Abstract
Summary
Background and objectives
An abnormal anion gap and an increased total protein and globulin are clues to the diagnosis of monoclonal gammopathy. We explored the utility of these markers in IgG, IgA, IgM, and free light chain monoclonal gammopathies.
Design, Setting, Participants, & Measurements
The anion gap, Na+ – (Cl– + HCO3–), corrected for hypoalbuminemia, was calculated in patients with monoclonal gammopathies. Exclusion criteria were serum calcium >10.5 mg/dl and/or creatinine >2 mg/dl.
Results
Among 287 patients, 242 remained after applying exclusion criteria (109 IgG, 64 IgA, 21 IgM, and 48 light chain); 36% of 242 patients required correction for hypoalbuminemia. The anion gap was decreased (<10) in 22% of IgG and increased (>15) in 31% of IgA monoclonal gammopathies. IgM did not affect the gap. In light chain gammopathies, the anion gap showed no consistent trend (15% increased, 17% decreased). Mean clonal IgG, IgA, and IgM concentrations were 10-fold higher than mean clonal free light chain concentrations in the respective monoclonal gammopathies (P < 0.001). These paraprotein level disparities were reflected in significantly increased mean serum total protein and globulin concentrations in IgG, IgA, and IgM versus free light chain monoclonal gammopathies, where mean total protein and globulin levels were within normal limits (P < 0.001).
Conclusions
The anion gap was significantly altered in IgG and IgA monoclonal gammopathies, but it was not a sensitive tool for suspecting the diagnosis. In light chain monoclonal gammopathies, the anion gap, total protein, and globulin did not provide reliable diagnostic clues.
Introduction
Multiple myeloma and other monoclonal gammopathies are characterized by a clonal proliferation of plasma cells producing a monoclonal Ig. The presence of a monoclonal protein is a major criterion for the diagnosis of multiple myeloma (1). Up to 20% of patients will produce only free light chains, and this subtype has a higher incidence of renal failure.
Monoclonal proteins often increase serum total protein and globulin levels, providing a clue to the diagnosis. In addition, monoclonal proteins may behave as cations or anions and alter the anion gap. A decreased anion gap has been associated with IgG monoclonal gammopathy. Perhaps less well known is the association of an increased anion gap with IgA monoclonal gammopathy. A correlation between paraprotein concentration and the anion gap has been reported (2–6). Evidence of these associations was first described in the 1970s and 1980s. However, early reports were limited by antiquated laboratory methods, relatively low patient numbers, analyses that did not separate IgG from IgA monoclonal gammopathy, and/or failure to exclude or correct for hypoalbuminemia, hypercalcemia, or renal failure (Table 1) (2–10).
Table 1.
Characteristics of previously published studies of the anion gap in monoclonal gammopathies
| Publication | Number of Controls | Patient Population | Analyzed IgG and IgA Separately | Exclusion or Correction for Hypercalcemia, Hypoalbuminemia, or Renal Failure | Normal Range Anion Gap | Percent of Gammopathies with Abnormal Anion Gap |
|---|---|---|---|---|---|---|
| Murray et al., 1975 (5) | 50 | 50 multiple myeloma | No | No | Mean 12.2a | 28% low |
| Frohlich et al., 1976 (3) | 100 | Multiple myeloma (n = 8), plasma cell leukemia (n = 1), polyclonal gammopathy (n = 1), all IgG | NA | No | 9 to 13 | NRb |
| Mikulski, 1976 (7) | 23 | 21 multiple myeloma | No | Analyzed hypoalbuminemia separately | Mean 12.3a | NR |
| De Troyer et al., 1977 (2) | 150 | 50 multiple myeloma (29 IgG, 18 IgA, 3 other) | Yes | No | 8 to 20c | 72% IgG low; all IgA normal |
| Schnur et al., 1977 (8) | 40 | 50 asymptomatic plasma cell dyscrasias (38 IgG, 11 IgA, 1 IgM) | No | Excluded albumin <2.5 g/dl, creatinine >2 mg/dl, calcium >11 mg/dl | 6 to 15 | 30% low |
| Paladini et al., 1979 (6) | 30 | 26 multiple myeloma (15 IgG, 11 IgA) | Yes | Excluded albumin <3 g/dl; no patient had renal failure or calcium >5 mEq/L | 10 to 14c | 100% low in IgG; NR in IgA but often increased |
| Paladini et al., 1981 (9) | 24 | 12 multiple myeloma (7 IgG, 5 IgA) | Yes | Excluded albumin <3 g/dl, renal impairment, calcium >5 mEq/L | ∼10 to 14c | 100% low in IgG; 60% high in IgA |
| Flanagan et al., 1988 (10) | 100 | 78 multiple myeloma (48 IgG, 30 IgA), 71 MGUS | Yes | No | Mean 9.4a | 63% IgG low; 34% of IgA low |
| Mansoor et al., 2007 (4) | 104 | 82 multiple myeloma (63 IgG, 13 IgA, 6 other) | Yes | No | ∼9 to 13 | 62% of IgG low, all IgA normal |
MGUS, monoclonal gammopathy of undetermined significance; NA, not applicable; NR, not reported.
Range not reported.
Only reported patients with low anion gap, therefore, by definition, 100%; all had IgG > 5 g/dl.
Anion gap was originally calculated as (Na+ + K+) − (Cl− − HCO3−); the range reported in the table represents the original range minus four.
Detection and quantification of monoclonal proteins has improved in recent decades. Agarose gels replaced cellulose acetate for routine serum protein electrophoresis, resulting in higher resolution and reproducibility (11). In 2001, assays became available, allowing quantification of serum free kappa and free lambda light chain levels and providing an over 50-fold increase in sensitivity over serum protein electrophoresis (12).
The anion gap in light chain monoclonal gammopathies has not been examined. Increased clonal serum free light chains levels are present in virtually all patients with light chain myeloma as well as most patients with IgG or IgA multiple myeloma and IgM-related disease (13,14). However, there is little correlation between serum concentrations of clonal intact Ig and free light chains (13). For example, some patients with IgG kappa myeloma will cosecrete large quantities of free kappa and present with light chain nephrotoxicity, whereas others will produce few light chains and have no renal impairment. The effect of circulating clonal free light chains on the anion gap in IgG and IgA monoclonal gammopathy is unknown.
The objectives of this study were to evaluate the anion gap in IgG, IgA, IgM, and light chain monoclonal gammopathies, and to explore the effect of clonal free light chains on the anion gap in IgG, IgA, and IgM monoclonal gammopathies. In addition, serum total protein and globulin concentrations were evaluated as a diagnostic clue to monoclonal gammopathy.
Materials and Methods
In this retrospective study, electronic laboratory results were obtained from two reference laboratories (Quest Diagnostics, Chantilly, Virginia, and Teterboro, New Jersey, and Laboratory Corporation of America, Burlington, North Carolina, and Raritan, New Jersey), representing records of outpatients referred to Hackensack University Hospital for evaluation of monoclonal gammopathy from 2003 through 2007. Because IgG gammopathies are most common, the study period for IgG monoclonal gammopathies was narrowed to between November 1, 2006 and December 31, 2007. At both reference laboratories, sodium and chloride were performed using the ion selective electrode method, and bicarbonate was performed using enzymatic spectrophotometry. At Quest Diagnostics, these analytes were measured on an Olympus 5400®. At LabCorp, these analytes were measured on a Roche Hitachi. Free kappa and free lambda assays were performed at both laboratories using a nephelometric immunoassay (Freelite®; The Binding Site Limited, Birmingham, United Kingdom) on a Siemens BN™II nephelometer. This protocol was approved by the Hackensack Hospital Institutional Review Board.
Electronic records within the study period were searched for patients with (a) first available laboratory results, (b) results obtained during routine follow-up of a stable monoclonal protein (applicable to patients with monoclonal gammopathy of undetermined significance [MGUS]), or (c) with results at relapse immediately pretreatment. Patients fulfilling the inclusion criteria were included if results of all of the following serum tests were available from a single blood draw: Na+, Cl–, HCO3–, free kappa, free lambda, albumin, globulin, total protein, creatinine, calcium, protein electrophoresis, immunofixation, IgG, IgA, and IgM. Patients with IgG, IgA, and IgM gammopathies needed to have a measurable monoclonal protein m-spike on serum protein electrophoresis (≥1 g/dl) and serum immunofixation positive for the corresponding heavy chain. Patients with light chain gammopathies were required to have measurable disease on 24-hour urine protein electrophoresis (monoclonal protein ≥200 mg) and serum or urine immunofixation positive for a clonal free light chain only (kappa or lambda), or with only a trivial heavy chain component (<1 g/dl on serum protein electrophoresis) (1).
Controls represented all patients identified in the database who had no evidence of monoclonal gammopathy by serum protein electrophoresis and serum immunofixation, no measurable disease by urine protein electrophoresis or serum free light chain analysis (free kappa <10 mg/dl and free lambda <10 mg/dl), and normal serum concentrations of IgG, IgA, IgM, creatinine, calcium, total protein, globulin, and albumin.
To establish a normal range, the anion gap (Na+– [Cl– + HCO3–]), expressed in mmol/L, was calculated in controls. Then the anion gap was calculated in patients with monoclonal gammopathies. Following raw data analysis, patients were excluded if serum calcium was >10.5 mg/dl and/or serum creatinine was >2 mg/dl. Then, the anion gap was corrected for hypoalbuminemia <3.6 g/dl using the formula (anion gap [adjusted] = anion gap + 2.3 [4 – albumin]) (15).
Statistical analyses were performed using MicroSoft EXCEL® and MedCalc® V11.1.1 software. Chi-squared tests were performed to assess significant associations. Correlation was estimated using Pearson's coefficient of correlation (r), and t tests were used to compare means. P values less than 0.05 were considered statistically significant.
Results
Normal Anion Gap
The mean age of the 40 controls was 56 years (range 33 to 75), and 38% were male. The anion gap ranged between 9 and 15. Ninety-seven percent of controls had an anion gap between 10 and 15; therefore, this was used as the normal range in the analysis. The mean (SD) anion gap at the two reference laboratories was similar (12.4 [1.5] and 12.7 [1.7], P = 0.56).
Anion Gap in Monoclonal Gammopathies
Data from 287 patients were collected. In the raw analysis, 30% of patients with IgG monoclonal gammopathies had a decreased anion gap, and 23% of patients with IgA monoclonal gammopathies had an increased anion gap. In IgM, 18% had a decreased anion gap. In free light chain gammopathies, 21% had an increased anion gap (Table 2).
Table 2.
Anion gap in controls and in IgG, IgA, IgM, and light chain monoclonal gammopathies in the raw analysis, after exclusions, and after correction for hypoalbuminemia
| n | Mean (SD) | Anion Gap <10 (n [%]) | Anion Gap >15 (n [%]) | Pa | |
|---|---|---|---|---|---|
| Normal | 40 | 12.5 (1.6) | 1 (2.5) | 0 | NA |
| IgG | |||||
| raw analysis | 122 | 10.6 (2.6) | 37 (30) | 4 (3) | <0.001 |
| after exclusions | 109 | 10.4 (2.6) | 33 (30) | 3 (3) | <0.001 |
| corrected | 109 | 11.3 (2.5) | 24 (22) | 8 (7) | 0.02 |
| IgA | |||||
| raw analysis | 71 | 13.3 (3.2) | 7 (10) | 16 (23) | 0.03 |
| after exclusions | 64 | 13.2 (3.3) | 7 (11) | 16 (25) | 0.03 |
| corrected | 64 | 14.0 (3.3) | 6 (9) | 20 (31) | 0.002 |
| IgM | |||||
| raw analysis | 22 | 11.5 (2.3) | 4 (18) | 1 (5) | 0.40 |
| after exclusions | 21 | 11.5 (2.3) | 4 (19) | 1 (5) | 0.40 |
| corrected | 21 | 11.7 (2.0) | 3 (14) | 1 (5) | 0.65 |
| Light chain | |||||
| raw analysis | 72 | 13.0 (3.0) | 8 (11) | 15 (21) | 0.07 |
| after exclusions | 48 | 12.5 (3.1) | 8 (17) | 8 (17) | 0.81 |
| corrected | 48 | 13.2 (2.6) | 7 (15) | 8 (17) | 0.62 |
Chi-squared test for trend.
Forty-five patients were excluded for serum calcium >10.5 mg/dl and/or creatinine >2 mg/dl. In IgG monoclonal gammopathy, 13 were excluded, 10 for increased creatinine and three for hypercalcemia. In IgA monoclonal gammopathy, seven were excluded: four for hypercalcemia and three for increased creatinine. In light chain processes, 24 were excluded: 22 for increased creatinine and two for hypercalcemia. In IgM, one patient was excluded for increased creatinine. After these exclusions, a trend remained toward a decreased anion gap in IgG and toward an increased anion gap in IgA, but there was no trend in either direction for IgM or light chain monoclonal gammopathies.
Hypoalbuminemia <3.6 g/dl was observed in 41% (45 of 109) of IgG, 45% (29 of 64) of IgA, 14% (3 of 21) of IgM, and 21% (10 of 48) of light chain monoclonal gammopathies. After correction for hypoalbuminemia, fewer IgG monoclonal gammopathies (22%) and more IgA monoclonal gammopathies (31%) had an abnormal anion gap. No association in either direction was identified for IgM or light chain monoclonal gammopathies.
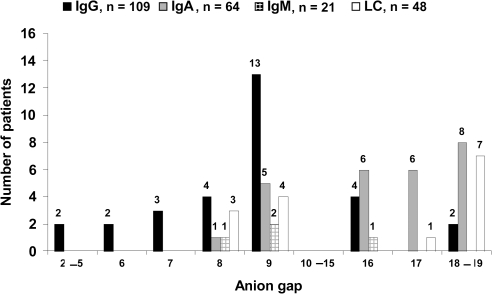
Distributions of the abnormal anion gap by monoclonal protein subtype are shown in Figure 1. No patient had a negative anion gap. The light chain subtype (kappa or lambda) had no significant effect on the anion gap in IgG, IgA, or light chain monoclonal gammopathies (Table 3). The low number of IgM gammopathies precluded meaningful statistical analysis.
Figure 1.
Distribution of abnormal anion gap in IgG, IgA, IgM, and light chain (LC) monoclonal gammopathies.
Table 3.
Effect of light chain subtype on the anion gap in IgG, IgA, and light chain monoclonal gammopathies
| Monoclonal Protein Subtype | Anion Gap <10 | Anion gap >15 | P |
|---|---|---|---|
| IgGK | 15 of 59 (25%) | Not applicable | 0.48 |
| IgGL | 9 of 50 (18%) | Not applicable | |
| IgAK | Not applicable | 10 of 38 (26%) | 0.92 |
| IgAL | Not applicable | 8 of 26 (31%) | |
| Free kappa | 3 of 24 (13%) | 5 of 24 (21%) | 0.70 |
| Free lambda | 4 of 24 (17%) | 3 of 24 (13%) |
Correlation of Anion Gap with Monoclonal Protein Concentration
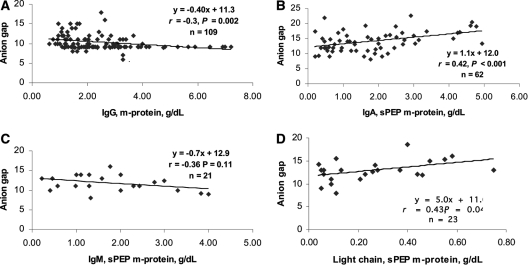
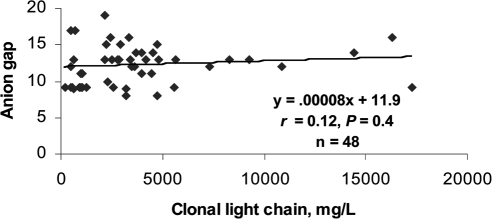
Modest to weak correlations were found between the anion gap and the monoclonal protein concentration on serum protein electrophoresis (Figure 2). Because only 23 of 48 light chain monoclonal gammopathies had measurable m-spikes on serum protein electrophoresis, an analysis of the correlation between the anion gap and the clonal free light chain concentrations was performed, but again, the correlation was modest (Figure 3). Although trend lines were sloped in the expected direction and some achieved statistically significance, the strongest correlation coefficient was only 0.43.
Figure 2.
Correlation of anion gap with monoclonal protein concentration by serum protein electrophoresis (sPEP) in IgG (A), IgA (B), IgM (C), and light chain (D) monoclonal gammopathies.
Figure 3.
Correlation of anion gap with clonal free light chain concentration.
Effect of Light Chains on the Anion Gap
The median clonal IgG, IgA, or IgM concentration was more than 100-fold higher than the concomitant median clonal free light chain concentration in IgG, IgA, and IgM monoclonal gammopathies (Table 4). In IgA, 55% (35 of 64) of patients had measurable circulating clonal free light chains (≥10 mg/dl). An increased anion gap (> 15) was observed in 31% (11 of 35) with, and 31% (9 of 29) without, measurable free light chains (P = 0.81). The mean clonal free light chain concentration was 52.9 mg/dl in patients with an increased gap versus 47.5 mg/dl in patients without an increased anion gap (P = 0.98). In IgG, 52 (48%) of 109 patients had measurable clonal free light chains (≥10 mg/dl). A decreased anion gap (< 10) was observed in 27% (14 of 52) of patients with, and 18% (10 of 57) of patients without measurable free light chains (P = 0.34). The mean clonal free light chain concentration was 27.4 mg/dl without a decreased anion gap and 41.3 mg/dl with a decreased anion gap (P = 0.92). Thus, coproduced clonal free light chains had no significant effect on the anion gap in IgA or IgG gammopathies. The number of patients with IgM gammopathies was too low for meaningful analysis.
Table 4.
Demographics and serum test results in IgG, IgA, IgM, and light chain monoclonal gammopathies
| IgG | IgA | IgM | Free Kappa | Free Lambda | |
|---|---|---|---|---|---|
| n | 109 | 64 | 21 | 24 | 24 |
| Number male (%) | 54 (50) | 35 (55) | 11 (65) | 11 (46) | 12 (50) |
| Median age (yr [range]) | 63 (36 to 89) | 64 (36 to 86) | 71 (36 to 89) | 67 (37 to 85) | 66 (36 to 86) |
| Median m-protein, sPEP (g/dl [range]) | 2.2 (0.62 to 7.2) | 1.7 (0.2 to 4.9) | 2.0 (0.22 to 4.00) | 0.25 (0.06 to 0.58); n = 12a | 0.11 (0.04 to 0.75); n = 11a |
| Median, mean clonal intact Ig (mg/dl [range]) | 3210, 3865 (961 to 10,107) | 2928, 3097 (507 to 10,044) | 2960, 3449 (743 to 7640) | Not clonal | Not clonal |
| Median, mean clonal free kappa light chain (mg/dl [range]) | 10.8, 22.0 (0.65 to 131.0); n = 59 | 7.0, 32.8 (0.8 to 214.0); n = 38 | 4.4, 25.7 (0.9 to 295.0); n = 16 | 341.0, 495.4 (50.3 to 1630.0) | Not clonal |
| Median, mean clonal free lambda light chain (mg/dl [range]) | 7.6, 40.4 (0.28 to 332.0); n = 50 | 21.0, 73.1 (0.11 to 554.0); n = 26 | 4.3, 6.7 (1.7 to 18.0); n = 5 | Not clonal | 241.0, 295.5 (22.5 to 173.0) |
| Median, mean serum total protein (g/dl [range]) | 9.0, 8.9 (6.4 to 14.0) | 8.0, 8.1 (4.5 to 13.3) | 8, 8.1 (5.7 to 10.5) | 6.6, 6.6 (5.4 to 8.1) | 6.3, 6.1 (4.4 to 8.2) |
| Median, mean serum globulin (g/dl [range]) | 5.0, 5.3 (2.4 to 12.3) | 5.0, 4.6 (2.2 to 10.4) | 4.0, 4.3 (1.9 to 6.9) | 2.3, 2.4 (1.6 to 4.3) | 2.2, 2.3 (1.9 to 4.0) |
| Median, mean serum albumin (g/dl [range]) | 4.0, 3.6 (2.1 to 5.0) | 4.0, 3.5 (1.7 to 4.4) | 4.0, 3.9 (3.1 to 4.6) | 4.2, 4.2 (3.4 to 4.9) | 3.9, 3.8 (2.3 to 4.9) |
Normal ranges in serum: IgA, 70 to 400 mg/dl; IgG, 700 to 1600 g/dl; IgM, 40 to 230 mg/dl; free kappa, 0.3 to 1.94 mg/dl; free lambda, 0.57 to 2.63 mg/dl; albumin, 3.6 to 5.1 g/dl; globulin, 2.0 to 3.5 g/dl; total protein, 6.2 to 8.3 g/dl. sPEP, serum protein electrophoresis.
Missing n due to serum protein electrophoreses that were normal or nonquantifiable.
Other Findings
Demographic and laboratory data are shown in Table 4. A measurable monoclonal protein spike on serum protein electrophoresis was identified in all IgG, IgA, and IgM gammopathies (by definition) but in only 48% (23 of 48) of light chain gammopathies. Serum free light chain analysis was diagnostic in all light chain gammopathies. Mean clonal IgG, IgA, or IgM concentrations were approximately 10-fold higher in these respective gammopathies than mean clonal free light chain concentrations in light chain gammopathies (P < 0.001). Mean serum total protein and globulin concentrations were significantly higher in IgG, IgA, and IgM compared with free light chain monoclonal gammopathies (P < 0.001), where these values were within normal limits. Correlations were high between IgG and total protein (r = 0.95, P < 0.001), IgA and total protein (r = 0.89, P < 0.001), and IgM and total protein (r = 0.88, P < 0.001) but poor between clonal free light chains and total protein (r = 0.11, P = 0.44) in these respective gammopathies (data not shown). Mean serum albumin concentrations were significantly higher in free light chain compared with IgG and IgA (P < 0.001) but were similar to those of IgM monoclonal gammopathies (P = 0.38).
Discussion
In this study—the largest series to examine the anion gap in monoclonal gammopathies and the only study that corrected the anion gap for hypoalbuminemia—we confirmed the associations of IgG monoclonal gammopathy with a decreased anion gap and IgA monoclonal gammopathy with an increased anion gap. Light chain and IgM gammopathies showed no consistent effect on the gap in either direction.
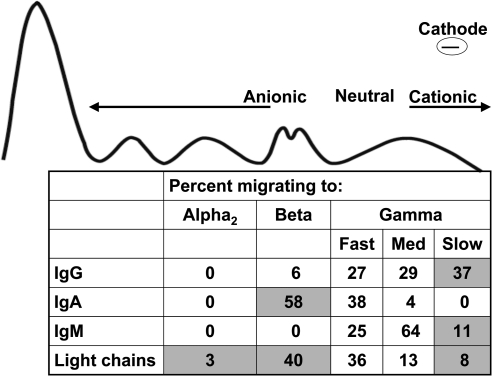
An abnormal anion gap is a clever tool to suspect a monoclonal gammopathy, but, overall, its sensitivity was low and there was only a weak to modest correlation between the anion gap and the monoclonal protein concentration. In part, the low sensitivity and weak correlation may be attributable to the variable charge properties of immunoglobulins. Most IgG monoclonal proteins are cationic, migrating to the gamma region of the electrophoretic gel (Figure 4). The gamma region is classically divided into slow (highly cationic), medium, and fast domains (16). Approximately one-third of IgG monoclonal proteins migrate to the slow gamma region (16). Thus, in high concentration, slow γ-migrating IgG monoclonal proteins decrease the anion gap. Similarly, approximately one-half of IgA paraproteins are anionic and migrate to the alpha or beta regions (16). Our observation of an abnormal gap in one-quarter of IgG gammopathies and one-third of IgA gammopathies is consistent with the migratory pattern of monoclonal proteins.
Figure 4.
Migration patterns of IgG, IgA, IgM, and light chain monoclonal proteins (n = 1742) (16). Estimated anionic, neutral, and cationic migration ranges are also provided. Shaded areas represent migratory patterns that may account for abnormalities in the anion gap (see text).
It has been reported that IgA kappa is relatively cationic versus IgA lambda, which is relatively anionic (17). Thus, IgA lambda may have been predicted to more frequently increase the anion gap, but no association was found.
Correlation coefficients between serum IgG monoclonal protein levels and the anion gap have been reported as high as −0.77 (2) and as low as −0.40 (4), but none corrected the anion gap for hypoalbuminemia. Hypoalbuminemia lowers the anion gap, and its correction increases the anion gap. Hypoalbuminemia is present in 40% to 50% of patients with multiple myeloma at presentation (18,19) and was observed in 36% of our cohort. Hypoalbuminemia accounted for the decreased anion gap in approximately one-third of our patients with IgG gammopathy. After correction for hypoalbuminemia, it is not surprising that the correlation coefficient we calculated between IgG monoclonal protein and the anion gap (0.3) was less robust than that of prior studies.
There is no standard reference range for the anion gap (20). Normal ranges for the anion gap depend, in part, on the population and analytic methodology (21,22). The normal ranges we calculated were similar to previously reported ranges (21,23). Optimal study design would be to compare the anion gap before development of disease to anion gap at presentation. However, before presentation with symptomatic myeloma, virtually all patients have a pre-existing MGUS (24,25). Monoclonal proteins have been demonstrated up to 14 years before symptomatic presentation with multiple myeloma (25). Thus, samples from patients with multiple myeloma would need to be available at least 15 years before diagnosis to establish a reliable paraprotein-free baseline.
There are four subclasses of IgG. IgG1 is the most common IgG monoclonal protein, found in one-half to two-thirds of IgG monoclonal gammopathies (26–29). IgG2 is the second most common, identified in approximately one-fourth of IgG gammopathies (26,28,29). IgG1 has the highest pI (8.0 to 9.5), whereas IgG2 has a pI of 7.0 to 7.5, and IgG4 has a pI <6 (30). The IgG subclass of the monoclonal proteins was not available for evaluation in this study, but subclass may conceivably affect the anion gap.
Free light chains had no consistent effect on the anion gap in light chain gammopathies. These findings were not unexpected given that mean clonal free light chain concentrations averaged 10-fold lower in light chain gammopathies compared with clonal IgG, IgA, and IgM in those gammopathies. Free light chains are measured in most laboratories in mg/L versus m-spikes in g/dl. Thus, a modest 1 g/dl m-spike is equivalent to 1000 mg/dl (or 10,000 mg/L); clonal free light chain concentrations >1000 mg/dl are extremely rare. Moreover, the electrophoretic migration patterns of free light chains are quite variable, and no consistent effect on the anion gap would be expected.
Increased serum total protein and globulin are clues to the diagnosis of multiple myeloma, but their means were within normal limits in light chain monoclonal gammopathies. This finding was not surprising, given that mean clonal free light chain levels in light chain disease were significantly lower than clonal IgG, IgA, and IgM concentrations. Indeed, serum protein electrophoresis failed to demonstrate a monoclonal spike in approximately one-half of light chain gammopathies, a finding that has been reported by others (31). Because nephrotoxicity is common in light chain-mediated gammopathies, and because abnormalities in the anion gap, serum protein electrophoresis, serum total protein, and globulin are uncommon in light chain gammopathy, a lower threshold is warranted to test for light chain disorders in patients presenting with acute renal injury.
Most patients in this study had malignant monoclonal gammopathies, but a few had MGUS. The size of the monoclonal spike alone does not differentiate multiple myeloma from MGUS. In a series of 884 newly diagnosed patients with multiple myeloma, 43% had a monoclonal protein concentration <3 g/dl and 28% had a monoclonal protein concentration <2 g/dl (32). Because paraprotein concentrations below 2 g/dl are less likely to affect the anion gap and are found in one-quarter of patients with multiple myeloma, the anion gap becomes a less reliable tool.
Conclusions
A decreased anion gap was seen in a minority of patients with IgG, and an increased anion gap was seen in a minority of patients with IgA monoclonal gammopathies. Overall, the anion gap was not a sensitive tool for suspecting a monoclonal gammopathy. Monoclonal protein concentrations did not strongly correlate with the anion gap. Concomitant clonal free light chain concentrations did not affect the anion gap in IgG or IgA monoclonal gammopathies. In light chain monoclonal gammopathy, routine measurements of serum total protein and globulin generally failed to provide useful clues to the diagnosis, and the anion gap was unreliable.
Disclosures
KvH is an employee of The Binding Site, Inc.
Acknowledgments
This work was presented in part as an abstract and poster at Renal Week, November 16 to 21, 2010, Denver, Colorado.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV. on behalf of the International Myeloma Working Group: International uniform response criteria for multiple myeloma. Leukemia 20: 1467–1473, 2006 [DOI] [PubMed] [Google Scholar]
- 2. De Troyer A, Stolarczyk A, De Beyl DZ, Stryckmans P: Value of anion-gap determination in multiple myeloma. N Engl J Med 296: 858–860, 1977 [DOI] [PubMed] [Google Scholar]
- 3. Frohlich J, Adam W, Golbey MJ, Bernstein M: Decreased anion gap associated with monoclonal and pseudomonoclonal gammopathy. CMAJ 114: 231–232, 1976 [PMC free article] [PubMed] [Google Scholar]
- 4. Mansoor S, Siddiqui I, Adil S, Nabi Kakepoto G, Fatmi Z, Ghani F: Anion gap among patients of multiple myeloma and normal individuals. Clin Biochem 40: 226–229, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Murray T, Long W, Narins RG: Multiple myeloma and the anion gap. N Engl J Med 292: 574–575, 1975 [DOI] [PubMed] [Google Scholar]
- 6. Paladini G, Sala PG: Anion gap in multiple myeloma. Acta Haematol 62: 148–152, 1979 [DOI] [PubMed] [Google Scholar]
- 7. Mikulski SM: Letter: Anion gap and myeloma. N Engl J Med 294: 111–112, 1976 [DOI] [PubMed] [Google Scholar]
- 8. Schnur MJ, Appel GB, Karp G, Osserman EP: The anion gap in asymptomatic plasma cell dyscrasias. Ann Intern Med 86: 304–305, 1977 [DOI] [PubMed] [Google Scholar]
- 9. Paladini G, Sala PG: Effect of chemotherapy on the anion gap in multiple myeloma. Acta Haematol 66: 31–34, 1981 [DOI] [PubMed] [Google Scholar]
- 10. Flanagan NG, Ridway JC, Irving AG: The anion gap as a screening procedure for occult myeloma in the elderly. J R Soc Med 81: 27–28, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jenkins MA: Serum and urine electrophoresis for detection and identification of monoclonal proteins. Clin Biochem Rev 30: 119–122, 2009 [PMC free article] [PubMed] [Google Scholar]
- 12. Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, Drew R: Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem 47: 673–680, 2001 [PubMed] [Google Scholar]
- 13. Mead GP, Carr-Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR: Serum free light chains for monitoring multiple myeloma. Br J Haematol 126: 348–354, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Leleu X, Moreau AS, Weller E, Roccaro AM, Coiteux V, Manning R, Nelson M, Leduc R, Robu D, Dupire S, Hatjiharissi E, Burwick N, Darre S, Hennache B, Treon SP, Facon T, Gertz MA, Ghobrial IM: Serum immunoglobulin free light chain correlates with tumor burden markers in Waldenstrom macroglobulinemia. Leuk Lymphoma 49: 1104–1107, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Feldman M, Soni N, Dickson B: Influence of hypoalbuminemia or hyperalbuminemia on the serum anion gap. J Lab Clin Med 146: 317–320, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Wang H, Gao C, Xu L, Yang Z, Zhao W, Kong X: Laboratory characterizations on 2007 cases of monoclonal gammopathies in East China. Cell Mol Immunol 5: 293–298, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suen KK, Lewis WH, Lai KN: Analysis of charge distribution of lambda- and kappa-IgA in IgA nephropathy by focused antigen capture immunoassay. Scand J Urol Nephrol 31: 289–293, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Snozek CL, Katzmann JA, Kyle RA, Dispenzieri A, Larson DR, Therneau TM, Melton LJ, 3rd, Kumar S, Greipp PR, Clark RJ, Rajkumar SV: Prognostic value of the serum free light chain ratio in newly diagnosed myeloma: Proposed incorporation into the international staging system. Leukemia 22: 1933–1937, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, Lahuerta JJ, Ludwig H, Morgan G, Powles R, Shimizu K, Shustik C, Sonneveld P, Tosi P, Turesson I, Westin J: International staging system for multiple myeloma. J Clin Oncol 23: 3412–3420, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kraut JA, Madias NE: Serum anion gap: Its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2: 162–174, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Roberts WL, Johnson RD: The serum anion gap. Has the reference interval really fallen? Arch Pathol Lab Med 121: 568–572, 1997 [PubMed] [Google Scholar]
- 22. Winter SD, Pearson JR, Gabow PA, Schultz AL, Lepoff RB: The fall of the serum anion gap. Arch Intern Med 150: 311–313, 1990 [PubMed] [Google Scholar]
- 23. Johnson RJ, Feehally JF, eds: Comprehensive Clinical Nephrology, 2nd ed, London, UK, Elsevier, 2003 [Google Scholar]
- 24. Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, Dispenzieri A, Kumar S, Clark RJ, Baris D, Hoover R, Rajkumar SV: Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood 113: 5412–5417, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM: A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 113: 5418–5422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fasullo FJ, Jr, Fritsche HA, Jr, Liu FJ, Hamilton RG: IgG heavy-chain subclass typing of myeloma paraproteins by isoelectric focusing immunoblot analysis. Clin Chem 35: 364–368, 1989 [PubMed] [Google Scholar]
- 27. Klouche M, Bradwell AR, Wilhelm D, Kirchner H: Subclass typing of IgG paraproteins by immunofixation electrophoresis. Clin Chem 41: 1475–1479, 1995 [PubMed] [Google Scholar]
- 28. Papadea C, Reimer CB, Check IJ: IgG subclass distribution in patients with multiple myeloma or with monoclonal gammopathy of undetermined significance. Ann Clin Lab Sci 19: 27–37, 1989 [PubMed] [Google Scholar]
- 29. Tichy M, Hrncir Z, Mracek J: Subclasses IgG1–IgG4 in 84 sera with IgG paraprotein. Neoplasma 25: 107–110, 1978 [PubMed] [Google Scholar]
- 30. Buis B, Wever PC, Koomen GC, van Acker BA, Groothoff JW, Krediet RT, Arisz L: Clearance ratios of amylase isoenzymes and IgG subclasses: Do they reflect glomerular charge selectivity? Nephron 75: 444–450, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Abraham RS, Clark RJ, Bryant SC, Lymp JF, Larson T, Kyle RA, Katzmann JA: Correlation of serum immunoglobulin free light chain quantification with urinary Bence Jones protein in light chain myeloma. Clin Chem 48: 655–657, 2002 [PubMed] [Google Scholar]
- 32. Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM, Greipp PR: Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 78: 21–33, 2003 [DOI] [PubMed] [Google Scholar]