Abstract
The present study was undertaken to evaluate the mucilage isolated from Spinacia oleracea L. leaves, commonly named spinach (family: Amaranthaceae) as an innovative suspending agent. Zinc oxide suspensions (20% w/v) were prepared using the mucilage of S. oleracea L. leaves as a suspending agent, and it was evaluated for its stability by using parameters like, sedimentation profile, degree of flocculation, and redispersibility. The effect of the tested mucilage on the suspension was compared with various commonly used suspending agents, such as, tragacanth, bentonite, and sodium carboxymethyl cellulose (NaCMC) at concentrations of 0.5, 1.0, and 2.0% w/v. The results obtained indicated that the mucilage of S. oleracea L. leaves could be used as a suspending agent, and the performance was found to be superior to both tragacanth and bentonite.
Keywords: Mucilage, Spinacia oleracea L. leaves, suspending agent, suspension
INTRODUCTION
Nowadays, the whole world is turning toward natural drugs and excipients. Natural materials do hold advantages over synthetic materials, because they are non-toxic, less expensive, and freely available. Furthermore, they can be modified to obtain tailor-made materials for the drug delivery system and they can compete with the synthetic agents available in the market.[1] Among them, plant mucilages are well known, since ancient times, for their medicinal use. In recent years, plant mucilages have evoked tremendous interest due to their diverse applications in pharmacy, for formulation of both solid and liquid dosage forms.[2] Plant mucilages are pharmaceutically important polysaccharides with a wide range of applications such as thickeners, binding agents, water retention agents, emulsion stabilizers, suspending agents, disintegrants, gelling agents, and film formers.[3] Thus, with the increased demand for these substances, it has been necessary to explore newer sources, to meet the industrial demands. Among the Asian countries, India, due to its geographical and environmental positioning has traditionally been a good source for such products.
A pharmaceutical suspension is thermodynamically unstable, thus, making it necessary to include in the dosage form, a suspending agent that reduces the rate of settling and permits easy redispersion of any settled particulate matter, both by increasing the consistency of the suspending medium and by protective colloidal action.[3] There are reports about the successful use of various plant mulcilages, as innovative suspending agents, like mucilages isolated from Hibiscus rosasinensis L. leaves,[1] Ocimum gratissimum seeds,[4] Cassia tora seeds,[5] Cassia roxburghii seeds,[6] Hibiscus cannabinus seed,[7] Chlorophytum borivilianum tuber,[8] Abelmoschus esculentus fruits,[9] Malava sylvestris, Pedalium murex fruits,[3] and so on. The purpose of this study is to search for a chip and effective natural excipients that can be used as new alternative suspending agents for the formulation of pharmaceutical suspensions.
The plant Spinacia oleracea L. (family: Amaranthaceae), commonly called spinach or palang (vernacular name), is an annual plant (rarely biennial).[10] It is native to central and southwestern Asia. Spinach may survive over winter in temperate regions. The leaves are alternate, simple, ovate to triangular-based, variable in size, from about 2 – 30 cm long and 1 – 15 cm broad, with larger leaves at the base of the plant and small leaves higher on the flowering stem. It is a favorite food vegetable among Indians in the winter season and is a dietary power house, full of vitamins and minerals.[10] However, no study has been reported so far on the use of mucilage isolated from S. oleracea L. leaves. The present study is an attempt to isolate and investigate the pharmaceutical properties of the isolated mucilage from S. oleracea L. leaves, to assess its suitability as a new innovative suspending agent in a pharmaceutical formulation.
MATERIALS AND METHODS
The materials used include zinc oxide (E. Merck, Mumbai, India); tragacanth and sodium carboxymethyl cellulose (Loba Chemie Mumbai, India); bentonite, potassium dihydrogen phosphate, sodium metabisulfite (Qualigens Fine Chemicals, Mumbai, India); and benzoic acid (SD Fine Chemicals, Mumbai, India). All the solvents and chemicals used were of analytical grade.
Isolation of Mucilage
Fresh, young (one-month-old), healthy leaves of spinach (S. oleracea L.) were collected from the local Jharpokharia market in the month of March and taxonomic identification was made by the Department of Pharmacognosy, Jadavpur University, Kolkata, West Bengal, India. The voucher specimen of each sample has been preserved in our laboratory for future reference (Ref no: DA1).
Leaves were cut into pieces, homogenized with demineralized water (1:5) containing 1% w/v sodium metabisulfite, and kept aside to release the mucilage into the water. The crude material was squeezed through muslin cloth to remove the marc from the filtrate. The mucilage was precipitated from the water using acetone. The precipitated mucilage was dried at room temperature for 24 hours and passed though sieve number # 80. The powdered mucilage was stored in desiccators for further use.[3,11]
Isolated S. oleracea L. leaves mucilage was subjected to some tests for confirmation of the isolated mucilage. The tests performed were to determine the presence of carbohydrates (Molisch's test), mucilage (Ruthenium red test), and starch (Iodine test).[12,13]
Preparation of zinc oxide suspension
Zinc oxide, which was sieved through sieve No #100 and retained on sieve No#200 was used to yield a 20% w/v suspension in water using S. oleracea L. leaves mucilage and various conventional suspending agents at concentrations of 0.5, 1.0, and 2.0% w/v. Zinc oxide was first levigated with glycerin (1:1). Next, the weighed amount of these suspending agents were added and triturated. The volume was made up with distilled water. The suspensions contained 0.1% w/v benzoic acid as a preservative. All the suspensions were deflocculated. To determine the degree of flocculation, flocculated suspensions were made, using potassium dihydrogen phosphate (0.004 M) as the flocculating agent.[4]
Evaluation of Zinc Oxide Suspension
Sedimentation volume: Sedimentation volume (F) was determined by keeping a measured volume of the suspension in a graduated cylinder at an undisturbed position for a definite period of time and noting the value of the ultimate height (Hu) of the sediment as the suspension settled and the initial height (Ho) of the total suspension (F = Hu / Ho).[1,3]
Degree of flocculation: The degree of flocculation (β) was determined from the following equation: β = (Vu)floc / (Vu)defloc, where (Vu)floc is the ultimate sedimentation volume in the flocculated suspension and (Vu)defloc is the ultimate sedimentation volume in the deflocculated suspension.[4]
Redispersibility: Fifty milliliters of various suspensions were kept in calibrated measuring cylinders, which were then stored at room temperature at various time intervals (1, 5, 10, 15, 20, 30, and 45 days). At regular intervals, one measuring cylinder was removed and shaken vigorously to redistribute the sediment and the presence of deposit was recorded, if any.[4]
RESULTS AND DISCUSSION
The yield of mucilage from S. oleracea L. leaves, isolated from fresh, young, healthy leaves was 12.62% w/w. Various confirmation tests were carried out on the isolated mucilage of S. oleracea L. leaves. The results of these tests on the isolated mucilage are summarized in Table 1.
Table 1.
Confirmation tests on the mucilage of isolated S. oleracea L. leaves

In these confirmation tests a violet ring was formed at the junction of the two liquids on reaction with Molisch's reagent, indicating the presence of carbohydrates. On treatment of the mucilage with ruthenium red, it showed a red color confirming the finding of mucilage.
To evaluate the suspending properties of the isolated mucilage from S. oleracea L. leaves, 20% w/v zinc oxide suspensions were prepared with a fixed concentration, with varying concentration (0.5, 1, and 2% w/v) of the test mucilage, as well as the commonly used suspending agents, like tragacanth, bentonite, and sodium carboxy methylcellulose (NaCMC). The sedimentation volume (F) of these formulated suspensions prepared with the mucilage of S. oleracea L. leaves were compared with the suspensions prepared using various commonly used suspending agents. The sedimentation volume (F) profile of these suspensions are presented in Figures 1 to 3.
Figure 1.
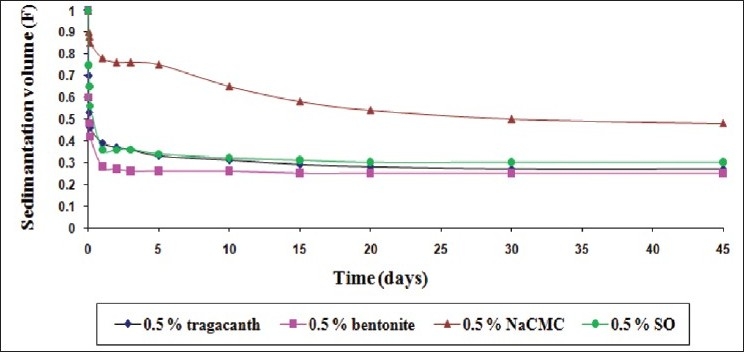
The comparative sedimentation volume (F) profile of the suspensions with the mucilage of S. oleracea L. leaves (SO) and traditional suspending agents (0.5% w/v suspending agent concentration)
Figure 3.
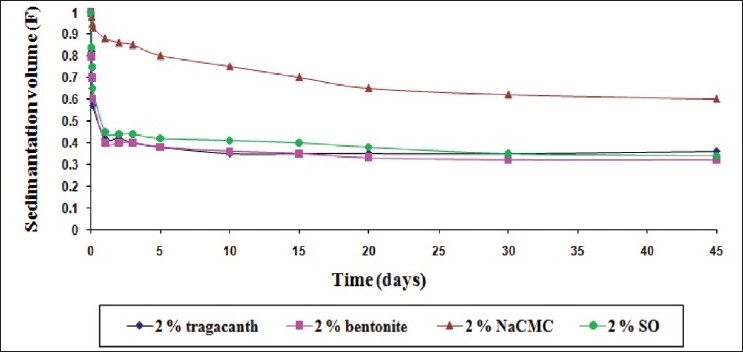
The comparative sedimentation volume (F) profile of the suspensions with mucilage of S. oleracea L. leaves (SO) and traditional suspending agents (2% w/v suspending agent concentration)
Figure 1 shows a comparison of the efficacy of the suspending agents when used in a lower concentration (0.5% w/v). Nevertheless, the dispersed particles sedimented at a faster rate in suspensions containing 0.5% w/v of the suspending agents in comparison of 1 and 2% w/v, as expected [Figures 2 and 3]. The mucilage of S. oleracea L. leaves showed its superiority over tragacanth and bentonite; while NaCMC exhibited a superior result in comparison with all the suspending agents used in this investigation.
Figure 2.
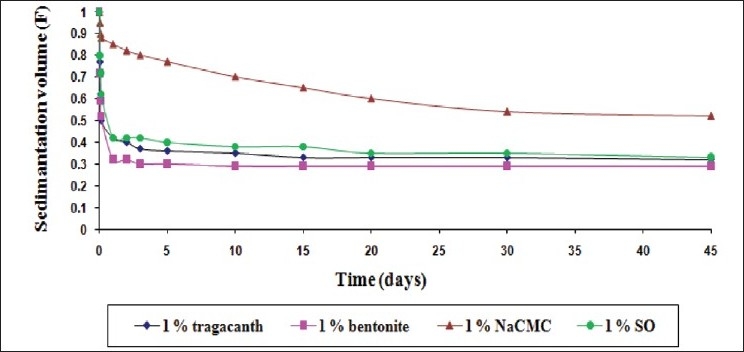
The comparative sedimentation volume (F) profile of the suspensions with mucilage of S. oleracea L. leaves (SO) and traditional suspending agents (1% w/v suspending agent concentration)
According to Martin et al.[14] the sedimentation volume provides only a qualitative account of the flocculation of a suspension, although, the degree of flocculation (β) is a more useful parameter, which is the ratio of the ultimate sedimentation volume in the flocculated and deflocculated systems. The degrees of flocculation (β) were determined for all the formulated suspensions using different concentrations of isolated S. oleracea L. leaves mucilage and the various commonly used suspending agents used in this investigation. The values of the degrees of flocculation for all formulated suspensions are presented in Table 2.
Table 2.
Degree of flocculation (β) of various suspending agents* (Mean±SD)
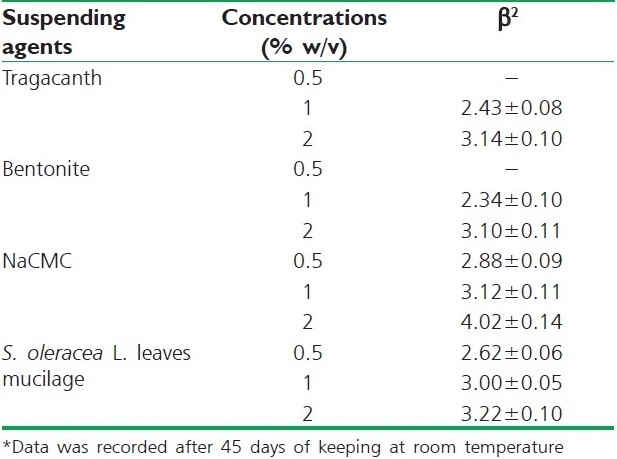
The comparative values of the degree of flocculation (β) indicated that NaCMC and the mucilage of S. oleracea L. leaves showed higher values when compared to other suspending agents (both tragacanth and bentonite). These observations showed that the test mucilage (mucilage isolated from S. oleracea L. leaves) was a better suspending agent than both tragacanth and bentonite.
As the suspension produces a sediment on storage, it must be readily dispersible so as to ensure the uniformity of the dose. If the sediment remains even after shaking vigorously for the specified time, the system is described as cake.[4] The suspensions with 0.5% w/v tragacanth and bentonite as suspending agents have caked after 30 days. However, the suspensions with the mucilage of S. oleracea L. leaves and NaCMC were found to be easily redispersible, irrespective of their concentrations.
CONCLUSION
The present investigation is a primary platform to indicate the suitability of the mucilage of S. oleracea L. leaves as a suspending agent. From the observations it can be concluded that the isolated mucilage from S. oleracea L. leaves has the potential of being used an innovative suspending agent, even at a low concentration, and can be used as a pharmaceutical adjuvant, considering the easy availability of spinach.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Edwin J, Edwin S, Dosi S, Raj A, Gupta S. Application of hibiscus leaves mucilage as suspending agent. Indian. J Pharm Educ Res. 2007;41:373–5. [Google Scholar]
- 2.Rao GC, Prasad PG, Srinivas K, Rao MF. Application of Moringa oleifera as a suspending agent in the formulation of sulphamethoxazole. Indian Pharm. 2005;5:75–7. [Google Scholar]
- 3.Yeole NB, Sandhya P, Chaudhari PS, Bhujbal PS. Evaluation of Malva sylvestris and Pedalium murex mucilage as suspending agent. Int J Pharm Tech Res. 2010;2:385–9. [Google Scholar]
- 4.Anroop B, Bhatnagar SP, Ghosh B, Parcha V. Studies on Ocimum gratissimum seed mucilage: Evaluation of suspending properties. Indian J Pharm Sci. 2005;67:206–9. [Google Scholar]
- 5.Mann AS, Jain NK, Kharya MD. Evaluation of the suspending properties of Cassia tora mucilage on sulphadimidine suspension. Asian J Exp Sci. 2007;21:63–7. [Google Scholar]
- 6.Kumaran KS, Vimalson CD, Palanisamy S, Jagadeesan M. Evaluation of suspending properties of Cassia roxburghii mucilage on sulphamethaoxazole suspension. Int J Pharma Bio Sci. 2010;1:1–10. [Google Scholar]
- 7.Palshikar GS, Jain BB, Pande VV, Katare YS. Study of Hibiscus cannabinus seed mucilage: Extraction and evaluation as suspending agent. J Pharm Res. 2010;3:462–4. [Google Scholar]
- 8.Deore SL, Khadabadi SS. Standardisation and pharmaceutical evaluation of Chlorophytum borivilianum mucilage. Rasayan J Chem. 2008;1:887–92. [Google Scholar]
- 9.Kumar R, Patil MB, Patil SR, Paschapur MS. Evaluation of Abelmoschus esculentus mucilage as suspending agent in paracetamol suspension. Int J Pharm Tech Res. 2009;1:658–65. [Google Scholar]
- 10.Das S, Guha D. CNS depressive role of aqueous extract of Spinacia oleracea L.leaves in adult male albino rats. Indian J Exp Biol. 2008;46:185–90. [PubMed] [Google Scholar]
- 11.Sepúlveda E, Sáenz C, Aliaga E, Aceituno E. Extraction and characterization of mucilage in Opuntia spp. J Arid Environ. 2007;68:534–45. Khandelwal KR Pharmacognosy, Techniques, and Experiments 9 th ed Pune, India: Nirali Prakashan; 2002. [Google Scholar]
- 12.Kokate CK, Purohit AP, Gokhale SB. 38th ed. Pune, India: Nirali Prakashan; 2000. Text Book of Pharmacognosy. [Google Scholar]
- 13.Martin A. Physical Pharmacy. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2001. Coarse dispersions. [Google Scholar]


