiology and progression of this disease.
Disclosures
None.
Acknowledgments
The work was supported by the National Institutes of Health (Grant RO1-AI-061739; to M.S.); the Deans Fellowship Child Health Research Program (to T.S. and M.S.); and the Sobrato Research Fund (S.H.W. and R.L.). We also thank Neeraja Kambham at Stanford Pathology for IHC and its interpretation, the Stanford Functional Genomics Center at Stanford University for the Axon scanner, and Van Dinh and the Sarwal Laboratory members for their help.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J: Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119: 1668–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berger J, Hinglais N: [Intercapillary deposits of IgA-IgG]. J Urol Nephrol (Paris) 74: 694–695, 1968 [PubMed] [Google Scholar]
- 3. Ibels LS, Gyory AZ: IgA nephropathy: Analysis of the natural history, important factors in the progression of renal disease, and a review of the literature. Medicine (Baltimore) 73: 79–102, 1994 [PubMed] [Google Scholar]
- 4. Schnack C, Hengerer B, Gillardon F: Identification of novel substrates for Cdk5 and new targets for Cdk5 inhibitors using high-density protein microarrays. Proteomics 8: 1980–1986, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Li L, Wadia P, Chen R, Kambham N, Naesens M, Sigdel TK, Miklos DB, Sarwal MM, Butte AJ: Identifying compartment-specific non-HLA targets after renal transplantation by integrating transcriptome and “antibodyome” measures. Proc Natl Acad Sci U S A 106: 4148–4153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meng L, Michaud GA, Merkel JS, Zhou F, Huang J, Mattoon DR, Schweitzer B: Protein kinase substrate identification on functional protein arrays. BMC Biotechnol 8: 22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Squarer A, Lemley KV, Ambalavanan S, Kristal B, Deen WM, Sibley R, Anderson L, Myers BD: Mechanisms of progressive glomerular injury in membranous nephropathy. J Am Soc Nephrol 9: 1389–1398, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Sigdel T, Kaushal A, Gritsenko M, Norbeck A, Qian W, Xiao W, Camp D, Smith R, Sarwal M: Novel shotgun proteomics approach identifies proteins specific for acute renal transplant rejection. Am J Transplant 10: 289–290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sutherland SM, Li L, Sigdel TK, Wadia PP, Miklos DB, Butte AJ, Sarwal MM: Protein microarrays identify antibodies to protein kinase Czeta that are associated with a greater risk of allograft loss in pediatric renal transplant recipients. Kidney Int 76: 1277–1283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sboner A, Karpikov A, Chen G, Smith M, Mattoon D, Freeman-Cook L, Schweitzer B, Gerstein MB: Robust-linear-model normalization to reduce technical variability in functional protein microarrays. J Proteome Res 8: 5451–5464, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Hiromura K, Pippin JW, Fero ML, Roberts JM, Shankland SJ: Modulation of apoptosis by the cyclin-dependent kinase inhibitor p27(Kip1). J Clin Invest 103: 597–604, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moreno-Manzano V, Ishikawa Y, Lucio-Cazana J, Kitamura M: Selective involvement of superoxide anion, but not downstream compounds hydrogen peroxide and peroxynitrite, in tumor necrosis factor-alpha-induced apoptosis of rat mesangial cells. J Biol Chem 275: 12684–12691, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Juang YT, Wang Y, Solomou EE, Li Y, Mawrin C, Tenbrock K, Kyttaris VC, Tsokos GC: Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J Clin Invest 115: 996–1005, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brambila-Tapia AJ, Gamez-Nava JI, Gonzalez-Lopez L, Sandoval-Ramirez L, Medina-Diaz J, Maldonado M, Gutierrez-Urena SR, Martinez-Bonilla G, Martin-Marquez BT, Vazquez Del Mercado M, Nava-Zavala A, Munoz-Valle JF, Salazar-Paramo M, Davalos-Rodriguez IP: FCGR3A V(176) polymorphism for systemic lupus erythematosus susceptibility in Mexican population. Rheumatol Int 31: 1065–1068, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Chen F, Putt M, Koo YK, Madaio M, Cambier JC, Cohen PL, Eisenberg RA: B cell depletion with anti-CD79 mAbs ameliorates autoimmune disease in MRL/lpr mice. J Immunol 181: 2961–2972, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chechlinska M, Siwicki JK, Gos M, Oczko-Wojciechowska M, Jarzab M, Pfeifer A, Jarzab B, Steffen J: Molecular signature of cell cycle exit induced in human T lymphoblasts by IL-2 withdrawal. BMC Genomics 10: 261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berglund L, Bjorling E, Oksvold P, Fagerberg L, Asplund A, Szigyarto CA, Persson A, Ottosson J, Wernerus H, Nilsson P, Lundberg E, Sivertsson A, Navani S, Wester K, Kampf C, Hober S, Ponten F, Uhlen M: A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol Cell Proteomics 7: 2019–2027, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Shan J, Wang P, Zhou J, Wu D, Shi H, Huo K: RIOK3 interacts with caspase-10 and negatively regulates the NF-kappaB signaling pathway. Mol Cell Biochem 332: 113–120, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Wang L, Kambham N, Montgomery K, Mason V, Vogelmann SU, Lemley KV, Brown PO, Brooks JD, van de Rijn M: Gene expression in the normal adult human kidney assessed by complementary DNA microarray. Mol Biol Cell 15: 649–656, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith AC, Molyneux K, Feehally J, Barratt J: O-glycosylation of serum IgA1 antibodies against mucosal and systemic antigens in IgA nephropathy. J Am Soc Nephrol 17: 3520–3528, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Jones CL, Powell HR, Kincaid-Smith P, Roberton DM: Polymeric IgA and immune complex concentrations in IgA-related renal disease. Kidney Int 38: 323–331, 1990 [DOI] [PubMed] [Google Scholar]
- 22. Reterink TJ, Schroeijers WE, Es LA, Daha MR: Dimeric and polymeric IgA, but not monomeric IgA, enhance the production of IL-6 by human renal mesangial cells. Mediators Inflamm 5: 191–195, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piecha D, Muratoglu S, Morgelin M, Hauser N, Studer D, Kiss I, Paulsson M, Deak F: Matrilin-2, a large, oligomeric matrix protein, is expressed by a great variety of cells and forms fibrillar networks. J Biol Chem 274: 13353–13361, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Cordin O, Banroques J, Tanner NK, Linder P: The DEAD-box protein family of RNA helicases. Gene 367: 17–37, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Nandi D, Tahiliani P, Kumar A, Chandu D: The ubiquitin-proteasome system. J Biosci 31: 137–155, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Bergmann J, Buchheidt D, Waldherr R, Maywald O, van der Woude FJ, Hehlmann R, Braun C: IgA nephropathy and hodgkin's disease: A rare coincidence. Case report and literature review. Am J Kidney Dis 45: e16–e19, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Mak SK, Wong PN, Lo KY, Wong AK: Successful treatment of IgA nephropathy in association with low-grade B-cell lymphoma of the mucosa-associated lymphoid tissue type. Am J Kidney Dis 31: 713–718, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Hubert D, Beaufils M, Meyrier A: [Immunoglobulin A glomerular nephropathy associated with inflammatory colitis. Apropos of 2 cases]. Presse Med 13: 1083–1085, 1984 [PubMed] [Google Scholar]
- 29. Dux S, Grosskopf I, Rosenfeld JB: Recurrent erythema nodosum, arthritis and IgA nephropathy. Dermatologica 176: 293–295, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Weiner NJ, Goodman JW, Kimmel PL: The HIV-associated renal diseases: Current insight into pathogenesis and treatment. Kidney Int 63: 1618–1631, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Corrado A, Quarta L, Di Palma AM, Gesualdo L, Cantatore FP: IgA nephropathy in systemic lupus erythematosus. Clin Exp Rheumatol 25: 467–469, 2007 [PubMed] [Google Scholar]
- 32. Takemura T, Okada M, Yagi K, Kuwajima H, Yanagida H: An adolescent with IgA nephropathy and Crohn disease: Pathogenetic implications. Pediatr Nephrol 17: 863–866, 2002 [DOI] [PubMed] [Google Scholar]