Abstract
Mucoadhesive drug delivery systems interact with the mucus layer covering the mucosal epithelial surface, and mucin molecules and increase the residence time of the dosage form at the site of absorption. The drugs which have local action or those which have maximum absorption in gastrointestinal tract (GIT) require increased duration of stay in GIT. Thus, mucoadhesive dosage forms are advantageous in increasing the drug plasma concentrations and also therapeutic activity. In this regard, this review covers the areas of mechanisms and theories of mucoadhesion, factors influencing the mucoadhesive devices and also various mucoadhesive dosage forms.
Keywords: Mucoadhesion, theories, mucoadhesive dosage forms
INTRODUCTION
Since the early 1980s, the concept of mucoadhesion has gained considerable interest in pharmaceutical technology.[1] Adhesion can be defined as the bond produced by contact between a pressure sensitive adhesive and a surface. The American Society of Testing and Materials has defined it as the state in which two surfaces are held together by interfacial forces, which may consist of valence forces, interlocking action or both. Mucoadhesive drug delivery systems prolong the residence time of the dosage form at the site of application or absorption. They facilitate an intimate contact of the dosage form with the underlying absorption surface and thus improve the therapeutic performance of the drug. In recent years, many such mucoadhesive drug delivery systems have been developed for oral, buccal, nasal, rectal and vaginal routes for both systemic and local effects.[2]
Dosage forms designed for mucoadhesive drug delivery should be small and flexible enough to be acceptable for patients and should not cause irritation. Other desired characteristics of a mucoadhesive dosage form include high drug loading capacity, controlled drug release (preferably unidirectional release), good mucoadhesive properties, smooth surface, tastelessness, and convenient application. Erodible formulations can be beneficial because they do not require system retrieval at the end of desired dosing interval. A number of relevant mucoadhesive dosage forms have been developed for a variety of drugs. Several peptides, including thyrotropin-releasing hormone (TRH), insulin, octreotide, leuprolide, and oxytocin, have been delivered via the mucosal route, albeit with relatively low bioavailability (0.1–5%),[3] owing to their hydrophilicity and large molecular weight, as well as the inherent permeation and enzymatic barriers of the mucosa.
The development of sustain release dosage form can achieve the aim of releasing the drug slowly for a long period but this is not sufficient to get sustained therapeutic effect. They may be cleared from the site of absorption before emptying the drug content. Instead, the mucoadhesive dosage form will serve both the purposes of sustain release and presence of dosage form at the site of absorption. In this regard, our review is high lighting few aspects of mucoadhesive drug delivery systems.
ADVANTAGES OF MUCOADHESIVE DRUG DELIVERY SYSTEM
Mucoadhesive delivery systems offer several advantages over other oral controlled release systems by virtue of prolongation of residence time of drug in gastrointestinal tract (GIT).
Targeting and localization of the dosage form at a specific site.
Also, the mucoadhesive systems are known to provide intimate contact between dosage form and the absorptive mucosa, resulting in high drug flux at the absorbing tissue.[4]
Mucus Membranes
Mucus membranes (mucosae) [Figure 1] are the moist surfaces lining the walls of various body cavities such as the gastrointestinal and respiratory tracts. They consist of a connective tissue layer (the lamina propria) above which is an epithelial layer, the surface of which is made moist usually by the presence of a mucus layer. The epithelia may be either single layered (e.g. the stomach, small and large intestines and bronchi) or multilayered/stratified (e.g. in the esophagus, vagina and cornea). The former contain goblet cells which secrete mucus directly onto the epithelial surfaces; the latter contain, or are adjacent to tissues containing, specialized glands such as salivary glands that secrete mucus onto the epithelial surface. Mucus is present either as a gel layer adherent to the mucosal surface or as a luminal soluble or suspended form. The major components of all mucus gels are mucin glycoproteins, lipids, inorganic salts and water, the latter accounting for more than 95% of their weight, making them a highly hydrated system.[5] The major functions of mucus are that of protection and lubrication.
Figure 1.
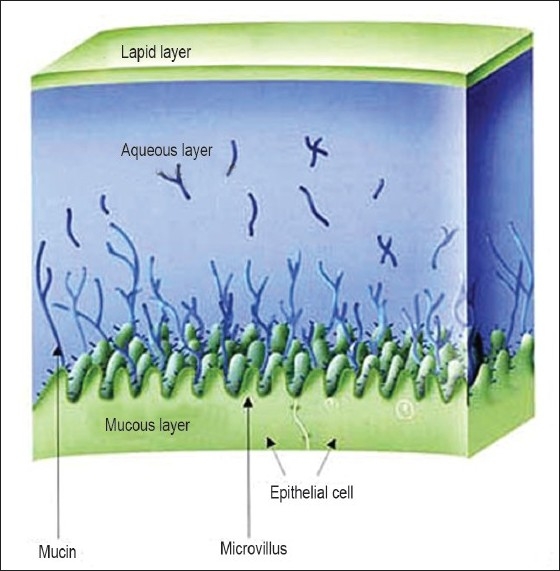
Mucus membrane structure
Mechanisms of Mucoadhesion
The mechanism of mucoadhesion is generally divided into two steps: the contact stage and the consolidation stage [Figure 2]. The first stage is characterized by the contact between the mucoadhesive and the mucus membrane, with spreading and swelling of the formulation, initiating its deep contact with the mucus layer.[6]
Figure 2.
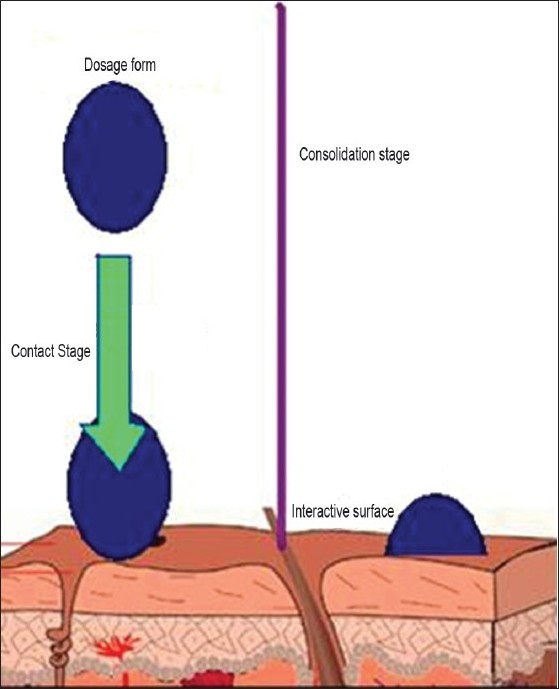
The process of contact and consolidation
In the consolidation step [Figure 2], the mucoadhesive materials are activated by the presence of moisture. Moisture plasticizes the system, allowing the mucoadhesive molecules to break free and to link up by weak van der Waals and hydrogen bonds. Essentially, there are two theories explaining the consolidation step: the diffusion theory and the dehydration theory. According to the diffusion theory, the mucoadhesive molecules and the glycoproteins of the mucus mutually interact by means of interpenetration of their chains and the building of secondary bonds. For this to take place, the mucoadhesive device has features favoring both chemical and mechanical interactions. For example, molecules with hydrogen bond building groups (–OH, –COOH), an anionic surface charge, high molecular weight, flexible chains and surface-active properties, which help in spreading throughout the mucus layer, can present mucoadhesive properties.[6]
Mucoadhesion Theories
Mucoadhesion is a complex process and numerous theories have been proposed to explain the mechanisms involved. These theories include mechanical interlocking, electrostatic, diffusion interpenetration, adsorption and fracture processes.
Wetting theory
The wetting theory applies to liquid systems which present affinity to the surface in order to spread over it. This affinity can be found by using measuring techniques such as the contact angle. The general rule states that the lower the contact angle, the greater is the affinity [Figure 3]. The contact angle should be equal or close to zero to provide adequate spreadability. The spreadability coefficient, SAB, can be calculated from the difference between the surface energies γB and γA and the interfacial energy γAB, as indicated in the equation given below.[5] This theory explains the importance of contact angle and reduction of surface and interfacial energies to achieve good amount of mucoadhesion.
Figure 3.
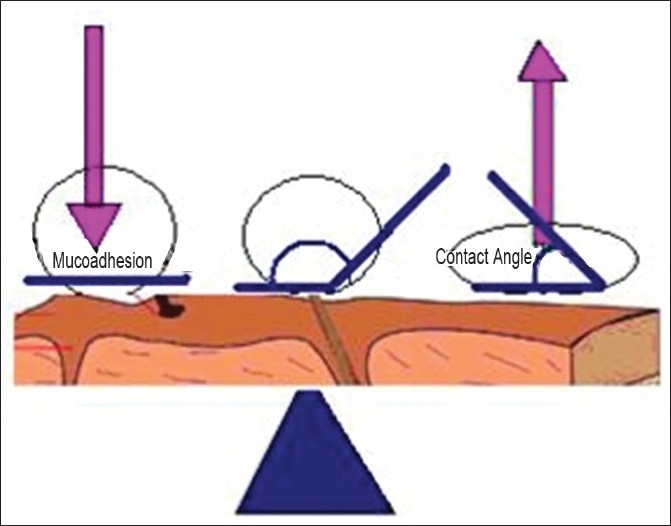
Influence of contact angle on mucoadhesion
SAB = γB – γA – γAB
Diffusion theory
Diffusion theory describes the interpenetration of both polymer and mucin chains to a sufficient depth to create a semi-permanent adhesive bond [Figure 4]. It is believed that the adhesion force increases with the degree of penetration of the polymer chains. This penetration rate depends on the diffusion coefficient, flexibility and nature of the mucoadhesive chains, mobility and contact time. According to the literature, the depth of interpenetration required to produce an efficient bioadhesive bond lies in the range 0.2–0.5 μm. This interpenetration depth of polymer and mucin chains can be estimated by the following equation:[5]
Figure 4.
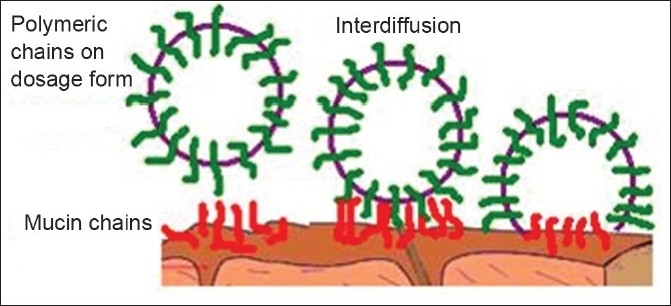
Secondary interaction between mucoadhesive device and of mucus
l = (tDb)½
where t is the contact time and Db is the diffusion coefficient of the mucoadhesive material in the mucus. The adhesion strength for a polymer is reached when the depth of penetration is approximately equivalent to the polymer chain size. In order for diffusion to occur, it is important that the components involved have good mutual solubility, that is, both the bioadhesive and the mucus have similar chemical structures. The greater the structural similarity, the better is the mucoadhesive bond.[5]
Fracture theory
This is perhaps the most used theory in studies on the mechanical measurement of mucoadhesion. It analyzes the force required to separate two surfaces after adhesion is established. This force, sm, is frequently calculated in tests of resistance to rupture by the ratio of the maximal detachment force, Fm, and the total surface area, A0, involved in the adhesive interaction
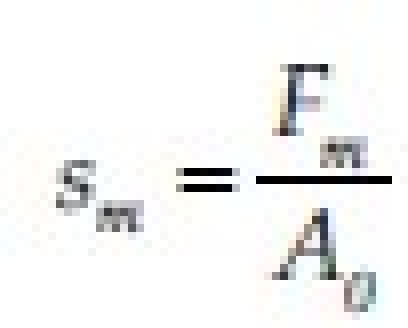
Since the fracture theory [Figure 5] is concerned only with the force required to separate the parts, it does not take into account the interpenetration or diffusion of polymer chains. Consequently, it is appropriate for use in the calculations for rigid or semi-rigid bioadhesive materials, in which the polymer chains do not penetrate into the mucus layer.[5,6]
Figure 5.
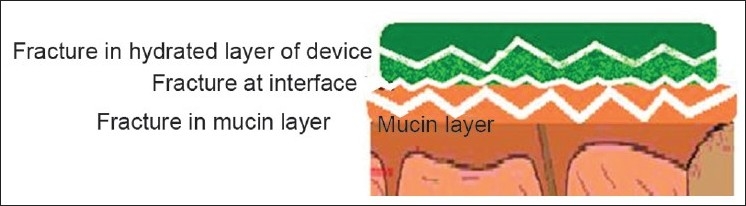
Fractures occurring for mucoadhesion
The electronic theory
This theory describes adhesion occurring by means of electron transfer between the mucus and the mucoadhesive system, arising through differences in their electronic structures. The electron transfer between the mucus and the mucoadhesive results in the formation of double layer of electrical charges at the mucus and mucoadhesive interface. The net result of such a process is the formation of attractive forces within this double layer.[7]
The adsorption theory
In this instance, adhesion is the result of various surface interactions (primary and secondary bonding) between the adhesive polymer and mucus substrate. Primary bonds due to chemisorptions result in adhesion due to ionic, covalent and metallic bonding, which is generally undesirable due to their permanency.[8] Secondary bonds arise mainly due to van der Waals forces, hydrophobic interactions and hydrogen bonding. Whilst these interactions require less energy to “break”, they are the most prominent form of surface interaction in mucoadhesion processes as they have the advantage of being semi-permanent bonds.[9]
All these numerous theories should be considered as supplementary processes involved in the different stages of the mucus/substrate interaction, rather than individual and alternative theories. Each and every theory is equally important to describe the mucoadhesion process. There is a possibility that there will be initial wetting of the mucin, and then diffusion of the polymer into mucin layer, thus causing the fracture in the layers to effect the adhesion or electronic transfer or simple adsorption phenomenon that finally leads to the perfect mucoadhesion. The mechanism by which a mucoadhesive bond is formed will depend on the nature of the mucus membrane and mucoadhesive material, the type of formulation, the attachment process and the subsequent environment of the bond. It is apparent that a single mechanism for mucoadhesion proposed in many texts is unlikely for all the different occasions when adhesion occurs.
Factors Affecting Mucoadhesion
Molecular weight
The mucoadhesive strength of a polymer increases with molecular weights above 100,000. Direct correlation between the mucoadhesive strength of polyoxyethylene polymers and their molecular weights lies in the range of 200,000–7,000,000.[10]
Flexibility
Mucoadhesion starts with the diffusion of the polymer chains in the interfacial region. Therefore, it is important that the polymer chains contain a substantial degree of flexibility in order to achieve the desired entanglement with the mucus.[11] The increased chain interpenetration was attributed to the increased structural flexibility of the polymer upon incorporation of polyethylene glycol. In general, mobility and flexibility of polymers can be related to their viscosities and diffusion coefficients, as higher flexibility of a polymer causes greater diffusion into the mucus network.[12]
Cross-linking density
The average pore size, the number and average molecular weight of the cross-linked polymers, and the density of cross-linking are three important and inter-related structural parameters of a polymer network. Therefore, it seems reasonable that with increasing density of cross-linking, diffusion of water into the polymer network occurs at a lower rate which, in turn, causes an insufficient swelling of the polymer and a decreased rate of interpenetration between polymer and mucin.[12]
Hydrogen bonding capacity
Hydrogen bonding is another important factor in mucoadhesion of a polymer. Desired polymers must have functional groups that are able to form hydrogen bonds, and flexibility of the polymer is important to improve this hydrogen bonding potential.[12] Polymers such as poly(vinyl alcohol), hydroxylated methacrylate, and poly(methacrylic acid), as well as all their copolymers, have good hydrogen bonding capacity.[13]
Hydration
Hydration is required for a mucoadhesive polymer to expand and create a proper macromolecular mes of sufficient size, and also to induce mobility in the polymer chains in order to enhance the interpenetration process between polymer and mucin. Polymer swelling permits a mechanical entanglement by exposing the bioadhesive sites for hydrogen bonding and/or electrostatic interaction between the polymer and the mucus network.[12] However, a critical degree of hydration of the mucoadhesive polymer exists where optimum swelling and mucoadhesion occurs.[13]
Charge
Some generalizations about the charge of bioadhesive polymers have been made previously, where nonionic polymers appear to undergo a smaller degree of adhesion compared to anionic polymers. Strong anionic charge on the polymer is one of the required characteristics for mucoadhesion.[13] Some cationic polymers are likely to demonstrate superior mucoadhesive properties, especially in a neutral or slightly alkaline medium.[14] Additionally, some cationic high–molecular-weight polymers, such as chitosan, have shown to possess good adhesive properties.[15] There is no significant literature about the influence of the charge of the membrane on the mucoadhesion but the pH of the membrane affects the mucoadhesion as it can influence the ionized or un-ionized forms of the polymers.[16]
Concentration
The importance of this factor lies in the development of a strong adhesive bond with the mucus, and can be explained by the polymer chain length available for penetration into the mucus layer. When the concentration of the polymer is too low, the number of penetrating polymer chains per unit volume of the mucus is small and the interaction between polymer and mucus is unstable. In general, the more concentrated polymer would result in a longer penetrating chain length and better adhesion. However, for each polymer, there is a critical concentration, above which the polymer produces an “unperturbed” state due to a significantly coiled structure. As a result, the accessibility of the solvent to the polymer decreases, and chain penetration of the polymer is drastically reduced. Therefore, higher concentrations of polymers do not necessarily improve and, in some cases, actually diminish mucoadhesive properties. One of the studies addressing this factor demonstrated that high concentrations of flexible polymeric films based on polyvinylpyrrolidone or poly(vinyl alcohol) as film-forming polymers did not further enhance the mucoadhesive properties of the polymer.[17]
Sites for Mucoadhesive Drug Delivery Systems
The common sites of application where mucoadhesive polymers have the ability to deliver pharmacologically active agents include oral cavity, eye conjunctiva, vagina, nasal cavity and GIT.
The buccal cavity has a very limited surface area of around 50 cm2 but the easy access to the site makes it a preferred location for delivering active agents. The site provides an opportunity to deliver pharmacologically active agents systemically by avoiding hepatic first-pass metabolism in addition to the local treatment of the oral lesions.
The sublingual mucosa is relatively more permeable than the buccal mucosa due to the presence of large number of smooth muscle and immobile mucosa. Hence, formulations for sublingual delivery are designed to release the active agent quickly while mucoadhesive formulation is of importance for the delivery of active agents to the buccal mucosa, where the active agent has to be released in a controlled manner. This makes the buccal cavity more suitable for mucoadhesive drug delivery.[18] The various mucoadhesive polymers used for the development of buccal delivery systems include cyanoacrylates, polyacrylic acid, sodium carboxymethylcellulose, hyaluronic acid, hydroxypropylcellulose, polycarbophil, chitosan and gellan. The delivery systems are generally coated with a drug and water impermeable film so as to prevent the washing of the active agent by the saliva.[19]
Like buccal cavity, nasal cavity also provides a potential site for the development of formulations where mucoadhesive polymers can play an important role. The nasal mucosal layer has a surface area of around 150–200 cm2. The residence time of a particulate matter in the nasal mucosa varies between 15 and 30 min, which has been attributed to the increased activity of the mucociliary layer in the presence of foreign particulate matter. The polymers used in the development of formulations for the development of nasal delivery system include copolymer of methyl vinyl ether, hydroxypropylmethylcellulose (HPMC), sodium carboxymethylcellulose, carbopol-934P and Eudragit RL-100.[20,21]
Due to the continuous formation of tears and blinking of eye lids, there is a rapid removal of the active medicament from the ocular cavity, which results in the poor bioavailability of the active agents. This can be minimized by delivering the drugs using ocular insert or patches. The mucoadhesive polymers used for the ocular delivery include thiolated poly(acrylic acid), poloxamer, celluloseacetophthalate, methyl cellulose, hydroxy ethyl cellulose, poly(amidoamine) dendrimers, poly(dimethyl siloxane) and poly(vinyl pyrrolidone).[22,23]
The vaginal and the rectal lumen have also been explored for the delivery of the active agents both systemically and locally. The active agents meant for the systemic delivery by this route of administration bypass the hepatic first-pass metabolism. Quite often, the delivery systems suffer from migration within the vaginal/rectal lumen, which might affect the delivery of the active agent to the specific location. The use of mucoadhesive polymers for the development of delivery system helps in reducing the migration of the same, thereby promoting better therapeutic efficacy. The polymers used in the development of vaginal and rectal delivery systems include mucin, gelatin, polycarbophil and poloxamer.[24–26]
GIT is also a potential site which has been explored for a long time for the development of mucoadhesive based formulations. The modulation of the transit time of the delivery systems in a particular location of the gastrointestinal system by using mucoadhesive polymers has generated much interest among researchers around the world. The various mucoadhesive polymers which have been used for the development of oral delivery systems include chitosan, poly(acrylic acid), alginate, poly(methacrylic acid) and sodium carboxymethyl cellulose.[27]
Each site of mucoadhesion has its own advantages and disadvantages along with the basic property of prolonged residence of dosage form at that particular site. In buccal and sublingual sites, there is an advantage of fast onset along with bypassing the first-pass metabolism, but these sites suffer from inconvenience because of taste and intake of food. In GIT, there is a chance for improved amount of absorption because of microvilli, but it has a drawback of acid instability and first-pass effects. Rectal and vaginal sites are the best ones for the local action of the drug but they suffer from inconvenience of administration. Nasal and ophthalmic routes have another drawback of mucociliary drainage that would clear the dosage form from the site.
Mucoadhesive Dosage Forms
Tablets
Tablets are small, flat, and oval, with a diameter of approximately 5–8 mm.[28] Unlike the conventional tablets, mucoadhesive tablets allow for drinking and speaking without major discomfort. They soften, adhere to the mucosa, and are retained in position until dissolution and/or release is complete. Mucoadhesive tablets, in general, have the potential to be used for controlled release drug delivery, but coupling of mucoadhesive properties to tablet has additional advantages, for example, it offers efficient absorption and enhanced bioavailability of the drugs due to a high surface to volume ratio and facilitates a much more intimate contact with the mucus layer. Mucoadhesive tablets can be tailored to adhere to any mucosal tissue including those found in stomach, thus offering the possibilities of localized as well as systemic controlled release of drugs. The application of mucoadhesive tablets to the mucosal tissues of gastric epithelium is used for administration of drugs for localized action. Mucoadhesive tablets are widely used because they release the drug for a prolonged period, reduce frequency of drug administration and improve the patient compliance. The major drawback of mucoadhesive tablets is their lack of physical flexibility, leading to poor patient compliance for long-term and repeated use.[29–31]
Films
Mucoadhesive films may be preferred over adhesive tablets in terms of flexibility and comfort. In addition, they can circumvent the relatively short residence time of oral gels on the mucosa, which are easily washed away and removed by saliva. Moreover, in the case of local delivery for oral diseases, the films also help protect the wound surface, thus helping to reduce pain, and treat the disease more effectively. An ideal film should be flexible, elastic, and soft, yet adequately strong to withstand breakage due to stress from mouth movements. It must also possess good mucoadhesive strength in order to be retained in the mouth for the desired duration of action. Swelling of film, if it occurs, should not be too extensive in order to prevent discomfort.[32]
Patches
Patches are laminates consisting of an impermeable backing layer, a drug-containing reservoir layer from which the drug is released in a controlled manner, and a mucoadhesive surface for mucosal attachment. Patch systems are similar to those used in transdermal drug delivery. Two methods used to prepare adhesive patches include solvent casting and direct milling. In the solvent casting method, the intermediate sheet from which patches are punched is prepared by casting the solution of the drug and polymer(s) onto a backing layer sheet, and subsequently allowing the solvent(s) to evaporate. In the direct milling method, formulation constituents are homogeneously mixed and compressed to the desired thickness, and patches of predetermined size and shape are then cut or punched out. An impermeable backing layer may also be applied to control the direction of drug release, prevent drug loss, and minimize deformation and disintegration of the device during the application period.[33,34]
Gels and ointments
Semisolid dosage forms, such as gels and ointments, have the advantage of easy dispersion throughout the oral mucosa. However, drug dosing from semisolid dosage forms may not be as accurate as from tablets, patches, or films. Poor retention of the gels at the site of application has been overcome by using mucoadhesive formulations. Certain mucoadhesive polymers, for example, sodium carboxymethylcellulose,[35] carbopol,[36] hyaluronic acid,[37] and xanthan gum,[38] undergo a phase change from liquid to semisolid. This change enhances the viscosity, which results in sustained and controlled release of drugs. Hydrogels are also a promising dosage form for buccal drug delivery. They are formed from polymers that are hydrated in an aqueous environment and physically entrap drug molecules for subsequent slow release by diffusion or erosion.[39] The application of mucoadhesive gels provides an extended retention time in the oral cavity, adequate drug penetration, as well as high efficacy and patient acceptability. A major application of adhesive gels is the local delivery of medicinal agents for the treatment of periodontitis, which is an inflammatory and infectious disease that causes formation of pockets between the gum and the tooth, and can eventually cause loss of teeth. It has been suggested that mucoadhesive polymers might be useful for periodontitis therapy when incorporated in antimicrobial-containing formulations that are easily introduced into the periodontal pocket with a syringe.[40–42] HPMC has been used as an adhesive ointment ingredient. Additionally, a highly viscous gel was developed from carbopal and hydroxypropylcellulose for ointment dosage forms that could be maintained on the tissue for up to 8 hours.[2]
CONCLUSION
This overview about the mucoadhesive dosage forms might be a useful tool for the efficient design of novel mucoadhesive drug delivery systems. Mucoadhesive drug delivery systems have applications from different angles, including development of novel mucoadhesives, design of the device, mechanisms of mucoadhesion and permeation enhancement. With the influx of a large number of new drug molecules due to drug discovery, mucoadhesive drug delivery will play an even more important role in delivering these molecules.
ACKNOWLEDGMENTS
The authors wish to thank the Management and HOD, Department of Pharmaceutics, Nalanda College of Pharmacy, Nalgonda, AP, India, and also Faculty of Pharmacy, Osmania University, for providing facilities to carry out this review work.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Chickering DE, III, Mathiowitz E. Fundamentals of bioadhesion. In: Lehr CM, editor. Bioadhesive drug delivery systems-Fundamentals, Novel Approaches and Development. New York: Marcel Dekker; 1999. pp. 1–85. [Google Scholar]
- 2.Ahuja A, Khar RK, Ali J. Mucoadhesive drug delivery systems. Drug Dev Ind Pharm. 1997;23:489–515. [Google Scholar]
- 3.Veuillez F, Kalia YN, Jacques Y, Deshusses J, Buri P. Factors and strategies for improving buccal absorption of peptides. Eur J Pharm Biopharm. 2001;51:93–109. doi: 10.1016/s0939-6411(00)00144-2. [DOI] [PubMed] [Google Scholar]
- 4.Punitha S, Girish Y. Polymers in mucoadhesive buccal drug delivery system: A review. Int J Res Pharm Sci. 2010;1:170–86. [Google Scholar]
- 5.Smart JD. The basics and underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev. 2005;57:1556–68. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Hägerström H, Edsman K, Strømme M. Low-frequency dielectric spectroscopy as a tool for studying the compatibility between pharmaceutical gels and mucus tissue. J Pharm Sci. 2003;92:1869–81. doi: 10.1002/jps.10451. [DOI] [PubMed] [Google Scholar]
- 7.Dodou D, Breedveld P, Wieringa P. Mucoadhesives in the gastrointestinal tract: Revisiting the literature for novel applications. Eur J Pharm Biopharm. 2005;60:1–16. doi: 10.1016/j.ejpb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Kinloch AJ. The science of adhesion. J Mater Sci. 1980;15:2141–66. [Google Scholar]
- 9.Jiménez-Castellanos MR, Zia H, Rhodes CT. Mucoadhe-sive drug delivery systems. Drug Dev Ind Pharm. 1993;19:143–94. [Google Scholar]
- 10.Tiwari D, Goldman D, Sause R, Madan PL. Evaluation of polyoxyethylene homopolymers for buccal bioadhesive drug delivery device formulations. AAPS Pharm Sci. 1999;1:13–21. doi: 10.1208/ps010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Leobandung W, Foss A, Peppas NA. Molecular aspects of muco- and bioadhesion: Tethered structures and site-specific surfaces. J Control Release. 2000;65:63–71. doi: 10.1016/s0168-3659(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 12.Gu JM, Robinson JR, Leung SH. Binding of acrylic polymers to mucin/epithelial surfaces: Structure–property relationships. Crit Rev Ther Drug Carrier Syst. 1998;5:21–67. [PubMed] [Google Scholar]
- 13.Peppas NA, Buri PA. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Control Release. 1985;2:257–75. [Google Scholar]
- 14.Park H, Amiji M, Park K. Mucoadhesive hydrogels effective at neutral pH. Proc Int Symp Control Release Bioact Mater. 1989;16:217–8. [Google Scholar]
- 15.Lehr CM, Bouwstra JA, Schacht EH, Junginger HE. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm. 1992;78:43–8. [Google Scholar]
- 16.Smart JD, Mortazavi SA. An investigation of the pH within the hydrating gel layer of a poly(acylic acid) compact. J Pharm Pharmacol. 1995;47:1099. [Google Scholar]
- 17.Solomonidou D, Cremer K, Krumme M, Kreuter J. Effect of carbomer concentration and degree of neutralization on the mucoadhesive properties of polymer films. J Biomater Sci Polym Ed. 2001;12:1191–205. doi: 10.1163/156856201753395743. [DOI] [PubMed] [Google Scholar]
- 18.Shojaei AH. Buccal mucosa as a route for systemic drug delivery: A review. J Pharm Pharm Sci. 1998;1:15–30. [PubMed] [Google Scholar]
- 19.Remuñán-López C, Portero A, Vila-Jato JL, Alonso MJ. Design and evaluation of chitosan/ethylcellulose mucoadhesive bilayered devices for buccal drug delivery. J Control Release. 1998;55:143–52. doi: 10.1016/s0168-3659(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 20.Semalty M, Semalty A, Kumar G. Formulation and characterization of mucoadhesive buccal films of glipizide. Indian J Pharm Sci. 2008;70:43–8. doi: 10.4103/0250-474X.40330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornof M, Weyenberg W, Ludwig A, Bernkop SA. Mucoadhesive ocular insert based on thiolated poly (acrylic acid): Development and in vivo evaluation in humans. J Control Release. 2003;89:419–28. doi: 10.1016/s0168-3659(03)00135-4. [DOI] [PubMed] [Google Scholar]
- 22.Sultana Y, Aqil M, Ali A. Ocular inserts for controlled delivery of pefloxacin mesylate: Preparation and evaluation. Acta Pharm. 2005;55:305–14. [PubMed] [Google Scholar]
- 23.Wagh VD, Inamdar B, Samanta MK. Polymers used in ocular dosage form and drug delivery systems. Asian J Pharmaceutics. 2008;2:12–7. [Google Scholar]
- 24.Elhadi SS, Mortada ND, Awad GA, Zaki NM, Taha RA. Development of in situ gelling and mucoadhesive mebeverine hydrochloride solution for rectal administration. Saudi Pharm J. 2003;11:150–71. [Google Scholar]
- 25.Neves JD, Amaral MH, Bahia MF. Vaginal drug delivery. In: Gad SC, editor. Pharmaceutical Manufacturing Handbook. NJ: John Willey and Sons Inc; 2007. pp. 809–78. [Google Scholar]
- 26.Choi HG, Kim CK. In situ gelling and mucoadhesive liquid suppository containing acetaminophen: Enhanced bioavailability. Int J Pharm. 1998;165:23–32. [Google Scholar]
- 27.Asane GS. Mucoadhesive gastro intestinal drug delivery system: An overview. Pharmainfo.net. 2007;5:1–5. [Google Scholar]
- 28.Schnürch AB. Mucoadhesive systems in oral drug delivery. Drug Discov Today Technol. 2005;2:83–7. doi: 10.1016/j.ddtec.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Rathbone MJ, Drummond BK, Tucker G. The oral cavity as a site for systemic drug delivery. Adv Drug Deliv Rev. 1994;13:1–22. [Google Scholar]
- 30.Rajput GC, Majmudar FD, Patel JK, Patel KN, Thakor RS, Patel BP, et al. Stomach specific mucoadhesive tablets as controlled drug delivery system: A review work. Int J Pharm Biol Res. 2010;1:30–41. [Google Scholar]
- 31.Remeth D, Sfurti S, Kailas M. In-vitro absorption studies of mucoadhesive tablets of acyclovir. Indian J Pharm Educ Res. 2010;44:183–8. [Google Scholar]
- 32.Shah D, Gaud RS, Misra AN, Parikh R. Formulation of a water soluble mucoadhesive film of lycopene for treatment of leukoplakia. Int J Pharm Sci Rev Res. 2010;12:6–11. [Google Scholar]
- 33.Biswajit B, Kevin G, Thimmasetty J. Formulation and evaluation of pimozide buccal mucoadhesive patches. Int J Pharm Sci Nanotechnol. 2010;2:32–41. [Google Scholar]
- 34.Wong CF, Yuen KH, Peh KK. Formulation and evaluation of controlled release Eudragit buccal patches. Int J Pharm. 1999;178:11–22. doi: 10.1016/s0378-5173(98)00342-1. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S, Haglund BO, Himmelstein KJ. In situ-forming gels for ophthalmic drug delivery. J Ocul Pharmacol. 1994;10:47–56. doi: 10.1089/jop.1994.10.47. [DOI] [PubMed] [Google Scholar]
- 36.Ishida M, Nambu N, Nagai T. Highly viscous gel ointment containing carbopol for application to the oral mucosa. Chem Pharm Bull. 1983;31:4561–4. doi: 10.1248/cpb.31.4561. [DOI] [PubMed] [Google Scholar]
- 37.Gurny R, Ryser JE, Tabatabay C, Martenet M, Edman P, Camber O. Precorneal residence time in humans of sodium hyaluronate as measured by gamma scintigraphy. Graefes Arch Clin Exp Ophthalmol. 1990;228:510–2. doi: 10.1007/BF00918481. [DOI] [PubMed] [Google Scholar]
- 38.Meseguer G, Gurny R, Buri P. Gamma scintigraphic evaluation of precorneal clearance in human volunteers and in rabbits. Eur J Drug Metab Pharmacokinet. 1993;18:190–4. [Google Scholar]
- 39.Martin L, Wilson CG, Koosha F, Uchegbu IF. Sustained buccal delivery of the hydrophobic drug denbufylline using physically cross-linked palmitoyl glycol chitosan hydrogels. Eur J Pharm Biopharm. 2003;55:35–45. doi: 10.1016/s0939-6411(02)00118-2. [DOI] [PubMed] [Google Scholar]
- 40.Jones DS, Woolfson AD, Brown AF, Coulter WA, McClelland C, Irwin CR. Design, characterisation and preliminary clinical evaluation of a novel mucoadhesive topical formulation containing tetracycline for the treatment of periodontal disease. J Control Release. 2000;67:357–68. doi: 10.1016/s0168-3659(00)00231-5. [DOI] [PubMed] [Google Scholar]
- 41.Vinholis AH, De Figueiredo LC, Marcantonio E, Marcantonio RA, Salvador SL, Goissis G. Subgingival utilization of a 1% chlorhexidine collagen gel for the treatment of periodontal pockets. A clinical and microbiological study. Braz Dent J. 2001;12:209–13. [PubMed] [Google Scholar]
- 42.Ikinci G, Kenel SS, AkVncVbay H, Kas S, Ercis S, Wilson CG, et al. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis. Int J Pharm. 2002;235:121–7. doi: 10.1016/s0378-5173(01)00974-7. [DOI] [PubMed] [Google Scholar]


