Abstract
In this study, the antibacterial activity of crude (aqueous and alcoholic) extracts of the bark and leaf of Croton roxburghii Balak. (Euphorbiaceae) was tested against enteric pathogens causing urinary tract infection (UTI) using the agar cup method, minimum inhibitory concentration (MIC), time kill kinetics and synergy study. The ethanol extract exhibited a significant and broad spectrum of inhibition as compared to the aqueous extract of both the bark and leaf. The highest antibacterial activity was observed against Staphylococcus aureus followed by enteropathogenic and enterotoxigenic Escherichia coli. The diameter of inhibition zones varied from 10 to 18 mm for both aqueous and alcoholic extracts. The MIC value ranged from 356 to 625 μg/ml. This could justify the traditional use of this plant in dysentery and other infections.
Keywords: Croton roxburghii, MIC, Similipal Biosphere Reserve, synergy, UTI
INTRODUCTION
In recent years, multiple resistance in human pathogenic microorganisms have developed due to the indiscriminate use of commercial antimicrobial drugs commonly employed in the treatment of infectious diseases. This situation, the undesirable side effects of certain antibiotic, and the emergence of previously uncommon infections have forced scientists into looking for new antimicrobial substances from various sources like medicinal plants.[1] The screening of plant extracts and plant products for an antimicrobial activity has shown that higher plants represent a potential source of new anti-infective agents.[2] Infectious diseases account for approximately one-half of all deaths in tropical countries. In industrialized nations, despite the progress made in the understanding of microorganisms and their control, the incidence of epidemics due to drug-resistant microorganisms and the emergence of unknown disease-causing microorganisms pose enormous public health concerns.[3]
Although more than 1000 species of Croton are reported, in India only five species are used in ethnomedicine for the treatment of various diseases, disorders, and ailments like antifertility, boils, bowel complaints, chicken pox, cholera, cold and cough, constipation, cuts and wounds, diarrhea, dysentery, eye diseases, epilepsy, fever, gastric disorders, insanity, jaundice, liver complaints, malaria, rheumatism, ringworms, scurvy, spasmolytic agent, snake bite, sprains, etc.[4] Tribal people in India used various parts of C. roxburghii against snake poisoning and to treat infertility, fever, and wounds.[5] The tribes of the Similipal Biosphere Reserve use the cold decoction of the root in sore throat. Two to three teaspoon decoction of leaves is given in dysentery. The paste of root-bark is heated and applied to boils for either subsiding or hastening suppuration. To the best of our knowledge, there is no report available on the antibacterial activity of C. roxburghii except our previous study on the preliminary screening of medicinal plants.[6] Therefore, a microbiological study was conducted to determine the antibacterial property of C. roxburghii bark and leaf extracts against enteric pathogens.
MATERIALS AND METHODS
The aqueous and the alcoholic extracts of the bark and leaf of C. roxburghii were prepared following the method of Panda et al.[7] The extraction was done by using cold percolation (steeping) by soaking the plant material in alcohol and distilled water for 3 days. Enteric pathogens like enteropathogenic and enterotoxigenic Escherichia coli (EPEC and ETEC, respectively), Pseudomonas aeruginosa, Salmonella typhimurium, Shigella flexneri, S. sonnei, Staphylococcus aureus and Vibrio cholerae were tested in the present study. The agar cup method and MIC were used to study the antibacterial activity.[7] Broth microdilution technique adopted using 96-well microtiter plates and tetrazolium salt, 2,3,5-triphenyltetrazolium chloride (TTC), was carried out to determine the MIC following the methods as described by Eloff et al.[8] Selected extracts were serially diluted in the 96-well plate with an overnight culture of microorganisms (0.5 McFarland) grown at 37°C to obtain the final concentration of extracts ranging from 78 to 5000 μg/ml. The microplate was sealed and incubated at 37°C at 130 rpm and observed for the growth of the microorganism.
The antibiotic in combination with the crude drug was serially diluted along with antibiotic and crude drugs. According to the NCCLS guidelines for broth microdilution, the MIC was defined as the lowest concentration of an antibiotic that completely inhibits the growth of the organism as detected with the naked eye. Synergy is more likely to be expressed when the ratio of the concentration of each antibiotic to the MIC of that antibiotic was same for all components of the mixture. The ∑FICs were calculated as follows: ∑FIC=FIC A+FIC B, where FIC A is the MIC of drug A in the combination/MIC of drug A alone, and FIC B is the MIC of drug B in the combination/MIC of drug B alone. The combination is considered synergistic when the ∑FIC is ≤0.5 and antagonistic when the ∑FIC is ≥2.[9]
RESULTS AND DISCUSSION
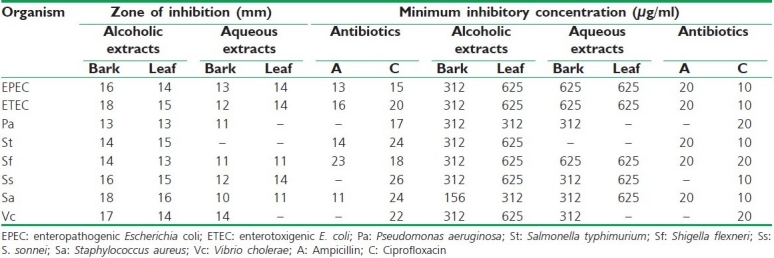
The broad-spectrum activity of aqueous and alcoholic extracts of the leaf and bark of C. roxburghii was observed against a battery of enteric pathogens as shown in Table 1. The alcoholic extract of C. roxburghii (bark and leaf) exhibited an antibacterial activity against all the reference bacterial strains while the aqueous extract of both bark and leaf did not show any activity against S. typhimurium. The highest antibacterial activity was observed against S. aureus followed by enteropathogenic and enterotoxigenic E. coli. Similar observations were also noticed during the determination of the MIC with leaf extracts [Table 1]. However, the alcoholic extract of bark did not show any remarakable difference for MIC. The growth inhibition of the test bacteria ranged from 156 to 625 μg/ml (w/v). The lowest MIC value was recorded against S. aureus at 156 μg/ml (w/v). It is interesting to note that the solvent extract, i.e. ethanol, has a highly pronounced antibacterial activity compared to the aqueous extract. This indicates the presence of more than one active principle in C. roxburghii.
Table 1.
Inhibition of the growth of enteric bacteria by alcoholic and aqueous extracts of Croton roxburghii
Even though the MIC for eight bacterial samples was determined, only five of them were considered for the synergism experiment [Table 2]. This was due to the loss of the resistance for few antibiotics observed in EPEC, ETEC, S. typhimurium, S. aureus and V. cholerae [Table 3], probably because of the loss of plasmids, where the resistance genes are usually located. Since the majority of bacteria were resistant to many antibiotics, only Amikacin was used in the synergism assays. This was because the resistance to at least one of these drugs was common in the entire test bacteria. In addition to this, only the aqueous extract of leaf was selected because this extract showed relatively less activity in comparison to the ethanol extract. The MIC of Amikacin was found to be ≥5000 μg/ml in all strains. The MIC of Amikacin in combination with the aqueous extract of leaf ranged from 48 to 192 μg/ml whereas the MIC for the aqueous extract alone ranged from 2500 to ≥5000 μg/ml. The FIC value was calculated in between 0.05–0.2. The time kill kinetics experimented for EPEC showed that the combination of Amikacin and C. roxburghii leaf (aqueous) remained in the lag phase for 1 h. After lag phase for the period of 1–4 h, it started multiplying at much slower rate as compared to the control strain as well as growth in the presence of Amikacin. During 4–8 h of incubation, this strain multiplied at a constant rate and it started dying out rapidly at the end 12 h. However, by the combination of aqueous extract and Amikacin went into lag phase much earlier as compared to the individual treatment of antibiotic or aqueous extract against EPEC [Figure 1].
Table 2.
Synergy study among aqueous extract of Croton roxburghii leaf
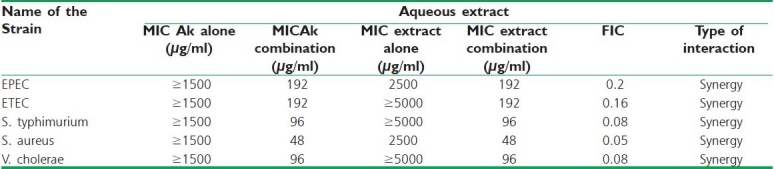
Table 3.
List of the bacteria used to assess the antibacterial activity
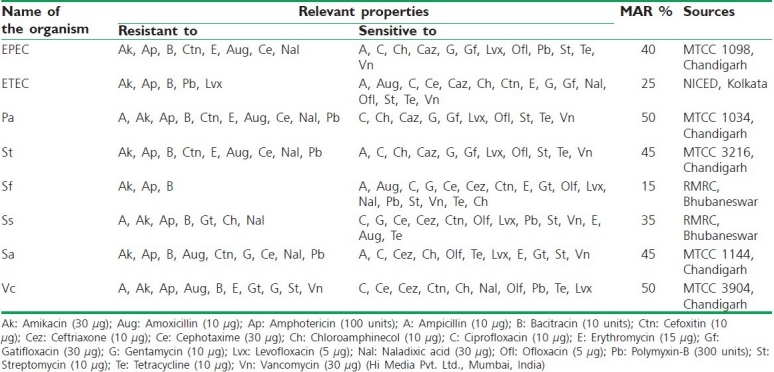
Figure 1.
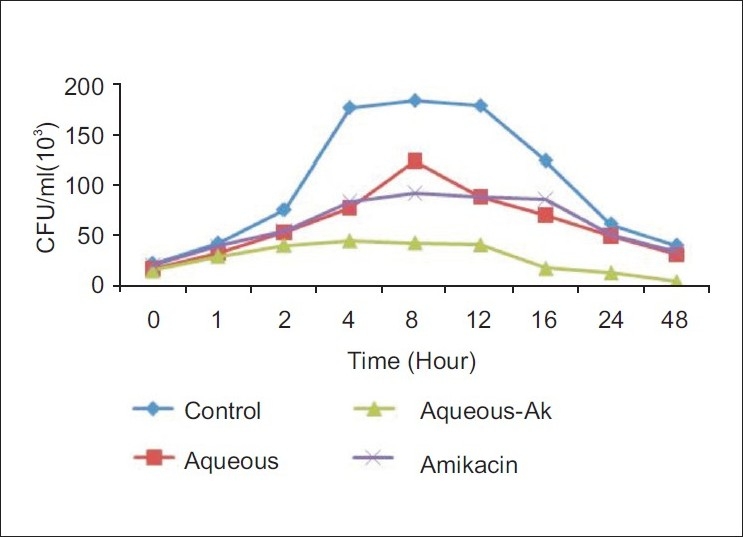
Time kill kinetics of the aqueous extract against EPEC
Antimicrobial resistance has been reported among pathogenic microorganisms like S. aureus, E. coli, P. aeruginosa, and S. typhimurium.[10] In the present experiment strains viz. P. aeruginosa and V. cholerae showed 50% MAR index, S. aureus and S. typhimurium 45% MAR index, EPEC 40% MAR index, S. sonnei showed 35%, ETEC 25%, and S. flexneri showed 15% MAR index [Table 3].
The combined action of Amikacin with the water extract of leaf of C. roxburghii showed a different mode of action. The prime reason for this selection is that higher plants are the rich source of antimicrobials and bioactive substances while traditional medicinal plants are the basic part of health care. A synergistic effect was observed for EPEC, ETEC, S. typhimurium, S. aureus, and V. cholerae which showed resistance to different antibiotics.
At this point, the exact mechanism of synergy is unknown but several hypothesis can be put forward to explain. First, water extract may disrupt the lipopolysaccharide layer of EPEC and helps in restoring protein channels thus facilitating the flow of antibiotic-Amikacin to target sites. Second, it may have negative effects on the efflux mechanism and lead to a sufficient concentration of Amikacin to remain in the bacterium, thus supporting its inhibitory activity. Third, C. roxburghii may be inhibiting protein synthesis in combination with Amikacin which may not possible to inhibit alone the antibiotic or the extract. Fourth, C. roxburghii may be blocking the inhibitory effects of the enzymes or additional inhibitory effects of the plant material.
Considerable work has been carried out on the chemistry and biological activity of the genus Croton which has been reviewed by several workers and is reported to have chiefly diterpenoids such as phorbol esters, clerodane, labdane, kaurane, trachylobane, pimarane, etc.[11] The antibacterial activity of the bark and leaf of C. roxburghii against the enteric pathogens has not been documented so far in the literature. The present work authenticates the scientific use of both aqueous and alcoholic extracts of the bark and leaf of C. roxburghii against a battery of enteric pathogens, causative agents of diarrhea and urinary tract infection.
CONCLUSION
Further studies are required to isolate the active compounds, from the aqueous and alcoholic extracts of C. roxburghii bark and leaf, responsible for the antibacterial property which may lead to compound(s) in the field of antimicrobial.
ACKNOWLEDGEMENTS
The present research has been funded by the Department of Science and Technology, Government of Orissa (Grant No. 2818/28.06.2006). We are also grateful to the authorities of North Orissa University for providing necessary facilities to carry out this research.
Footnotes
Source of Support: Department of Science and Technology, Government of Orissa (Grant No.2818/28.06.2006)
Conflict of Interest: Nil.
REFERENCES
- 1.Marchese A, Shito GC. Resistance patterns of lower respiratory tract pathogens in Europe. Int J Antimicrob Agents. 2001;16:25–9. doi: 10.1016/s0924-8579(00)00302-2. [DOI] [PubMed] [Google Scholar]
- 2.Arias ME, Gomez JD, Cudmani NM, Vattuone MA, Isla MI. Antibacterial activity of ethanolic and aqueous extract of Acacia aroma Gill ex Hook et Arn. Life Sci. 2004;75:191–202. doi: 10.1016/j.lfs.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Iwu MM, Duncan AR, Okunji CO. New antimicrobials of plant origin. In: Janick J, editor. Perspectives on new crops and new uses. Alexandria: ASHS Press; 1999. [Google Scholar]
- 4.Salatino A, Maria L, Salatino F, Negri G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae) J Braz Soc Chem. 2007;18:11–33. [Google Scholar]
- 5.Gupta M, Mazumber UK, Vanisi ML, Sivakumar T, Kandar CC. Antisteriogenic activity of the two Indian medicinal plants in mice. J Ethnopharmacol. 2004;90:21–5. doi: 10.1016/j.jep.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Thatoi HN, Panda SK, Rath SK, Dutta SK. Antimicrobial activity and ethnomedicinal uses of some medicinal plants from Similipal Biosphere Reserve, Orissa. Asian J Plant Sci. 2008;7:260–7. [Google Scholar]
- 7.Panda SK, Dubey D, Dutta SK. Anticandidal activity of Diospyros melanoxylon Roxb. bark from Similipal Biosphere Reserve, Orissa, India. Int J Green Pharm. 2010;4:42–7. [Google Scholar]
- 8.Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of the plant extracts for bacteria. Planta Med. 1998;64:711–3. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 9.Orhan G, Bayram A, Zer Y, Balci I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J Clin Microbiol. 2005;43:140–3. doi: 10.1128/JCM.43.1.140-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox KK, Knapp JS, Holmes KK, Hook EW, Judson FN, Thompson SE, et al. Antimicrobial resistance in Neisseria gonorrhoeae in the United States, 1988-1994: The emergence of decreased sus-ceptibility to the fluoroquinolones. J Infect Dis. 1997;175:1396–403. doi: 10.1086/516472. [DOI] [PubMed] [Google Scholar]
- 11.Block S, Baccelli C, Tinant B, Van Meervelt L, Rozenberg R, Habib Jiwan JL, et al. Diterpenes from the leaves of Croton zambesicus. Phytochemistry. 2004;65:1165–71. doi: 10.1016/j.phytochem.2004.02.023. [DOI] [PubMed] [Google Scholar]


