Abstract
Some novel substituted pyramidinopyrazoles and pyrimidinotriazoles have been synthesized by using 6-anisyl-5-cyan-2-hydrazine-3-N-methyl-3, 4-dihydropyrimidine-4-one as the starting material. Structures of these compounds have been established by IR, 1H NMR, Mass and elemental analysis data and have been evaluated for their antimicrobial activity. Significant antimi.crobial activity was observed for some members of these series.
Keywords: Antimicrobial activity, Pyramidinopyrazoles, Pyrimidinotriazoles
INTRODUCTION
The presence of pyrimidine base in thymine, cytosine and Uracil, which are the essential building blocks of nucleic acids DNA and RNA, is one possible reason for their wide spread therapeutic applications. The literature survey indicated that a wide range of pharmacological activities is exhibited by the compounds encompassing pyrimidine nucleus. For example, 5-flonrouracil is used as anticancer agent; idoxuriduie and isifluoriduie as antiviral; zidovudine and stavudine as antiHIV, trimethoprim, sulphamethiazine as antibacterial, sulphadoxin as antimalarial and antibacterial etc. In addition to this, various analogs of pyrimidines have been found to possess antibacterial[1–4], antifungal[5–6], antileishmanial[7] and anti-inflmmatory[8] activities. Tegafur[9] is an anti-cancer agent encompassing pyrimidine nucleus, discovered after formulation of the antimetabolite theory by Woods and Fildes in 1940. A large number of 2, 4-diaminopyrimidines have been synthesized as antifolates. It was eventually proved that these pyrimidines are inhibitors of the enzyme dihydrofolate reductase (DHFR)[10–11]. Pyrimidine derivatives of sulfa drugs, namely sulfadiazine, sulfamerazine and sulfadimidine are superior to many other sulfonamides and are used in some acute urinary tract infections (UTI), cerebrospinal meningitis and for patients allergic to penicillins[9]. Epirazole[9], another non steroidai anti-inflammatory drug containing pyrimidine derivative with pyrazole, is suggested to be a COX-2 inhibitor. A novel series of pyrazolo [1, 5-b] pyridazines[12] have been synthesized and identified as cyclin dependant kinase inhibitors potentially useful for the treatment of solid tumors. To exploit the biological potency of pyrimidine moiety it was thought of synthesizing compounds encompassing pyrimidine with pyrazole and pyrimidine with triazolo moiety.
MATERIAL AND METHODS
Experimental section
Reactions and purity of compounds were monitored by TLC (silica gel G60) using ethylacetate: chloroform (5:5) solvent system and the spots were identified by iodine vapor chamber. Melting points were determined in open capillary using paraffin bath and are uncorrected. The IR spectra of the compounds were recorded on a 300 MHz Shimadzu FT-IR-8400S spectrophotometer using KBr pellets[1]. H NMR spectra were recorded in DMSO on a 300 MHz Shimadzu FT-NMR (δ in PPM) relative to TMS as internal standard. The mass spectra were recorded on Triple Quadropole LC-MS with ESI source. Mfg. SCIEX at 70 eV.
Preparation of 6-anisyl-5-cyano-2-(5’-amino-4’-ethylcarboxylate-pyrazole-1’-yl)-3-N-methyl-3, 4 dihydropyrimidine-4-one 2
A mixture of compound 1 (0.271g, 0.01 mol) and ethyl-2-cyno-3-ethylacrylate (0.169g, O.Olmol) in alcohol and few drops of acetic acid was refluxed for six h. Excess of solvent was removed under reduced pressure and the reaction mixture was added to crushed ice with stirring. The product that separated was filtered, washed with water, dried and recrystallized from DMF to get pure yellow solid.
2. IR: 3270cm-1 (NH2), 1650cm-1 (C=O), 1750cm-1 (C=O), 2250cm-1 (CN); 1H NMR: 1.2δ (t, 3H, CH3) 3.4δ (s, 3H, N-CH3) 3.8δ (s, 3H, O-CH3) 4.2δ (q, 2H, CH2), 7.1δ (s, 1H, N=CH) 7.2 δ (d, 2H, Ar-H) 8.0 δ (d, 2H, Ar-H); Mass: miz 395, 349, 240, 83.
Preparation of 6-anisyl-5-cyano-2-(5’-methoxybenzylideneamino-4’-ethylcarboxylate-pyrazole-1’-yl)-3-N-methyl-3, 4-dihydropyrimidine-4-ones 3a
Compound 2 (0.394g, O.Olmol) and anisaldehyde (0.136g, O.Olmol) was taken in alcohol (20mL) and the reaction mixture was refluxed for half an hour. A catalytic quantity of NaOH was added and refluxing was continued for six h. The reaction mixture was then added to crush ice to get the desired Schiff's base 3a. It was filtered, washed with water and then dried. Similarly, various Schiff's bases 3b-j were prepared.
3a. IR: 3170cm-1 (NH), 1670cm-1 (C=0), 1745cm-1 (C=0), 2255cm-1 (CN); 1H NMR: 1.3 δ (t, 3H, CH3), 3.2 δ (s, 3H, N-CH3), 3.6 δ (s, 3H, O-CH3), 4.5 δ (q, 2H, CH2), 7.2 δ (s, 1H, N=CH), 7.6 δ (d, 2H, Ar-H), 7.7 δ (d, 2H, Ar-H ), 7.8 δ (d, 2H, Ar-H), 8.0 δ (d, 2H, Ar-H),11.2 δ (s, 1H, N=CH).
Preparation of 6-cyano-5-(p-methoxyphenyl)-8-methyl-7-oxo-7, 8-dihydro [1, 2, 4] triazolo [4, 3-a] pyrimidines 4a
The compound 1 (0.271g, 0.01 mol) was refluxed with formic acid molar ratio in slight excess for seven h in presence of catalytic amount of concentrated sulphuric acid and the reaction mixture was poured into crushed ice. The solid was separated was filtered, dried and recrystallized from DMF. Similarly, compounds 4b and 4c were prepared by refluxing compound with acetic anhydride and benzoyl chloride respectively.
4b. IR: 3210cm-1 (NH), 1690cm-1 (C=O), 2255cm-1(CN); 1H NMR: 2.0 δ (s, 3H, CH3), 2.6 δ (s, 3H, N-CH3), 3.6 δ (s, 3H, O-CH3), 7.7 δ (d, 2H, Ar-H) 7.9 δ (d, 2H, Ar-H).
Preparation of 2-(5’, 3’-diphenyl-4’, 5’-dihydro-1’H-pyrazol-1’-yl)-5-cyano-6-(p-methoxyphenyl)-3-N-methyl-3, 4-dihydropyrimidine-4-one 5a
A mixture of compound 1 (0.271g, 10 mol) and 3-(phenyl)- 1 -phenylprop-2- en--1-one (0.238g, 0.01mol) in acetic acid was refluxed for seven h. Excess of solvent was removed under reduced pressure and the reaction mixture was added to crushed ice. The product separated was filtered, washed with water, dried and recrystallized from DMF. Similarly, 5b and 5c compounds were prepared by refluxing the compound 1 with substituted chalcones separately.
5b. IR: 1685cm-1 (C=0), 2250cm-1 (CN), 1450cm-1 (C=N); 1H NMR: 2.6 δ (s, 3H, N-CH3), 2.8 δ (t, 1H, CH), 3.6 δ (s, 3H, O-CH3), 3.8 δ (s, 3H, O-CH3), 3.8 δ (d, 2H, CH2),7.7 δ (d, 2H, Ar-H) 7.9 δ (d, 2H, Ar-H).8.0 to 8.4 δ (m, 9H, Ar-H).
Antimicrobial activity
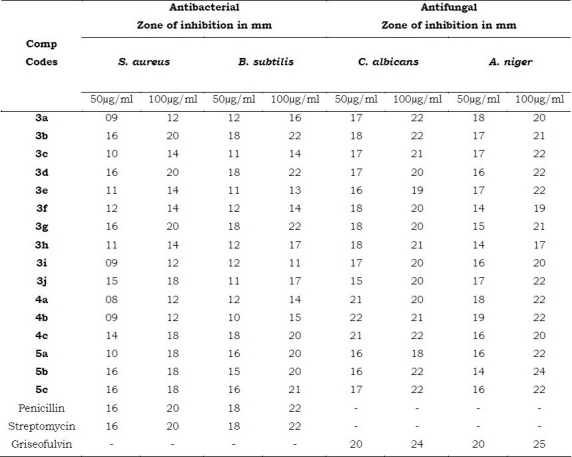
The in vitro antimicrobial activity was carried out against 24 h old cultures of bacteria and fungi using cup-plate method[14]. All the newly synthesized compounds were screened for the antimicrobial activity against S. arureus and B. subtilis and antifungal activity against C. albicans and A. niger. The compounds were tested at two concentrations namely 50΅g/ ml and 100΅g/ml in DMF against all organisms. The zone of inhibition was compared with procaine penicillin and streptomycin for antibacterial activity after 24 h of incubation at 25°C and Griseofulvin for antifungal activity after 48 h of incubation at 30°C.
RESULTS AND DISCUSSION
The most general and widely employed route to prepare pyrimidines involves the combination of a reagent containing the N-C-N skeleton with C-C-C unit. These methods are typical examples of the bis-nucleophilic plus bis-electrophilic method of constructing heterocycles. Both the nitrogen atoms of the N-C-N reagent act as nucleophiles and both the terminal carbon atoms of C-C-C reagent are electrophiles. Urea, thiourea and guanidine are commonly used as N-C-N reagents and 1, 3-diketones, diesters and dinitriles are the typical C-C-C reagents.
Thus, ethylcyanoacetate and anisaldehyde C-C-C unit on condensation with thiourea N-C-N unit in presence of K2CO3 produced 6-anisyl-5-cyano-thiouracil. Which was subsequently treated with methyl iodine and hydrazine hydrate resulted in the formation of 6-anisyl-5-cyano-2- hydrazine-3-N-methyl-3, 4-dihydro pyrimidine-4-one (6) 1 and it served as the starting material for the synthesis of pyrazole and traiazole derivatives.
The IR spectrum of compound 1 exhibited peaks at 3310, 2225, 1680 cm-1 due to NH, CN and C=O groups respectively. 1H NMR spectrum of the same compound recorded in DMSO exhibited a singlet corresponding to one proton at δ 10.5 and two doublets at 6 7.9 to 7.1 for four aromatic protons. It also exhibited two sharp singlets at 8 3.8 to 3.4 due to O-methyl and N-methyl protons and also another broad singlet at δ 1.2 due to NH 2protons. The structure of 1 was further confirmed by the appearance of molecular ion peak at m/z 271 in its mass spectrum. This served as starting material for the synthesis of the title compounds as shown in Scheme-I.
Scheme I.
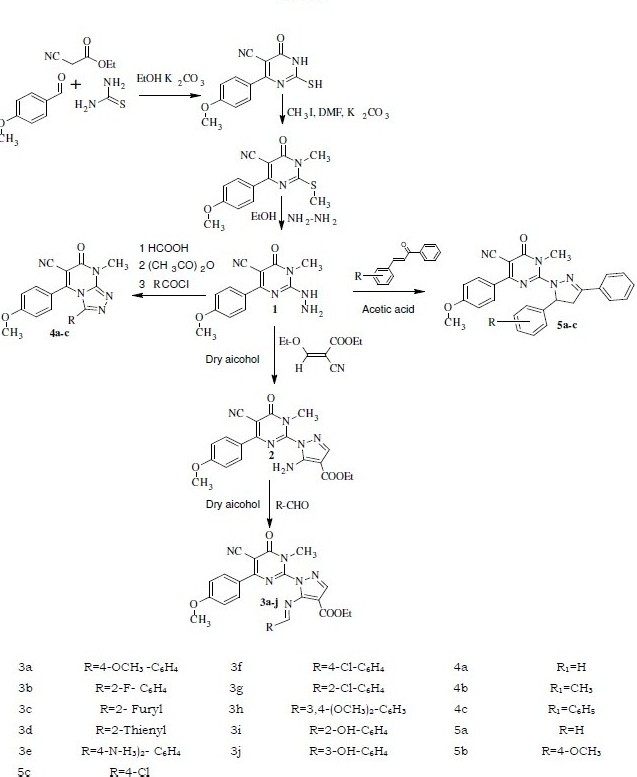
A mixture of compound 1 and ethyl-2--cyano-3-ethylacrylate in dry alcohol was refluxed for 6 h, in presence of catalytic amount of HCl to get 6-anisyl-5-cyano-2-(5’-amino-4’-ethyl carboxylate-pyrazole-1’-yl)-3-N-methyl-3,4 dihydropyrimidin-4-one 2. The completion of the reaction was monitored by TLC. The formation of the compound 2 was obvious by the presence of peak at 1705 cm-1 due to C=O stretching of ester group. The 1H NMR spectrum of 2 recorded in DMSO exhibited two doublets, each corresponding to two protons, at δ 8.0 and 7.2 due to four aromatic protons and broad peak at δ 7.1 for amine group. A singlet integrating for one proton at δ 7.05 due to -CH proton of pyrazole ring, quartet and triplet at δ 4.2 and 1.2 due to protons of ethyl group, two sharp singlets at δ 3.8 and 3.4 for O-methyl and N-methyl groups respectively were also observed. The formation of the compound 2 was also confirmed by its mass spectral data, which exhibited a molecular ion peak at m/z 394.
A mixture of compound 2 and anisaldehyde was refluxed in dry alcohol, in presence of catalytic quantity of NaOH. After the completion of reaction, the reaction mixture was decomposed in crushed ice to get desired Schiff's base, 3a. The selection of aromatic aldehydes was dependent upon the presence of electron donating and electron withdrawing groups, which would help in drawing some conclusions regarding the effect of these groups on various biological and pharmacological activities. The various other Schiff's bases 3b-j were synthesized in similar manner.
The formation of Schiff's base 3a was conspicuous by the absence of NH peak stretching at 3270cm-1in the IR spectrum. In addition, the 1H NMR spectrum of 3a recorded in DMSO exhibited singlet integrating for one proton at δ 12.50 due to -CH proton of Schiff base, a multiplet in between δ 8.0 to δ 7.0 due to aromatic protons, a quartet at δ 4.6 and triplet at 1.4 due to ethyl protons.
In order to fuse pyrimidine with triazole, the compound 1 was refluxed with formic acid, acetic anhydride and benzoyl chloride separately in presence of catalytic amount of concentrated sulfuric acid[13]. Completion of reaction was monitored by TLC, after the completion of reaction; the reaction mixture was added to crush ice to get desired triazoles 4a-c. Subsequently, the formation of triazole 4b was an agreement with its IR spectrum by the absence of NH stretching. The 1H NMR spectrum showed the absence of NH--NH 2peaks.
The compound 1 was also utilized attributed for the synthesis of substituted pyrazoles. The compound 1 was refluxed with various chalcones separately in presence of glacial acetic acid, to get pyrazole derivatives 5a-c. The formation of pyrazole 5a-c was confirmed by absence of NH stretching in its IR spectrum and by the absence of, NH-NH2 peaks in 1H NMR spectrum.
All the newly synthesized compounds melting point, yield and elemental analysis are tabulated in the Table-I. Some of the selected compounds have been tested for antibacterial and antifungal activity. The results of antimicrobial activity are shown in Table-II, the compounds 3b, 3d, 3g, 3j, 4c, 5b, and 5c showed moderate activity against S. arureus and B. subtilis but all compounds showed significant activity against C. albicans and A. niger at 50 μg/ml and 100 μg/ml respectively.
Table 1.
Characterization data of synthesized compounds
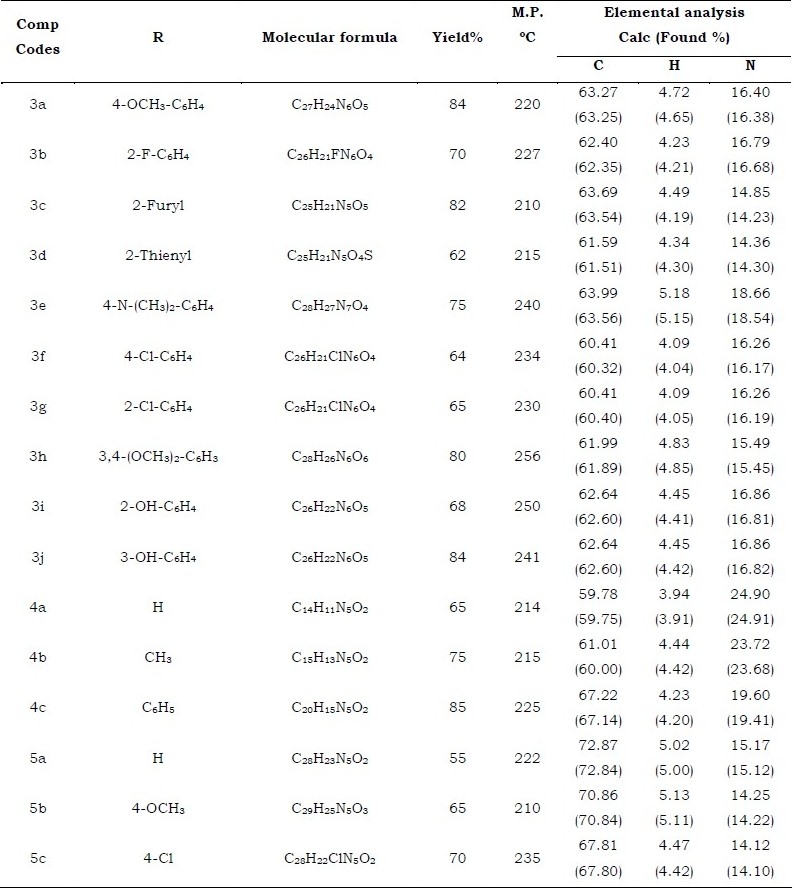
Table 2.
Antimicrobial activity of the synthesized compounds
CONCLUSION
In conclusion, we have prepared some novel pyramidinopyrazoles and pyTimidinotriazoles. Preliminary antimicrobial screening has indicated that some of the tested compounds have found to possess significant antimicrobial activity. They may also possess other biological profile as they are found in many pharmaceutical lead molecules.
ACKNOWLEDGEMENT
Authors are thankful to Sri. Shivamurthy Murugha Sharanaru, President, S.J.M. Vidyapeetha, Chitradurga, for providing necessary facilities though the Principal, S.J.M. College of Pharmacy, Chitradurga to carryout this work, for providing laboratory facilities to carry out the synthesis and pharmacological activity of the compounds. They are also thankful to Sophisticated Analytical Instrument Facility, Chandigarh for providing spectral data.
REFERENCES
- 1.Rane N, Gurram. V.K. Synthesis and QSAR studies of pyrimido [4, 5-d] pyrimidine-2, 5-dione derivatives as potential antimicrobial agents. Bioorg Med Chem. Lett. 2004:14–4185. doi: 10.1016/j.bmcl.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Prakash O., Bhardwaj. V., Kumar. R., Tyaagi. P., Areja. K.R. Organoiodine (III) mediated synthesis of 3-aryl/hetryl-5,7-dimethyl-1,2,4-triazolo[4,3-a]pyrimidines as antibacterial agents. Eur J Med Chem. 2004;39:1073. doi: 10.1016/j.ejmech.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Botta M., Artico. M., Massa. S., Gamvacorta. A., Maronigu. M.E., Pani. A., Lacolla. P. Synthesis, antimicrobial and antiviral activities of isotrimethoprim and some related, derivatives. Eur J Med Chem. 1992;27:251. [Google Scholar]
- 4.Agarwal N, Srivastava. P., Raghuwanshi. S K., Upadhyay. D.N., Shukla. P.K., Ram. V.J. Chloropyrimidines as a new class of antimicrobial agents. Bioorg Med Chem. 2002;10:869. doi: 10.1016/s0968-0896(01)00374-1. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal N.P., Raghuwanshi. S.K., Upadhyay. D.N., Shukla. P.K., Ram. V.J. Suitably functionalized pyrimidines as potential antimycotic agents. Bioorg Med Chem lett. 2000;10:703. doi: 10.1016/s0960-894x(00)00091-3. [DOI] [PubMed] [Google Scholar]
- 6.Basavaraja H.S., Sreenivasa. G.M., Jayachandran. E. Synthesis and biological activity of novel pyrimidino imidazolinones. Ind Journal of Heter Chem. 2005;15:69. [Google Scholar]
- 7.Ram V. J., Haque. N., Guru. P.Y. Chemotherapeutic agents XXV: synthesis and leishmanicidal activity of carbazolylpyrimidines. Euro J Med Chem. 1972;27:851. [Google Scholar]
- 8.Amir. M., Javed. S.A., kumar Harish. Pyrimidine as antiinflammatory agent: A review. Ind J Pharma Sci. 2007;68:337. [Google Scholar]
- 9.Jain K.S., Chitre. T.S., Miniyar. P.B., Kathiravan. M.K., Bendre. V.S. Biological and medicinal significance of pyrimidines. Current Sci. 2006;90:793. [Google Scholar]
- 10.Futterman S J. Enzymatic reduction of folic acid and dihydrofolic acid to tetrahydro-folic acid. J Biol Chem. 1957;228:1031. [PubMed] [Google Scholar]
- 11.Werkheiser W.C. Synthesis and evaluation of pyrazolo[1,5-b]pyridazines as selective cyclin dependent kinase inhibitors. J biol chem. 1961;236:888. doi: 10.1016/j.bmcl.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 12.Kirk. L., Michael Stevens, Reno. J., Jennifer B. Synthesis and evaluation of pyrazolo [1, 5-b] pyridazines as selective cyclin dependent kinase inhibitors. Bio Med Chem. lett. 2008;18:5758. doi: 10.1016/j.bmcl.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 13.Azza. M., Kadr Eatedal H., Ahdel Aal Hanan A., Ahdel-Fattah., Aman M., AJ-Mahmond. Synthesis and evaluation of pyrazolo [1, 5-b] pyridazines as selective cyclin dependent kinase inhibitors. ARKIVOC. 2008;X:127. doi: 10.1016/j.bmcl.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 14.Biological assay. Indian Pharmacopoeia. published by Govt. of India. 1966;2:A-88. [Google Scholar]


