Abstract
The formazan derivatives (FM1–FM5) were synthesized by the reaction of benzaldehyde phenylhydrazone with substituted aromatic and hetero aromatic amines. The structures of the synthesized compounds were then elucidated using UV, IR, 1H NMR and mass spectral data. The synthesized derivatives were screened for anticonvulsant, antibacterial and antiviral activities. All the compounds showed remarkable antibacterial activity at 250 μg/ml, but FM4 and FM3 did not show any inhibition on Staphylococcus aureus and Vibriocholera, respectively. All the compounds showed significant anticonvulsant effect at 100 mg/kg p.o. and the experimental data were statistically significant at P<0.001 level. But none of the compounds was effective against Japanese encephalitis virus.
Keywords: Anticonvulsant, antibacterial, antiviral, formazan, Ranikhet disease virus
INTRODUCTION
Formazans have been found to possess important medical applications due to their various activities such as antiviral,[1] antimicrobial,[2,3] anti-inflammatory, analgesic,[4] antifungal,[5] anticancer, anti-HIV,[6] etc. Several formazans showed promising anti-fertility,[7] anti-parkinsonian[8] and anticonvulsant activities.[9] The antiviral activity has been shown by formazan derivatives in both plants and animals. Various authors have reported that this class of compounds is active against the Ranikhet disease virus, Tobacco mosaic virus (TMV) and Gomphrena mosaic virus (GMV).[10] The antiviral properties of some formazan derivatives have been reported in which the antiviral effect is attributed to the presence of an intact C=NNH grouping and C–N=N grouping [Figure 1].[11] Formazan derivatives have been widely used to evaluate cell viability and to screen anti-HIV agents and the cytotoxicity of these agents. Synthetic formazan effectively inhibited the replication of laboratory-adapted and primary HIV-1 isolates and cell-to-cell fusion, with low cytotoxicity. It blocks the six-helix bundle formation between peptides derived from the N- and C-terminal heptad repeat regions of the gp41 ectodomain of HIV-1.[12,13] It has been reported that members of a series of formazan are used for detecting and quantifying the presence of a target molecule, such as an antigen, an antibody or a polynucleotide. The crown formazan derivative, used as a replicase inhibitor specific to the bovine viral diarrhea virus, has been recently identified.[14] Moreover, they have been studied extensively because of their ready accessibility, diverse chemical reactivity and broad spectrum of biological activities.[15,16]
Figure 1.
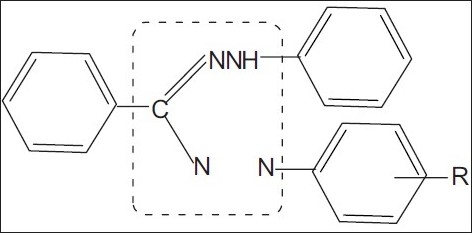
Formazan basic skeleton
Taking into consideration the referred important biological activities of formazan derivatives, our study was designed to synthesize some potent molecule for biological screening.
MATERIALS AND METHODS
Instruments and Equipments
The melting points were determined using open capillary tubes and are uncorrected. The lambda max of the compounds was measured by UV–visible spectrophotometer (UV-Pharma Spec 1700, Shimadzu,Kyoto,Japan). The infrared (IR) spectra were recorded on FT-IR8400S, Fourier Transform (Shimadzu) Infrared spectrophotometer using KBr disk method. The proton magnetic resonance spectra were recorded on Perkin Elmer spectrophotometer [300 MHz in dimethyl sulfoxide (DMSO)-d6] using Tetramethylsilane as an internal standard and chemical shifts were expressed in δ ppm. The mass spectra were recorded on a JEOL SX-102 (FAB) spectrometer.
Chemicals and Drugs
All the chemicals and reagents were of synthetic grade and commercially procured from s.d. Fine Chem. Ltd. (Mumbai, India). Diazepam (Calmpose inj. Ranbaxy,gurgaon India) was purchased from local medical stores (Majhitar, East Sikkim). Streptomycin was obtained from Alkem Pvt. Ltd. (East Sikkim, India) as a gift sample.
Animals
Male Swiss Albino mice weighing 22–25 g were used for the pharmacological and toxicological studies. Animals were housed in groups of 6–8 per cage at a temperature of 25±1°C and a relative humidity of 45–55%. A 12:12 dark:light cycle was followed during the experiments. Animals had free access to food and water ad libitum. During the study period, guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Institutional Animals Ethics Committee (IAEC), were followed for the maintenance of animals.
Preparation of benzaldehyde phenylhydrazone derivatives
0.01 mol of phenyl hydrazine was added dropwise to a well-stirred mixture of 0.01 mol benzaldehyde in dilute acetic acid (2 ml in 10 ml water) in a 100 ml conical flask at room temperature. The reaction mixture was further stirred for 1 hour and kept at room temperature for 30 minutes. The precipitated yellow crystalline mass was filtered and dried in an oven at 60°C. The crude product was recrystallized from rectified spirit with charcoal treatment.[17] Benzaldehyde phenylhydrazone was obtained as fine colorless needles, with melting point 156°C, and % yield was 80%.
Synthesis of 1-phenyl-3-phenyl-5-[aryl/heteroaryl] formazan derivatives
0.01 mol of substituted aryl amine was dissolved in a mixture of 5 ml concentrated hydrochloric acid and 5 ml water taken in a 100 ml conical flask, with constant stirring. The reaction mixture was cooled in ice bath until the temperature fell below 5°C. Separately, 1.6 g of sodium nitrite was dissolved in 7.5 ml of water and chilled in an ice bath below 5°C. The sodium nitrite solution was filtered to obtain a clear solution and then added dropwise to the aniline mixture with vigorous shaking and the temperature was not allowed to rise above 10°C. This diazonium salt solution of aryl and heteroaryl amine was filtered to obtain a clear solution and then added dropwise with continuous stirring to a solution of benzaldehyde phenyl hydrazone (0.01 mol) in pyridine (20 ml), maintaining the temperature below 10°C. The reaction mixture was allowed to stand for about 4 hours and was then poured into 250 ml of ice-cold water with continuous stirring. The dark colored solid which separated out was filtered, washed successively with cold water followed by hot water, finally with methanol and dried in air as well. The formazans thus synthesized were recrystallized from the mixture of chloroform and petroleum ether.[18] The scheme of synthesis is given in Figure 2 and the physicochemical data of the title compounds are presented in Table 1.
Figure 2.
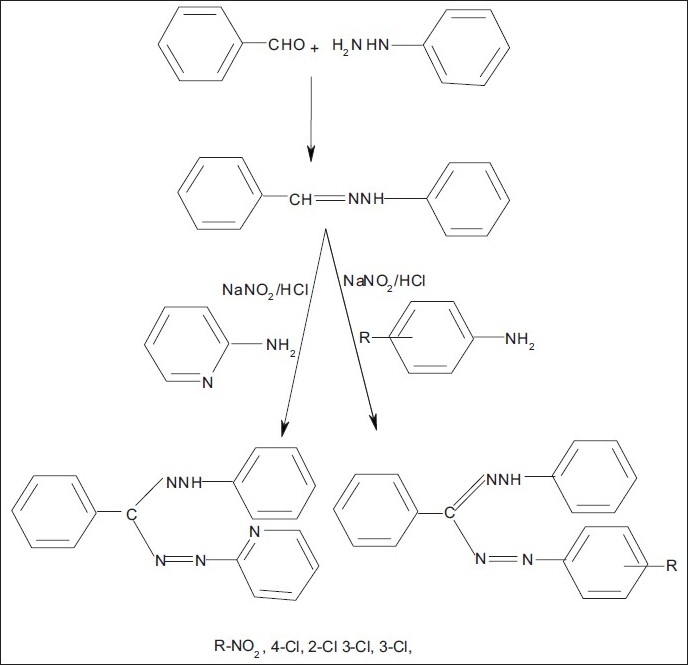
Scheme of synthesis
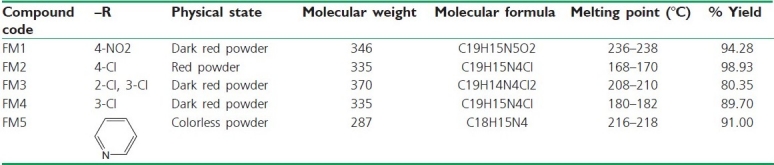
Table 1.
Physicochemical data of the synthesized compounds
FM1: 1-phenyl-3-phenyl-5-[4-nitrophenyl] formazan
λ max (nm) ethanol: 468; IR (KBr, cm-1): 3470 (N–H), 1635 (C=N), 1593 (N=N); 1H NMR (CDCl3): d 14.6 (s, 1H, NH), 7.25–7.51 (m, 5H, Ar–H), 7.94–8.91 (m, 5H, Ar–H), 8.24–8.27 (m, 4H, Ar–H); MS: m/z 346, 346 [M]+, 345 [M – 1]+, 344 [M – 2]+.
FM2: 1-phenyl-3-phenyl-5-[4-chlrophenyl] formazan
λ max (nm) ethanol: 484; IR (KBr, cm-1): 3446 (N–H str.), 1364 (C=N str.), 1593 (N=N); 1H NMR (CDCl3): δ 15.35 (s, 1H, NH), 7.05–7.45 (m, 5H, Ar–H), 7.55–7.83 (m, 5H, Ar–H), 7.91–8.21 (m, 5H, Ar–H); MS: m/z 335 [M]+, [M – 2]+, 333.
FM3: 1-phenyl-3-phenyl-5-[2,3-dichlorophenyl] formazan
λ max (nm): ethanol 344; IR (KBr, cm-1): 3313 (N–H str.), 1655 (C=N str.), 1593 (N=N str.); 1H NMR (CDCl3): δ 14.25 (s, 1H, NH), 6.84–6.96 (m, 4H, Ar–H), 7.08–7.3 (m, 5H, Ar–H), 7.33–7.37 (m, 5H, Ar–H); MS: m/z 287 [M]+, 286 [M – 1]+, 285 [M – 2]+.
FM4: 1-phenyl-3-phenyl-5-[3-chlrophenyl] formazan
λ max (nm) ethanol: 476; IR (KBr,cm-1): 3450 (N–H str.), 1654 (C=N str.), 1581 (N=N str.); 1H NMR (CDCl3): δ 15.31 (s, 1H, NH), 7.90–8.31 (m, 5H, Ar–H), 7.01–7.62 (m, 5H, Ar–H), 6.6–6.9 (m, 3H, Ar–H); MS: m/z 370 [M]+, 369 [M – 1]+.
FM5: 1-phenyl-3-phenyl-5-[2-pyridyl] formazan λ max (nm) ethanol: 473; IR (KBr, cm–1)
3502 (N–H str.), 1653 (C=N str.), 1589 (N=N str.); 1H NMR (CDCl3): δ 16.23 (s, 1H, NH), 7.71–8.27 (m, 5H), 7.24–7.61 Ar–H (m, 5H, Ar–H), 6.73–7.50 (m, 4H, Ar–H); MS: m/z 335 [M]+, 333[M – 2]+.
Acute Toxicity Studies
Groups of six albino mice, weighing 20–25 g, were fasted overnight and treated perorally with the test compounds.[19,20] The dosage was varied from 100 to 1000 mg/kg body weight. The animals were observed for 24 hours for any signs of acute toxicity such as increased or decreased motor activity, tremors, convulsion, sedation, lacrimation, etc. All the animal experiments were performed on obtaining the approval of Institutional Animal Ethics Committee, Himalayan Pharmacy Institute, East Sikkim, India (IAECNo: HPI/10/59/IAEC/0081).
Antibacterial Activity
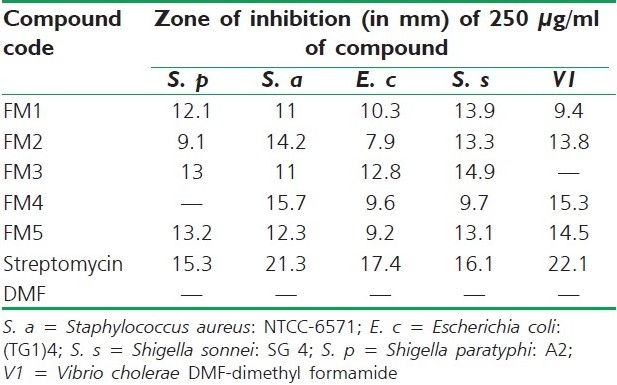
The antibacterial activity was determined using agar cup-plate method.[21] Exactly 20 ml of sterile nutrient agar medium was poured into sterile petri dishes and allowed to solidify. The petri dishes were incubated at 37°C for 24 hours to check for sterility. The medium was seeded with the micro-organisms by pour plate method using sterile top agar (4 ml) containing 1 ml culture. The bores were made on the medium using sterile borer. Test compounds were dissolved in DMSO and 0.1 ml (250 μg/ml) of the different test compounds was added to the respective bores. 0.1 ml of streptomycin at a concentration of 250 μg/ml was taken as standard reference and 0.1 ml of DMSO was used as control. The plates were incubated overnight at 37°C with appropriate positive and negative controls. The petri dishes were kept in refrigerator at 4°C for half an hour for diffusion. Then, the petri dishes were incubated at 37°C for 24 hours and zone of inhibition were observed and measured in millimeters. The results of determination of antibacterial activity are summarized in Table 2 with that of the standard drug.
Table 2.
Zone of inhibition (in mm) of 250 μg/ml of the compound against bacteria
Anticonvulsant Activity (Maximum Electroshock Method)
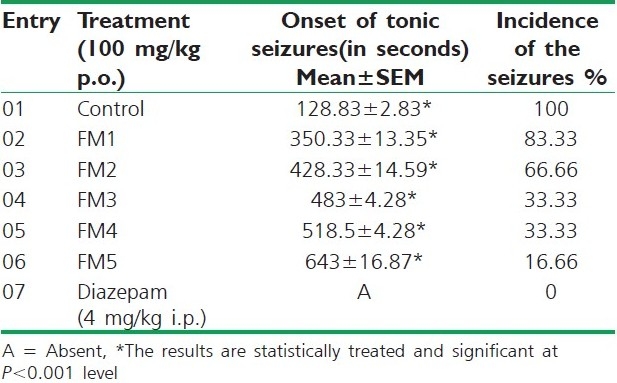
Several animal models of convulsions have been developed to evaluate anti-seizure activity. Many drugs that increase the brain contents of gamma aminobutyric acid (GABA) have exhibited anticonvulsant activity against seizures induced by maximum electroshock (MES), pentylenetetrazol (PTZ) and lithium-pilocarpine (Li-Pilo). The MES is probably the best validated method for assessment of anti-epileptic drugs in generalized tonic–clonic seizures.[22,23] Male Swiss albino mice (22–25 g) of either sex, maintained in standard conditions for temperature, relative humidity, light/day cycles and fed with normal diet and water ad libitum, were used. All the synthesized compounds were screened for their anticonvulsant activity using electroconvulsiometer. Maximal seizures were induced by application of an electrical current across the brain via corneal electrodes. Diazepam 4 mg/kg i.p. was used as standard drug. It was administered at a dose of 100 mg/kg p.o. in 0.5% Carboxymethyl cellulose to groups of mice (n=6), 30 minutes before the application of electric shock (42 mA, 0.2 seconds) using corneal electrodes. After 30 minutes and 4 hours of drug administration, electroshock was applied using corneal electrodes. The disappearance of the hind leg extensor component of convulsion was used as a positive criterion. The incidence and onset of tonic seizures were noted. The results are summarized in Table 3.
Table 3.
Anticonvulsant activity of synthesized compounds
Antiviral Activity
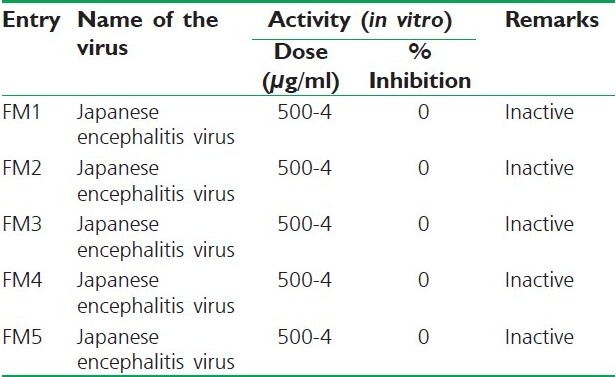
All the compounds were tested for their antiviral activity against Japanese encephalitis virus [P20778].[24,25] The observed results are presented in Table 4.
Table 4.
Antiviral activities of synthesized compounds
RESULTS AND DISCUSSION
Almost all the compounds are active against Gram-positive organism S. aureus, except FM4. The compound FM5 exhibited significant zone of inhibition (13.2 mm) followed by FM3 (13 mm). Compounds FM1 and FM2 had moderate to fewer zones of inhibition but FM4 did not show inhibition on S. aureus. In the case of Gram-negative bacteria, all the compounds had shown remarkable antibacterial activities except FM3 on V. cholerae. Comparatively, all the compounds had shown lesser antibacterial activities than that of streptomycin at 250 μg/ml.
All the compounds were screened for their anticonvulsant activity by maximum electroshock method. Compound FM5 was found to be most active among all the synthesized compounds, whereas rest of the compounds were found to be moderate or less active. But none of the compounds showed neurotoxicity. An imbalance between the excitatory and inhibitory neurotransmitters is responsible for seizures.[26,27] At neuronal level, seizure activity often occurs when glutamatergic excitatory neurotransmitters override GABA mediated inhibition.[28] Several drugs are thought to inhibit seizures by regulating GABA mediated synaptic inhibition through an action at distinct sites of the synapse.[29] Hence, it can be expected that formazan may produce anticonvulsant effect by regulating GABA mediated synaptic inhibition. From the SAR point of view, it is interesting to observe that the formazan derived from hetero aniline FM5 displayed profound antibacterial and anticonvulsant activities. From the antiviral activity data, it was found that none of the compounds was effective.
ACKNOWLEDGMENTS
The authors are grateful to the Director, Dr. H. P. Chhetri, Himalayan Pharmacy Institute, Majhitar, East Sikkim, for providing laboratory facilities and to the Director, IICB Kolkata, India, for providing spectral data. The authors are also thankful to Dr. A. K. Goel, Deputy Director, CDRI, Lucknow, for providing antiviral data.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Misra VS, Dhar S, Chowdhary BL. sSynthesis of some newer formazans and tetrazolium salts as antiviral agents. Pharmazies. 1978;33:790–2. [PubMed] [Google Scholar]
- 2.Desai RM, Desai JM. Synthesis and antimicrobial activity of some new formazan derivatives. Indian J Heterocycl Chem. 1999;8:329–31. [Google Scholar]
- 3.Desai JM, Shah VH. Synthesis and antimicrobial profile of 5-imidazolinones, sulphonamides, azomethines, 2-azetidinones and formazans derived from 2-amino-3-cyano-5-(5-chloro-3-methyl-1-phenyl pyra¬zol-4-yl vinyl)-7,7-dimethyl-6,7-dihydro ben¬¬¬zo (b) thiophenes. Indian J Chem. 2003;42:631–6. [Google Scholar]
- 4.Kalsi R, Pande K, Bhalla TN, Parmar SS, Barthwal JP. Novel formazans as potent anti-inflammatory and analgesic agents. Pharmacology. 1988;37:218–24. doi: 10.1159/000138469. [DOI] [PubMed] [Google Scholar]
- 5.Desai KG, Desai KR. Microbial screening of novel synthesized formazans having amide linkages. J Heterocycl Chem. 2006;43:1083–9. [Google Scholar]
- 6.Bhardwaj SD, Jolly VS. Synthesis, anti HIV and anticancer activities of some new formazans. Chem Asian J. 1997;9:48–51. [Google Scholar]
- 7.Mazaahir K, Negi N, Gupta SD. Synthesis and antifertility activity of 1, 5-diaryl-3-(3’-indolyl) formazans. Chem. Pharm Bull. 1994;42:2363–4. doi: 10.1248/cpb.42.2363. [DOI] [PubMed] [Google Scholar]
- 8.Kumar P, Nath C, Shanker K. Newer dopamine quinazolones as anti-parkinsonian agents. Pharmazie. 1985;40:267–8. doi: 10.1002/chin.198531244. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava A, Kumar AV. Synthesis of newer formazon potential anticonvulsant agents. Indian J Pharma Sci. 2001;65:358–62. [Google Scholar]
- 10.Misra VS, Dhar S. Synthesis of some newer formazans and tetrazolium salts and their effect on Ranikhet disease virus and the vaccinia virus. Pharmazie. 1980;35:585–6. [PubMed] [Google Scholar]
- 11.Davis JN, Nineham BM, Ashley W, Slack R. Synthesis of some new formazans as potential antiviral agents. J Chem Soc. 1953;3:881–8. [Google Scholar]
- 12.Barltrop JA, Owen TC, Cory AH, Cory JG. 5-(3-Carboxymethoxyphenyl) - 2- (4, 5-dimethylthiazolyl)-3-(4 sulfophenyl) tetrazolium inner salt (MTS) and related analogs of 3-(4, 5- dimethylthiazolyl)-2, 5 diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators. Bioorg Med Chem Lett. 1991;1:611–4. [Google Scholar]
- 13.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–33. [PubMed] [Google Scholar]
- 14.Frysova I, Slouka J, Hlavac J. The synthesis of condensed imidazoles II. A simple Synthesis of some 1, 5-diaryl-3-[2-(naphtho [2,3-d]imidazol-2-yl] formazans and its derivatives. Arkivoc. 2006;2:207–12. [Google Scholar]
- 15.Nineham AW. The chemistry of formazans and tetrazolium salts. Chem Rev. 1955;55:355–483. [Google Scholar]
- 16.Stefănescu E, Dorneanu M, Dănilă C, Aprotosoaie C, Grosu G, Pavelescu M. Hydrazones and formazans with possible biological activity. Rev Med Chir Soc Med Nat Iasi. 1997;101:178–82. [PubMed] [Google Scholar]
- 17.Mann FG, Saunders BC. Practical Organic Chemistry. 4th ed. New Delhi: Orient Longmann Ltd Inc; 1998. p. 229. [Google Scholar]
- 18.Pande A, Saxena VK. Synthesis and antiviral activity of 1-aryl-3 (3, 4- dimethoxy-6-nitrophenyl)-5-phenylformazans as antiviral agents. Indian Drugs. 1986;23:423–6. [Google Scholar]
- 19.Raghavan PV. Expert Consultant, CPCSEA, OECD, Guideline No 2000. :420. [Google Scholar]
- 20.Litchfield JT, Jr, Wilkoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–110. [PubMed] [Google Scholar]
- 21.Mandal SC, Nandy A, Pal M, Saha BP. Evaluation of antibacterial activity of Asparagus racemous wild root. Phytother Res. 2000;14:118–9. doi: 10.1002/(sici)1099-1573(200003)14:2<118::aid-ptr493>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 22.Fisher RS. Animal models of epilepsies. Brain Res Brain Res Rev. 1989;14:245–78. doi: 10.1016/0165-0173(89)90003-9. [DOI] [PubMed] [Google Scholar]
- 23.Löscher W, Fassbender CP, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. II. Maximal electroshock seizure models. Epilepsy Res. 1991;8:79–94. doi: 10.1016/0920-1211(91)90075-q. [DOI] [PubMed] [Google Scholar]
- 24.Reed LJ, Muench H. A simple, method of estimating 50 percent end point. Am Ind Hyg Assoc J. 1938;27:493–7. [Google Scholar]
- 25.Sidwell RW, Huffman JH. Use of disposable micro tissue culture plates for antiviral and interferon induction studies. Appl Microbiol. 1971;22:797–801. doi: 10.1128/am.22.5.797-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNamara JO. Drugs effective in the treatment of the epilepsies. In: Hardman JG, Limbird JE, Molinoff PB, Ruddon RW, Gillman AG, editors. Goodman and Gillman's The Pharmacological Basis of Therapeutics. 9th ed. New York: McGraw Hill; 1996. pp. 461–86. [Google Scholar]
- 27.Rang HP, Dale MM, Ritter JM. Pharmacology. Edinburg: Churchill Livingstone; 1999. pp. 575–87. [Google Scholar]
- 28.Smolders I, Van Belle K, Ebinger G, Michotte Y. Hippocampal and cerebellar extracellular amino acids during pilocarpine-induced seizures in freely moving rats. Eur J Pharmacol. 1997;319:21–9. doi: 10.1016/s0014-2999(96)00830-8. [DOI] [PubMed] [Google Scholar]
- 29.Macdonald RL, Kelly KM. Mechanisms of action of currently prescribed and newly developed antiepileptic drugs. Epilepsia. 1993;35:41–50. doi: 10.1111/j.1528-1157.1994.tb05955.x. [DOI] [PubMed] [Google Scholar]


