Abstract
The aqueous and ethanolic extracts of C. grandis (Family: Leguminosae) were evaluated for antidiabetic activity by a glucose tolerance test, in normal rats and alloxan-induced diabetic rats. The aqueous and ethanolic extracts showed that they significantly lowered the blood glucose levels to normal in the glucose tolerance test. In alloxan-induced diabetic rats the maximum reduction in blood glucose was observed after three hours, at a dose level of 150 mg/kg of body weight. The percentage of protection given by the aqueous and ethanolic extracts was 32.72 and 46.42%, respectively. In the long-term treatment of alloxan-induced diabetic rats, the degree of protection was determined by measuring the blood glucose, cholesterol, and triglycerides on the tenth day. Both the extracts showed a significant antidiabetic activity comparable to that of glibenclamide. These results showed that the Cassia grandis possessed significant antidiabetic activity.
Keywords: Alloxan-induced diabetes, C. grandis, hypoglycemic activity
INTRODUCTION
Diabetes mellitus is a complex and a multifarious group of disorders that disturbs the metabolism of carbohydrates, fats, and proteins. It results from shortage or lack of insulin secretion or reduced sensitivity of the tissue to insulin. Several drugs such as biguanides and sulfonylureas are presently available to reduce hyperglycemia in diabetes mellitus. These drugs have side effects like diarrhea, lactic acidosis, weight gain, fetal hypoglycemia, and so on, and thus searching for a new class of compounds is essential to overcome diabetic problems.[1] Management of diabetes without any side effect is still a challenge to the medical community. There is continuous search for alternative drugs. Therefore, it is prudent to look for options in herbal medicines for diabetes as well. Herbal medicines have long been used effectively in treating diseases in Asian communities and throughout the world.
Diabetes mellitus is a major endocrine disorder affecting nearly 10% of the population all over the world.[2] Diabetes is one of the leading causes of death in humans and animals. In animals it occurs most frequently in dogs, with an incidence of approximately 0.2%.[3] In the indigenous Indian system of medicine, a good number of plants have been mentioned for the cure of diabetes and some of them have been experimentally evaluated and the active principles have been isolated.[4] The World Health Organization (WHO)[5] has also recommended the evaluation of effective plants in conditions where there are no safe modern drugs.[6] The ethnobotanical information reports state that about 800 plants may possess antidiabetic potential.[7] In recent times the medicinal values of various plant extracts have been studied by many scientists in the field of diabetic research.[8] Traditional antidiabetic plants may provide new oral hypoglycemic compounds, which can counter the high cost and poor availability of the current medicines / present day drugs, for many rural populations in developing countries. India is well-known for its herbal wealth.
Cassia, is a large genus of around 500 species of flowering plants in the family Leguminosae and is widely distributed throughout Asia including India, Mauritius, China, East Africa, South Africa, America, Mexico, West Indies, and Brazil. There are hundreds of species of Cassia that occur, with more than 1000 names. Some important species are Cassia fistula, Cassia grandis, Cassia hirsutica, Cassia sieberiena, Cassia alata, Cassia tora, Cassia occidentalis, C. auriculata, and C. Nigricans.[9] From a pharmaceutical perspective the presence of anthranoides with strong laxative and purgative effects is of particular interest and a characteristic of this genus. Cassia species have been of medical interest due to their good therapeutic value in folk medicine. Abo and Eluojoba have shown that the leaves and pods of Cassia fistula, Cassia spectabilis, and Cassia podocarpa possess laxative and antimicrobial activities.[10,11] The extracts of the flowers and seeds of C. auriculata have been found to possess antidiabetic activity.[12] Manonmania has evaluated the antioxidant activity of C. Fistula.[13] Considering the above-mentioned findings it is observed that C.grandis has been less explored. Hence, the current study was performed to evaluate the antidiabetic potential of the aqueous and ethanolic extracts of C. Grandis.
MATERIALS AND METHODS
Plant Material
The complete plant was obtained from Nasik and authenticated by Dr. S. C. Pal, Head of Pharmacognosy Department, College of Pharmacy, Nasik, India. The plant was vacuum dried.
Preparation of Extract
The dried stem (2 kg) was macerated in 1000 ml of 80% ethanol (ethanol / water ratio 4 / 1) for seven days. Next, the extract was filtered and concentrated in a rotary evaporator. The resulting extract, after drying, gave 96.81 g (i.e., a 4.84% yield) of brownish extract. The extracts were dissolved in 0.2% tween 80 in normal saline (vehicle) for pharmacological experiments.
Test Animals
Adult albino rats weighing about 150 – 180 g were used in the present investigation. All the rats were given a period of acclimatization for 15 days before starting the experiment. They were fed ad libitum every day with a standard chow diet and were given free access to water. The animals described as fasting were deprived of food for at least 16 hours, but were allowed free access to drinking water.
Effect of C. grandis Extracts on Glucose Tolerance in Rats
Fasted rats were divided into three groups of six rats each. Group I served as a control, received vehicle. Groups II and III received a single dose of aqueous and ethanolic extracts, respectively, at a dose of 150 mg/kg body weight (less than one-tenth of LD50, based on the preliminary study conducted at our laboratory) as a fine aqueous suspension, orally. Group IV received glibenclamide (10 mg/kg, p.o.). The rats of all groups were given glucose (2 g/kg body weight, p.o) 30 minutes after administration of the extract. Blood samples were collected from the tail vein just prior to glucose administration and at 30 and 90 minutes after the glucose loading, for estimation of blood glucose levels.[14]
Effect of the C. grandis Extracts on Alloxan-induced Diabetic Rats
Male Wistar rats (180 – 200 g) were made diabetic by a single i.p injection of 120 mg/kg body weight of alloxan monohydrate in sterile normal saline. The rats were maintained on 5% glucose solution for the next 24 hours to prevent hypoglycemia.[15] Five days later blood samples were drawn from the tail vein and glucose levels were determined to confirm the development of diabetes (350 mg/dl). The diabetic rats were divided into four groups, each containing six animals. The controls rats (Group I) were given distilled water orally, while C. grandis aqueous ethanolic extracts were given to groups II and III, respectively, at a dose of 150 mg/kg, orally. Group IV received glibenclamide at a dose of 10 mg/kg. Blood samples were collected from the tail vein just prior to and one, three, and five hours after extract administration.
The effect of C. grandis extracts was also tested for prolonged treatment. The diabetic male Wistar rats (160 – 180g) were divided into four groups of eight rats each. Group I served as the diabetic control received distilled water instead of extracts. The rats of groups II and III received aqueous and ethanolic extracts, respectively, at a dose of 150 mg/kg body weight, as fine aqueous suspension, orally. Group IV received glibenclamide at a dose of 10 mg/kg. The administration of extracts was continued for 10 days, once daily. On the tenth day, after extract administration, the blood glucose, total cholesterol, and triglyceride levels were determined for all the samples.
Statistical Analysis
All the values were expressed as mean ± Standard error mean (SEM). The differences were compared using two-way analysis of variance (ANOVA) followed by Dunnet's t test. P values < 0.05 were considered as significant.
RESULTS
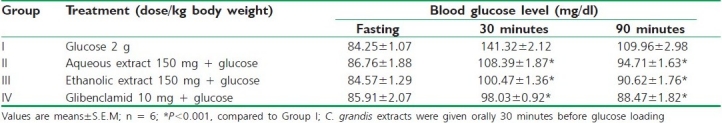
Table 1 shows the changes in the levels of blood glucose in diabetic control and experimental groups after oral administration of glucose (2 g/kg). In animals treated with ethanolic and aqueous extracts of C. grandis, the blood glucose concentration was significantly decreased (P < 0.001) after 30 minutes and 90 minutes, which was comparable to the standard drug glibenclamide. The maximum effect was observed for the ethanolic extract of C. grandis.
Table 1.
Effects of C.grandis extracts on oral glucose tolerance in rats
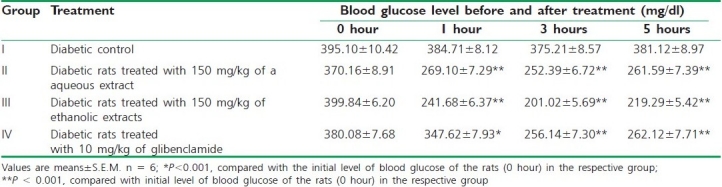
The antihyperglycemic effect of different extracts of C. grandis on fasting blood glucose levels in diabetic rats were assessed at different time intervals. The percentage blood glucose reductions with aqueous (150 mg/kg) and ethanolic extracts (150 mg/kg) of C. grandis at three hours were 32.72 and 46.42%, respectively. Glibenclamide 10 mg/kg dose produced 39.73% blood glucose reduction and the results are shown in Table 2.
Table 2.
Effect of C. grandis extracts on blood glucose levels (mg/dl) of alloxan-induced diabetic rats
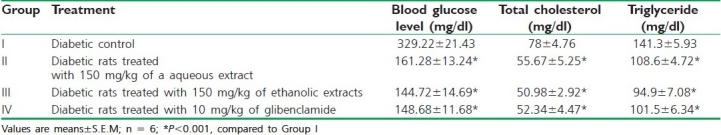
As shown in the Table 3 Groups II and III significantly reduced (P < 0.01) the blood glucose level, total cholesterol, and triglyceride level when compared with the diabetic control (Group I) on the tenth day of alloxan-induced diabetic rats. The percentage of total cholesterol reduction with aqueous and ethanolic extracts of C. grandis on the tenth day was 28. 62 and 34.64%, respectively, while triglyceride reduction was found to be 23.14 and 32.83%, respectively. Glibenclamide produced 32.89% reduction in total cholesterol and 28.16% reduction in the triglyceride level.
Table 3.
Effect of C. grandis extracts on blood glucose levels, total cholesterol, and triglyceride level on the tenth day of alloxan-induced diabetic rats
DISCUSSION
The present study was conducted to evaluate the hypoglycemic and antidiabetic activity of C. grandis. The plasma glucose levels of normal rats reached a peak at 30 minutes, after the oral administration of glucose, and gradually decreased to the pre-glucose load level [Table 1]. These results showed that both the extracts had a hypoglycemic effect at a specific time interval. Among the two extracts, the hypoglycemic effect was observed more consistently in the case of the ethanolic extract. This would suggest the presence of hypoglycemic components in the ethanolic extract.
Diabetes mellitus is one of the most common chronic diseases and is associated with hyperlipidemia and comorbidities such as obesity and hypertension. Hyperlipidemia is a metabolic complication of both clinical and experimental diabetes.[16] Alloxan, a beta cytotoxin, induces ‘chemical diabetes’ in a wide variety of animal species, by damaging the insulin secreting pancreatic β-cell, resulting in a decrease in endogenous insulin release, which suggests ways for the decreased utilization of glucose by the tissues.[17–19] In vitro studies have shown that alloxan is selectively toxic to pancreatic beta cells, leading to the induction of cell necrosis.[20] It is well-established that sulfonylureas produce hypoglycemia by increasing the secretion of insulin from the pancreas, and these compounds are active in mild alloxan-induced diabetes, but they are inactive in intense alloxan diabetes.[21,22] As our results show that glibenclamide reduces blood glucose levels in hyperglycemic animals, the state of diabetes is not intense. The acute antihyperglycemic effects of the aqueous and ethanolic extracts of C. grandis are similar to those of glibenclamide.
Continuous treatment with aqueous and ethanolic extracts of C. grandis for a period of 10 days produces a significant decrease in the blood glucose levels of diabetic rats. It is possible that the drug may be acting by potentiating pancreatic secretion or increasing the glucose uptake. Hypercholesterolemia and hypertriglyceridemia have been reported to occur in alloxan diabetic rats.[23,24] Increase in glycogen in the liver can be brought about by an increase in glycogenesis and / or a decrease in glycogenolysis. In our study, the diabetic rats have shows hypercholesterolemia and hypertriglyceridemia. The treatment with plant extracts significantly decreases both cholesterol and triglyceride levels. Therefore, the aqueous and ethanolic extracts of C. grandis may have stimulated glycogenesis and / or inhibited glycogenolysis in diabetic rat liver and may prevent or be helpful in reducing the complications of lipid profile seen in some diabetes with hyperglycemia and hypercholesterolemia coexisting.[25]
In conclusion, the plant has shown potential activity in decreasing the serum glucose level and other biochemical changes associated with experimental diabetes. This research supports the inclusion of this plant in traditional antidiabetic preparations. Further research is needed to find the exact active constituents responsible for the antidiabetic activity.
ACKNOWLEDGMENT
The authors are grateful to Principal Dr. Dinesh R. Shah and Dr. Shailesh A Shah, Maliba Pharmacy College, India, and Principal Mr. Shashikant N. Dhole, Modern College of Pharmacy (for ladies), India, for their unending support during the preparation of this research article.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Noor AS, Gunasekaran AS, Manickam, Vijayalakshmi MA. Antidiabetic activity of Aloe vera and histology of organs in streptozotocin induced diabetic rats. Curr Sci. 2008;94:1070–6. [Google Scholar]
- 2.Burke JP, Williams K, Narayan KM, Leibson C, Haffner SM, Stern MP. A population perspective on diabetes prevention: Whom should we target for preventing weight gain? Diabetes Care. 2003;26:1999–2004. doi: 10.2337/diacare.26.7.1999. [DOI] [PubMed] [Google Scholar]
- 3.Avizeh R, Najafzadeh H, Pourmahdi M, Mirzaee M. Effect of glibenclamide and fruit extract of zizyphus spina-christi on alloxan-induced diabetic dogs. Int J Appl Res Vet Med. 2010;8:109–13. [Google Scholar]
- 4.Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes mellitus. 2nd rep, WHO Technical report series. Geneva: WHO; 1980. World Health Organization; p. 646. [PubMed] [Google Scholar]
- 6.Upadhaya VP, Pandey K. Ayurvedic approach to diabetes mellitus and its management by indigenous resources.Diabetes mellitus in developing countries. In: Bajaj JS, editor. New Delhi: Interprint, New Delhi; 1984. pp. 357–7. [Google Scholar]
- 7.Aguilara FJ, Ramos RR, Gutierrez SP, Contreras AA, Weber CC, Saenz JL. Study of the antihyperglycemic effect of plants used as antidiabeties. J Ethanopharmacol. 1998;61:101–10. [Google Scholar]
- 8.Daisy P, Eliza J. Hypoglycemic property of polyherbal formulation in streptozotocin induced diabetic rats. Biochem Cell Arch. 2007;7:135–40. [Google Scholar]
- 9.Ayol RG, Amupitan JO, Yimin Z. Cytotoxicity and antimicrobial studies of 1, 6, 8- trihydroxy-3-methyl-anthraquinone (emodin) isolated from the leaves of Cassia nigricans. Afr J Biotech. 2007;6:1276–9. [Google Scholar]
- 10.Abo KA, Adeyemi AA, Jegede IA. Spectrophotometric estimation of anthraquinone content and antimicrobial potential of extracts of some Cassia species used in herbal medicine in Ibadan. Sci Forum. 2000;3:57–63. [Google Scholar]
- 11.Eluojoba AA, Abere AT, Adelusi SA. Laxative activities of Cassia pods sourced from Nigeria. Niger J Nat Prod Med. 1999;3:51–3. [Google Scholar]
- 12.Jalalpure SS, Patil MB, Aruna P, Shah BN, Salahuddin MD. Antidiabetic activity of Cassia auriculata seeds in alloxan induced diabetic rats. Niger J Nat Prod Med. 2004;8:22–3. [Google Scholar]
- 13.Manonmani G, Bhavapriyaa V, Kalpanaa S, Govindasamya S, Apparananthamb T. Antioxidant activity of Cassia fistula (Linn.) flowers in alloxan induced diabetic rats. J Ethnopharmacol. 2005;97:39–42. doi: 10.1016/j.jep.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 14.Whittington KB, Solomon S, Lu N. Islets allograft in cryptochd tests of spontaneous diabetic BBI wor dp rats.Response, glypizide and arginine. Endocrinology. 1991;128:2671–7. doi: 10.1210/endo-128-6-2671. [DOI] [PubMed] [Google Scholar]
- 15.Gupta NP, Solis NG, Avella ME, Sanchez E. Hypoglycaemic activity of Neurollena lobata. J Ethanopharmacol. 1984;10:323–7. doi: 10.1016/0378-8741(84)90020-5. [DOI] [PubMed] [Google Scholar]
- 16.Bierman EL, Amaral JA, Balknap BH. Hyperlipidemia and diabetes mellitus. Diabetes. 1975;25:509–15. [Google Scholar]
- 17.Yamamoto H, Uchigata Y, Okamoto H. STZ and alloxan induces DNA strand breaks and poly (ADPribose) synthatase in pancreatic islets. Nature. 1981;294:284–6. doi: 10.1038/294284a0. [DOI] [PubMed] [Google Scholar]
- 18.Lenzen S, Panten U. Alloxan: History and mechanism of action. Diabetologia. 1988;31:337–42. doi: 10.1007/BF02341500. [DOI] [PubMed] [Google Scholar]
- 19.Oberley LW. Free radicals and diabetes. Free Radic Biol Med. 1988;5:113–24. doi: 10.1016/0891-5849(88)90036-6. [DOI] [PubMed] [Google Scholar]
- 20.Jorns A, Munday R, Tiedge M, Lenzen S. Comparative toxicity of alloxan, N-alkyl-alloxans and ninhydrin to isolated pancreatic islets in vitro. J Endocrinol. 1997;155:283–93. doi: 10.1677/joe.0.1550283. [DOI] [PubMed] [Google Scholar]
- 21.Yallow RS, Black H, Villazan M, Berson SA. Comparison of plasma insulin levels following administration of tolbutamide and glucose. Diabetes. 1960;9:356–62. doi: 10.2337/diab.9.5.356. [DOI] [PubMed] [Google Scholar]
- 22.Grodsky GM, Epstein GH, Fanska R, Karam JH. Pancreatic action of sulphonylureas. Fed Proc. 1971;36:2719–28. [PubMed] [Google Scholar]
- 23.Resmi CR, Aneez F, Sinilal B, Latha MS. Anti-diabetic effect of a herbal drug in alloxan-diabetic rats. Indian Drugs. 2001;38:319–22. [Google Scholar]
- 24.Joy KL, Kuttan R. Anti-diabetic activity of Picrorrhiza kurroa extract. J Ethanopharmacol. 1999;67:143–8. doi: 10.1016/s0378-8741(98)00243-8. [DOI] [PubMed] [Google Scholar]
- 25.Sharma SB, Nasir A, Prabhu KM, Murthy PS, Dev G. Hypoglycaemic and hypolipidemic effect of ethanolic extract of seeds of Eugenia jambolana in alloxaninduced diabetic rabbits. J Ethnopharmacol. 2003;85:201–6. doi: 10.1016/s0378-8741(02)00366-5. [DOI] [PubMed] [Google Scholar]


