Abstract
Nymphaea stellata Willd. (Syn. Nymphaea nouchali Burman f.) (Nymphaeaceae) is an important and well-known medicinal plant, widely used in the Ayurveda and Siddha systems of medicines for the treatment of diabetes, inflammation, liver disorders, urinary disorders, menorrhagia, blenorrhagia, menstruation problem, as an aphrodisiac, and as a bitter tonic. There seems to be an agreement between the traditional use and experimental observations, such as, hepatoprotective, anti-inflammatory, and particularly antidiabetic activity. Nymphayol, a steroid isolated from the flowers has been scientifically proved to be responsible for the traditionally claimed antidiabetic activity; it reverses the damaged endocrine tissue and stimulates secretion of insulin in the β-cells. However, taking into account the magnitude of its traditional uses, the studies conducted are still negligible. This review is an attempt to provide the pharmaceutical prospective of Nymphaea stellata.
Keywords: Bitter, indian blue water lily, Nymphaea nouchali, nymphayol
INTRODUCTION
In Ayurveda, for the highest heat, fever, and pitta conditions — bitter, fire purging, and heat dispelling herbs are used, that is, bitters. Bitters are the strongest herbs for cooling pitta, for sedating, detoxifying the liver, and for reducing the deep-seated heat / fever in the interior of the body. Bitters also increase agni (Fire) by their drying action; in addition they do not aggravate pitta.[1] According to contemporary knowledge, bitters are a group of botanicals with a predominantly bitter taste, due to the presence of chemical constituents like alkaloids, monoterpenes (iridoid and secoiridoids), sesquiterpene lactones, diterpenes, triterpenes, and rarely flavanones, acyl phloroglucides, and steroids (pregnane type).[2] Bitters stimulate the gastric reflex, increase the flow of digestive juices from the pancreas and duodenum, increase the nerve tone of the entire digestive tract muscles and enhance the liver for better assimilation of the nutrients. Bitters produce a diuretic effect and also regulate the secretion of the pancreatic hormones (insulin and glucagon) that regulate blood sugar. Bitters can also be supportive in reducing stress, anxiety, and regenerating the nervous system. The intensity of all these effects is considered directly proportional to the strength of its bitterness. Nymphaea stellata Willd. (Nymphaeaceae) is one such traditionally recommended bitter for various ailments, in India. N. stellata is an ingredient of many ayurvedic formulations and its morphological parts are used by traditional healers for treating various diseases. In the last few decades there has been an increasing interest in the study of medicinal plants, as knowledge on ethnopharmacology, its holistic system approach, supported by the experiential base, can serve as an innovative and powerful discovery engine for newer, safer, and affordable medicines.[3] This review is an attempt to assess the available scattered literatures and compile them under different categories in a systematic way, to provide the pharmaceutical prospective of Nymphaea stellata.
NYMPHEACEAE AND NYMPHEA
Paleobotanical studies[4–7] support the view that the so-called ANITA clads (Amborellaceae, Nymphaeales, Illiciales, Trimeniaceae, Austrobaileyaceae) were the first line to diverge from the main branch of the angiosperm phylogenetic tree. Nympheaceae is classified under the order Nymphaeales, in the group of the ‘basal families,’ in the recent molecular-based angiosperm phylogeny.[8] Nympheaceae is a primitive family; the fossil record goes back to the early cretaceous period.[5] Nympheaceae Salisb. is cosmopolitan with about six genera and 75 species.[9] The genus Nymphaea includes approximately 40 species found in tropical and temperate climates on both hemispheres. Nymphaea is divided into two main groups, which in turn is divided into five subgenera. Group Apocarpiae includes the subgenera Anecphya, Brachyceras, and group Syncarpiae consists of subgenera Hydrocallis, lotos, and Nymphaea.
NYMPHAEA STELLATA
Nymphaea stellata Willdenow (syn. Nymphaea nouchali Burman f.) belongs to the family Nympheaceae [Figure 1]. In Greek nymphala refers to water nymph and stellata in Latin means star-shaped.[10] For a variety of reasons a lot of synonymy occurs for N. stellata.[11,12] The synonyms are; Nymphaea cyanea Roxb., Nymphaea malabarica Poir., Nymphaea minima F. M. Bailey, Nymphaea punctata Edgew, and Nymphaea versicolor Sims.[13] N. caerula is also considered as a synonym by some botanists, while some include as a variety, Nymphaea nouchali Burm.f. var. caerulea (Savigny) Verdc.[14] Other varieties recorded are, N. nouchali var. cyanea (Hooker F. and Thomson), Almeida (syn. N. stellata var. cyanea), and N. nouchali var. versicolor (Roxburgh) Hooker f. and Thomson (syn. N. stellata var. vesicolor).[15] Verdcourt has quoted that N. nouchali should be a synonym for N. stellata and not for N. pubescens as some have stated.[16,17] However, in some literature and books N. stellata and N nouchali have been differentiated as two species.[18] To date there exists a controversy among botanists regarding the synonymy and the varieties. Although, this review is constructed considering N. nouchali as a synonym of N. stellata.
Figure 1.

Nymphaea stellata
N. stellata is commonly known as Indian blue water lily / Indian water lily in English and has different vernacular names in India [Table 1].[19] Sometimes this water lily is often referred as ‘blue lotus of India’, but it is not a lotus.[15] Many reports specify that ‘blue lotus of the nile’ and the ‘blue lotus of India’ are N. caerulea and N. nouchali, respectively, while others report ‘sacred blue lily’ as Nymphaea nouchali var. caerulea. In India the vernacular names used for N. nouchali, besides the correct local name ‘Nilotpalam’, include ‘Allithamarai’ and ‘Vellambal’ (Tamil), which are in fact applicable to N. pubescens.[20] Another name ‘Nilotpala’ refers to three plants – N. stellata, N. rubra, and Monochoria hastate. N. stellata alone has 17 names including Indivar, Nilakamala, Nilotpala, and Utpala.[21] Lotus has no blue colored flowers in India, the name ‘Neelathamara’ is applicable only to the water lily with bluish flowers, which is N. stellata. These vernacular names used for N. stellata in India are sometimes conclusive, but mostly diverge dramatically, making identification of the plant complicated.
Table 1.
Different vernacular names for N. stellata in India[19]
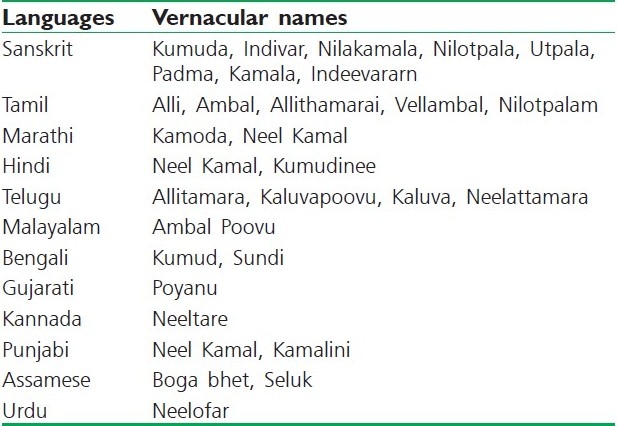
Karyotype analysis on N. stellata showed 2n = 28.[22] Another study on the chromosome number of N. nouchali was seen to be euploidy in nature. Three types have been identified, N. nouchali (Type 1) 2n = 56, N. nouchali (Type 2) 2n = 84, and N. nouchali (Type 3) 2n= 70. N. nouchali (Type 1) 2n = 56 (4x) chromosomes may have evolved by the doubling of a chromosome (2n = 28) from the ancestral species. Similarly, N. nouchali (Type 2) 2n = 84 (6x) may have evolved from the chromosome doubling of N. daubeniana 2n = 42 (3x). N. nouchali (Type 3) 2n = 70 (5x) might have originated by the crossing of N. pubescens 2n = 84 (6x) and N. nouchali (Type 1) 2n = 56 (4x). Most of the species in Nymphea hybridize freely among themselves naturally, and thereby, generate uncertainty regarding their identity.[23] A lot of study remains to be done to improve the understanding of this wide-ranging and highly variable taxon and its relationship, to help the related taxa.
Genotypic studies reveal that carnivory is polyphyletic.[24] Phylogenetic trees prepared on the basis of taxonomy suggest a strong evolutionary linkage between some carnivorous families such as Nepanthaceae and Sarraceniaceae to Nympheaceae, which is conclusive from N. stellata as it indulges in a primitive form of insectivory. No insectivorous flowering plant has been reported and N. nouchali may be the missing link in the evolutionary history of other highly evolved carnivorous plant families.[25] N. stellata is also reported for its allelopathic potential, being more toxic to the growth of hyacinth.[26]
GEOGRAPHICAL SOURCE
N. stellata is a perennial aquatic rooting herb, wild / cultivated, generally found in tanks and ponds throughout the warmer parts of India, particularly the Eastern Ghats. For centuries it has been cultivated in Southeast Asia, especially around temples.[15,27] Native to Borneo, Philippines, Srilanka, Myanmar, Afganistan, Pakistan, Bangladesh, Nepal, Cambodia, Malaysia, Laos, Thailand, Vietnam, New Guinea, Indochina to China, Taiwan and Indonesia, distribution also reported in Africa and Australia.[10,19,28–32]
CULTIVATION
N. stellata is cultivated in portions of paddy fields left uncultivated during the Southwest monsoon. Seed / roots are first selected, then air dried and stored. The mud is first prepared and manured with old weeds or stubble, or special compost. Seed / roots are propagated in shallow mud and later transplanted to positions in rows three feet apart, and two-to-three feet apart in the rows. The blue water lily may be grown from seeds, but it takes three to four years to flower. The seed can be sown in spring and during summer. Finely sieved clean loam soil without any organic matter or fertilizer is used. Seeds should be sown thinly, covered lightly with soil and then plunged into shallow water, no deeper than 2.5 cm, and placed in a sunny position. Germination should take three-to-four weeks. When the first two or three floating leaves appear the seedling should be picked out and planted into individual containers and immersed back in the water. They may be submerged into deeper water and larger containers as they grow and lengthen. The easiest method of propagation is division. Plants are left in one place for two years, but pot-grown plants are best lifted, divided just before new growth commences in the spring and planted in fresh soil. The fleshy roots are pulled or cut and replanted immediately in a fresh soil mixture. Each new plant should have at least one bud at the tip of the rhizome.[33,34]
ANATOMY, HISTOLOGY, AND MICROSCOPY
N. stellata is a rather weak or stout herb from a cone-like tuberous rhizome, bearing small white pithy roots; stems are often reddish violet. Rhizomes are short, erect, stout, fleshy, unbranched, pyriform, and about the size of an egg. The rhizomes are enclosed in a thin covering, which turns horny on drying. The covering is itself covered with a cottony substance, especially at the apex. The rhizome is full of starch and is very palatable when boiled or prepared with curry, being more palatable than the Yam (Dioscorea) and Cocoyam (Colocasia).[33] The fruit is about 2.5 – 3 cm in diameter, globose, 6.5 cm, glabrous, and contains round, flask-shaped seeds, less than 1 mm in diameter. It has many seeds, berries, which are ellipsoid-globose, black with a white aril, 0.5 – 1.3 mm, with longitudinal rows of hairs.[13,35,36] Table 2 shows the anatomy, histology, and powder microscopy of the flower and leaf of N. stellata.[15,19,32,37–44]
Table 2.
Anatomy, histology, and powder microscopy of different parts of N. stellata

The Ayurvedic pharmacopeia of India has specified the proximate analytical parameters, namely, foreign matter (Not More Than 2%), total ash (NMT 8%), acid insoluble ash (NMT 0.5%), alcohol soluble extractive (Not Less Than 5%), and water soluble extractive values (NLT 22%) for identity, purity, and strength of flowers of N. stellata. The TLC pattern of the alcoholic flower extract in chloroform : ethylacetate : formic acid (5 : 4 : 1) showed three visible spots at Rf 0.59, 0.68, and 0.81 (all bluish-gray) and on spraying with 10% aqueous ferric chloride solution two spots appeared at Rf 0.68 and 0.81 (both blue color).[19]
TRADITIONAL USES
The Indian system of medicines, particularly Ayurveda and Siddha, uses N. stellata as a single drug or in combination with other drugs. It is also known as Utpala in Sanskrit, but this name refers only to dried flowers. N. stellata are ingredients of many ayurvedic formulations like, Asokarista, Arvindasava, Usirasava, Candanasava, Kalyanaka Ghrta, Samangadi Curna, Kanaka Taila, Jatyadi Taila, Tungadrumadi Taila, Manjesthadi Taila, Candanadi Lauha, and Triphala Ghrta.[19] It is also an ingredient of many polyherbal formulations for anti-aging, rejuvenation, and menstrual irregularities. The traditional uses of different parts of N. stellata are given in Table 3. The rhizome, fruit, leaf petiole, roots, flowers, tubers, and seed are used as edible parts in different ways by people.[45–50] It has also been cultivated for food in Srilanka as the rhizomes are full of starch and reputedly quite tasty when boiled.[15] The roots and rhizomes are considered to be nutritious when eaten either raw or roasted.[51] Flowers are used in temples, rhizomes in medicine, flower and flower stalks as vegetables, green manure, and fodder. N. stellata is considered as one of the ten most common noxious aquatic weeds in India.[52]
Table 3.
Traditional uses of different parts of N. stellata

CHEMISTRY
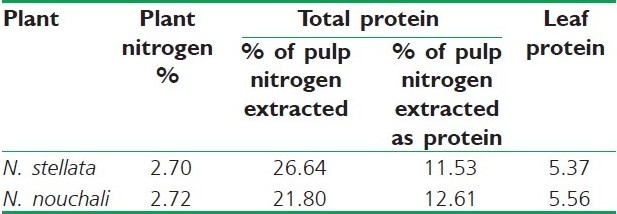
Different solvent extracts of the entire plant have shown the presence of sterols, alkaloids, saponins, tannins, and flavonoids. Nymphayol (25,26-dinorcholest-5-en-3b-ol) [Figure 2], a new sterol has been isolated from the successive chloroform extract of the flower.[53] Protein, pentosan, mucilage, and tannins are reported in the seeds.[54,55] Astragalin, corilagin, gallic acid, gallic acid methyl ester, isokaempferide, kaempferol, quercetin-3-methyl ether, quercetin, 2,3,4,6-tetra-o-galloyl dextroglucose, and 3-o-methylquercetin-3’-o-beta dextroxylopyranoside have been identified in the flowers.[56,57] The HPTLC method for quantitative determination of gallic acid from hydroalcoholic dried flower extract has been reported.[58] The leaves and shoots of N. stellata (Blue water lily) and N. nouchali (Red water lily) as different species have been studied for their chemical composition. The proximate analysis showed dry matter -8.4%, crude protein-16.8, ash-18.7, crude fat-2.8, crude fiber-26.3, and nitrogen free extract-35.4 for N. nouchali; and dry matter-7, crude protein-16.7, Ash-14.1, crude fat-2.6, crude fiber-24, and nitrogen free extract-42.6 for N. stellata, respectively. Mineral content showed sodium-1.19, potassium-2.23, calcium-0.52, phosphorus-0.32, and calcium / phosphorus ratio 1.63 for N. nouchali; and sodium-0.93, potassium-1.30, calcium-0.95, phosphorus-0.21, and calcium / phosphorus ratio-4.52 for N. stellata. Alkaloids had been detected in fraction A (extracted with chloroform from an ammonical solution) for both, while N. nouchali and N. stellata differed in their nitrate content with 2 and 0.9%, respectively. N. nouchali showed polyphenols total-8.7%, free-5.9%, and bound-2.8%; and N. stellata showed polyphenols total-10.2%; free-9.3%, and bound-0.9%.[59] On account of the nitrogen and protein content [Table 4] of N. stellata and N. nouchali, they have also been reported as two different species.[60] There is not much difference quantitatively between the two and a trivial difference in plant content is familiar. N. stellata (Blue water lily) and N. nouchali (Red water lily) may be still the same for the reason that blue, pink, mauve or white blue flower colors are also recorded in N. stellata. It is also possible that they may be two varieties. Apomorphine, nuciferine, and nornuciferine have been reported[61] from N. stellata, in fact, they have been isolated from N. caerulea.
Figure 2.
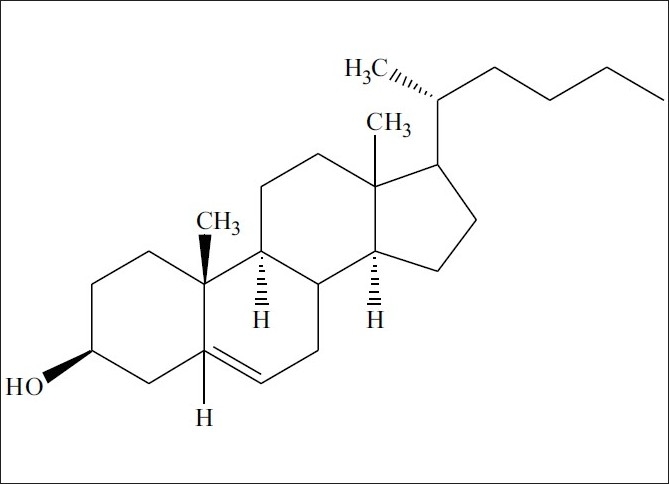
Chemical structure of Nymphayol
Table 4.
Nitrogen and protein content of N. stellata and N. nouchali [60]
PHARMACOLOGY
Antidiabetic Activity
The defatted ethanolic leaf extract (14.26 %w/w) at a dose of 100 and 200 mg/kg was studied for hypoglycemic activity in alloxan-induced diabetic rats (Wistar, 150 – 220 g). Oral treatment significantly and dose-dependently reduced the hyperglycemia. Moreover, it decreased the levels of cholesterol (CHL) and triglycerides (TGL) that had been increased by the alloxan treatment. On the contrary, no effect was seen in normal rats, both in the glucose and lipid plasma levels. The hypocholesterolemic effect of the ethanolic extract of the leaves of N. stellata could possibly be related to its amino acid and saponin composition.[62] The hydroalcoholic extract (yield: 6.8% w/w) of flowers at 200, 300, and 400 mg/kg (oral) were studied in normoglycemic and alloxan-induced diabetic rats (Male, Wistar strain, 150 – 200 g). It showed no hypoglycemic effect in normoglycemic animals, but showed statistically significant antihyperglycemic activity by improvement seen on the oral glucose tolerance test (OGTT). The flower extract caused significant reduction in the blood glucose level of diabetic rats. The dose of 300 mg/kg showed significant blood glucose level reduction (45%), fours hours after administration of the flower extract. The hydroalcoholic extract also showed a dose-dependent response.[63]
The antidiabetic effect of hydroalcoholic extract of the N. stellata flower could be linked to more than one mechanism. The possible mechanism includes the stimulation of β-cells and a subsequent release of insulin and activation of the insulin receptors. The antihyperglycemic action may be due to the potentiation of pancreatic secretion of insulin, which is clearly evident from the increased level of insulin in the treated rats. N. stellata also acts as a hepatoprotective agent,[64] therefore, it could have improved the function of the liver and maintained glucose uptake, enhanced transport of blood glucose to the peripheral tissue, and also enhanced utilization, which may be another mechanism of action. The extract might have also have stimulated glycogenesis and / or inhibited glycogennolysis in the diabetic rat liver. Administration of the extract reduced TC, TG, LDL, VLDL, and also improved the HDL level. Serum phospholipid was elevated, whereas, the phospholipids in the liver and kidney were decreased. Treatment could have restored the normal metabolism by shifting the balance from lipid metabolism to carbohydrate metabolism.[65]
Oral administration of N. stellata flower extract for 30 consecutive days to diabetic rats also decreased their food consumption and improved body weight. This could be due to a better control of the hyperglycemic state in the diabetic rats. Administration of te flower extract to diabetic rats significantly increased the level of total hemoglobin, which might be due to the decreased level of blood glucose. Oral administration of the flower extract improved the total protein concentration in the serum. Administration of N. stellata flower extract to diabetic rats reversed the changes and improved the HDL levels. These results unmistakably indicate that the flower extract could effectively manage the diabetic complications such as body weight maintenance, hyperlipidemia, cardiovascular complications in diabetes mellitus and progression of atherosclerosis.[66] Nymphayol (25,26-dinorcholest-5-en-3b-ol), a new sterol isolated from the bioactive successive chloroform flower extract has been reported for its antidiabetic activity at 20 mg/kg bw in streptozotocin-induced diabetic rats. Oral administration of Nymphayol for 45 days significantly restored the plasma glucose levels and increased the plasma insulin levels to near normal in STZ-diabetic rats. Light microscopy and immunocytochemical staining of Nymphayol-treated diabetic pancreas revealed an increased number of insulin positive β-cells. The mode of action of Nymphayol may be due to the reversal of the damaged endocrine tissue, thereby stimulating the secretion of insulin in β-cells, as revealed by insulin assay. The active principle of Nymphayol enhances the antioxidant defense against the reactive oxygen species (ROS) produced under hyperglycemic conditions and thus protects the pancreatic β-cells against loss.[53]
Tumor Inhibition Studies
The methanolic extract of Nymphaea nouchali roots at 200 μg/ml were screened for their inhibitory activity toward tumor promoter 12-O-hexadecanoylphorbol-13-acetate-induced Epstein-Barr virus activation in the Raji cells. The extract was inactive with zero inhibition rate.[67]
Antihepatotoxic Effect
The alcoholic extract (yield, 9% w/w) of the N. stellata flowers was evaluated against carbon tetrachloride-induced hepatic damage in albino Wistar rats (8 – 10 weeks, 100 – 120 g) at 250, 500, and 750 mg/kg (orally), in the form of an aqueous suspension, once a day, for 10 days. The hepatoprotective activity exerted by the extract could be due to cell membrane stabilization, hepatic cell regeneration, and activation of antioxidative enzymes such as glutathione reductase, glutathione peroxidase, superoxide dismutase, and catalase.[64] The petroleum ether extracts of N. stellata seeds were tested against carbon tetrachloride (CCl4)-induced hepatotoxicity in rats and mice at a dose of 300 mg/kg i.p. The extract markedly reduced the prolongation of sleeping time and significantly prevented the CCl4-induced increase in weight and volume of the liver, and the mortality. The extract also prevented necrosis of the liver and promoted, to some extent, liver generation.[68]
Cholinergic Activity
The alcohol extract of the defatted fruits of N. stellata produced mild sedation and ataxia, potentiated hexobarbitone-induced hypnosis in mice, and also produced a sharp and transient hypotension blocked by pretreatment with atropine. If large doses were administered after atropinization, a rise in blood pressure and also a stimulant effect was observed on guinea pig ileum, indicating the presence of some unstable cholinergic principle.[31]
Analgesic and Anti-inflammatory Activity
The extract had a significant analgesic activity as revealed by the aconitine-induced writhing in mice and the antipyretic activity against carrageenin-induced rat paw edema. The anti-inflammatory activity exhibited was comparable to that of hydrocortisone.[69]
Antimicrobial Activity
Flowers of N. nouchali were effective against Pseudomonas aeruginosa, Bacillus cereus, and Staphylococcus aureus.[70] N. stellata also demonstrated a broad spectrum of activity against phytopathogenic bacteria. The ethanolic extract of Nymphaea stellata leaves has shown considerable antibacterial activity against E. coli.[44]
Other Activities
The LD50 of 50% ethanol extract of N. stellata was found to be 681 mg/kg in albino mice. N. stellata was found to be inactive as an antibacterial, antifungal, antiprotozoal, antiviral, diuretic, with no effect on the cardiovascular system and central nervous system.[71]
CONCLUDING REMARKS AND FUTURE POTENTIAL
Regarding the copious synonymy, a lot more study remains to be done, to precisely differentiate the closely related species and varieties. The numerous vernacular names used complicate the identification of the traditionally claimed species. However, anatomical studies will provide details for establishing the identity and the degree of purity. The traditional usage will give an idea of how people treat different health problems with the aid of N. stellata parts. Taking into account the magnitude of its traditional uses, the studies conducted are still too scant.
To date sterols, alkaloids, saponins, tannins, and flavonoids are reported from different parts. Bitter principles are known for a variety of biological responses like blood sugar regulation, stimulating the gastric reflex, and increasing the secretion of enzymes. Although traditionally N. stellata is considered as bitter, no particular bitter principle has been identified. However, there is very good agreement between the traditional use and experimentally observed effect — the hepatoprotective, anti-inflammatory, and particularly antidiabetic activity. Nymphayol, an isolated steroid reverses the damaged endocrine tissue and stimulates secretion of insulin in β-cells, accentuating the fact that traditional uses of medicinal plants are frequently coherent with the pharmacological effects of the main active principles. On the other hand, experimental studies have uncovered the potential uses of N. stellata in hyperlipidemia, cardiovascular complications in diabetes, and also in the progression of atherosclerosis.
Some trends in N. stellata should be reassessed. It is evident from the reference list of the present study that extracts have been the object of relatively many investigations, while fractions of bioactive extract / pure compounds have so far been neglected by phytochemists and pharmacologists. Future phytochemical investigation may be focused on identifying bioactive moieties, such as the unstable cholinergic principle reported and the constituents responsible for the anti-inflammatory and antihepatotoxic effect. Part of the future pharmacological investigation should center their focus on exhaustive studies on unexplored claims like aphrodisia and their effectiveness in urinary disorders, menorrhagia, blenorrhagia, and menstruation problems. It is expected that many novelties will rapidly enlarge the current knowledge about N. stellata, their constituents, and corresponding pharmacological effects.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Lad V, Frawley D. Wisconsin: Lotus Press; 1986. The Yoga of Herbs: An Ayurvedic Guide to Herbal Medicine. [Google Scholar]
- 2.Wagner H, Bladt S. New Delhi: Springer India Pvt. Ltd; 1996. Plant Drug Analysis – A Thin Layer Chromatography Atlas. [Google Scholar]
- 3.Patwardhan B. Ethnopharmacology and Drug Discovery. J Ethnopharmacol. 2005;100:50–2. doi: 10.1016/j.jep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Buzgo M, Soltis PS, Kim S, Soltis DE. The making of the flower. Flower Evolution. 2005;52:149–54. [Google Scholar]
- 5.Friis EM, Pedersen KR, Crane PR. Fossil evidence of water lilies (Nymphaeales) in the early cretaceous. Nature. 2001;410:357–60. doi: 10.1038/35066557. [DOI] [PubMed] [Google Scholar]
- 6.Soltis PS, Soltis DE, Zanis Phylogeny, classification and floral evolution of water lilies (Nymphaeaceae; Nymphaeales): A synthesis of non-molecular, rbcl, mat K, and 18s r DNA data. Syst Bot. 1999;24:279–92. [Google Scholar]
- 7.Yoo MJ, Bell CD, Soltis PS, Soltis DE. Divergence times and historical biogeography of Nymphaeales. Syst Bot. 2006;30:693–704. [Google Scholar]
- 8.Anonymous. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG II. Bot J Linn Soc. 2003;141:399–436. [Google Scholar]
- 9.Mabberley DJ. Canbridge: University Press; 1997. The Plant-Book: A portable dictionary of the vascular plants. [Google Scholar]
- 10.Wiart C. New York: CRC Press; 2006. Medicinal plants of Asia and the Pacific. [Google Scholar]
- 11.Danin A. The nomenclature news of flora Palaestina. Flora Mediterranea. 2000;10:109–72. [Google Scholar]
- 12.Merlin MD. Archaeological evidence for the tradition of psychoactive plant use in the old world. Econ Bot. 2003;57:295–323. [Google Scholar]
- 13.Stephens KM, Dowling RM. Collingwood: CSIRO publishing; 2002. Wetland Plants of Queensland. [Google Scholar]
- 14.Viljoen C, Notten A. Cape Town: Kirstenboch National Botanical Gardens; 2002. Nymphaea nouchali Burm. F. var. caerulea (Sav.) Verdc. [Google Scholar]
- 15.Slocum PD. Portland: Timber Press; 2005. Waterlilies and Lotuses: Species, Cultivars and New hybrids. [Google Scholar]
- 16.Simmonds MS, Howes MJ. Plants used in the treatment of diabetes. In: Soumyanath A, editor. Traditional Medicines for Modern Times: Antidiabetic Plants. New York: CRC press; 2006. [Google Scholar]
- 17.Verdcourt B. The typification of Nymphaea lotus L. Kew Bull. 1989;44:179–80. [Google Scholar]
- 18.Singh HB, Sandhu JS. New Delhi: Daya Books; 2003. Herbal Medicine of Manipur - A Colour Encyclopaedia. [Google Scholar]
- 19.Vol. 3. New Delhi: Government of India press; 2001. Anonymous. The Ayurvedic Pharmacopeia of India, part I. [Google Scholar]
- 20.Sanjappa M, Venu P, Albertson D. Sasyabharati (Dakshinamnaya): A database on plants of South India. Curr Sci. 2005;88:825–6. [Google Scholar]
- 21.Nair RV. Hyderabad: Orient Longman Pvt. Ltd; 2004. Controversial Drug Plants. [Google Scholar]
- 22.Ping-he W, Wei-pei C. Study on the karyotype analysis of nymphaeacee and its taxonomic position. Acta Phytotaxonomica Sinica. 1994;32:293–300. [Google Scholar]
- 23.Hossain A, Kabir G, Ud-deen MM, Alam AM. Cytological studies of Nymphaea species available in Bangladesh. J Bio Sci. 2007;15:7–13. [Google Scholar]
- 24.Albert VA, Williams SE, Chase MW. Carnivorous plants, phylogeny and structural evolution. Science. 1992;257:1491–5. doi: 10.1126/science.1523408. [DOI] [PubMed] [Google Scholar]
- 25.Tetali P, Sutar S, Tetali S. Selective insectivory in Nymphaea nouchali Burm. f. Nature Proceedings. 2008 hdl:10101/npre.2008.1817.1: Posted on 20 Apr 2008. [Google Scholar]
- 26.Gupta J, Saxena MK. Allelopathic potential of three aquatic macrophytes on growth of water hyacinth. In: Kumar A, editor. Water Pollution. New Delhi: APH Publishing; 2004. [Google Scholar]
- 27.Johannesburg: Jacana Media; 2004. Anonymous. Lowveld and Kruger Guide. [Google Scholar]
- 28.Vol. 6. Taipei: National Taiwan University; 2003. Anonymous. Flora of Taiwan. [Google Scholar]
- 29.Kirtikar KR, Basu BD. Vol. 1. Dehradun: Oriental Enterprises; 2001. Indian Medicinal Plants. [Google Scholar]
- 30.Pullaiah T, Rao DM. Vol. 1. New Delhi: Regency Publications; 2002. Flora of Eastern Ghats: Hill ranges of south east India. [Google Scholar]
- 31.Satyavati GV. Medicinal Plants of India. Vol. 2. New Delhi: Cambridge Printing Works; 1987. Plant descriptions. [Google Scholar]
- 32.Shui LK, Dezhi F, Wiersema JH. Nymphaeaceae. Flora China. 2001;6:115–8. [Google Scholar]
- 33.Irvine FR, Trickett RS. Waterlilies as Food. Kew Bull. 1953;8:363–70. [Google Scholar]
- 34.Soyza J de. Nymphaea stellata (Water lily) as an Economic Crop. Trop Agriculturalist. 1936;87:371–6. [Google Scholar]
- 35.Butchart D. Cape Town: Struik Publishers; 2000. Wildlife of the Okavango. [Google Scholar]
- 36.Pullaiah T, Prabhakar C, Rao BR. New Delhi: Daya Books; 1998. Flora of Medak District, Andhra Pradesh, India. [Google Scholar]
- 37.Dassanyake MD. Nymphaeaceae. In: Dassanayake MD, editor. A Revised Handbook to the Flora of Ceylon 10. New Delhi: Oxford and IBH Publishing Co. Pvt. Ltd; 1996. [Google Scholar]
- 38.Dhanabal SP, Mohan Maruga Raja MK, Paramakrishnan N, Suresh B. Pharmacognostical evaluation of Nymphaea stellata Willd. Aryavaidyan. 2006;20:8–13. [Google Scholar]
- 39.Farooqui P. Ontogeny of stomata in some Nymphaeaceae. Proc Indian Acad Sci. 1980;89:437–42. [Google Scholar]
- 40.Gupta RC, Shome U, Khanna RK, Sharma HP. Pharmacognostic studies on ‘Nilotpala’ (Nymphaea stellata Willd.) I - Vegetative parts. New Bot. 1980;7:127–43. [Google Scholar]
- 41.Pullaiah T, Silar Mohammed M. New Delhi: Daya Books; 2000. Flora of Ranga Reddi District, Andhra Pradesh, India. [Google Scholar]
- 42.Verdcourt B. New York: CRC Press; 1989. Nymphaeaceae. [Google Scholar]
- 43.Yakandawala D, Paebotuwage I. ‘Tel-olu’ – is it really an ‘olu’.Cey. J Sci. 2007;36:88–99. [Google Scholar]
- 44.Mohan Maruga Raja MK, Dhanabal SP, Patil MJ. Pharmacognostical investigation and antibacterial activity of Nymphaea stellata Willd.Leaves. Hamdard Med. 2008;51:139–45. [Google Scholar]
- 45.Kumar A, Bohra C. Waning wetlands: A need for its conservation. In: Kumar A, editor. Ecological Studies. New Delhi: Daya Books; 2005. [Google Scholar]
- 46.Ngugi G. Kenya: Lokichoggio; 1999. Case study from Kenya on indigenous wild vegetables.Proceedings of workshop: Exploring the potential of indigenous wild food plants in Southern Sudan. [Google Scholar]
- 47.Patiri B, Borah A. Guwahati: Geetakhi Printers and Publishers; 2007. Wild Edible Plants of Assam. [Google Scholar]
- 48.Sarma H, Sarma AM, Sarma CM. Traditional knowledge of weeds: A study of herbal medicines and vegetables used by the Assamese people (India) Herba Polonica. 2008;54:80–8. [Google Scholar]
- 49.Sharma M. Effect of environmental upheavals in Manipur. In: Thomas J, Gopalakrishnan R, Ranjan R, Singh RK, editors. Constraints in Development of Manipur. New Delhi: Daya Books; 2001. [Google Scholar]
- 50.Venu P. Environmental impact assessment: Some considerations on evaluation of flora – an overview. PINSA. 1999;5:257–74. [Google Scholar]
- 51.Michler I. Cape Town: Struik Publishers; 2004. Bostwana: The Insider's Guide. [Google Scholar]
- 52.Varshney CK, Rzoska J. New York: Springer; 1976. Aquatic Weeds in South East Asia. [Google Scholar]
- 53.Subash Babu P, Ignacimuthu S, Agastian P, Varghese B. Partial regeneration of β-cells in the islets of langerhans by Nymphayol a sterol isolated from Nymphaea stellata (Willd.) flowers. Bioorgan Med Chem. 2009;17:2864–70. doi: 10.1016/j.bmc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Gujral ML, Saxena PN, Mishra SS. An experimental study of the comparative activity of indigenous diuretics. J Indian Med Assoc. 1955;25:49–51. [PubMed] [Google Scholar]
- 55.Kapoor VP, Khan PS, Raina RM, Farooqui MI. Chemical analysis of seeds from 40 non- leguminous species, part III. Sci Cult. 1975;41:336. [Google Scholar]
- 56.Kizu H, Tamimori Phenolic constituents from the flowers of Nymphaea stellata. Nature Med. 2003;57:118–9. [Google Scholar]
- 57.Mukherjee KS, Bhattacharya P, Mukheriee RK, Ghosh PK. Chemical examination of Nymphaea stellata Willd. J India Chem Soc. 1986;513:530–1. [Google Scholar]
- 58.Rakesh SU, Salunkhe VR, Dhabale PN, Burande KB. HPTLC method for quantitative determination of gallic acid in hydroalcoholic extract of dried flowers of Nymphaea stellata Willd. Asian J Res Chem. 2009;2:131–4. [Google Scholar]
- 59.Banerjee A, Matai S. Composition of Indian aquatic plants in relation to utilization as animal forage. J Aquat Plants Manage. 1990;28:69–73. [Google Scholar]
- 60.Dewanji A, Chanda S, Si L, Barik S, Matai S. Extractability and nutritional value of leaf protein from tropical aquatic plants. Plant Foods Hum Nutr. 1997;50:349–57. doi: 10.1007/BF02436081. [DOI] [PubMed] [Google Scholar]
- 61.Perry EK, Ashton H, Young AH. Amsterdam: John Benjamins Publishing Company; 2002. Neurochemistry of consciousness: Neurotransmitters in mind. [Google Scholar]
- 62.Dhanabal SP, Mohan Maruga Raja MK, Ramanathan M, Suresh B. Hypoglycemic activity of Nymphaea stellata leaves ethanolic extract in alloxan induced diabetic rats. Fitoterapia. 2007;78:288–91. doi: 10.1016/j.fitote.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Rajagopal K, Sasikala K, Ragavan B. Hypoglycemic and antihyperglycemic activity of Nymphaea stellata flowers in normal and alloxan diabetic rats. Pharm Biol. 2008;46:654–9. [Google Scholar]
- 64.Bhandarkar M, Khan A. Antihepatotoxic effect of Nymphaea stellata Willd.Against carbon tetrachloride-induced hepatic damage in albino rats. J Ethnopharmacol. 2004;91:61–4. doi: 10.1016/j.jep.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 65.Rajagopal K, Sasikala K. Antidiabetic activity of hydro-ethanolic extracts of Nymphaea Stellata flowers in normal and alloxan induced diabetic rats. Afr J Pharm Pharmacol. 2008;2:173–8. [Google Scholar]
- 66.Rajagopal K, Sasikala K. Antihyperglycaemic and antihyperlipidaemic effects of Nymphaea stellata in alloxan-induced diabetic rats. Singapore Med J. 2008;49:137. [PubMed] [Google Scholar]
- 67.Murakami A, Ali AM, Mat-Salleh K, Koshimizu K, Ohigashi H. Screening for the in vitro anti-tumor-promoting activities of edible plants from Malaysia. Biosci Biotechnol Biochem. 2000;64:9–16. doi: 10.1271/bbb.64.9. [DOI] [PubMed] [Google Scholar]
- 68.Singh N, Nath R, Gupta ML, Kohli RP, Singh DR. An experimental evaluation of protective effects of some indigenous drugs on carbon-tetra chloride hepatotoxicity in mice and rats. Quart J Crude Drug Res. 1978;16:8–16. [Google Scholar]
- 69.Singh N, Nath R, Kohli RP. Pharmacological study on Nymphaea stellata Nilkamal. J Resi Indian Med Yoga Homoe. 1977;12:53–7. [Google Scholar]
- 70.Vasu K, Singaracharya MA. Antimicrobial activity of certain aquatic angiosperms against some pathogenic bacteria. Asian J Microbiol Biotechnol Environ Sci. 2008;10:609–13. [Google Scholar]
- 71.Aswal BS, Bhakuni DS, Goel AK, Kar K, Mehrotra N, Mukherjee KC. Screening of Indian plants for biological activity, part X. Indian J Exp Biol. 1984;22:312–22. [PubMed] [Google Scholar]
- 72.Cridland JS, Koonin S. Use of traditional medicines towards a classification. S Afr Med J. 2001;91:489–91. [PubMed] [Google Scholar]
- 73.Deutschlander MS, Lall N, Van de Venter M. Plant species used in the treatment of diabetes by South African traditional healers: An inventory. Pharma Biol. 2009;47:348–65. [Google Scholar]
- 74.Kirtikar KR, Basu BD. Vol. 1. Dehradun: International Book Distributors; 1999. Indian Medicinal Plants. [Google Scholar]
- 75.Nadkarni KM. Vol. 1. Bombay: Popular Prakashan Pvt. Ltd; 1982. Indian Materia Medica. [Google Scholar]
- 76.Sharma PV. Varanasi: Caukhambha Visvabharati; 1998. Puspayurvedah. [Google Scholar]
- 77.Tirkey A, Khan F, Khan SS, Saify T. Medicinal plants used in treatment of indigestion in Raigarh district of Chhattisgarh. Proceedings of national conference: Biodiversity and sustainable utilization of biological resources, Sagar, Madhya Pradesh, India. 2001 [Google Scholar]
- 78.Tyagi DK. New Delhi: Atlantic Publishers and Distributors; 2005. Pharma Forestry: Field guide to Medicinal Plants. [Google Scholar]
- 79.Kaul MK. New Delhi: Indus Publishing; 1997. Medicinal Plants of Kashmir and Ladakh. [Google Scholar]
- 80.Lawrence WR. Lahore: Shirkat Printing Press; 1991. The Valley of Kashmir. [Google Scholar]
- 81.Manjunatha BK, Krishna V, Pullaiah T. New Delhi: Daya Books; 2004. Flora of Davanagere District, Karnataka, India. [Google Scholar]
- 82.Watt JM, Breyer-Brandwijk MG. London: Livingston; 1962. The Medicinal and Poisonous Plants of Southern and Eastern Africa. [Google Scholar]
- 83.Arnold HJ, Gulumian M. Pharmacopeia of traditional medicine in Venda. J Ethnopharmacol. 1984;12:35–74. doi: 10.1016/0378-8741(84)90086-2. [DOI] [PubMed] [Google Scholar]
- 84.Singh P, Sisodia S, Shah J. Angiospermous seeds medicinal importance in Gujarat state. In: Chopra AK, Khanna R, Prasad G, Malik DS, Bhutiani, editors. Medicinal Plants: Conservation Cultivation and Utilization. New Delhi: Daya Books; 2007. [Google Scholar]
- 85.Achariya RK, Upadhyay BN, Dwivedi LD. Dietary management in prameha. Ancient Sci Life. 1996;115:176–89. [PMC free article] [PubMed] [Google Scholar]
- 86.Subbulakshmi G, Naik M. Indigenous foods in the treatment of diabetes mellitus. Bombay Hosp J. 2001;43:548–61. [Google Scholar]
- 87.Crevost C, Lemarie CH, Petelot A. Vol. 1. Hanoi: Impr. d’Extreme-Orient; 1917. Catalogue des Produits de l’Indochine. [Google Scholar]
- 88.Partha P, Enayet Hossain AB. Ethnobotanical investigation into the Mandi ethnic community in Bangladesh. Bangladesh J Plant Taxon. 2007;14:129–45. [Google Scholar]


