Abstract
Summary
Background and objectives
Rituximab and intravenous Ig (IVIG) are commonly used for desensitization of HLA and blood group–incompatible (ABOi) transplants. However, serious infections have been noted in association with rituximab administration. In this study, we retrospectively compared infectious outcomes in those who received rituximab plus IVIG for HLA or ABOi transplants (RIT group) with a group of nonsensitized, ABO-compatible transplant recipients (non-RIT group).
Design, setting, participants, & measurements
Patients undergoing kidney transplantation at Cedars-Sinai Medical Center were included in the analysis. A total of 361 patients were identified. All received antimicrobial prophylaxis and viral surveillance. The primary outcome was infection.
Results
Overall patient survival was 97 and 96%, and graft survival was 91 and 89% in the RIT and non-RIT groups, respectively, after an average follow-up of 18 months. There were equal rates of bacterial (34.7% versus 39.1%), viral (21.8% versus 25.1%), fungal (5.9% versus 5.2%), and serious infections (22.9% versus 25.5%) in the RIT and non-RIT groups respectively. Urinary tract infection was the most common infection, accounting for 50% of all bacterial infections. Cytomegalovirus viremia was nonsignificantly more common in the nonrituximab-treated group (15.2% versus 10%), whereas BK viremia was marginally more frequent in the rituximab-treated group (10.6% versus 5.8%). There were no graft losses caused by BK-associated nephropathy. There were two deaths in each group related to infection (1%).
Conclusion
Rituximab does not increase infection risk when used with intravenous Ig for desensitization.
Introduction
An increasing portion of the kidney transplant waiting list consists of broadly HLA-sensitized (HS) patients and patients with blood type–incompatible (ABOi) living donors. HS patients are more likely to remain on the kidney transplant waiting list and may never receive a transplant. Those with blood type–incompatible living donors contribute to the growth of the wait list and must now wait years for a deceased-donor allograft. Rituximab (anti-CD20, anti-B cell) with intravenous Ig (IVIG) is used in HLA and blood type–incompatible desensitization protocols and improves transplantation rates in HS patients (1–6). In addition, ABOi protocols using rituximab have demonstrated short- and long-term outcomes similar to ABO-compatible living donor allografts (6–9).
There are conflicting reports regarding the rate and severity of infections in patients who receive rituximab for transplant-related diagnoses (6,10). A trend toward an increased rate of bacterial, viral, and fungal infections has been reported after rituximab administration in kidney transplant patients who received rituximab for ABOi or positive crossmatch transplantation (11–16). The safety of rituximab use has also been reported in kidney transplant recipients (9,17–20). For HS patients who received rituximab with IVIG and alemtuzumab induction, no increased rate of infection was seen (19). Here we report infectious outcomes in kidney transplant recipients who received rituximab and IVIG for desensitization compared with a group of nonsensitized patients who received ABO compatible allografts without rituximab or IVIG.
Materials and Methods
The study was approved by the Institutional Review Board at Cedars-Sinai Medical Center. We included all patients who underwent kidney transplantation at Cedars-Sinai Medical Center between January 2007 and February 2010 (n = 361). One group consisted of low-risk patients who were not HS and did not receive IVIG or rituximab before transplantation (non-RIT group). Outcomes were then compared with a second group that consisted of HS patients who received desensitization for HLA antibodies or ABOi transplantation (RIT group). The primary outcome was the occurrence of any bacterial, viral, or fungal infection. Bacterial and fungal infections were defined by a positive culture or symptomatic presentation. We defined cytomegalovirus (CMV) viremia as greater than 50 copies/PCR (500 ng of total DNA/PCR) and polyomavirus BK (BKV) viremia as greater than 2500 copies/ml plasma or when treatment was initiated despite a lower viral load. CMV disease was diagnosed by the presence of viral symptoms or organ involvement in the presence of CMV viremia. BKV-associated nephropathy (BKAN) was diagnosed by the presence of viral inclusions or a positive SV-40 stain on allograft biopsy. Serious infections were considered as those associated with a positive blood culture or those requiring hospitalization.
Infection Monitoring and Prophylaxis
Antimicrobial prophylaxis was administered as described previously (3). All recipients who received rituximab, lymphocyte depleting induction, or were D+/R− received valganciclovir for CMV prophylaxis. Acyclovir was administered to all other patients for viral prophylaxis. Pneumocystis jirovecii pneumonia (PCP) and viral prophylaxis were given for 3 months post transplant. This was extended to 6 months post transplant in 2008. Plasma BKV and whole blood CMV viral surveillance was done by PCR at 1, 2, 3, 6, 9, and 12 months post transplant. Additional viral testing was done when clinically indicated.
Desensitization Protocols
Patients designated as HS received two doses of IVIG (2 g/kg; maximum, 140 g), 1 month apart, with one dose of rituximab (1 g) given between the IVIG doses (3). Patients receiving an ABOi allograft received immunosuppression with mycophenolate mofetil 1 month before transplant, rituximab (1 g) 3 weeks before transplant, and five sessions of plasmapheresis (PE) followed by one dose of IVIG (2 g/kg; maximum, 140 g) after the final PE. The number of PE sessions did not vary with the degree of sensitization. All of the patients with an acceptable crossmatch (CXM) and anti-A/B titer ≤1:8 were transplanted as described previously (2,3,21).
Immunosuppression
All patients received induction immunosuppression. Maintenance immunosuppression consisted of a calcineurin inhibitor, mycophenolate mofetil, and prednisone. Target tacrolimus levels for those that received a lymphocyte-depleting agent were 7 to 9 ng/ml for the first 6 months, 5 to 7 ng/ml for months 7 to 12, and 4 to 6 ng/ml thereafter. Target levels for those who received an IL-2 receptor antagonist were 8 to 10 ng/ml for the first 3 months, 7 to 9 ng/ml for months 4 to 6, 5 to 7 ng/ml for months 7 to 12, and 4 to 6 ng/ml thereafter. Patients treated with a lymphocyte-depleting agent received an initial dose of 500 mg twice daily of mycophenolate mofetil, whereas patients who received an IL-2 receptor antagonist received 1000 mg twice daily as tolerated. Prednisone was tapered to 5 mg daily over 2 to 4 weeks. Cell-mediated rejection (CMR) and antibody-mediated rejection (AMR) were treated as described previously (3). Those patients with severe CMR (Banff grade 2) or refractory to methylprednisolone received anti-thymocyte globulin (cumulative dose, 6 mg/kg).
Statistical Methods
Demographic variables were collected on all patients. Other variables collected included patient follow-up, dialysis vintage, induction type, and transplant type (living versus deceased). Univariate analysis was done for continuous variables using unpaired, two-sample t tests. Univariate analysis for categorical variables was done using the chi-squared test. Univariate and multivariate logistic regression was done to test for association of variables between the groups. Survival estimates were determined by Kaplan-Meier product limit method.
Results
Baseline Characteristics
A total of 361 patients were included in the analysis, 170 (46%) of whom underwent desensitization with rituximab and IVIG (RIT group). Among those in the RIT group, 26 received an ABOi allograft. Six of the ABOi recipients were also designated as HS. Baseline characteristics are shown in Table 1. Recipients undergoing desensitization were more likely to be female and have a living donor. More recipients undergoing desensitization received a lymphocyte-depleting agent, mostly alemtuzumab, for induction immunosuppression.
Table 1.
Baseline demographic and transplant characteristics
| RIT Group (n = 170) | Non-RIT Group (n = 191) | P | |
|---|---|---|---|
| Age (years)a | 47.5 [36 to 57] | 53 [42 to 63] | <0.001 |
| Male gender, n (%) | 69 (41.0) | 141 (73.8) | <0.001 |
| Cause of ESRD, n (%) | <0.001 | ||
| DM | 31 (18.2) | 71 (37.2) | |
| HTN | 21 (12.4) | 28 (14.7) | |
| glomerulonephritis | 64 (37.6) | 36 (18.8) | |
| cystic disease | 6 (3.5) | 18 (9.4) | |
| other | 28 (16.5) | 36 (18.8) | |
| unknown | 20 (11.8) | 2 (1.1) | |
| PRA, n (%) | |||
| <10% | 26 (15.3) | 174 (91.1) | <0.001 |
| 10 to 80% | 51 (30.0) | 17 (8.9) | |
| >80% | 93 (54.7) | 0 (0) | |
| Type of donor, n (%) | |||
| living | 80 (47.1) | 68 (35.6) | 0.03 |
| deceased | 90 (52.9) | 123 (64.4) | |
| Lymphocyte depletion, n (%) | |||
| no | 47 (27.6) | 110 (57.6) | <0.001 |
| yes | 123 (72.4) | 81 (42.4) | |
| Race/ethnicity, n (%) | 0.02 | ||
| Caucasian (non-Hispanic) | 73 (42.9) | 92 (48.1) | |
| Hispanic | 44 (25.9) | 64 (33.5) | |
| African American | 29 (17.1) | 22 (11.5) | |
| Asian | 15 (8.8) | 12 (6.3) | |
| other | 9 (5.3) | 1 (<1) | |
| Follow-up (days)a | 403.5 [283 to 653] | 598 [368 to 819] | <0.001 |
| Dialysis vintage (days)a | 1223 [420 to 2537] | 815 [322 to 1617] | <0.001 |
DM, diabetes mellitus; HTN, hypertension; PRA, panel reactive antibodies; RIT, rituximab.
Median [interquartile range].
The degree of sensitization is shown in Table 1. Eighty-five percent of the RIT group had a panel reactive antibodies level of >10%, with over half having a panel reactive antibodies level of >80%. Sixty-five (38%) patients in the RIT group had a positive T-flow CXM. Seven HS patients had a persistently unacceptable CXM and received PE as part of their desensitization. Donor specific antibody (DSA) data were available for 142 HS patients, 92 (65%) of whom had DSA present. Fifty-four had both Class I and II, 21 had only Class I, and 17 had only Class II. Twenty-one patients had strong binding DSA by luminex single antigen beads (Mean Fluorescence Intensity >10,000). In addition, 61 had multiple DSA present at the time of transplant.
Patient and Graft Survival
Overall patient and graft survival were 97 and 90%, respectively. There was no difference in patient and graft survival in the two groups (Table 2). Five deaths occurred in the RIT group, and seven occurred in the non-RIT group. Infection was the cause of death in four patients (1%), two from each group. One death from each group resulted from fungal infection and bacterial infection. One death in the non-RIT group was secondary to Epstein-Barr virus negative post transplant lymphoproliferative disorder. There were no patients with post transplant lymphoproliferative disorder in the RIT group. Death-censored graft survival was 93% and was not significantly different in the groups.
Table 2.
Patient survival, graft survival, and number of patients who developed various infections in the RIT and non-RIT groups
| RIT Group (n = 170) | Non-RIT Group (n = 191) | P | |
|---|---|---|---|
| Overall patient survival, n (%) | 165 (97.1) | 183 (96.3) | 0.70 |
| Overall graft survival, n (%) | 154 (90.6) | 170 (89.0) | 0.62 |
| Rejection, n (%) | 47 (27.7) | 36 (18.9) | 0.05 |
| Any infection, n (%) | 88 (51.8) | 103 (53.9) | 0.68 |
| Bacterial infections, n (%) | 59 (34.7) | 75 (39.1) | 0.27 |
| All viral infections, n (%) | 37 (21.8) | 48 (25.1) | 0.45 |
| CMV viremia | 17 (10.0) | 29 (15.2) | 0.14 |
| BKV viremia | 18 (10.6) | 11 (5.8) | 0.09 |
| Fungal infections, n (%) | 10 (5.9) | 10 (5.2) | 0.46 |
| Serious infections, n (%) | 39 (22.9) | 49 (25.5) | 0.62 |
CMV, cytomegalovirus; BKV, polyomavirus BK; RIT, rituximab.
Overall Infections
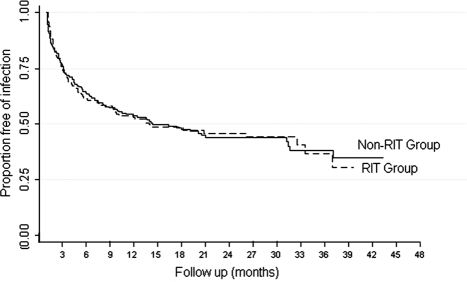
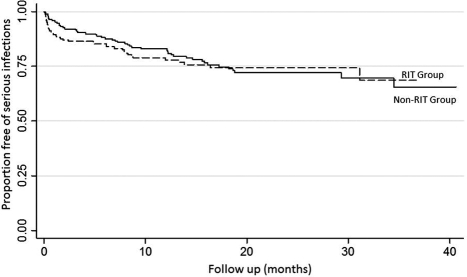
There were 88 patients (52%) in the RIT group and 103 patients (54%) in the non-RIT group diagnosed with an infection during the follow-up period (Table 2). The cumulative incidence of infection was similar in both groups, and most infections occurred during the first 3 months post transplant (Figure 1). A total of 163 infections in the RIT group and 208 infections in the non-RIT group were identified. Urinary tract infection (UTI) was the most common, accounting for 50% of all infections. There were comparable rates of bacterial, viral, and fungal infections between the groups (Table 2). There was no difference in the rate of serious infections (Figure 2). A total of 51 serious infections in 39 (23%) patients and 67 serious infections in 49 (26%) patients were seen in the RIT and non-RIT groups, respectively (Table 3).
Figure 1.
Kaplan-Meier survival curve showing freedom from any infection in the rituximab (RIT) group versus the non-RIT group; log rank P = 0.98.
Figure 2.
Kaplan-Meier survival curve showing freedom from serious infections in the rituximab (RIT) group versus the non-RIT group; log rank P = 0.73.
Table 3.
Total number and types of serious infections observed in the RIT and non-RIT groups
| RIT | Non-RIT | |
|---|---|---|
| Bacterial | ||
| UTI/pyelonephritis | 14 | 15 |
| sepsis | 10 | 24 |
| abscess | 7 | 6 |
| pneumonia | 3 | 4 |
| cellulitis | 1 | 2 |
| cholecystitis | 2 | 0 |
| diverticulitis | 1 | 0 |
| peritonitis | 1 | 0 |
| appendicitis | 2 | 0 |
| wound infection | 0 | 3 |
| Fungal | ||
| UTI | 5 | 0 |
| pulmonary aspergillosis | 2 | 0 |
| cryptococcal pneumonia | 0 | 2 |
| coccidioidomycosis | 1 | 0 |
| PCP | 0 | 2 |
| esophagitis | 0 | 1 |
| peritonitis | 0 | 1 |
| wound infection | 0 | 1 |
| Viral | ||
| varicella zoster | 1 | 0 |
| influenza A | 1 | 2 |
| myocarditis | 0 | 1 |
| CMV pneumonia | 0 | 1 |
| EBV(+) PTLD | 0 | 1 |
| CMV colitis | 0 | 1 |
A total of 118 serious infections occurred in 88 patients. The frequency of sepsis was greater in the non-RIT group (P = 0.04), and the frequency of fungal UTIs was greater in the RIT group (P = 0.03). CMV, cytomegalovirus; EBV, Epstein-Barr virus; PCP, Pneumocystis carinii pneumonia; PTLD, post transplant lymphoproliferative disorder; RIT, rituximab; UTI, urinary tract infection.
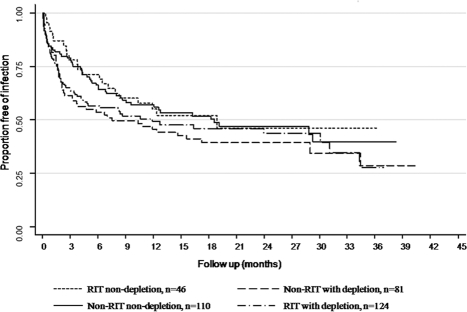
Univariate regression for association of variables with infection showed deceased donor and age to be the only variables associated with infection (Table 4). However, these variables were NS in the multivariate model. Lymphocyte depletion was not associated with infection in the univariate or multivariate model. There was a nonsignificant trend for age, gender, and rejection to be associated with infection. We also performed a subgroup analysis for the use of lymphocyte-depleting agents in the RIT and non-RIT groups. There was no difference in infectious outcomes among the four groups (Figure 3).
Table 4.
Univariate and multivariate logistic regression for association of variables with occurrence of any infection as the outcome
| Unadjusted OR | P | 95% CI |
||
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Univariate | ||||
| non-RIT versus RIT | 0.92 | 0.68 | 0.61 | 1.39 |
| PRA category | 0.96 | 0.77 | 0.76 | 1.23 |
| follow-up (days) | 1.00 | 0.64 | 1.00 | 1.00 |
| vintage | 1.00 | 0.55 | 1.00 | 1.00 |
| DD versus LD | 1.53 | 0.05 | 1.01 | 2.34 |
| induction agent | 1.00 | 0.98 | 0.79 | 1.26 |
| lymphocyte depletion | 1.34 | 0.16 | 0.89 | 2.04 |
| race | 0.90 | 0.27 | 0.74 | 1.09 |
| age (years) | 1.02 | 0.02 | 1.00 | 1.03 |
| gender | 1.45 | 0.08 | 0.95 | 2.21 |
| DM and/or HTNa | 1.12 | 0.07 | 0.99 | 1.28 |
| acute rejection | 1.57 | 0.08 | 0.95 | 2.59 |
| Multivariate | ||||
| lymphocyte depletion | 1.18 | 0.47 | 0.75 | 1.87 |
| DD versus LD | 1.37 | 0.16 | 0.88 | 2.12 |
| age (years) | 1.02 | 0.06 | 1.00 | 1.03 |
| gender | 1.52 | 0.06 | 0.98 | 2.34 |
| DM and/or HTNa | 1.09 | 0.27 | 0.94 | 1.25 |
| acute rejection | 1.64 | 0.06 | 0.98 | 2.76 |
The reference group for diabetes mellitus and/or hypertension is all other kidney diseases (glomerulonephritis, cystic, other, and unknown). The reference group for gender is male. DD, deceased donor; LD, living donor; OR, odds ratio; PRA, panel reactive antibodies; RIT, rituximab; DM, diabetes mellitus; HTN, hypertension; CI, confidence interval.
The presence of diabetes mellitus and/or hypertension as the cause of end-stage renal disease.
Figure 3.
Kaplan-Meier survival curve showing freedom from any infection in the four subgroups; log rank P = 0.34. RIT, rituximab.
Bacterial Infections
Bacterial infections were the most commonly observed, occurring in 53% of patients. There was no difference in the rate of bacterial infections in the RIT and non-RIT groups (Table 2). UTI accounted for 50% of all bacterial infections. Other causes of bacterial infections included sepsis (urinary and nonurinary source) and wound infections. Serious infections included hospital admission for pyelonephritis, sepsis, and abscess (Table 3). Twenty-four (13%) cases of sepsis were seen in the non-RIT group, whereas 10 (6%) occurred in the RIT group. Cholecystitis, diverticulitis, and appendicitis were diagnosed in the RIT group but not in the non-RIT group (Table 3). One death in each group was related to bacterial infection from sepsis.
Viral Infections
The rate of viral infections was similar in the two groups. Twenty-two percent of patients in the RIT group were diagnosed with viral infections compared with 25% in the non-RIT group (Table 2). There were more patients with serious viral infections in the non-RIT group compared with the RIT group (Table 3). There were no patients with reactivated hepatitis B or hepatitis C. There were no patients with progressive multifocal leukoencephalopathy. The most common viral infections were CMV and BKV viremia.
CMV infections were reported in 10% of the RIT group and 15% of the non-RIT group. CMV was the most common viral infection diagnosed in the non-RIT group. We compared the rate of CMV viremia among those in the non-RIT group who received valganciclovir with those who received acyclovir for antiviral prophylaxis. There were more patients with CMV viremia in the subgroup that received valganciclovir within the non-RIT group (P = 0.009). We also compared the rate of CMV viremia in patients who received valganciclovir in the RIT and non-RIT groups. There were 17 (10%) patients in the RIT group and 22 (22%) in the non-RIT group who developed CMV infection (P = 0.01). There was one patient with tissue invasive CMV disease in the RIT group and 13 patients in the non-RIT group. Average time to CMV infection was 178 days.
We found BKV to be the most common viral infection in the RIT group, accounting for 40% of all viral infections. There were 18 patients (11%) with BKV infection in the RIT group compared with 11 (6%) in the non-RIT group. There appeared to be a trend toward more BKV infection in the RIT group (Table 2). However, there were only two patients with BKAN in the RIT group and one in the non-RIT group. No graft losses caused by BKAN were seen. Overall, five patients with low grade BKV viremia (<2500 copies/ml plasma) were identified and treated by lowering immunosuppression. One case of BKV viremia (3200 copies/ml plasma) resolved without intervention.
Fungal Infections
The rate of fungal infection was 6% in the RIT group and 5% in the non-RIT group (P = 0.46). The most common fungal infections were UTI and oral candidiasis. A total of 12 patients developed a serious fungal infection: five in the RIT group and seven in the non-RIT group. Serious fungal infections are detailed in Table 3. Two patients with PCP were identified in the non-RIT group. There were two deaths related to fungal infection: one in each group (<1%). One death was related to coccidioidomycosis in the RIT group and cryptococcus in the non-RIT group. Most fungal infections occurred within the first 6 months after transplantation. Overall, fungal infections were more common before changing the fungal prophylaxis strategy; however, this was not statistically significant.
Rejection
There were more rejections observed in the RIT group. The rate of rejection is shown in Table 2. In the RIT group, 26 had AMR, 14 had CMR, and seven had evidence of both. Fourteen were treated with PE, IVIG, and rituximab for AMR, and two received anti-thymocyte globulin for CMR. Sixteen patients had multiple episodes of rejection requiring additional treatment. Rejection episodes in the non-RIT group were primarily CMR, 31 of 36 (86%). Twelve patients received anti-thymocyte globulin for CMR.
We assessed the effect of rejection on the development of subsequent infection in both groups. Seventeen (36%) patients developed an infection after rejection in the RIT group. In the non-RIT group, 15 (42%) patients had an infection after rejection. There was no statistical difference in the two groups with regards to the development and time of infection after rejection. The time to rejection was compared because the risk of infection decreases post transplant (Figure 1). Patients in the RIT group developed rejection 143 days on average after transplant compared with 281 days in the non-RIT group (P = 0.03).
Discussion
Here we report infectious outcomes in a cohort of patients who received rituximab with IVIG for desensitization compared with a group of those who did not receive rituximab. Patient survival and the rate of infection was similar in the two groups. The infection rates observed are similar to what is reported in the literature (20,22). This is the largest cohort of rituximab-treated kidney transplant recipients examined to date for the purpose of identifying infectious complications. We note that rituximab was administered in combination with IVIG in the RIT group. This differs from other studies that have examined infection risks associated with rituximab administration in kidney transplant recipients who were already on maintenance immunosuppression and did not receive IVIG. Thus, the anti-infective properties of IVIG might account for reduced infections in this group.
Overall exposure to immunosuppression was different in the two groups. A subgroup analysis to compare outcomes on the basis of exposure to immunosuppression was performed to account for this (Figure 3). We also examined the additional exposure to anti-rejection therapies and found no difference. This finding is influenced by risk factors that include increased exposure to lymphocyte-depleting agents in the non-RIT group and earlier timing of rejection in the RIT group. Additionally, protective factors such as more treatment with IVIG for rejection in the RIT group must be taken into account.
Kamar et al. (16) reported a fungal infection rate of 16.9% in kidney transplant recipients receiving rituximab for various indications. The fungal infections reported in their cohort were serious in nature. This contrasts the lower rate of fungal infection seen in the RIT group, which was comparable to the non-RIT group (Table 2). Furthermore, the risk of serious fungal infection in the RIT group was 4%, and risk of death from fungal infection was 0.6%. Kamar et al. used rituximab in multiple dose regimens for several post transplant diagnoses. Here, most patients received one dose of rituximab before transplantation. In addition, all patients received PCP prophylaxis for 6 months and fungal prophylaxis for 1 month after transplant. We changed our fungal prophylaxis in patients who received rituximab and/or a lymphocyte-depleting agent and found that 85% of the fungal infections observed occurred before switching to fluconazole. However, our study was not designed for the purpose of evaluating fungal prophylaxis.
CMV infection has been reported in association with rituximab administration (14,15). Our findings showed no difference in the rate of CMV infection in the two groups, despite greater use of lymphocyte depletion in the RIT group. CMV prophylaxis and surveillance is a critical part of preventing complications related to CMV in the post transplant period. All of the patients in the RIT group received valganciclovir for prophylaxis by protocol. In contrast, some patients in the non-RIT group received a high dose of acyclovir for viral prophylaxis. The finding of fewer CMV infections among those in the non-RIT group who received acyclovir prophylaxis is expected because acyclovir was administered in patients at low risk for CMV. There were statistically more CMV infections in the non-RIT group when limiting our analysis to patients receiving valganciclovir prophylaxis (P = 0.01). IVIG contains anti-CMV IgG, and this may help explain the overall lower number of CMV infections in the RIT group (23).
BKV viremia is reported to occur in approximately 10 to 15% of kidney transplant recipients with a subset progressing to BKAN (24). Kamar and colleagues found BKV infection to be the most common viral infection in rituximab-treated patients, accounting for 64% of all viral infections. In their study, all patients diagnosed with BKV viremia developed BKAN (12%) with three graft losses. Here, we also found BKV to be the most common viral infection, accounting for 40% of all viral infections. However, there was little progression and no graft losses related to BKAN. Although there was a trend toward more BKV viremia in the RIT group, the rate was similar to what is reported (24). The development of BKV viremia in the RIT group is likely influenced by the use of lymphocyte depleting induction as a risk factor. However, the administration of IVIG may be protective. It is therefore difficult to establish the influence that rituximab has on the development of BKV viremia in this group. Surveillance for BKV is critical for preventing the development of BKAN. Early intervention with reduction in immunosuppression can improve low grade BKV viremia. IVIG is known to contain anti-BKV antibodies and has been reported to clear BKAN and improve BKV viremia (25,26).
The retrospective nature of this study limited our ability to provide a randomized control. In addition, the cohort is from a single center and may not reflect outcomes at other centers. The baseline characteristics, exposure to immunosuppression, and prophylaxis in the two groups are different, making a comparison difficult. However, these characteristics are representative of HS transplant recipients.
In conclusion, we did not find an increased risk of infection in patients that received rituximab with IVIG for desensitization. Antimicrobial prophylaxis, viral surveillance, and close monitoring are critical components for minimizing infectious complications. The additive anti-infective role of IVIG must also be considered.
Disclosures
None.
Acknowledgments
We would like to acknowledge the support of the Sister Renal Program of the International Society of Nephrology for making this collaboration possible.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Kahwaji J, Vo AA, Jordan SC: ABO blood group incompatibility: A diminishing barrier to successful kidney transplantation? Expert Rev Clin Immunol 6: 893–900, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, Peng A, Villicana R, Jordan SC: Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med 359: 242–251, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Vo A, Peng A, Toyoda M, Kahwaji J, Cao K, Lai C, Reinsmoen N, Villicana R, Jordan S: Use of intravenous immune globulin and rituximab for desensitization of highly HLA-sensitized patients awaiting kidney transplantation. Transplantation 89: 1095–1102, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Sonnenday CJ, Warren DS, Cooper M, Samaniego M, Haas M, King KE, Shirey RS, Simpkins CE, Montgomery RA: Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. Am J Transplant 4: 1315–1322, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Genberg H, Kumlien G, Wennberg L, Berg U, Tydén G: ABO-incompatible kidney transplantation using antigen-specific immunoadsorption and rituximab: A 3-year follow-up. Transplantation, 85: 1745–1754, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Kahwaji J, Tong C, Jordan S, Vo A: Rituximab: An emerging therapeutic agent for kidney transplantation. Transplant Res Risk Management 1: 15–29, 2009 [Google Scholar]
- 7. Montgomery RA, Locke JE, King KE, Segev DL, Warren DS, Kraus ES, Cooper M, Simpkins CE, Singer AL, Stewart ZA, Melancon JK, Ratner L, Zachary AA, Haas M: ABO incompatible renal transplantation: A paradigm ready for broad implementation. Transplantation 87: 1246–1255, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Tanabe K: Japanese experience of ABO-incompatible living kidney transplantation. Transplantation 84: S4–S7, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Genberg H, Kumlien G, Wennberg L, Tydén G: Long-term results of ABO-incompatible kidney transplantation with antigen-specific immunoadsorption and rituximab. Transplantation 84: S44–S47, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Kelesidis T, Daikos G, Boumpas D, Tsiodras S: Does rituximab increase the incidence of infectious complications? A narrative review. Int J Infect Dis, 15: e2–e16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grim S, Pham T, Thielke J, Sankary H, Oberholzer J, Benedetti E, Clark N: Infectious complications associated with the use of rituximab for ABO-incompatible and positive cross-match renal transplant recipients. Clin Transplant 21: 628–632, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Basse G, Ribes D, Kamar N, Mehrenberger M, Sallusto F, Esposito L, Guitard J, Lavayssiere L, Oksman F, Durand D, Rostaing L: Rituximab therapy for mixed cryoglobulinemia in seven renal transplant patients. Transplant Proc 38: 2308–2310, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Faguer S, Kamar N, Guilbeaud-Frugier C, Fort M, Modesto A, Mari A, Ribes D, Cointault O, Lavayssiere L, Guitard J, Durand D, Rostaing L: Rituximab therapy for acute humoral rejection after kidney transplantation. Transplantation 83: 1277–1280, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Aksoy S, Harputluoglu H, Kilickap S, Dede DS, Dizdar O, Altundag K, Barista I: Rituximab-related viral infections in lymphoma patients. Leuk Lymphoma 48: 1307–1312, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Usui M, Isaji S, Mizuno S, Sakurai H, Uemoto S: Experiences and problems pre-operative anti-CD20 monoclonal antibody infusion therapy with splenectomy and plasma exchange for ABO-incompatible living-donor liver transplantation. Clin Transplant 21: 24–31, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Kamar N, Milioto O, Puissant-Lubrano B, Esposito L, Pierre M, Mohamed A, Lavayssière L, Cointault O, Ribes D, Cardeau I, Nogier M, Durand D, Abbal M, Blancher A, Rostaing L: Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am J Transplant 10: 89–98, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Zarkhin V, Li L, Kambham N, Sigdel T, Salvatierra O, Sarwal MM: A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. Am J Transplant 8: 2607–2617, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Munoz AS, Rioveros AA, Cabanayan-Casasola CB, Danguilan RA, Ona ET: Rituximab in highly sensitized kidney transplant recipients. Transplant Proc 40: 2218–2221, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Vo AA, Wechsler EA, Wang J, Peng A, Toyoda M, Lukovsky M, Reinsmoen N, Jordan SC: Analysis of subcutaneous (SQ) alemtuzumab induction therapy in highly sensitized patients desensitized with IVIG and rituximab. Am J Transplant 8: 144–149, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Scemla A, Loupy A, Candon S, Mamzer MF, Martinez F, Zuber J, Sberro R, Legendre C, Thervet E: Incidence of infectious complications in highly sensitized renal transplant recipients treated by rituximab: A case-controlled study. Transplantation 90: 1180–1184, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Lai CH, Cao K, Ong G, Naim M, Wang Q, Mirocha J, Vo A, Jordan SC, Reinsmoen NL: Antibody testing strategies for deceased donor kidney transplantation after immunomodulatory therapy. Transplantation 92: 48–53, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Dharnidharka V, Caillard S, Agodoa L, Abbott K: Infection frequency and profile in different age groups of kidney transplant recipients. Transplantation 81: 1662–1667, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Leroy F, Sechet A, Abou Ayache R, Thierry A, Belmouaz S, Desport E, Bauwens M, Bridoux F, Touchard G: Cytomegalovirus prophylaxis with intravenous polyvalent immunoglobulin in high-risk renal transplant recipients. Transplant Proc 38: 2324–2326, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Wiseman A: Polyomavirus nephropathy: A current perspective and clinical considerations. Am J Kidney Dis 54: 131–142, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Randhawa P, Schonder K, Shapiro R, Farasati N, Huang Y: Polyomavirus BK neutralizing activity in human immunoglobulin preparations. Transplantation 89: 1462–1465, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sener A, House AA, Jevnikar AM, Boudville N, McAlister VC, Muirhead N, Rehman F, Luke PP: Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: One-year follow-up of renal allograft recipients. Transplantation 81: 117–120, 2006 [DOI] [PubMed] [Google Scholar]