Abstract
Arthritis is an auto immune disorder characterized by pain, swelling and stiffness. Its prevalence depends upon age. It occurs more frequently in women than in men. It is an inflammation of synovial joint due to immuno mediated response. All anti inflammatory drugs are not anti arthritic because it does not suppress T-cell and B-cell mediated response. Epidemiological studies overall show a female to male ratio of about 3:1. There are many class of anti-arthritic drugs are available like NSAIDS, Monoclonal anti-bodies, uricosuric agents, gold compounds, anti-cytokinine immunosuppressant like glucocorticoids, etc. But this all class of drugs is responsible for symptomatic relief. To evaluate the drug which actually prevent cause of arthritic or act during various step of arthritis there is requirement of evaluative model which produce arthritis in (vial same that produce in humans. Animal models of arthritis are used to study pathogenesis of disease and to evaluate potential anti-arthritic drugs for clinical use. Therefore morphological similarities to human disease and capacity of the model to predict efficacy in humans are important criteria in model selection.
Keywords: Monoclonal anti-bodies, uricosuric agents, gold compounds, anti-cytokinine immunosuppressant
INTRODUCTION
Evaluation of Antiarthritic parameter is that it gives sympathetic relief main prevent Inflammation and pain. So the must posses Analgesic, Anti inflammatory property, arthritis is immune. So drug should be immune modulators. For that parameters Antiarthritic activity is evaluate.
The American College of Rheumatology Subcommittee on Rheumatoid Arthritis (ACRSRA) recommends that baseline laboratory evaluations include a complete blood cell count with differential, rheumatoid factor, and erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP). Joint fluid evaluation Baseline evaluation of renal and hepatic function also is recommended because these findings will guide medication choices[9]. on their guideline various experimental parameters are create to anti-arthritic activity in animal like , Paw edema, Body weight, Arthritic index, Erythrocyte Sedimentation Rate (ESR), Quantitative determination of the Rheumatoid Factors (RF),Histopathology of synovial joints, Radiology (x-Ray measurements), Photographic parameter.
(I) Arthritis induced by hyper immunization
Collagen type ii induced arthritis in rats
Principle
Collagen-induced arthritis (CIA) is among the most widely used animal models of RA. CIA is genetically controlled by Class II major histocompatibility complex (MHC) molecules. CIA an excellent model for studying the mechanisms underlying immune responses to an auto antigen potentially involved in human disease. Collagen arthritis can be induced readily in many strains of rats by immunizing them with heterogonous or homologous native type II collagen emulsified in incomplete Freund's adjuvant. This disease, which is characterized by the development of both cellular and humoral immune response to type II collagen, can be passively transferred by sensitized spleen and lymph node cells as well as IgG antibodies to type II collagen. These findings are consistent with the proposal that collagen arthritis is the relt of immunologic hypersensitivity to type II collagen. In a recent report, showed that rats with adjuvant arthritis exhibited both humoral and cellular sensitivities to homologous type II collagen. When introduced into the dermis, CII is immediately captured by antigen-presenting cells (APCs).Disease involves activation of both T & B cells that are antigen-specific & auto reactive. T cell & T cell-derived cytokines promote differentiation & activation of macrophages, osteoclasts & fibroblast, leading to an aggressive erosive arthritis[1].
Procedure
Collagen was dissolved in a concentration of 2.0 mg/ml in 0.1 M acetic acid overnight at 4°C. This solution is added drop wise to an equal volume of chilled incomplete Freund's adjuvant. On day zero, each rat receives a total of 0.5 mg collagen in 0.5 ml, equally divided, in 5 sites. All injections are intradermal, one at the base of each appendage and one in the nape of the neck. Seven days post-immunization, the animals receive identical booster injections. Control animals receive only the incomplete Freund's adjuvant diluted with 0.1 M acetic acid. The volume of both hind paws is measured plethysmographically on day 20. To minimize the possibility of including animals with minimal transient disease, only animals with a paw volume of 1.8 ml or greater are used for further testing. From days 20-40, the animals receive the test compounds p.o. once a day. On day 41, the paw volumes are recorded again.
Fig. 1.
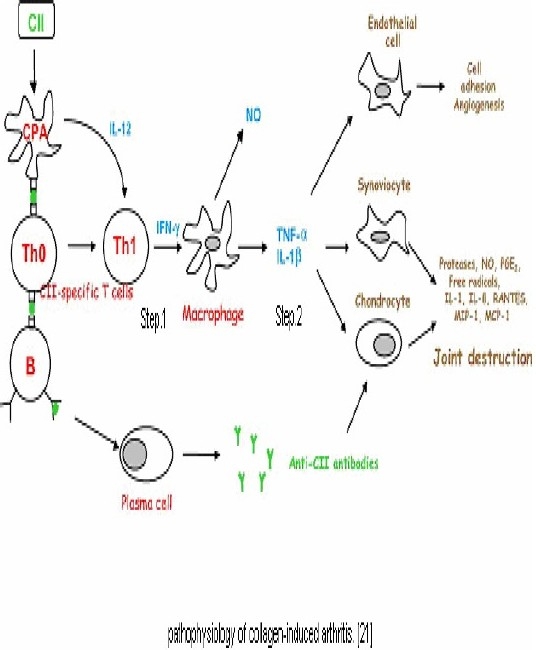
Describes pathophysiology of collagen-induced arthritis[1].
Complete Freund's adjuvant induce arthritis in rat
Principle
Freund's complete adjuvant induced arthritis in rat model which is the best and most widely used experimental model for arthritis with clinical and laboratory features which closely mimic the clinical features of human rheumatoid disease. This model is sensitive to anti inflammatory and immune inhibiting medicines and considers being relevant for the study of phathophysiological and pharmacological control of inflammation process as well as for the evaluation of anti nociceptive potential of drugs[2,3].
Procedure
On day zero, Animals are injected into the sub plantar region of the left hind paw with o.1 ml of complete Freunds adjuvant (FA). This consist of 6mg Mycobacterium butyricum suspended in heavy paraffin oil by through grinding with motor and pestle to give a concentration of 6mg/ml. Dosing with the test and standard compounds are administered on the same day and continued for 12 days according to the following schedule. Purposely from day 13th to 21st, the animals were not dosed with the test compound or the standard. The following parameters are measure[4].
Cartilage oligomeric matrix protein (COMP) induced arthritis
Immunization with COMP in IFA induces severe arthritis in susceptible rat strains, such as DA and LEW. Although the peripheral joint arthritis clinically resembles RA, COMP-induced arthritis, however, does not result in the permanent destruction of joints. Disease development appears to be dependent on an immune response to autologous COMP and not on cross-reactivity to other cartilage rat collagens[2,5,6,7].
Proteoglycan-induced Arthritis
Principle
Proteoglycan (PG) aggrecan, a major macromolecular component of cartilage, is highly immunogenic; it induces arthritis in genetically susceptible BALE/c mice. PGcore protein-speciWc synthetic peptides to prime and hyper-immunize BALB/c mice[8].
Procedure
High buoyant density cartilage proteoglycans are prepared from fetal and adult human, canine or bovine articular cartilages as well as from 1-week-old mouse epiphyseal cartilage. 100 μg of deglycosylated PG protein[9,10,11] was injected intraperitoneally (i.p.) on days 0, 21, and 42. The First injection is given in complete Freund's adjuvant, whereas the second and third boosters contained antigen in incompleteFreund's adjuvant. Typically, PGimmunizedBALB/c mice develop arthritis 10–14 days .The animals are treated with test drug or vehicle from 20-40days.Sera from mice with progressive polyarthritis are tested for antibodies to arthritogenic proteoglycans during weeks 12-18 of immunization[11,12,13].
Monosodium urate crystal-induced arthritis
Principle
Unmetabolised product of purine are deposited as uric acid in synovial tissue joint which evoke activation of kinin leukotriene B4, Accumulation of neutrophil granulocytes characterized by intermittent attack of Acute Arthritis or Gout. On this this principle are injected interadermaly at knee joint produce arthritis[14]. The importance of urate in gout and the deposition of sodium urate in gouty tophi is well known. By Injecting 20 mg sodium urate crystal suspensions in their own knee joint 12. They experienced severe pain and prostration which resembled an acute gouty attack[15].
Procedure
Inflammation was induced by intradermal injection of 0.2 ml (4 mg) of endotoxin free monosodium urate crystal suspension into the right foot pad. Day considered as day 0-3. Treatment with standard & test is started from day zero before half hour of MSU & continue for three day[16].
Preparation of monosodium urate crystals
About 4 g of uric acid was dissolved and heated in 800 ml H2O with NaOH (9 ml/ 0.5 N), adjusted to pH 8.9 at 60°C; cooled over night in a cold room; washed and dried. Needle-like crystals were recovered and were suspended in sterile saline (20 mg/ml). MSU crystals were checked before administration for bacterial endotoxin contamination using a commercial test kit of limulus amebocyte lysate (LAL) as indicated by the supplier[17].
Carrageen induce paw odema
Carrageen an (CRR), a sulphated polysaccharide, is often used in pain models. CRR produces acute and chronic inflammatory responses. The acute response appears to be similar to rheumatoid arthritic lesions, which are characterized by sustained cellular emigration[15,18]. Hydrolyzed carrageen an induce ileocecal inflammation by inhibiting deoxyribonucleic acid synthesis it also effects on chromium release, and cell morphology retarded cell growth and eventually caused cell death.
Procedure
Acute inflammation is produce in all animals by sub plantar injection of freshly prepared suspension of 1% carrageen an in normal saline on the left hind paw of rats. Paw thickness was measured using a plethysmograph before and after carrageen a challenge in each group. In all the above models, the degree of edema formation is determined as increase in paw thickness. Increase in paw thickness and per cent inhibition is calculate as follows: Increase in paw thickness in control/treatment PC/PT = Pt – P0. Per cent inhibition) * 100?= (PC – PT /PC .Where Pt is paw thickness at time t, P0 is initial paw thickness, PC is increase in paw thickness of the control group and PT is the increase in paw thickness of the treatment groups[16,19].
Type II Collagen is Best Model for Evaluation of Anti-Arthritic Drugs
Collagen Type II is best model for evaluation of anti-arthritic drugs than other model because The CIA model demonstrates that autoimmunity to CII can generate autoimmune arthritis, which encompasses inflammation of synovial joints, destruction of cartilage, and bone erosion. Type II collagen as an auto antigen in human RA and collagen-induced arthritis (CIA), Most autoimmune diseases involve the development of autoimmunity to autologous proteins and have been probed for auto reactive T cells and antibodies in both human disease and animal models. The high incidences of anti-CII antibodies and CII-specific T cells indicate that CII is one of the major auto antigens of human RA 38. The role of the major histocompatibility complex region was the discovery that only certain strains were susceptible to CIA[19]. The most susceptible haplotypes after immunization with heterologous collagens like chicken, human, bovine and rat CII were the H-2r and H-2q haplotypes. Interestingly, these haplotypes were also permissive for the induction of arthritis with mouse CII and development of a strong autoantibody response to CII[20]. From the anti-CII immunity to joint inflammation, the engagement of DCs and T cells also companies the activation of naïve B cells. The anti-CII antibodies in CIA are mainly of the IgG2 subclass, both IgG2a and IgG2b, both the IgG2a and IgG2b subclasses are capable of activating the complement cascade, the binding and accumulation of anti-CII antibodies in the articular region may initiate inflammatory responses[21]. The production of proinflammatory cytokines, such as IL- 1β, TNF-α, IL-6, and IL-8, by synovial macrophage-like cells and infiltrating immune cells induces many other cytokines, including IL-23 and IL-17, chemokines, and extra cellular matrix (ECM)-degrading enzymes also initiate destruction. In this way, the higher prevalence's of CII-specific antibodies and T cells noted during the early phase of RA indicate that CII-specific immunity plays an important role in the initiation of inflammation in the articular joints[22].
CONCLUSION
Arthritis is an auto immune disorder characterized by pain, swelling and stiffness. Its prevalence depends upon age. It occurs more frequently in women than in men. It is an inflammation of synovial joint due to immuno mediated response. The high incidences of anti-CII antibodies and CII-specific T cells indicate that CII is one of the major auto antigens of human RA. In this way, the higher prevalence's of CII-specific antibodies and T cells noted during the early phase of RA indicate that CII-specific immunity plays an important role in the initiation of inflammation in the articular joints. So, collagen type-II is best model for evaluation of anti-arthritic drugs compare to other models.
REFERENCES
- 1.Doncarli A, Stasiuk LM, Fournier C, Abehsira-AlnarO Conversion in vivo from an early dominant Th0/Th1 response to a Th2 phenotype during the development of collagen-induced arthritis. Eur. J. Immunol. 1997;27:1451–8. doi: 10.1002/eji.1830270623. [DOI] [PubMed] [Google Scholar]
- 2.Newbould BB. Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. J Pharmacol. 1963;21:127–36. doi: 10.1111/j.1476-5381.1963.tb01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson CM, Wood FD. Studies of arthritis and other lesions induced in rats by the injection of mycobacterial adjuvant. VIII. Pathologic details of the arthritis and spondylitis. Am J Pathol. 1963;43:73–95. [PMC free article] [PubMed] [Google Scholar]
- 4.Butler SH, Godefroy F, Besson JM. A limited arthritic model for chronic pain studies in the rats Pain. Weil-Fugazza J. 48;1992:73–81. doi: 10.1016/0304-3959(92)90133-V. [DOI] [PubMed] [Google Scholar]
- 5.Di Rosa M. Biological properties of carrageenan. J Pharm Pharmacol. 1972;24:89–102. doi: 10.1111/j.2042-7158.1972.tb08940.x. [DOI] [PubMed] [Google Scholar]
- 6.Ling KY, Bhalla D, Hollander D. Mechanisms of carrageen an injury of IEC18 small intestinal epithelial cell monolayer. 1988 Dec;95(6):1487–95. doi: 10.1016/s0016-5085(88)80067-2. [DOI] [PubMed] [Google Scholar]
- 7.Carlsen S. Cartilage oligomeric matrix protein (COMP)-induced arthritis in rats. Clin. Exp. Immunol. 1998;114:477–484. doi: 10.1046/j.1365-2249.1998.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edit I Buzás, Anikó Végvári, Yanal M Murad, Alison Finnegan, Katalin Mikecz, Tibor T. Glant T-cell recognition of diVerentially tolerated epitopes of cartilage proteoglycan aggrecan in arthritis Cellular Immunology. 2005;235:98–108. doi: 10.1016/j.cellimm.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Glant TT, Bárdos T, Vermes, Chandrasekaran R, Valdéz JC, Otto JM, Gerard D, Velins S, Lovász G, Zhang J, Mikecz K, Finnegan A. Variations in susceptibility to proteoglycan-induced arthritis and spondylitis among C3H substrains of mice, Evidence of genetically acquired resistance to autoimmune disease. Arthritis Rheum. 2001;44:682–692. doi: 10.1002/1529-0131(200103)44:3<682::AID-ANR118>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 10.Giant TT, Cs-Szabó G, Nagase H, Jacobs JJ, Mikecz K. Progressive polyarthritis induced in BALB/c mice by aggrecan from human osteoarthritic cartilage. Arthritis Rheum. 1998;41:1007–1018. doi: 10.1002/1529-0131(199806)41:6<1007::AID-ART7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Glant TT, Finnegan A, Mikecz K. Proteoglycan-induced arthritis: immune regulation, cellular mechanisms and genetics. Crit Rev Immunol. 2002;23:199–250. doi: 10.1615/critrevimmunol.v23.i3.20. [DOI] [PubMed] [Google Scholar]
- 12.Glant TT, Mikecz K, Arzoumanian A, Poole AR. Proteoglycan- induced arthritis in BALB/c mice, Clinical features and histopathology. Arthritis Rheum. 1987;30:201–212. doi: 10.1002/art.1780300211. [DOI] [PubMed] [Google Scholar]
- 13.Glant TT, Mikecz K. Proteoglycan (aggrecan)-induced arthritis, A murine autoimmune model of rheumatoid arthritis. In: Perl A., editor. Autoimmunity: Methods and Protocols. Totowa, NJ: Humana Press; 2003. pp. 313–338. [DOI] [PubMed] [Google Scholar]
- 14.Rang HP, Dale MM, Ritter JM, Moore PK. 5th edition. Newdelhi: charchill livngstone; 2006. pharmacology. [Google Scholar]
- 15.Faires JS, McCarty DJ. Acute arthritis in man and dog after intrasynovial injection of sodium urate crystals. Lancet. 1962;2:682–685. doi: 10.1016/s0140-6736(62)91050-4. [DOI] [PubMed] [Google Scholar]
- 16.Rasool M, Varalakshmi P. Suppressive effect of Withania somnifera root powder on MSU crystal-induced Inflammation – an in vivo and in vitro study. 2006:776–84. doi: 10.1016/j.vph.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Sabina Evan Prince, Khan Rasool Mahaboob, Mathew Lazar, EzilRani Panneerselvam, Indu Haridas. Food and Chemical Toxicology, 2010;48:229–235. doi: 10.1016/j.fct.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Kim HY, Kim WU, Cho ML, Lee SK, Yours J, Kim SI, et al. Enhanced T cell proliferative response to type II collagen and synthetic peptide CII (255-274) in patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:2085–93. doi: 10.1002/1529-0131(199910)42:10<2085::AID-ANR8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.Wooley P.H., Luthra H.S., Stuart J.M., David C.S. Type II collagen induced arthritis in mice. I. Major histocompatibility complex (Iregion) linkage and antibody correlates. J. Exp. Med. 1981;154:688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmdahl R., Jansson L., Andersson M., Larsson E. Immunogenetics of type II collagen autoimmunity and susceptibility to collagen arthritis. Immunology. 1988;65:305–310. [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming SD, Tsokos GC. Complement, natural antibodies, auto antibodies and tissue injury. Autoimmune Rev. 2006;5:89–92. doi: 10.1016/j.autrev.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Kim WU, Yoo WH, Park W, Kang YM, Kim SI, Park JH, et al. IgG antibodies to type II collagen reflect inflammatory activity in patients with rheumatoid arthritis. J Rheumatol. 2000;27:575–81. [PubMed] [Google Scholar]


