Abstract
Background
Iron deficiency anemia is the most common form of anemia in India. Hemoglobin A1c (HbA1c) is used in diabetic patients as an index of glycemic control reflecting glucose levels of the previous 3 months. Like blood sugar levels, HbA1c levels are also affected by the presence of variant hemoglobins, hemolytic anemias, nutritional anemias, uremia, pregnancy, and acute blood loss. However, reports on the effects of iron deficiency anemia on HbA1c levels are inconsistent. We conducted a study to analyze the effects of iron deficiency anemia on HbA1c levels and to assess whether treatment of iron deficiency anemia affects HbA1c levels.
Methods
Fifty patients confirmed to have iron deficiency anemia were enrolled in this study. HbA1c and absolute HbA1c levels were measured both at baseline and at 2 months after treatment, and these values were compared with those in the control population.
Results
The mean baseline HbA1c level in anemic patients (4.6%) was significantly lower than that in the control group (5.5%, p<0.05). A significant increase was observed in the patients' absolute HbA1c levels at 2 months after treatment (0.29 g/dL vs. 0.73 g/dL, p<0.01). There was a significant difference between the baseline values of patients and controls (0.29 g/dL vs. 0.74 g/dL, p<0.01).
Conclusions
In contrast to the observations of previous studies, ours showed that HbA1c levels and absolute HbA1c levels increased with treatment of iron deficiency anemia. This could be attributable to nutritional deficiency and/or certain unknown variables. Further studies are warranted.
Keywords: Iron deficiency anemia, Hemoglobin A1c, HbA1c, Glycated hemoglobin
INTRODUCTION
Hemoglobin A1c (HbA1c) is a glycated hemoglobin that can be used as an indicator of a patient's glycemic status over the previous 3 months [1]. Glycated hemoglobins, including HbA1c and other hemoglobins, constitute the HbA1 fraction of adult hemoglobin (HbA) [1]. HbA1c is the predominant hemoglobin found in HbA1 fractions. According to the American Diabetes Association Guidelines published in 2007, HbA1c levels should be maintained below 7% in all diabetic patients in order to prevent the development of microvascular complications [2]. HbA1c levels are not affected by blood glucose levels alone. They are also altered in hemolytic anemias [3], hemoglobinopathies [4], acute and chronic blood loss [5, 6], pregnancy [7-9], and uremia [10-12]. Vitamin B12, folate, and iron deficiency anemias have also been shown to affect HbA1c levels.
Iron deficiency anemia is the most common form of anemia in India [13]. Initial studies by Brooks et al. [14], Sluiter et al. [15], and Mitchell et al. [16] revealed a relationship between iron deficiency anemia and HbA1c levels and attempted to explain the alteration in HbA1c levels in iron deficiency anemia on the basis of both modifications to the structure of hemoglobin and levels of HbA1c in old and new red blood cells. Later, Heyningen et al. [17] and Hansen et al. [18] reported that there were no differences between the HbA1c levels of anemic patients and controls. These observations were strikingly different from those of previous studies. Rai et al. [19] investigated different methods to assay HbA1c levels and found no differences in HbA1c levels detected when using colorimetric assays, ion exchange chromatography, and affinity chromatography. Further studies showed that HbA1c levels were higher in patients with iron deficiency anemia and decreased significantly upon treatment with iron [20, 21]. The results of these studies are conflicting, and the exact mechanism underlying the effects of iron deficiency anemia on HbA1c levels is not yet known. Therefore, both because of this lack of corresponding evidence and since no such studies have been conducted on the Indian population, we were prompted to conduct the current study to investigate the effects of iron deficiency anemia on HbA1c levels in Indian patients.
METHODS
Fifty patients (both men and women) aged between 12 and 50 yr with confirmed iron deficiency-type anemia [Hb<12 g/dL in women and <13 g/dL in men] were included in this study. The patients were from both outpatient and inpatient departments of Lok Nayak Hospital, Delhi, India, and were enrolled after they provided written consent. Patients with a history of acute blood loss, hemolytic anemia, hemoglobinopathies, kidney disease, pregnancy, established diabetes, impaired fasting glucose, or impaired glucose tolerance were excluded. Those with no history of glucose intolerance, but with fasting blood glucose levels greater than 100 mg/dL at the time of enrollment were also excluded. Women of child-bearing age who had amenorrhea were excluded if found to be pregnant after a urinary pregnancy test. Since hemolytic anemias, hemoglobinopathies, and uremia can be present in asymptomatic individuals, all patients were screened to rule out these disorders. Those with a reticulocyte production index greater than 2.5 at baseline in the absence of overt bleeding were considered to have hemolytic anemia and were excluded from the study. Hemoglobin electrophoresis was performed to rule out hemoglobinopathies. Kidney function tests, including analysis of blood urea and serum creatinine levels, were conducted to determine baseline values by using a chemistry analyzer (CX 5 PRO SYNCHRON Clinical System, Beckman Coulter, Brea, CA, USA). Patients with blood urea levels greater than 20 mg/dL or with serum creatinine levels greater than 1.2 mg/dL (male patients) or 0.9 mg/dL (female patients) were excluded.
All patients were asked to provide a detailed history and were subjected to a physical examination. The levels of hemoglobin, mean corpuscular hemoglobin (MCH), hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), platelet count, total leucocyte count (TLC), and differential leucocyte count (DLC) were measured by an automated counter (SYSMEX, Japan) at baseline and then again at 1 week, 1 month, and 2 months following treatment. Peripheral blood smear examinations to define the anemia type, as well as all other investigations, were conducted in our Pathology Department. On the basis of hemoglobin levels, patients were categorized as having mild, moderate, or severe anemia: mild anemia (male patients, 12-12.9 g/dL and female patients, 11-11.9 g/dL); moderate anemia (male patients, 9-11.9 g/dL and female patients, 8-10.9); and severe anemia (male patients, less than 9 g/dL and female patients, less than 8 g/dL) [22]. Those with predominantly microcytic indices (MCV<80 fL) and hypochromic indices (MCH<26 pg/cell) were considered to have iron deficiency anemia, which was then also confirmed by low serum ferritin levels (<10 ng/mL in female and <29 ng/mL in male patients). Serum ferritin levels were measured by an ELISA test kit (Biochek Inc., India). Serum ferritin was measured again at 1 and 2 months after treatment. HbA1c levels (%) were measured at the time of enrollment and then again at 1 and 2 months following the start of treatment. HbA1c levels were measured using the Glycohemoglobin Reagent Kit (TECO Diagnostics, India) on the basis of the principle that glycohemoglobin (HbA1a, HbA1c) moves as a fast fraction and thus elutes first during cation exchange column chromatography. After separation of glycohemoglobin from non-glycosylated hemoglobin by cation exchange, the absorbance values for glycohemoglobin and total hemoglobin were measured at 415 nm. These values, along with values of standards provided in the kit, were used for calculating the percent glycohemoglobin and HbA1c according to instructions provided by the manufacturer. Absolute HbA1c levels were calculated from the measured HbA1c levels by using the following formula:
Absolute HbA1c (g/dL)= [Glycated hemoglobin (%)×Hemoglobin (g/dL)]/100
Fifty healthy participants were enrolled to serve as controls after they provided written consent. All the laboratory parameters analyzed for patients were analyzed for the control group as well. However, in the case of the control group, the readings were recorded just once, at the time of enrollment. The exclusion criteria for the control group were the same as for patients. The data are presented as mean±SD for continuous variables. A Student's t-test was applied for comparison of group means. Pearson's coefficient of correlation was calculated to determine the correlation between 2 variables. P value of less than 0.05 was considered statistically significant.
RESULTS
Of 50 patients, 34 (68%) were female and 16 (32%) were male. In the control group, 21 participants (42%) were female and 29 (58%) were male. The mean age of the patients was 30.3 yr (range, 12-50 yr). The mean age of the control participants was 36.2 yr (range, 13-50 yr). Severe anemia was seen in 38 (76%) patients, and moderate anemia, in 12 (24%) patients. We found no cases of mild anemia. With regards to symptoms, weakness was seen in 48 (96%) patients; malaise, in 46 (92%); lack of interest in work, in 30 (60%); dyspnea, in 17 (34%); pica, in 4 (8%); and presence of worms in stool, in 4 (8%). Examination revealed pallor in all patients (50/50, 100%) and right inguinal lymphadenopathy, which on fine-needle aspiration cytology (FNAC) showed reactive lymphoid hyperplasia, in 1 patient. Nail changes were seen in 6 patients, 5 of whom had platonychia and 1 of whom had koilonychia. Mild splenomegaly (<2 cm) was noted in 12 patients (24%). Ejection systolic murmur in the aortic area was observed in 40 patients (80%).
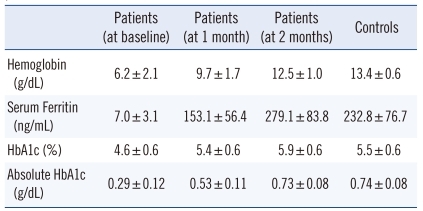
The mean hemoglobin levels of patients at baseline and after 1and 2 months were 6.2 g/dL, 9.7 g/dL, and 12.5 g/dL, respectively (Table 1). The mean hemoglobin level of the control group was 13.4 g/dL. These data provided evidence that hemoglobin was indeed lower in anemic patients than in healthy controls, and the observed difference was statistically significant (p<0.01). After 2 months of treatment for iron deficiency anemia, there was a significant increase in the hemoglobin levels of anemic patients (p<0.01). However, the mean hemoglobin levels of patients after 2 months of treatment was still lower than that of the control group at baseline, and this difference was still statistically significant (p<0.01).
Table 1.
Comparison of hemoglobin, ferritin, and HbA1c levels in patients and controls
Data are presented as mean±SD values.
The mean serum ferritin levels of anemic patients at baseline and after 1 and 2 months were 7.0 ng/mL, 153.1 ng/mL, and 279.1 ng/mL, respectively, and that of controls was 232.8 ng/mL (Table 1). The mean baseline serum ferritin levels were significantly lower in patients than in controls (p<0.01). However, at 2 months after treatment, the mean serum ferritin levels were significantly higher in patients than in the control group (p<0.01).
The mean HbA1c levels in anemic patients were 4.6%, 5.4%, and 5.9% at baseline and after 1 and 2 months, respectively, while that in the controls was 5.5% (Table 1). The baseline HbA1c levels were significantly lower in patients than in controls; however, there was a significant increase in HbA1c levels in patients after 2 months of treatment for iron deficiency anemia (p<0.01). Furthermore, the mean HbA1c levels were significantly higher in patients after 2 months of treatment than in the controls (p<0.01). The mean absolute HbA1c level in patients at baseline and after 1 and 2 months were 0.29 g/dL, 0.53 g/dL, and 0.73 g/ dL, respectively, while that in controls was 0.74 g/dL (Table 1). A significant difference was observed between the baseline values of patients and controls (p<0.01). Additionally, there was a significant increase in absolute HbA1c levels over the 2-month treatment period (p<0.01). However, after 2 months of treatment, there was no significant difference between the absolute HbA1c levels of patients and controls (p>0.05).
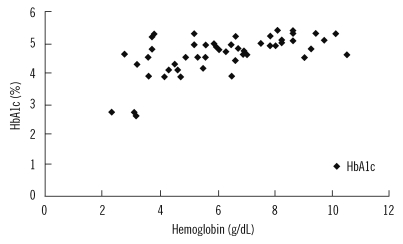
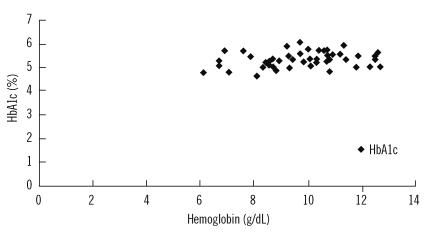
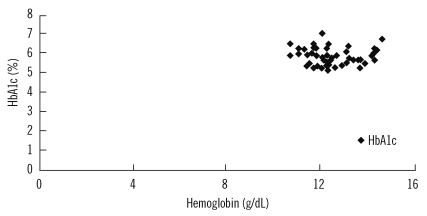
We observed a significant correlation between hemoglobin and HbA1c levels in patients at baseline (coefficient of correlation=0.593; p<0.01; Fig. 1) and after 1 month of treatment (coefficient of correlation=0.490; p<0.01; Fig. 2). However, there was no positive correlation between hemoglobin and HbA1c levels at the end of the 2-month treatment period (coefficient of correlation=-0.63; p >0.05; Fig. 3).
Fig. 1.
Correlation between hemoglobin and HbA1c levels in patients at baseline.
Abbreviation: HbA1c, hemoglobin A1c.
Fig. 2.
Correlation between hemoglobin and HbA1c levels in patients after 1 month of treatment
Abbreviation: HbA1c, hemoglobin A1c.
Fig. 3.
Correlation between hemoglobin and HbA1c levels in patients after 2 months of treatment.
Abbreviation: HbA1c, hemoglobin A1c.
DISCUSSION
Iron deficiency anemia is the most common form of anemia. HbA1c is a glycated hemoglobin that can be used to assess the glycemic status of diabetic patient for the previous 3 months. Besides blood sugar, other conditions such as hemolytic anemias, hemoglobinopathies, acute and chronic blood loss, pregnancy, and uremia have been shown to affect HbA1c levels [3-12]. Recently, researchers have become interested in studying HbA1c levels in more commonly encountered anemias like iron deficiency anemia.
The earliest study to investigate the effects of iron deficiency anemia on HbA1c levels was conducted by Brooks et al. [14] who assessed HbA1 levels in 35 non-diabetic patients having iron deficiency anemia both before and after treatment with iron. They observed that HbA1 levels were significantly higher in iron deficiency anemia patients and decreased after treatment with iron. The mechanisms leading to increased glycated HbA1 levels were not clear. It was proposed that, in iron deficiency, the quaternary structure of the hemoglobin molecule was altered, and that glycation of the globin chain occurred more readily in the relative absence of iron [14]. Sluiter et al. [15] tried to provide an explanation for the above findings. They proposed that the formation of glycated hemoglobin is an irreversible process and hence, the concentration of HbA1 in 1 erythrocyte will increase linearly with the cell's age. For example, they found that in patients with normal blood glucose levels, but with very young red cells, as would be found after treatment of iron deficiency anemia, HbA1 concentration was reduced. However, if iron deficiency has persisted for a long time, the red cell production rate would fall, leading not only to anemia but also to a higher-than-normal average age of circulating erythrocytes and, therefore, increased HbA1 levels [15]. In 1980, Mitchell et al. [16] commented on the study done by Brooks et al. [14]. They calculated the absolute amount of HbA1 in each red cell (i.e., the mean corpuscular HbA1) and found that there were no differences in HbA1 levels before and after iron treatment. They also analyzed the study done by Sluiter et al. and suggested that red cell age was unlikely to be a significant factor in explaining the changes in HbA1 levels during the treatment of iron deficiency anemia [15].
Later van Heyningen et al. [17] reported no differences in HbA1c concentrations when comparing non-diabetic patients with iron deficiency anemia before and after iron treatment to healthy controls. They believed that the reported differences in HbA1c concentrations before and after iron supplementation were due to differences in the laboratory methods used for measuring HbA1c [17]. Hansen et al. also demonstrated that there were no significant differences in HbA1c concentrations in irondeficient patients, vitamin B12-deficient patients, and healthy controls [18]. They were of the opinion that in iron deficiency anemia, the erythrocyte survival rate is normal, while in vitamin B12 deficiency, the red cell survival rate decreases, but the hemolytic component is often minor and affects both mature and immature erythrocytes. Hence, they thought that normal levels of glycated hemoglobin are expected. They found that HbA1c levels decreased upon treatment of the anemia, which was probably due to increased bone marrow erythropoiesis caused by the treatment, leading to production of new immature erythrocytes. In a study by Rai et al., colorimetric assays, ion exchange chromatography, and affinity chromatography were compared as methods for measuring HbA1c, and no significant differences were found in HbA1c values measured by any of these methods [19]. Further studies by El-Agouza et al. [20] and Cogan et al. [21] showed that HbA1c levels were higher in patients with iron deficiency anemia and decreased significantly upon treatment with iron. They argued that elevated HbA1c levels in iron deficiency anemia could be explained by the assumption that if serum glucose remains constant, a decrease in the hemoglobin concentration might lead to an increase in the glycated fraction [20, 21]. As evident from the above studies, the exact mechanism through which iron deficiency anemia affects HbA1c levels still remains unclear. The explanations provided in the studies quoted above are merely speculation. Due to the variation in results obtained from these multiple studies, we were prompted to conduct our own study to investigate the affects of iron deficiency anemia on HbA1c levels.
Among the 50 patients studied, 34 were female, suggesting that iron deficiency anemia is more common in women. Mild splenomegaly was seen in 12 patients, all of whom had severe anemia. Splenomegaly is most commonly seen in severe, persistent, and untreated iron deficiency anemia [23]. As expected, the mean hemoglobin and mean serum ferritin levels increased in anemia patients over 2 months of iron treatment. None of our patients had non-responsive iron deficiency anemia. However, in stark contrast to previous studies, in our study, the HbA1c levels were found to be significantly lower in patients with iron deficiency anemia than in the controls. Moreover, the HbA1c levels increased after treatment, which had not been reported in any previous study. The absolute HbA1c levels, which were significantly lower in patients than in controls, were also found to increase significantly after iron treatment. The hemoglobin and HbA1c levels were positively correlated in anemia patients before treatment, but no positive correlation was apparent at 2 months after treatment.
Our observation of decreased HbA1c levels at baseline and the subsequent rise in HbA1c with iron supplementation was strikingly different from the findings in previous studies. In fact, no other studies in the literature support our findings. We used accepted methodology (ion-exchange chromatography) to estimate HbA1c levels, and the analysis was validated in our laboratory. Strict quality control was ensured, and samples were tested in an assorted manner (i.e., the control samples and the test samples were not analyzed in separate batches, but rather in mixed batches). Our observations can be attributable to the fact that our population belonged mainly to a lower socio-economic strata. Here, the causes of iron deficiency are not just bleeding and malabsorption, but also nutritional deficiency which may play an important role in the cause of iron deficiency. These factors (or other unknown variables) may have a bearing on our results. Further studies to confirm and elucidate the roles of these factors are warranted.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Telen MJ, Kaufman RE. The mature erythrocyte. In: Greer JP, Forester J, et al., editors. Wintrobe's clinical hematology. 11th ed. Lippincot: Williams and Wilkins; 2004. p. 230. [Google Scholar]
- 2.American Diabetes Association. Position statement: Standards of medical care in diabetes-2007. Diabetes Care. 2007;30(S1):S9. [Google Scholar]
- 3.Horton BF, Huisman TH. Studies on the heterogeneity of hemoglobin. VII. Minor hemoglobin components in haematological diseases. Br J Haematol. 1965;11:296–304. doi: 10.1111/j.1365-2141.1965.tb06589.x. [DOI] [PubMed] [Google Scholar]
- 4.Eberentz-Lhomme C, Ducrocq R, Intrator S, Elion J, Nunez E, Assan R. Haemoglobinopathies: a pitfall in the assessment of glycosylated haemoglobin by ion-exchange chromatography. Diabetologia. 1984;27:596–598. doi: 10.1007/BF00276975. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein RE. Glycosylated hemoglobins: hematologic considerations determine which assay for glycohemoglobin is advisable. Clin Chem. 1980;26:174–175. [PubMed] [Google Scholar]
- 6.Starkman HS, Wacks M, Soeldner JS, Kim A. Effect of acute blood loss on glycosylated hemoglobin determinations in normal subjects. Diabetes Care. 1983;6:291–294. doi: 10.2337/diacare.6.3.291. [DOI] [PubMed] [Google Scholar]
- 7.Lind T, Cheyne GA. Effect of normal pregnancy upon the glycosylated haemoglobins. Br J Obstet Gynaecol. 1979;86:210–213. doi: 10.1111/j.1471-0528.1979.tb10595.x. [DOI] [PubMed] [Google Scholar]
- 8.Hanson U, Hagenfeldt L, Hagenfeldt K. Glycosylated hemoglobins in normal pregnancy: sequential changes and relation to birth weight. Obstet Gynecol. 1983;62:741–744. [PubMed] [Google Scholar]
- 9.Phelps RL, Honig GR, Green D, Metzger B, Frederiksen MC, Frienkel N. Biphasic changes in hemoglobin A1c concentrations during normal human pregnancy. Am J Obstet Gynecol. 1983;147:651–653. doi: 10.1016/0002-9378(83)90443-x. [DOI] [PubMed] [Google Scholar]
- 10.de Boer MJ, Miedema K, Casparie AF. Glycosylated haemoglobin in renal failure. Diabetologia. 1980;18:437–440. doi: 10.1007/BF00261697. [DOI] [PubMed] [Google Scholar]
- 11.Flückiger R, Harmon W, Meier W, Loo S, Gabbay KH. Hemoglobin carbamylation in uremia. N Engl J Med. 1981;304:823–827. doi: 10.1056/NEJM198104023041406. [DOI] [PubMed] [Google Scholar]
- 12.Paisey R, Banks R, Holton R, Young K, Hopton M, White D, et al. Glycosylated haemoglobin in uraemia. Diabet Med. 1986;3:445–448. doi: 10.1111/j.1464-5491.1986.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 13.Shendurnikaref N, editor. Iron deficiency is preventable. [Updated on Apr 2007]. http://www.indiaparenting.com/raisingchild/data/raisingchild063.shtml.
- 14.Brooks AP, Metcalfe J, Day JL, Edwards MS. Iron deficiency and glycosylated haemoglobin A. Lancet. 1980;2:141. doi: 10.1016/s0140-6736(80)90019-7. [DOI] [PubMed] [Google Scholar]
- 15.Sluiter WJ, van Essen LH, Reitsma WD, Doorenbos H. Glycosylated haemoglobin and iron deficiency. Lancet. 1980;2:531–532. doi: 10.1016/s0140-6736(80)91853-x. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell TR, Anderson D, Shepperd J. Iron deficiency, haemochromatosis, and glycosylated haemoglobin. Lancet. 1980;2:747. doi: 10.1016/s0140-6736(80)91969-8. [DOI] [PubMed] [Google Scholar]
- 17.van Heyningen C, Dalton RG. Glycosylated haemoglobin in iron-deficiency anaemia. Lancet. 1985;1:874. doi: 10.1016/s0140-6736(85)92234-2. [DOI] [PubMed] [Google Scholar]
- 18.Gram-Hansen P, Eriksen J, Mourits-Andersen T, Olesen L. Glycosylated haemoglobin (HbA1c) in iron- and vitamin B12 deficiency. J Intern Med. 1990;227:133–136. doi: 10.1111/j.1365-2796.1990.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 19.Rai KB, Pattabiraman TN. Glycosylated haemoglobin levels in iron deficiency anaemia. Indian J Med Res. 1986;83:234–236. [PubMed] [Google Scholar]
- 20.El-Agouza I, Abu Shohla A, Sirdah M. The effect of iron deficiency anaemia on the levels of haemoglobin subtypes: possible consequences for clinical diagnosis. Clin Lab Haematol. 2002;24:285–289. doi: 10.1046/j.1365-2257.2002.00464.x. [DOI] [PubMed] [Google Scholar]
- 21.Coban E, Ozdogan M, Timuragaoglu A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol. 2004;112:126–128. doi: 10.1159/000079722. [DOI] [PubMed] [Google Scholar]
- 22.Rastogi T, Mathers C, editors. Global burden of iron deficiency anaemia in the year 2000. [Updated on Apr 2007]. http://www.who.int/entity/healthinfo/statistics/bod_irondeficiencyanaemia.pdf.
- 23.Conrad ME, editor. Iron deficiency. [Updated on Apr 2007]. http://www.emedicine.com/med/topic1188.htm.