Abstract
Swarna makshika (chalcopyrite) bhasma (SMB) has been used for different therapeutic purposes since long in Ayurveda. The present study is conducted to evaluate the effect of conventionally prepared SMB on different bio-chemical parameters in experimental animals, for providing scientific data base for its logical use in clinical practice. The genuine SMB was prepared by following classical techniques of shodhana and marana most commonly used by different Ayurvedic drug manufacturers. Shodhana was done by roasting raw swarna makshika with lemon juice for three days and marana was performed by 11 putas. The experimental animals (rats) were divided into two groups. SMB mixed with diluted honey was administered orally in therapeutic dose to Group SMB and diluted honey only was administered to vehicle control Group, for 30 days. The blood samples were collected twice, after 15 days and after 30 days of drug administration and different biochemical investigations were done. Biochemical parameters were chosen based on references from Ayurvedic classics and contemporary medicine. It was observed that Hb% was found significantly increased and LDL and VLDL were found significantly decreased in Group SMB when compared with vehicle control group. This experimental data will help the clinician for the logical use of SMB in different disease conditions with findings like low Hb% and high LDL, VLDL levels.
Keywords: Bio-chemical parameters, makshika bhasma, marana, puta, shodhana
INTRODUCTION
In Ayurveda, Swarna makshika (chalcopyrite) bhasma (SMB) is a very popular drug since hundreds of years.[1] It is used singly or as an important ingredient of many compound formulations for treating pandu (anemia), hridaya roga (cardiac diseases), agnimandya (impaired digestive capacity)[2] etc. The manufacturing of SMB involves two main processes i.e. shodhana and marana. Shodhana includes different techniques like roasting, heating and quenching. marana is puta system of heating (please go through the glossary at the end). SMB can be manufactured using different putas like varaha puta, kukkuta puta, gaja puta etc. or kupipakwa method of heating can be used. Various processing materials of herbal origin like lemon juice and mineral origin like gandhaka (sulfur) are used during manufacturing process of SMB.
Biochemical parameters provide key points to diagnose and treat diseases for contemporary medicine. Though SMB is being used since long on the basis of Ayurvedic classical parameters, development of biochemical science can provide tools to strengthen its usage. The study was planned with an intention to enhance acceptability of SMB as a potent medicine by cross disciplinary scientists and practitioners. A database of biochemical values justifying its use in certain disease conditions was generated by this study on experimental animals.
MATERIALS AND METHODS
Genuine SMB was prepared in the laboratory of Dept. of Rasa Shastra, Faculty of Ayurveda, Banaras Hindu University by following classical references,[5] and earlier research work done in this line.[6] Standard operative procedures were strictly followed for manufacturing of SMB. Shodhana was done by roasting technique with frequent addition of lemon juice in it. Marana was done by puta system of heating using incineration of 04 kg cow dung cakes[1,6] each time. The incineration process was repeated 11 times (11 putas).
Steps in Swarna Makshika Bhasama manufacturing process
Raw Swarna Makshika (Chalcopyrite)
Powderization of Swarna Makshika using Mortar and Pestle
Shodhana Process: Bharjana (Roasting with lemon juice at about 750°C for three days 24h)
Swangashita (allowed to self cool)
Shodita Swarna Makshika
Bhawana (Wet trituration with lemon juice)
Chakrika Nirman (Pelletization)
Sharava Samputa (Sealing the dried pellets in earthen casseroles using cloth and fuller's earth)
Putapaka (Firing the sharava samputa in puta system of heating using cow dung fuel and allowed to self cool)
The processes of Bhawana, Putapaka and Swangashita were repeated 11 times
Swarna Makshika Bhasma (Finished product)
Ayurvedic standardization of SMB
The finished product SMB was assessed on following quality control parameters advocated in Ayurvedic classical texts.[7,8]
Rekhapurnatvam
A pinch of SMB was rubbed between thumb and index finger. It was observed that the bhasma enters into the lines of the finger which was not easily cleansed out from the cleavage of finger lines.
Varitaratavam
Small amount of the SMB was taken and sprinkled over the silent water taken in a glass beaker. It was found that the bhasma particles float over the surface of the water.
Uttama
The SMB was sprinkled over water in a glass beaker and a rice grain was placed over it. Bhasma and the rice grain were found floating on water surface.
Nisvadutvam
The SMB was found tasteless when a small amount was kept over the tongue.
Amla Parikshya
A pinch of SMB was mixed with little amount dadhi (curd) taken in a clean and dry petri dish, kept for 24 h and then observed for any color change. No color change of dadhi (curd) was observed. The same procedure was followed with lemon juice taken in a test tube and same observation was found.
Dantagre na cha kacha kacha bhava iti (without gridding sensation on teeth)
When a small amount of the SMB was placed between the teeth, no sandy feeling was observed.
Avami
Intake of very small amount of the SMB did not produce any nausea / vomiting.
The SMB was also subjected to different modern quality control parameters like XRD (X-ray diffraction), TEM (transmission electron microscope) and EDAX (energy dispersive X-ray analysis), and reports were documented.
XRD of raw material was showing all the major peaks that of CuFeS2 (chalcopyrite) and for finished product the peaks were indicating mixture of many compounds viz. Cu2O, FeSO4, Fe2O3, SiS2, and Cu2S.
TEM study showed that the particle size of the raw material was 5-10 μ and for the SMB it was 50-200 nm.
Analyzing both raw Swarna Makshika and SMB, using EDAX study revealed that, both contain, iron, copper and sulfur. In addition to it, finished product SMB contains potassium, magnesium, aluminum and silicon in small amount.
Dose determination
Dose of the trial drug was extrapolated (human dose to animal dose) using extrapolation factor and honey was used as vehicle for administration of the drug.[9]
Animal dose = total clinical dose (a) ×(extrapolation factor (b) 0.018 = (c) per 200 g of rat
Total clinical dose considered was 250 mg /60 kg of human per day[10]
250 mg /60 kg of human per day × 0.018 = 4.5 mg/200 g of rat/day
The actual dose administered to the animal was 4.5 mg/200 g of rat/day
Vehicle honey
Honey was used as the vehicle control as directed by the reference of the study. Other than this, honey is described as one of the best yogavahi substances, which facilitates assimilation of other substance in body, in Ayurvedic classics. Also most of the time, Ayurvedic clinicians use SMB with honey. Hence it was used as the vehicle. Honey was diluted with de-ionized water for the convenience of administration.
Caring of animals
The study was conducted on Charles's foster strain, male albino rats weighing between 100 and 150 g. All the animals were kept in colony cages at an ambient temperature of 25± 2°C, with relative humidity 45–55% and 10:14 h’ light and dark conditions. The animals were kept on standard rodent feed and water was allowed ad libitum. Principles of laboratory animal care and use were followed throughout the study.[11] Animals were acclimatized for seven days before starting the experiment. The study was designed with due permission of Animal ethics committee.
Grouping of animals
The experimental animals were divided into two groups, with 6 animals in each group. SMB mixed with diluted honey was administered orally in therapeutic dose to Group SMB and diluted honey only was administered to vehicle control Group, for 30 days.
Group SMB
SMB was administered to the animals using rubber catheter in therapeutic dose, i.e. 4.5 mg/200 g of rat orally for 30 days with diluted honey (3 ml honey + 4.5 ml de-ionized water). The actual amount administered was adjusted between 0.5 ml and 1 ml containing calculated dose of the drug for individual animal.
Control group
Only diluted honey (3 ml honey + 4.5 ml de-ionized water) was administered to the animals in control group.
Evaluation of the effect of trial drug on biochemical parameters
As the trial drug is indicated for its use in pandu (anemia), hridayaroga (heart disease) and agnimandya (impaired digestive capacity) in classical texts,[3,4] biochemical parameters related to liver function, lipid metabolism and Hb% were chosen to evaluate in experimental animals.[12]
Biochemical parameters related to liver function
SGOT (serum glutamic oxaloacetic transaminase), SGPT (serum glutamic pyruvate transaminase), ALP (alkaline phosphatase), serum bilirubin (total), serum bilirubin (direct), serum protein, serum albumin.
Biochemical parameters related to lipid metabolism
Serum cholesterol, serum triglycerides, HDL (high density lipoproteins), LDL (low density lipoproteins), VLDL (very low density lipoprotein)
Hematological parameter:
Hemoglobin%
Collection of blood samples: Rationale and method
With an intention to study the effect of the drug intimately, the parameters related to liver function and Hb% were assessed twice i.e. after 15 days and after 30 days. However, the parameters related to lipid metabolism were studied only after 30 days because the parameters are not expected to change in a short duration of 15 days.
All the animals from both the groups were anesthetized using ketamine hydrochloride 24 mg/kg body wt.[11] and blood samples were collected from retro-orbital plexus. The samples were sent for biochemical analysis. The procedure was repeated both the times, after 15 days and after 30 days. After 30 days, the animals were sacrificed and disposed as per standard protocol for animal ethics.
The results of biochemical investigations were documented and analyzed statistically by using Student's paired ‘t’ test.
OBSERVATIONS AND RESULTS
Comparison between biochemical values of both the groups SMB and control revealed following observation [Table 1].
Table 1.
Effect of SMB on various biochemical parameters after 15 and 30 days of administration and comparison between the groups (number of animals in each group: 6)
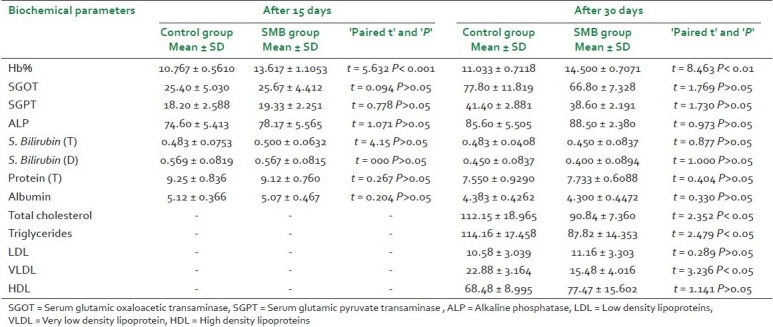
Statistically significant increase in Hb% was observed in Group S in comparison with Group V. The observation was similar for both the phases of experiment.
The changes in all the biochemical parameters related to liver function i.e. SGOT, SGPT, ALP, serum bilirubin (total), serum bilirubin (direct), albumin and protein were observed statistically insignificant. The observations were similar for both the phases of experiment.
Serum cholesterol, triglyceride and VLDL were found significantly decreased after administration of trial drug for 30 days.
After 30 days of administration, increased HDL level was observed in animals of treated group, but it was statistically insignificant.
After 30 days of administration, the changes in LDL level were also found to be statistically insignificant.
DISCUSSION
Administration of SMB in therapeutic dose for 15 days significantly increased hemoglobin%. The observation advocates the logical use of the drug to achieve therapeutic effect even in short-term administration in disease conditions with low Hb%. This may be due to the presence of Fe (iron) in makshika bhasma as one of the major ingredients.
The changes in biochemical parameters like SGOT, SGPT, ALP, serum bilirubin (total), serum bilirubin (direct), serum protein, and serum albumin after 15 days were found to be statistically insignificant. It indicates that in short-term administration, the drug has no significant effect on parameters related to liver function.
Administration of SMB in therapeutic dose for 30 days showed significant increase in Hb% and significant decrease in serum cholesterol, triglycerides and VLDL level. It also showed increase in HDL levels, though it was not statistically significant.
Significant increase in serum cholesterol, triglycerides and VLDL levels elucidates that the SMB may be used very effectively in different health campaigning programs in the subject of high cholesterol, triglycerides and VLDL level leading to supportive treatment for obesity and cardiac problems.
Significant increase in Hb% in both the phases of study with liver function test values within the normal limits shows that the conventionally prepared SMB can be used very effectively in various disease conditions. The results advocate its use in conditions with hemoglobin deficiency e.g. anemia due to blood loss, malnutrition, pregnancy etc. which may be of great importance in public health perspectives.
CONCLUSIONS
The current research work justifies the therapeutic use of SMB in pandu (anemia), claimed in ancient texts, in experimental animals both for long-term and shot-term administration. Significant decrease in serum cholesterol, triglycerides and VLDL level in treated group rationalizes the thousands-years clinical use of SMB in hridayaroga in long-term administration.
This study gives explanation for the sayings of ancient Acharyas that the long-term use of the bhasma can provide strength and stout body[13] instead of causing untoward effect. It has no significant changes on parameters related to liver function, which indicates that the uses of traditional metal/mineral bhasmas are safe, even in long-term administration. Hence the conventionally prepared bhasma is a noble dosage form of Ayurvedic treatment and can be used very effectively if manufactured following the standard operative procedures and following the traditional quality control parameters strictly.
Glossary of important terms used in this article
Bhasma
Bhasma is a herbo-mineral manufactured from metal/mineral after typical Ayurvedic pharmaceutical processes like shodhana and marana documented in ancient texts. The finished product is expected to follow the standardization parameters quoted in texts and can be used as a safe drug if all the guidelines for its usage are followed.
Shodhana
Various pharmaceutical processes like heating and quenching in herbal juices, boiling in animal products like urine, roasting in pan are advocated with an intention to convert a metal-mineral into drug. The shodhana generates a product suitable for next pharmaceutical process or it may generate a finished product. e.g. shodhana of Abhraka (mica) is done by heating till red hot and quenching it in triphala decoction.
Marana
The technique usually followed by shodhana is used to convert the metal/mineral into a potent therapeutic drug using ancient Puta system of heating
Puta
It is the measure of amount of heat required to convert or transform any metal or mineral into bhsama. This amount of heat is substance specific and measured in terms of fuel used (number of cow dungs or its weight).
Sharava
An earthen petri dish having specific measurements.
Bhavana
The pharmaceutical process of trituration of the drug with liquid medium e. g. Hingula with fresh Zinzibar officinalis juice.
Chakrika
These are disc-like pellets having approx. 1.5-2 cm in diameter and 0.5-8 cm in thickness, prepared during the marana process, after bhavana process.
ACKNOWLEDGEMENT
We acknowledge Dr. A.C. Kar, Head, Dept. of Vikrit Vigyan, Faculty of Ayurveda, Banaras Hindu University, Varanasi for his cordial support during different investigations in the Vikrit Vigyan Laboratory, IMS, BHUProf. D. Dash, Head, Dept. of Biochemistry, Faculty of Modern Medicine, Banaras Hindu University, Varanasi- for his extensive cooperation during the biochemical investigation and during the writing and revising of the current manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mohapatra S, Jha CB. Vol. 1. New Delhi: IJAR, Dept. of AYUSH; 2010. Physico-chemical characterization of Ayurvedic Bhasma (Swarna makshika bhasma): An approach to standardization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta RK, Vijay Lakshmi, Mohapatra S, Jha CB. Vol. 31. Jamnagar, Gujarat: AYU, Gujarat Ayurveda University; 2010. Therapeutic uses of Swarna makshika bhasma: A critical Review. [Google Scholar]
- 3.Sharma S. Varanasi: Motilal Banarasi Das Publication; 2004. Rasa Tarangini Chapter 21, Verse 28. [Google Scholar]
- 4.Upadhya M. Varanasi: Chaukhamba Bharati Academy; 1999. Ayurveda Prakasha.Chapter 4, Verse 9. [Google Scholar]
- 5.Sharma S, Rasa Tarangini. Varanasi: Motilal Banarasi Das Publication; 2004. Chapter 21, Verse 7-11; pp. 19–20. [Google Scholar]
- 6.Mohapatra S, Jha CB. Vol. 21. Kottakal: Aryavaidyan, Aryavaidyasala; 2007. Standardization of Swarna makshika bhasma- A pharmaceutical Study. [Google Scholar]
- 7.Acharya Vagbhata. Chapter-8/26-30: 10/48-50. In: Kulkarni Dattatreya Ananta, Sri, Ratna Rasa, Samucchhaya, editors. New Delhi: Meharchand Lachmandas Publication; 1998. [Google Scholar]
- 8.Mohapatra S, Jha CB. Varanasi: Dept. of Rasa Shastra, Banaras Hindu University; 2006. Process Standardization of Makshika Bhasma and its experimental evaluation for hypnotic and behavioral activities on experimental animal. [Google Scholar]
- 9.Paget GE, Branes JM. Evaluation of Drug activities in pharmacometrics. In: Laurence DR, Bocharach AL, editors. Vol. 1. New York: Academic Press; 1964. p. 135. [Google Scholar]
- 10.Sharma S. Varanasi: Motilal Banarasi Das Publication; 2004. Rasa Tarangini Chapter 21, Verse 7-29. [Google Scholar]
- 11.New Delhi: 1992. Indian National Science Academy guidelines for care and use of Animals, Govt. of India. [Google Scholar]
- 12.Godkar PB, Godkar DP. 2nd ed. Mumbai: Bhalani Publishing House; 2000. A Text book of Medical Laboratory Technology; p. 265. (267, 347, 349, 350, 371, 372, 375, 726). [Google Scholar]
- 13.Acharya Vagbhata, Samuchchaya Rasa Ratna, Kulkarni Dattatreya Ananta., Sri, editors. New Delhi: Meharchand Lachmandas Publication; 1998. Chapter 5/139. [Google Scholar]


