Abstract
Objectives:
To assess incidence, burden of illness, and risk factors for human rhinoviruses (HRVs) in a cohort of very low birth weight (VLBW) infants.
Methods:
A 2-year prospective cohort study was conducted among VLBW premature infants in Buenos Aires, Argentina. Infants were enrolled in the NICU from June 1, 2003, to May 31, 2005, and managed monthly and with every acute respiratory illness (ARI) during the first year of life. Nasal wash samples were obtained during every respiratory episode and tested for HRV, respiratory syncytial virus (RSV), human parainfluenza viruses, influenza viruses, and human metapneumovirus using reverse transcriptase-polymerase chain reaction.
Results:
Of 119 patients, 66 (55%) had HRV-associated ARIs. The incidence of HRV-associated ARI was 123 events per 100 child-years of follow-up. Of those infants experiencing an episode of bronchiolitis, 40% had HRV versus 7% with RSV. The incidence of HRV-associated bronchiolitis was 75 per 100 infant-years of follow-up. HRV was associated with 12 of 36 hospitalizations (33%), and RSV was associated with 9 of 36 hospitalizations (25%). The incidence of HRV-associated hospitalization was 12 per 100 infant-years of follow-up. The risk of HRV-associated hospitalization was higher for infants with bronchopulmonary dysplasia and those who were not breastfed.
Conclusions:
HRV is an important and frequent pathogen associated with severe respiratory infections in VLBW infants. Bronchopulmonary dysplasia and the absence of breastfeeding are risk factors for hospitalization. The results of our study reveal that HRV is the predominant pathogen of respiratory infections in premature infants.
KEY WORDS: premature infants, rhinovirus, very low birth weight
What’s Known on This Subject:
Human rhinovirus infections are common in children. Although historically associated with upper respiratory tract illness, rhinoviruses are increasingly recognized for their role in the exacerbation of asthma. Their role in bronchiolitis and severe lung disease in premature infants is unclear.
What this study adds:
The authors of this study prospectively explore the role of rhinoviruses in premature infants using molecular techniques and identify these agents as the most frequent cause of hospitalization in this population.
Very low birth weight (VLBW) infants are at high risk for severe lower respiratory tract infections (LRTIs), with attack rates up to 25%.1–5 For decades, the agent most frequently linked to these severe episodes has been respiratory syncytial virus (RSV). RSV is responsible for 2% to 18% of severe LRTI cases in VLBW infants year-round.6–10 Passive prophylaxis against it using a humanized monoclonal antibody (palivizumab) prevents ∼50% of RSV-associated hospitalizations,11 so numerous cases of severe LRTI in VLBW infants are caused by other infections. Yet the general impression has been that these infections are elicited by a variety of viruses as relatively infrequent events. Initiatives against these agents have been relatively scarce. In fact, the viruses responsible for numerous cases of acute respiratory illness (ARI) in VLBW infants are unclear.12
Although once thought to cause only the common cold, it is now known that human rhinoviruses (HRVs) are associated with LRTIs.13–22 During recent years, in studies using more sensitive reverse transcriptase-polymerase chain reaction, HRVs have been associated with a significant burden of disease in infants and young children.12,14 We investigated whether HRV infections are a common and underappreciated cause of disease during the first year of life in a prospective cohort of VLBW infants in Buenos Aires, Argentina.
Methods
Study Design
We enrolled infants and children at high risk for pulmonary disease prospectively from June 1, 2003, through May 31, 2005, at the Garrahan Children’s Hospital and the Maternidad Sarda High Risk Clinics in Buenos Aires, Argentina.4 Families of infants who were leaving the NICUs were invited to participate during their first visit to the clinics between June 1, 2003, and November 30, 2004. Written, witnessed informed consent was obtained from all mothers, fathers, or guardians (all participating families had at least 1 literate parent). Families received oral and written instructions for recognizing respiratory signs and symptoms in special workshops at the time of infant discharge from the NICU or at study enrollment. Participating parents or guardians were asked to visit the clinics every time their child developed changes in baseline respiratory status. Respiratory status was established using a validated score based on oxygen saturation, respiratory rate, and pulmonary clinical signs.23 Travel expenses to the clinic and a small meal allowance for accompanying siblings were provided for every visit because the clinics serve a population of low socioeconomic status (33% of families below the poverty line). Participating children were examined in the clinics monthly, and families were contacted by telephone every 2 weeks by a pediatrician with a standard questionnaire to inquire about changes in respiratory status. The clinics provide highly specialized care for children that is not widely available in other public institutions in Buenos Aires, and infants were assigned a primary care provider on enrollment; patients were unlikely to receive additional care in other centers. A primary care provider was available every day, guaranteeing the accessibility of participating infants to a study pediatrician. In 2 instances, participating children were admitted to a nonparticipating institution; however, a study pediatrician visited them during that hospitalization, and data were included in the analysis. Children who attended the participating clinics did not receive a humanized monoclonal antibody against RSV (palivizumab) during the winter respiratory viral season (May 1–August 31) because of cost constraints. No public hospital had palivizumab available in Argentina at that time. Participating children were ≤8 months of corrected gestational age (9 of 119 infants ≥5 months of corrected gestational age), had a birth weight of <1500 g, and had reached the corrected gestational age of 1 year before May 31, 2005, to enroll in the study. Inclusion criteria were based on previous studies of VLBW infants.4,23,24 Families lived within 70 km (44 miles) of the clinics. Children were excluded from the study if they had <6 months of life expectancy, known bleeding disorders, immune deficiencies, or orofacial malformations. Families who did not have a home telephone number (n = 4) were contacted routinely through scheduled telephone appointments at a neighbor’s home or a relative’s home. The study was approved by the institutional review boards of all participating institutions.
Clinical Assessments
ARI was defined as the sudden onset of ≥1 of the following signs or symptoms: rhinorrhea, pharyngitis, cough, retractions, wheezing, or crackles with or without fever. Bronchiolitis was defined as the acute onset of low-grade fever, coryza, cough, tachypnea, chest retractions, and wheezing.25 Severe acute lung disease was defined as need for rehospitalization (determined on the basis of changes in baseline oxygen requirement and the development of respiratory distress). The evaluations were conducted by 1 of 3 pediatricians trained in the study protocol by using a modification of the validated score designed to detect changes in oxygen saturation, respiratory rate, and pulmonary signs in children at high risk.23 This score discriminated upper from lower respiratory tract illness. Infants were managed clinically as inpatients or outpatients until symptoms resolved. Breastfeeding was categorized as exclusive and nonexclusive. Exclusive breastfeeding was infrequent in this population (4 of 119 infants), because most VLBW infants require supplemental nutrition provided by the state as preterm formula. Because World Health Organization recommendations emphasize the importance of unrestricted breastfeeding whenever the infant shows signs of hunger26 (and these episodes can be brief and intermittent), establishing the number of daily episodes of lactation was not possible. Data regarding birth weight, gestational age, length of NICU stay, length of ventilatory support, mother’s age, ≥1 family member smoking in the home, diagnosis of asthma in 1 or both parents, detection of viral RNA from nasal secretions, presence of siblings <10 years of age in the household, maternal education, household income, and presence and severity of bronchopulmonary dysplasia (BPD, defined as oxygen supplementation for ≥28 days after birth27) were collected and considered as potential confounders.23,28 Baseline severity for BPD was established at 36 weeks of gestational age.27
Viral Detection
Nasal secretions were assayed for HRV by real-time reverse transcriptase-polymerase chain reaction with the Smart Cycler II (Cepheid, Sunnyvale, CA) by using primers and probe sequences directed at a highly conserved HRV 5′-non-coding region.29 Nasal secretions also were tested for the presence of RSV; human metapneumovirus; human parainfluenza viruses 1, 2, and 3; and influenza virus by reverse transcriptase-polymerase chain reaction by use of the Hexaplex Plus assay (Prodesse, Waukesha, WI), as described previously.24,30
Statistical Analysis
Incidence of HRV-associated disease was computed by using negative binomial regression. Similarly, risk factors for HRV-associated episodes were assessed by using relative risks computed through the use of negative binomial regression. χ2 or Fisher’s exact tests were used as appropriate for contingency table analyses of symptoms and signs associated with HRV. Comparisons of the age and duration of symptoms for the different viral etiologies were calculated by using Wilcoxon rank-sum tests. All analyses used 2-sided tests with P < .05 indicating statistical significance. Analyses were performed by using R statistical software, version 2.10.0 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). Analysis scripts are available at http://biostat.mc.vanderbilt.edu/ArchivedAnalyses.
Results
Study Population
From June 2003 through May 2005, 207 VLBW infants attended the high-risk clinics for the first time. Among these infants, 68 were excluded from participation in this study (61 did not reach 1 year of corrected gestational age before completion of the study, 2 were infected with HIV, and 5 resided >70 km from the clinics). Of the remaining 139 patients, 9 parents did not consent to their infants’ participation, and 11 children were lost to follow-up or moved away before completion of the study. One hundred nineteen VLBW eligible infants participated in our cohort and were managed from enrollment. Epidemiologic characteristics of participating and nonparticipating infants were similar (data not shown). Median corrected gestational age at enrollment was 2 months (interquartile range, 0–2 months). Infants were managed until a corrected gestational age of 12 months. The total number of infant-years of follow-up was 101.8.
Among the 119 participating infants, 40 (34%) were younger than 1 month of corrected age at enrollment, 92 (77%) were younger than 3 months, and 110 (92%) were younger than 5 months. Median gestational age in the cohort population was 30 weeks.31 Eighty infants (67%) were born at participating hospitals, and 39 infants (33%) were transferred from other institutions to participating NICUs. Mothers were young, and more than one-third of infants lived in extreme poverty (Table 1). Smoking at home was frequent (37%). Fifty-eight percent of infants were breastfed. Among infants with BPD, 36 (77%) persisted with an oxygen requirement at 36 weeks of gestational age. Of these infants, 22 required ≥30% fraction of inspired oxygen.
TABLE 1.
Demographics of Infants Who Had at Least 1 HRV-Associated ARI Episode Versus Infants With No HRV-Associated Episodes
| HRV-Negative Status (n = 52) | HRV-Positive Status (n = 67) | Pa | |
|---|---|---|---|
| Corrected gestational age, mob | 2 (0–2) | 1 (0–2) | 0.37 |
| Gestational age, wk | 30 (28–31) | 30 (28.0–31.5) | 0.67 |
| Wt, g | 1210 (972.5–1360.0) | 1180 (932.5–1335.0) | 0.73 |
| Maternal age, y | 26 (22–33) | 24 (20.0–30.5) | 0.17 |
| Maternal education , y | 9 (7–12) | 9 (7–12) | 0.93 |
| Ventilator days | 5 (3–21) | 11 (2.25–41.75) | 0.75 |
| NICU days | 65 (48.75–95.00) | 69 (49.0–90.5) | 0.89 |
| No. of coinhabitants <10 y old | 0 (0–1) | 1 (0–1) | 0.08 |
| Basic needs unsatisfied, no. (%)49 | 15 of 49 (31) | 24 of 66 (36) | 0.66 |
| Smoking at home, no. (%) | 23 of 46 (50) | 21 of 63 (33) | 0.12 |
| Asthma in parents, no. (%) | 7 of 46 (15) | 8 of 63 (13) | 0.92 |
| Presence of BPD, no. (%) | 16 (31) | 30 (45) | 0.17 |
| Exclusively breastfed, no. (%) | 2 of 50 (4) | 2 of 62 (3) | 0.77 |
| Breastfed (any), no. (%) | 27 (52) | 42 (63) | 0.32 |
| Female gender, no. (%) | 24 (46) | 30 (45) | 0.97 |
Based on Wilcoxon rank-sum test for continuous variables; χ2 test for categorical.
Median (interquartile range) shown for all continuous variables.
Human Rhinoviruses Were the Most Frequent Cause of ARI in VLBW Infants
Three hundred three episodes of ARI were identified in 119 infants during their participation in the study. One hundred twenty-five (41%) of these episodes were associated with HRV, 20 (7%) with RSV, 12 (4%) with human parainfluenza virus type 3, 7 (2%) with human metapneumovirus, 5 (2%) with seasonal influenza virus A, and 3 (1%) with human parainfluenza virus 1. Human parainfluenza virus 2 was not detected. Among the 125 HRV infections, there were 11 coinfections: 7 with RSV, 2 with human parainfluenza virus 3, and 2 with human metapneumovirus. Thirty-five infants had at least 2 repeat HRV-associated episodes.
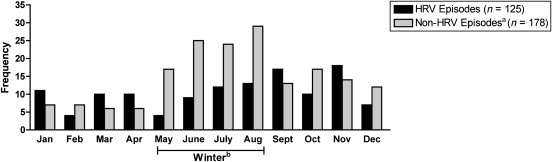
No significant demographic differences were detected in the unadjusted analysis of infants who had at least 1 HRV-associated ARI compared with those did not (Table 1). The incidence of HRV-associated ARI was 123 events per 100 infant-years of follow-up (95% confidence interval [CI]: 100–152). The median age of infants with HRV-associated ARI was not different from those with ARIs caused by other viruses (median interquartile range: 6 [CI: 3.25–8] versus 6 [CI: 4–8] for HRV-positive status and HRV-negative status, respectively). Episodes of HRV infection were more likely to occur during the nonwinter months than episodes not associated with HRV (P < .001; Fig 1). In a multivariable negative binomial regression model using the number of HRV-associated episodes and including data on weight, BPD, breastfeeding, parental asthma, smoking in home, and maternal age, BPD was associated with nearly an 80% increase in the risk for HRV-negative–associated ARI episodes (relative risk [RR]: 1.78; 95% CI: 1.11–2.84; P = .017).
FIGURE 1.
Frequency of HRV versus non-HRV episodes, June 2003 to May 2005 (including HRV coinfections). aNon-HRV episodes include other or no study viruses. bJune, July, and August are winter months in Argentina.
Human Rhinoviruses Are the Most Frequent Viral Etiology of Bronchiolitis and Hospitalization in VLBW Infants
Eighty-eight infants had an LRTI, with 46 infants experiencing moderate to severe symptoms, and 33 infants requiring hospitalization. One hundred ninety of 303 infants (63%) who experienced ARI episodes were diagnosed with bronchiolitis by a pediatrician. Of those infants with bronchiolitis, 40% had HRV versus 7% with RSV (Table 2). The incidence of HRV-associated bronchiolitis was 75 per 100 infant-years of follow-up (95% CI: 55–102). In a multivariable analysis including data regarding BPD, weight, breastfeeding status, parental asthma, smoking in the home, and maternal age, only BPD was associated with a higher risk for HRV-associated bronchiolitis (RR: 2.18; 95% CI: 1.12–4.24; P = .022).
TABLE 2.
Acute Respiratory Episode Type by Viral Etiology
| Viral Etiology | ARI Episodes, n = 303 | Bronchiolitis, n = 190 | Hospitalizations, n = 36 |
|---|---|---|---|
| Rhinovirus | 125 (41) | 76 (40) | 12 (33) |
| RSV | 20 (7) | 13 (7) | 9 (25) |
| Human metapneumovirus | 7 (2) | 8 (4) | 1 (3) |
| Adenovirus | 0 | 0 | 0 |
| Influenza virus | 5 (2) | 4 (2) | 0 |
| Parainfluenza virus 1 | 3 (1) | 2 (1) | 1 (3) |
| Parainfluenza virus 2 | 0 | 0 | 0 |
| Parainfluenza virus 3 | 12 (4) | 8 (4) | 1 (3) |
| Study virus-negative | 142 (47) | 79 (42) | 19 (53) |
All values are presented as no. (%). Percent episodes may exceed 100%, because coinfections are included under individual viruses.
Thirty-three infants were hospitalized 36 times, and an etiologic agent was identified in 24 (67%). HRV was associated with 12 (33%), and RSV was associated with 9 (25%) (Table 2). Of the 36 hospitalizations, 4 were coinfections: 4 with HRV and RSV. Of the 11 infants with 12 HRV-associated hospitalizations, 3 had a previous HRV-associated ARI episode: 1 with previous HRV-positive hospitalization (>15 days previously at age 3 months), the other 2 with previous HRV-positive outpatient ARI visits (1 with HRV-associated ARI at 3 and 5 months, hospitalized at age 6 months with HRV; and the second with HRV-associated ARI at 3 months, then HRV hospitalization at 7 months). The incidence of HRV-negative–associated hospitalization was 12 per 100 infant-years of follow-up (95% CI: 7–21). Infants with any HRV-positive hospitalizations are compared with those with no HRV-positive hospitalizations in Table 3. Significantly more infants with any HRV-positive hospitalization had BPD (73% versus 35% with no HRV-positive hospitalization, P = .035), and fewer infants with any HRV-positive hospitalization were breastfed (18% versus 62% of infants with no HRV-positive hospitalization, P = .013). In a poisson regression model including weight, BPD, and breastfeeding, the adjusted RRs for HRV-associated hospitalizations were increased for BPD (RR: 5.59; 95% CI: 1.36–23; P = .017) and marginally decreased for breastfed infants (RR: 0.27; 95% CI: 0.07–1.00; P = .051). In secondary analyses, gender, maternal age, and parental asthma were not associated with the number of HRV hospitalizations (P = .73, 0.25, and 0.2, respectively). Interestingly, an association was observed between severity of BPD and severity of HRV-positive episodes (rank correlation: 0.38; P = .005), which remained significant after adjusting for weight and maternal age (adjusted rank correlation: 0.3; P = .03). Conversely, no association was present between severity of BPD and severity of HRV-negative episodes (rank correlation: −0.03; P = .84; adjusted rank correlation: −0.01; P = .92).32
TABLE 3.
Demographics of Infants With at Least 1 HRV-Associated Hospitalization Versus Infants With No HRV-Associated Hospitalizations
| No HRV Hospitalization, n = 108 | ≥1 HRV Hospitalization, n = 11 | Pa | |
|---|---|---|---|
| Corrected gestational age, mob | 2 (0–2) | 2 (0.5–2) | 0.85 |
| Gestational age , wk | 30 (28–31) | 30 (27.5–30.5) | 0.42 |
| Wt , g | 1195 (972.5–1360.0) | 1185 (907.5–1298.0) | 0.71 |
| Maternal age , y | 25 (20.00–32.75) | 22 (20.0–26.5) | 0.18 |
| Maternal education , y | 9 (7–12) | 7 (7.0–10.5) | 0.09 |
| Ventilator days | 8.5 (2.00–37.25) | 12 (5.5–39.0) | 0.3 |
| NICU days | 66.5 (48–91) | 90 (60.5–115.0) | 0.19 |
| No. of coinhabitants <10 y old | 0 (0–1) | 1 (0.5–1.0) | 0.19 |
| Basic needs unsatisfied, no. (%) | 34 of 104 (33) | 5 of 11 (45) | 0.61 |
| Smoking at home, no. (%) | 43 of 98 (44) | 1 of 11 (9) | 0.057 |
| Asthma in parents, no. (%) | 12 of 98 (12) | 3 of 11 (27) | 0.36 |
| Presence of BPD, no. (%) | 38 of 108 (35) | 8 of 11 (73) | 0.035 |
| Exclusively breastfed, no. (%) | 4 of 101 (4) | 0 of 11 (0) | 0.85 |
| Breastfed, no. (%) | 67 of 108 (62) | 2 of 11 (18) | 0.013 |
| Female gender, no. (%) | 49 of 108 (45) | 5 of 11 (45) | 0.75 |
Based on Wilcoxon rank-sum test for continuous variables; χ2 test for categorical.
Median (interquartile range) shown for continuous variables.
Among all infants with HRV, adjusted RRs for HRV-associated hospitalizations versus HRV-associated episodes were lower for breastfed infants (RR: 0.26; 95% CI: 0.07–0.98; P = .047). In secondary analyses, excluding coinfection episodes, none of the 7 patients who had at least 1 HRV-only episode and were hospitalized was breastfed, whereas 40 of 58 infants with at least 1 HRV-only–associated episode who were not hospitalized were breastfed (P < .001). Six of the 7 patients who had at least 1 HRV-only –associated episode and were hospitalized had BPD, whereas 23 of 58 infants who had at least 1 HRV-only–associated episode and were not hospitalized had BPD (P = .039).
Comparison of HRV- and RSV-Associated Disease
Sixty-seven percent of the episodes occurred during the winter. Coinfections affected younger infants (median: 3 months old; interquartile range: 1–5 months old) and also occurred more frequently in the winter (82%). Episodes associated with only HRV versus only RSV were compared. Twenty-five percent (29 of 114) of HRV-positive episodes occurred during winter months, compared with 92% (12 of 13) of RSV episodes (P < .001). Median age at episode with only HRV versus only RSV was similar among these preterm infants (HRV-mediated at 6 months [interquartile range: 3.3–8 months] versus RSV-mediated at 6 months [interquartile range: 4–11; P = .6]). Among infants with only HRV, the rate of hospitalization was lower than among infants with only RSV (6% [7 of 114 infants] versus 38% [5 of 13 infants]; P = .001), and infants hospitalized with only HRV were in the hospital for fewer days than infants with only RSV (median: 6 days; interquartile range: 5–8.5 versus median: 13 days; interquartile range: 11–15; P = .034). Similarly, 2 of 5 infants (40%) hospitalized with only RSV were ventilated, compared with 0 of 7 infants hospitalized with only HRV (P = .29). Two of 13 infants (15%) with an RSV-only episode ended up needing a ventilator at the hospital, compared with 0 of 114 infants with an HRV-only episode (P = .003).
Discussion
In this article, we show that HRV was the most frequent viral agent associated with LRTIs in a cohort of VLBW infants in Argentina. The virus was associated with 41% of ARIs and 33% of hospitalizations. Although RSV infections were often severe, their frequency was significantly lower, so HRV was the most frequently detected agent in the absolute number of moderate to severe cases. These findings highlight the importance of an underappreciated threat for a vulnerable population.
Several interesting questions and conclusions emerge from these observations. First, our study population did not receive palivizumab to reduce RSV disease severity. Although an estimate of the benefit of palivizumab for protection against RSV disease in developing countries is unknown, the comparative impact of HRV in the number of severe cases when infants receive anti-RSV prophylaxis probably would be greater. Second, more than 150 genotypes of HRV have been identified to date.33–36 Diversity complicates passive and active prophylactic interventions. Further characterization of individual serotypes associated with severe disease should inform preventive strategies in the future. In the meantime, our findings provide yet another reason to minimize risk factors for BPD.30,37–39 Infants with BPD had higher risks of HRV-associated ARI, bronchiolitis, and hospitalization. The suspected role of HRV in the inception of asthma19,40 and the high rates of asthma in children who had BPD1–3 stress the potentially severe predictive implications and pathologic consequences of this combination. Third, here again, the emergence of molecular diagnostics redefines our understanding of the epidemiology of a severe pediatric disease.41,42 Finally, in the current study we detected more cases of HRV in nonwinter months, underscoring the importance of conducting studies of ARI year-round.
HRVs have been known historically as viruses of “older children” and thought not to be a significant contributor to illness in infants.17,21,43 In recent years, improved detection techniques have increased its detection in younger children.14 The study of HRV in premature babies has been limited. A retrospective analysis in the NICU in the Netherlands during a 12-year period found viral infections in 51 of 5396 infants, 2% with positive culture results for HRV.44 Other reports included small numbers of premature infants before the widespread availability of specific reverse transcriptase-polymerase chain reaction assays.45–47 In our article, 56% of VLBW infants had an HRV-associated ARI, and 41% of ARIs were associated with HRV.
Our prospective cohort study has limitations. We did not concurrently test healthy control subjects to explore the prevalence of asymptomatic HRV infection to suggest causality more strongly; however, we performed molecular testing for a spectrum of viruses known to cause lung disease in infants and did not detect a coinfecting virus in 91% of HRV-positive cases, suggesting that HRV was the causative pathogen. Our low-income study population had higher rates of BPD than those often reported in industrialized nations or for higher-income groups in developing countries.27 In contrast, they had very high rates of partial breastfeeding for infants discharged from the NICU, and, as previously discussed, the relative impact of HRV versus RSV may be underestimated compared with populations receiving palivizumab. All of these factors stress the importance of conducting similar studies in populations with different demographic characteristics.
Importantly, the study also has several strengths. We gathered data prospectively by frequent and standardized clinical monitoring of all infants in the cohort, predefined criteria for evaluation of respiratory signs and symptoms and severity of illness, and monitored infants until resolution of symptoms. In addition, the seasonality of respiratory viruses in Buenos Aires resembles that of industrialized nations,4,23,24,30 making certain generalizations possible.
Conclusions
We report HRV as an important pathogen associated with severe respiratory infections in VLBW infants and show that BPD and the absence of breastfeeding are risk factors for hospitalization. Furthermore, because certain interventions can reduce relapses of HRV-associated wheezing in young infants48 and HRV-mediated wheezing during the first 3 years of life is a significant risk factor for childhood asthma,40 this study should foster initiatives for the development of specific therapies, promote stringent enforcement of infection control recommendations in NICUs, and contribute to risk stratification in a vulnerable population.
Acknowledgments
This study was funded by the Director\x{2019}s Challenge Award from the National Institute of Environmental Health Sciences (Drs Kleeberger and Polack) and the Thrasher Research Fund (Dr Polack). Dr Bugna was supported by the National Institutes of Health, Office of the Director, Fogarty International Center through the International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988). Dr Miller received support from NIH KL2 RR24977-03 and NIH 1K23AI091691-01.
The authors thank the patients and their families for participation in this study.
Glossary
- ARI
acute respiratory illness
- BPD
bronchopulmonary dysplasia
- CI
confidence interval
- HRV
human rhinovirus
- LRTI
lower respiratory tract infection
- RR
relative risk
- RSV
respiratory syncytial virus
- VLBW
very low birth weight
Footnotes
Drs Miller, Bugna, and Libster contributed equally to this article.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
References
- 1.Branum AM, Schoendorf KC. Changing patterns of low birthweight and preterm birth in the United States, 1981-98. Paediatr Perinat Epidemiol. 2002;16(1):8–15 [DOI] [PubMed] [Google Scholar]
- 2.Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118(4):1566–1573 [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Kochanek KD, Strobino DM, Guyer B, MacDorman MF. Annual summary of vital statistics—2003. Pediatrics. 2005;115(3):619–634 [DOI] [PubMed] [Google Scholar]
- 4.Klein MI, Coviello S, Bauer G, et al. The impact of infection with human metapneumovirus and other respiratory viruses in young infants and children at high risk for severe pulmonary disease. J Infect Dis. 2006;193(11):1544–1551 [DOI] [PubMed] [Google Scholar]
- 5.Fariña D, Rodríguez SP, Bauer G, et al. Respiratory syncytial virus prophylaxis: cost-effective analysis in Argentina. Pediatr Infect Dis J. 2002;21(4):287–291 [DOI] [PubMed] [Google Scholar]
- 6.Pedraz C, Carbonell-Estrany X, Figueras-Aloy J, Quero J, IRIS Study Group Effect of palivizumab prophylaxis in decreasing respiratory syncytial virus hospitalizations in premature infants. Pediatr Infect Dis J. 2003;22(9):823–827 [DOI] [PubMed] [Google Scholar]
- 7.Henckel E, Luthander J, Berggren E, et al. Palivizumab prophylaxis and hospitalization for respiratory syncytial virus disease in the Stockholm infant population, 1999 through 2002. Pediatr Infect Dis J. 2004;23(1):27–31 [DOI] [PubMed] [Google Scholar]
- 8.Kusuda S, Koizumi T, Sakai T, Fujimura M, Nishida H, Togari H. Results of clinical surveillance during the Japanese first palivizumab season in 2002-2003. Pediatr Int. 2006;48(4):362–368 [DOI] [PubMed] [Google Scholar]
- 9.Liese JG, Grill E, Fischer B, Roeckl-Wiedmann I, Carr D, Belohradsky BH, Munich RSV Study Group Incidence and risk factors of respiratory syncytial virus-related hospitalizations in premature infants in Germany. Eur J Pediatr. 2003;162(4):230–236 [DOI] [PubMed] [Google Scholar]
- 10.Pedersen O, Herskind AM, Kamper J, Nielsen JP, Kristensen K. Rehospitalization for respiratory syncytial virus infection in infants with extremely low gestational age or birthweight in Denmark. Acta Paediatr. 2003;92(2):240–242 [DOI] [PubMed] [Google Scholar]
- 11.Romero JR. Palivizumab prophylaxis of respiratory syncytial virus disease from 1998 to 2002: results from four years of palivizumab usage. Pediatr Infect Dis J. 2003;22(suppl 2):S46–S54 [DOI] [PubMed] [Google Scholar]
- 12.Midulla F, Scagnolari C, Bonci E, et al. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child. 2010;95(1):35–41 [DOI] [PubMed] [Google Scholar]
- 13.Bertino JS. Cost burden of viral respiratory infections: issues for formulary decision makers. Am J Med. 2002;112(suppl 6A):42S–49S [DOI] [PubMed] [Google Scholar]
- 14.Miller EK, Lu X, Erdman DD, et al. New Vaccine Surveillance Network Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195(6):773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden FG. Rhinovirus and the lower respiratory tract. Rev Med Virol. 2004;14(1):17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gern JE. Rhinovirus respiratory infections and asthma. Am J Med. 2002;112(suppl 6A):19S–27S [DOI] [PubMed] [Google Scholar]
- 17.Jartti T, Lehtinen P, Vuorinen T, et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10(6):1095–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venarske DL, Busse WW, Griffin MR, et al. The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking. J Infect Dis. 2006;193(11):1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemanske RF, Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116(3):571–577 [DOI] [PubMed] [Google Scholar]
- 20.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ, The Group Health Medical Associates Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332(3):133–138 [DOI] [PubMed] [Google Scholar]
- 21.Heymann PW, Carper HT, Murphy DD, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114(2):239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peltola V, Waris M, Osterback R, Susi P, Hyypiä T, Ruuskanen O. Clinical effects of rhinovirus infections. J Clin Virol. 2008;43(4):411–414 [DOI] [PubMed] [Google Scholar]
- 23.Groothuis JR, Simoes EA, Levin MJ, et al. The Respiratory Syncytial Virus Immune Globulin Study Group Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. N Engl J Med. 1993;329(21):1524–1530 [DOI] [PubMed] [Google Scholar]
- 24.Laham FR, Israele V, Casellas JM, et al. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J Infect Dis. 2004;189(11):2047–2056 [DOI] [PubMed] [Google Scholar]
- 25.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917–1928 [DOI] [PubMed] [Google Scholar]
- 26.Vinther T, Helsing E. Breastfeeding: How to Support Success—A Practical Guide for Health Care Workers. Copenhagen, Denmark: World Health Organization, Regional Office for Europe; 1997:27 [Google Scholar]
- 27.Ehrenkranz RA, Walsh MC, Vohr BR, et al. National Institutes of Child Health and Human Development Neonatal Research Network Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360 [DOI] [PubMed] [Google Scholar]
- 28.Groothuis JR, Simoes EA, Hemming VG, Respiratory Syncytial Virus Immune Globulin Study Group Respiratory syncytial virus (RSV) infection in preterm infants and the protective effects of RSV immune globulin (RSVIG). Pediatrics. 1995;95(4):463–467 [PubMed] [Google Scholar]
- 29.Lu X, Holloway B, Dare RK, et al. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol. 2008;46(2):533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein MI, Bergel E, Gibbons L, et al. Differential gender response to respiratory infections and to the protective effect of breast milk in preterm infants. Pediatrics 2008;121(6). Available at: www.pediatrics.org/cgi/content/full/121/6/e1510 [DOI] [PMC free article] [PubMed]
- 31.Ferreira A, Williams Z, Donninger H, van Schalkwyk EM, Bardin PG. Rhinovirus is associated with severe asthma exacerbations and raised nasal interleukin-12. Respiration. 2002;69(2):136–142 [DOI] [PubMed] [Google Scholar]
- 32.Li C, Shepherd BE. Test of association between two ordinal variables while adjusting for covariates. J Am Stat Assoc. 2010;105(490):612–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelon W, Mogabgab WJ, Phillips IA, Pierce WE. A cytopathogenic agent isolated from naval recruits with mild respiratory illnesses. Proc Soc Exp Biol Med. 1957;94(2):262–267 [DOI] [PubMed] [Google Scholar]
- 34.Hamparian VV, Colonno RJ, Cooney MK, et al. A collaborative report: rhinoviruses—extension of the numbering system from 89 to 100. Virology. 1987;159(1):191–192 [DOI] [PubMed] [Google Scholar]
- 35.Kaplan NM, Dove W, Abd-Eldayem SA, Abu-Zeid AF, Shamoon HE, Hart CA. Molecular epidemiology and disease severity of respiratory syncytial virus in relation to other potential pathogens in children hospitalized with acute respiratory infection in Jordan. J Med Virol. 2008;80(1):168–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplan NM, Dove W, Abu-Zeid AF, Shamoon HE, Abd-Eldayem SA, Hart CA. Evidence of human metapneumovirus infection in Jordanian children. Saudi Med J. 2006;27(7):1081–1083 [PubMed] [Google Scholar]
- 37.Sinha A, Madden J, Ross-Degnan D, Soumerai S, Platt R. Reduced risk of neonatal respiratory infections among breastfed girls but not boys. Pediatrics. 2003;112(4). Available at: www.pediatrics.org/cgi/content/full/112/4/e303 [DOI] [PubMed] [Google Scholar]
- 38.Chantry CJ, Howard CR, Auinger P. Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics. 2006;117(2):425–432 [DOI] [PubMed] [Google Scholar]
- 39.Libster R, Bugna Hortoneda J, Laham FR, et al. Breastfeeding prevents severe disease in full term female infants with acute respiratory infection. Pediatr Infect Dis J. 2009;28(2):131–134 [DOI] [PubMed] [Google Scholar]
- 40.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller EK, Edwards KM, Weinberg GA, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol 2009;123:98–104. [DOI] [PMC free article] [PubMed]
- 42.Palmenberg AC, Spiro D, Kuzmickas R, et al. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science. 2009;324(5923):55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan WC. Viruses in asthma exacerbations. Curr Opin Pulm Med. 2005;11(1):21–26 [DOI] [PubMed] [Google Scholar]
- 44.Verboon-Maciolek MA, Krediet TG, Gerards LJ, Fleer A, van Loon TM. Clinical and epidemiologic characteristics of viral infections in a neonatal intensive care unit during a 12-year period. Pediatr Infect Dis J. 2005;24(10):901–904 [DOI] [PubMed] [Google Scholar]
- 45.Vocel J, Jezková Z, Strízová V, Brůcková M. Rhinovirus infection caused by Rhinovirus type 19 in premature and small infants in a premature infant ward [in Czech]. Cesk Pediatr. 1980;35(7):343–344 [PubMed] [Google Scholar]
- 46.Krasikova VA, Ritova VV, Schastnyĭ EI, Poddubnaia EI. Mixed viral infections in premature newborn infants [in Russian]. Vopr Okhr Materin Det. 1975;20(1):21–26 [PubMed] [Google Scholar]
- 47.Abzug MJ, Beam AC, Gyorkos EA, Levin MJ. Viral pneumonia in the first month of life. Pediatr Infect Dis J. 1990;9(12):881–885 [DOI] [PubMed] [Google Scholar]
- 48.Jartti T, Lehtinen P, Vanto T, et al. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25(6):482–488 [DOI] [PubMed] [Google Scholar]
- 49.Instituto Nacional de Estadística y Censos. Glosario. Available at: www.indec.gov.ar/glosario/textos_glosario.asp?id=21 Accessed August 11, 2011