Abstract
Genetic defects in like-glycosyltransferase (LARGE) cause congenital muscular dystrophy with central nervous system manifestations. The underlying molecular pathomechanism is the hypoglycosylation of α-dystroglycan (α-DG), which is evidenced by diminished immunoreactivity to IIH6C4 and VIA4-1, antibodies that recognize carbohydrate epitopes. Previous studies indicate that LARGE participates in the formation of a phosphoryl glycan branch on O-linked mannose or it modifies complex N- and mucin O-glycans. In this study, we overexpressed LARGE in neural stem cells deficient in protein O-mannosyltransferase 2 (POMT2), an enzyme required for O-mannosyl glycosylation. The results showed that overexpressing LARGE did not lead to hyperglycosylation of α-DG in POMT2 knockout (KO) cells but did generate IIH6C4 and VIA4-1 immunoreactivity and laminin-binding activity. Additionally, overexpressing LARGE in cells deficient in both POMT2 and α-DG generated laminin-binding IIH6C4 immunoreactivity. These results indicate that LARGE expression resulted in the glycosylation of proteins other than α-DG in the absence of O-mannosyl glycosylation. The IIH6C4 immunoreactivity generated in double-KO cells was largely removed by treatment either with peptide N-glycosidase F or with cold aqueous hydrofluoric acid, suggesting that LARGE expression caused phosphoryl glycosylation of N-glycans. However, the glycosylation of α-DG by LARGE is dependent on POMT2, indicating that LARGE expression only modifies O-linked mannosyl glycans of α-DG. Thus, LARGE expression mediates the phosphoryl glycosylation of not only O-mannosyl glycans including those on α-DG but also N-glycans on proteins other than α-DG.
Keywords: congenital muscular dystrophy, dystroglycan, dystroglycanopathy, like-glycosyltransferase, neural stem cells
Introduction
Mutations in genes encoding glycosyltransferases (or putative glycosyltransferases), including POMT1 (protein O-mannosyltransferase 1; de Beltran-Valero et al. 2002; Currier et al. 2005), POMT2 (van Reeuwijk et al. 2005), POMGnT1 (protein O-mannose N-acetylglucosaminyltransferase 1; Yoshida et al. 2001), LARGE (like-glycosyltransferase; Longman et al. 2003; van Reeuwijk et al. 2007; Clarke et al. 2011; Vuillaumier-Barrot et al. 2011), FKTN (fukutin; Kobayashi et al. 1998; de Bernabe et al. 2003) and FKRP (fukutin-related protein; Brockington et al. 2001; de Beltran-Valero et al. 2004), cause congenital muscular dystrophies (CMDs) with manifestations in the central nervous system and the eye, such as Walker–Warburg syndrome (WWS), muscle–eye–brain disease (MEB), Fukuyama CMD (FCMD) and CMD type 1D. Some of these genes are involved in the synthesis of O-linked mannosyl glycans, e.g. Siaα2,3Galβ1,4GlcNAcβ1,2Man-Ser/Thr (Chiba et al. 1997; Sasaki et al. 1998; Smalheiser et al. 1998), which account for one-third of O-linked glycans in the mammalian brain (Finne et al. 1979; Krusius et al. 1986; Chai et al. 1999; Kogelberg et al. 2001). POMT1 and POMT2 form a mutually indispensible enzyme complex and catalyze the initiation step of the O-mannosyl glycosylation pathway to form O-linked mannose (Manya et al. 2004; Akasaka-Manya et al. 2006). POMGnT1 transfers N-acetylglucosamine (GlcNAc) to O-linked mannose forming a β1,2 linkage (Yoshida et al. 2001; Zhang et al. 2002). The biochemical functions of fukutin, FKRP and LARGE are not yet fully elucidated.
α-Dystroglycan (α-DG) is a functional target of O-mannosyl glycosylation. It is an extracellular matrix receptor that binds with high affinity to several extracellular matrix components, including laminin (Ervasti and Campbell 1993; Gee et al. 1993; Yamada et al. 1994; Montanaro et al. 1999; Smalheiser and Kim 1995), agrin (Gee et al. 1994; Yamada et al. 1996), perlecan (Peng et al. 1998; Talts et al. 1999), neurexin (Sugita et al. 2001) and pikachurin (Sato et al. 2008). α-DG interacts with the transmembrane β-DG (Ervasti and Campbell 1991; Ibraghimov-Beskrovnaya et al. 1992), which in turn binds to the cytoskeleton (Ervasti and Campbell 1991; Winder 2001). Functional glycosylation of α-DG is essential in its interaction with the extracellular matrix. To evaluate the glycosylation status of α-DG, the monoclonal antibodies IIH6C4 and VIA4-1 have been widely used because they recognize carbohydrate epitopes (Ervasti and Campbell 1991, 1993). Hypoglycosylation is revealed by loss (or reduction) of IIH6C4 (or VIA4-1) immunoreactivity (Kano et al. 2002; Michele et al. 2002; Kim et al. 2004; Liu et al. 2006). Mutations in CMD-causing glycosyltransferases lead to the hypoglycosylation of α-DG with markedly reduced IIH6C4 (or VIA4-1) immunoreactivity and reduced laminin-binding activity (Grewal et al. 2001; Kano et al. 2002; Michele et al. 2002; Takeda et al. 2003; Kim et al. 2004; Liu et al. 2006) and pikachurin-binding activity (Kanagawa et al. 2010; Hu, Li, Zhang, et al. 2011) by α-DG.
LARGE is a putative glycosyltransferase (Peyrard et al. 1999). It contains two transferase-like domains (Grewal et al. 2001). The Largemyd mice bear a spontaneous deletion in the Large gene and exhibit phenotypes similar to CMD in humans (Grewal et al. 2001; Holzfeind et al. 2002). Site-directed mutagenesis of the transferase-like domains abolishes its glycosylation capability, suggesting that LARGE may indeed function as a glycosyltransferase (Brockington et al. 2005; Aguilan et al. 2009). Overexpressing LARGE or its homolog LARGE2 dramatically increases IIH6C4 immunoreactivity and the apparent molecular weight of α-DG on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) gels (Barresi et al. 2004; Brockington et al. 2005; Fujimura et al. 2005; Grewal et al. 2005). The IIH6C4 immunoreactive protein(s) confer laminin binding. The only known IIH6C4 immunoreactive protein is α-DG. However, we recently showed that LARGE expression can glycosylate proteins other than α-DG to confer IIH6C4 immunoreactivity and laminin-binding activity (Zhang et al. 2011). The increased glycosylation caused by LARGE overexpression occurs in cells isolated from not only Largemyd mice, but also patients with WWS, MEB and FCMD (Barresi et al. 2004). These studies raise the hope of using LARGE in gene therapy for all CMDs caused by defective α-DG glycosylation.
The biochemical function of LARGE remains elusive. Early evidence suggested that LARGE modifies O-linked mannosyl glycans, complex N-glycans, and mucin-type O-glycans (Patnaik and Stanley 2005; Aguilan et al. 2009). More recent data indicate that LARGE is involved in the extension of an unidentified phosphoryl glycosylation branch on O-linked mannose (Yoshida-Moriguchi et al. 2010). In this report, we overexpressed LARGE in POMT2-deficient and in POMT2/DG double-deficient cells and analyzed LARGE-mediated glycosylation by immunoblot with the IIH6C4 antibody. Our results indicate that LARGE can mediate phosphoryl glycosylation on N-linked glycans of non-α-DG proteins to generate IIH6C4 immunoreactive epitopes capable of laminin binding. However, the glycosylation of α-DG by LARGE is dependent on POMT2-mediated O-mannosyl glycosylation.
Results
Establishment and characterization of POMGnT1, POMT2-deficient and POMT2/DG double-deficient neural stem cells
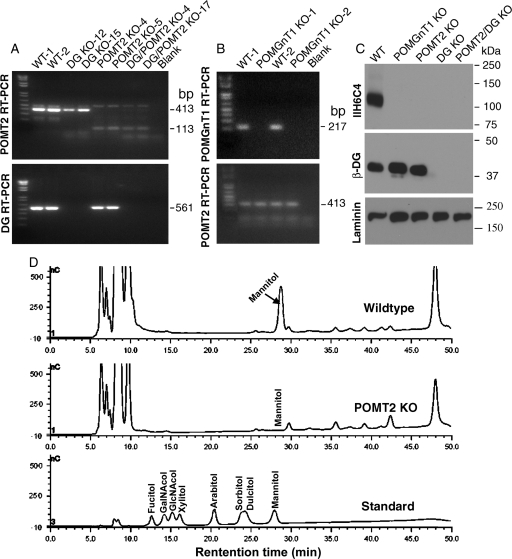
Neural stem cells were extracted from Dag1f/fPOMT2f/f;Emx1-Cre+ fetuses obtained from crosses of Dag1+/fPOMT2+/f;Emx1-Cre+ animals with Dag1+/fPOMT2+/f;Emx1-Cre− animals. From these cultures, 22 clones were isolated. Genotyping for POMT2 and DG knockout (KO) primers showed that nine clones were double KO. POMT2-deficient (Hu, Li, Gagen, et al. 2011) and DG-deficient (Zhang et al. 2011) neural stem cells were described previously. POMGnT1-deficient neural stem cells were isolated from fetuses of POMGnT1 KO mice similarly (Liu et al. 2006). To confirm the non-expression of POMT2 in POMT2 and DG/POMT2 double-KO clones, reverse transcription (RT)–polymerase chain reaction (PCR) with POMT2-specific primers that flank the deleted exons were carried out (Figure 1A). The expected RT–PCR fragments for wild-type POMT2 mRNA (413 bp) were detected in wild-type neural stem cells but not in POMT2 KO or in double-KO clones. Instead, the expected RT–PCR fragments for POMT2 KO mRNA (113 bp) were detected. To confirm the non-expression of DG in DG/POMT2 double-KO cells, RT–PCR with forward primer from exon 1 and reverse primer from floxed exon 2 was carried out. Although an expected RT–PCR fragment (561 bp) was detected from the wild type, it was not detected in the double-KO clones. To confirm the non-expression of POMGnT1 mRNA in POMGnT1 KO neural stem cells, RT–PCR was performed as described previously (Liu et al. 2006). The expected fragment for wild-type POMGnT1 mRNA was observed only in the wild-type but not in POMgnT1 KO neural stem cells (Figure 1B). Additionally, western blot analysis with the β-DG antibody did not detect β-DG in DG KO and in double-KO cells (Figure 1C). Similarly, western blot with the IIH6C4 antibody did not detect glycosylated α-DG in either DG or double-KO cells. As a loading control, endogenous laminin was comparably detected in all samples. These results indicate that POMT2 KO and DG/POMT2 double-KO cells were completely deficient in POMT2 and in DG and POMT2, respectively.
Fig. 1.
Characterization of POMGnT1, DG, POMT2 and DG/POMT2 double-KO neural stem cells. RT–PCR (A and B) and western blot analysis (C) were carried out to confirm the loss of DG, POMGnT1 and POMT2 expression in respective clones. Mannitol analysis was carried out by HPAEC-PAD on proteins isolated from wild-type and POMT2 KO neural stem cells after reductive β-elimination (D). (A) RT–PCR with DG or POMT2 primers. For POMT2, the 413 bp amplicon expected for wild-type mRNA was only observed for wild-type and DG KO clones. In contrast, the 113 bp amplicon expected for the KO mRNA was only observed for POMT2 KO and double-KO clones. For DG, the 561 bp amplicon expected for the wild-type mRNA was detected only in wild-type and POMT2 KO cells but not in DG KO and double-KO cells. (B) POMGnT1 RT–PCR. The 217 bp amplicon expected for the wild-type mRNA was present in wild-type but not in POMGnT1 KO cells. (C) Western blot analysis with IIH6C4, β-DG and laminin antibodies. IIH6C4 immunoreactivity was detected at 120 kDa in wild-type neural stem cells, but not in any other KO cell lines. β-DG immunoreactivity was detected only in wild-type, POMGnT1 KO and POMT2 KO neural stem cells, but not in DG KO or DG/POMT2 double-KO cells. As a loading control, laminin expressed by neural stem cells was readily detected in all cells. (D) Mannitol analysis. Mannitol peak was present in wild-type neural stem cells but only background was observed for POMT2 KO neural stem cells.
Mutually indispensable nature of POMT1 and POMT2 for protein O-mannosyl transferase activity (Manya et al. 2004) suggests that deficiency of POMT1 or POMT2 alone would result in diminished O-mannose. To evaluate O-mannose levels in POMT2-deficient neural stem cells, we released O-linked carbohydrate by reductive β-elimination and analyzed the reduced sugar alcohols by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD). Although mannitol was readily detected in wild-type neural stem cells, it was not detectable in POMT2-deficient neural stem cells (Figure 1D). These results suggest that protein O-mannosylation is abolished in POMT2-deficient cells.
LARGE overexpression in POMT2-deficient cells generated glycoproteins that bind to laminin and are recognized by IIH6C4 antibody
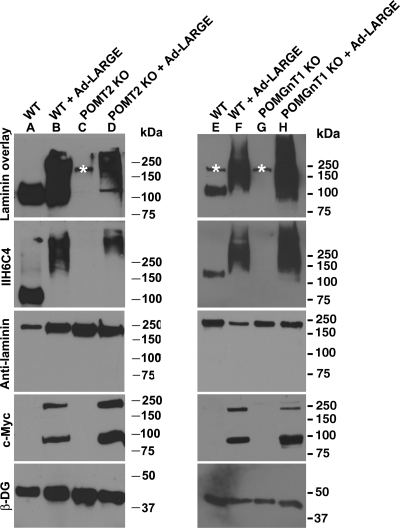
Previous studies suggest that LARGE modifies O-linked mannosyl glycans, complex N-glycans, mucin-type O-glycans of α-DG (Patnaik and Stanley 2005; Aguilan et al. 2009) or participate in the formation of a phosphoryl glycosylation branch on O-linked mannose (Yoshida-Moriguchi et al. 2010). To determine whether LARGE modifies glycans in addition to O-mannosyl glycans in mouse neural stem cells, we overexpressed LARGE in POMT2-deficient neural stem cells. Glycoproteins were isolated by wheat germ agglutin (WGA)-gel and immunoblotted with IIH6C4 antibody, an antibody that recognizes a carbohydrate epitope on α-DG (Figure 2). In wild-type neural stem cells, IIH6C4 immunoreactivity was detected at ∼120 kDa (lanes A and E). Laminin overlay assay indicated laminin binding at the same location. Overexpressing LARGE in wild-type neural stem cells markedly increased IIH6C4 immunoreactivity and laminin-binding activity (lanes B and F). In POMT2 KO neural stem cells, IIH6C4 immunoreactivity was not detectable (lane C). The laminin overlay assay did not show laminin-binding activity. However, overexpressing LARGE in POMT2 KO neural stem cells resulted in IIH6C4 immunoreactivity (lane D). The laminin overlay assay indicated that these IIH6C4 immunoreactive species bound to laminin. Protein O-mannose should be present in POMGnT1 KO cells that can serve as acceptors of LARGE modification. Indeed, LARGE overexpression in POMGnT1 KO cells generated IIH6C4 immunoreactivity as well as laminin binding (lane H). As expected, β-DG immunoreactivity was similar in all samples and c-Myc-tagged LARGE was detected in all samples infected by the Ad-LARGE virus. These results indicate that LARGE overexpression can lead to glycosylation of some proteins in the absence of POMT2-mediated O-mannosyl glycosylation.
Fig. 2.
LARGE overexpression in POMT2 KO neural stem cells generated IIH6C4 immunoreactive proteins capable of laminin binding. POMT2 KO neural stem cells were infected with Ad-LARGE and cultured for 2 days. Glycoproteins were isolated from the cell lysates by WGA-gel and analyzed by western blot with the IIH6C4 antibody and the laminin overlay assay. (A and E) Wild-type neural stem cells show IIH6C4 immunoreactivity and laminin binding at 125 kDa location. (B and F) Overexpression of LARGE in wild-type neural stem cells increased IIH6C4 immunoreactivity and laminin-binding activity at molecular weight ranges higher than 120 kDa. (C) In POMT2 KO neural stem cells, IIH6C4 immunoreactivity and laminin-binding activity could not be detected. (D) LARGE overexpression in POMT2 KO neural stem cells generated IIH6C4 immunoreactivity and laminin-binding activity. (G) POMGnT1 KO exhibit diminished IIH6C4 immunoreactivity and laminin binding. (H) LARGE overexpression in POMGnT1 KO neural stem cells generated IIH6C4 immunoreactivity and laminin-binding activity. Asterisks indicate endogenous laminin that is sometimes detected in the laminin overlay assay. The expected molecular mass of LARGE is 90 kDa. On western blot, a band corresponding to the dimer of LARGE was detected frequently.
LARGE overexpression rescued laminin-binding activity and IIH6C4 immunoreactivity independent of α-DG in POMT2-deficient cells
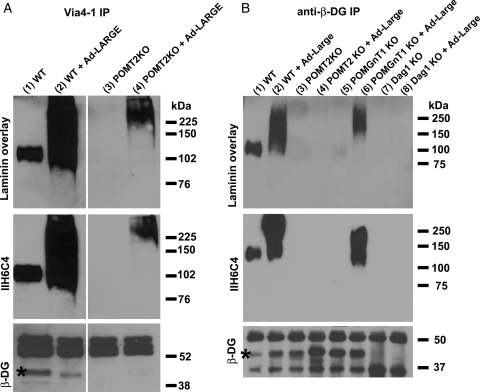
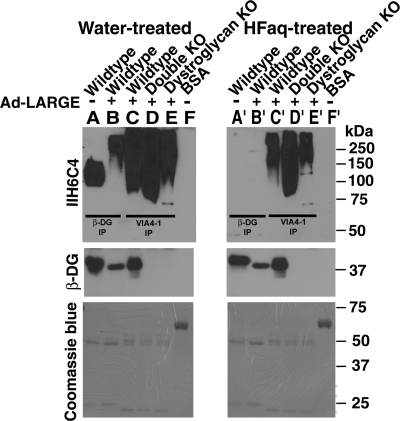
To determine whether the hyperglycosylated proteins in LARGE-overexpressing POMT2 KO neural stem cells are α-DG, we carried out immunoprecipitation assays with VIA4-1, an antibody that recognizes the glycosylated form of α-DG. VIA4-1 immunoprecipitates were blotted with the IIH6C4 antibody, and a β-DG antibody, and overlayed with laminin (Figure 3A). Interestingly, immunoblotting with anti-β-DG showed that β-DG was present in VIA4-1 immunoprecipitates of wild-type samples with and without LARGE overexpression. In contrast, it was not present in VIA4-1 immunoprecipitates of POMT2 KO neural stem cells with or without LARGE overexpression. IIH6C4 immunoreactivity was dramatically increased in wild-type cells overexpressing LARGE. Although IIH6C4 immunoreactivity was not detected in VIA4-1 immunoprecipitates of POMT2 KO cells, it was detected in LARGE- overexpressing POMT2 KO cells. Laminin overlay experiments indicated that IIH6C4 immunoreactive species generated by LARGE overexpression in POMT2 KO cells also bound laminin. These results indicate that LARGE overexpression did not result in addition of carbohydrate epitope that is recognized by IIH6C4 and VIA4-1 to α-DG in POMT2 KO cells.
Fig. 3.
LARGE overexpression in POMT2 KO neural stem cells did not functionally glycosylate α-DG. POMT2 KO neural stem cells were infected with Ad-LARGE and cultured for 2 days. Immunoprecipitation with VIA4-1 (A) and anti-β-DG (B) antibodies was carried out, followed by immunoblot with anti-β-DG and IIH6C4 antibodies and laminin overlay assays. (A, lane 1) VIA4-1 immunoprecipitate of wild-type cells showed immunoreactivity to anti-β-DG (asterisk) and IIH6C4 antibodies and bound to laminin. (A, lane 2) VIA4-1 immunoprecipitate of wild-type cells overexpressing LARGE showed immunoreactivity to anti-β-DG (asterisk) and dramatically increased immunoreactivity to IIH6C4 and laminin-binding activity. (A, lane 3) VIA4-1 immunoprecipitate from POMT2 KO cells showed no immunoreactivity to anti-β-DG and IIH6C4 with no laminin-binding activity. (A, lane 4) Overexpressing LARGE in POMT2 KO cells generated IIH6C4 immunoreactivity at higher molecular weight and laminin-binding activity but showed no β-DG in VIA4-1 immunoprecipitate. (B, lane 1) Anti-β-DG immunoprecipitate from wild-type cells showed IIH6C4 immunoreactivity and laminin-binding activity. (B, lane 2) Overexpressing LARGE in wild-type cells increased IIH6C4 immunoreactivity and laminin-binding activity in anti-β-DG immunoprecipitate. (B, lanes 3 and 4) IIH6C4 immunoreactivity and laminin-binding activity were not detected in anti-β-DG immunoprecipitate from POMT2 KO cells with or without LARGE overexpression. (B, lane 5) Anti-β-DG immunoprecipitate from POMGnT1 KO cells showed very weak IIH6C4 immunoreactivity at ∼70 kDa with very weak laminin-binding activity in this assay. (B, lane 6) Overexpressing LARGE in POMGnT1 KO cells increased IIH6C4 immunoreactivity and laminin binding in anti-β-DG immunoprecipitate. (B, lanes 7 and 8) As a control, anti-β-DG did not precipitate any IIH6C4 immunoreactivity and laminin-binding activity in DG KO cells with or without LARGE overexpression. No β-DG was detected in DG KO cells as expected.
Next, we overexpressed LARGE in wild-type, POMT2 null, and POMGnT1 null neural stem cells and carried out immunoprecipitation with anti-β-DG followed by IIH6C4 immunoblot and laminin overlay assays (Figure 3B). LARGE overexpression increased IIH6C4 immunoreactivity and laminin-binding capacity in both wild-type (lane 2) and POMGnT1 null neural stem cells (lane 6). However, no IIH6C4 immunoreactivity and laminin binding were detected for POMT2 null cells with or without overexpressing LARGE (lanes 3 and 4). As a negative control, no β-DG, IIH6C4 immunoreactivity and laminin-binding activity were detected in immunoprecipitate from DG KO neural stem cells with or without LARGE overexpression (lanes 7 and 8). Thus, the hyperglycosylation of α-DG by LARGE was not observed in POMT2-deficient cells. These results indicate that while LARGE overexpression led to the glycosylation of α-DG in the wild-type and POMGnT1 KO neural stem cells, it did not glycosylate α-DG in POMT2 KO neural stem cells. The IIH6C4 immunoreactive protein species in LARGE-overexpressing POMT2 KO neural stem cells likely represents non-α-DG proteins.
LARGE overexpression in Dag1/POMT2 double KO conferred IIH6C4 immunoreactivity and laminin-binding activity
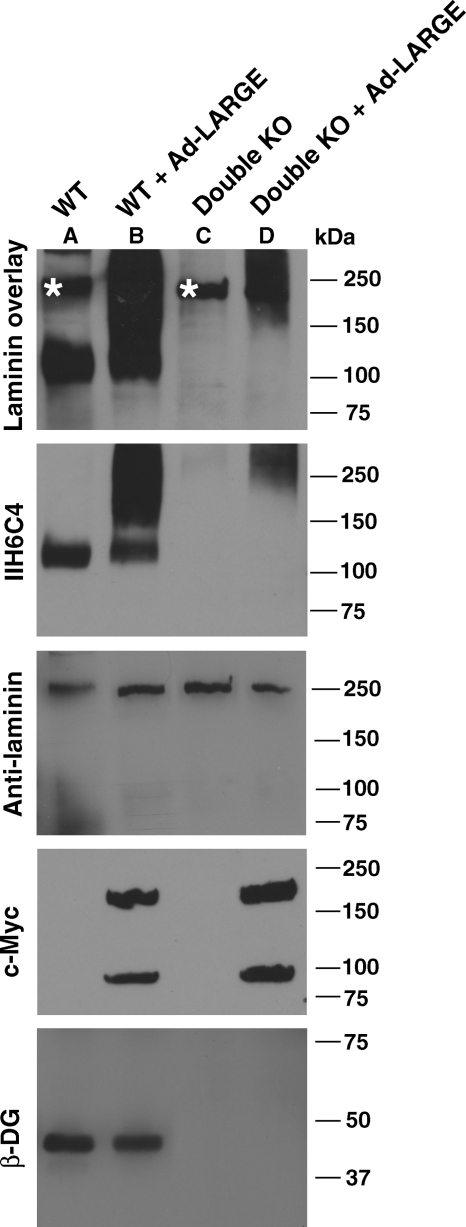
To confirm that LARGE overexpression glycosylated non-α-DG glycoproteins in the absence of O-mannosyl glycans, neural stem cells double deficient in DG and POMT2 (double KO) were generated and infected with Ad-LARGE. IIH6C4 immunoblot and laminin overlay assays were carried out on glycoproteins isolated from the lysate with WGA-gel (Figure 4). In wild-type neural stem cells, overexpressing LARGE increased IIH6C4 immunoreactivity and laminin-binding activity as expected. Although IIH6C4 and laminin-binding activity were not detected in double-KO neural stem cells, LARGE overexpression in these cells generated IIH6C4 immunoreactivity and laminin-binding activity. In laminin overlay assays, the endogenous laminin expressed by neural stem cell was observed in wild type, double KO and double KO with LARGE overexpression, indicating an equivalent amount of glycoproteins isolated by WGA-gel. β-DG immunoblot detected β-DG in wild-type and LARGE-overexpressing wild-type cells, but not in double-KO cells. Thus, overexpressing LARGE in Dag1/POMT2 double-KO cells glycosylated non-α-DG glycoproteins, indicating that LARGE-mediated glycosylation can occur in the absence of O-linked mannose and that this glycosylation can confer both IIH6C4 immunoreactivity and laminin binding to a protein(s) other than α-DG.
Fig. 4.
LARGE overexpression in POMT2/DG double-KO cells generated IIH6C4 immunoreactive proteins capable of laminin binding. POMT2/DG double-KO neural stem cells were infected with Ad-LARGE and cultured for 2 days. Glycoproteins were then isolated by WGA-gel and immunoblotted with anti-β-DG and IIH6C4 and assayed for laminin binding by overlay experiment. (A) Wild-type cells showing IIH6C4 immunoreactivity and laminin-binding activity at ∼120 kDa. (B) Overexpressing LARGE in wild-type cells dramatically increased IIH6C4 immunoreactivity and laminin-binding activity at higher-molecular-weight ranges. (C) No IIH6C4 immunoreactivity and laminin binding could be detected in POMT2/DG double-KO cells. (D) Overexpressing LARGE in double-KO cells generated IIH6C4 immunoreactive species capable of laminin-binding activity. Note that β-DG was detected only in wild-type cells but not double-KO cells. Asterisks indicate endogenous laminin that is sometimes detected in the laminin overlay assay.
LARGE overexpression modified N-glycans of non-α-DG glycoproteins and conferred laminin-binding activity
To identify a possible acceptor of LARGE-mediated glycosylation on non-α-DG glycoproteins, we initially considered N-linked glycans. VIA4-1 immunoprecipitate isolated from lysates of LARGE-overexpressing double-KO cells was treated with peptide N-glycosidase F (PNGase F), a glycosidase that cleaves N-linked glycans (Figure 5, lane C). The activity of PNGase F was evident in the reduced apparent molecular weight of Coomassie blue-stained VIA4-1 heavy chain in all treated samples (compare Figure 5 lanes C with B, E with D, C′ with B′ and E′ with D′, arrow indicates the location of heavy chain). PNGase F treatment significantly reduced IIH6C4 immunoreactivity in VIA4-1 immunoprecipitate from LARGE-overexpressing DG/POMT2 double-KO cells by 68.9% (compare Figure 5 lanes C with B; 95% confidence interval, 48.2–89.6%), indicating the presence of LARGE-modified glycosylation on N-linked glycans. Interestingly, when VIA4-1 immunoprecipitate isolated from DG KO cells overexpressing LARGE was treated with PNGase F, IIH6C4 immunoreactivity was only reduced by 12.4% (compare Figure 5 lanes E with D; 95% confidence interval, 3.7–21%). Together, these results indicate that LARGE can modify N-linked glycans. They also indicate that O-mannosyl glycosylation is present and contributes significantly to LARGE modifications on proteins other than α-DG.
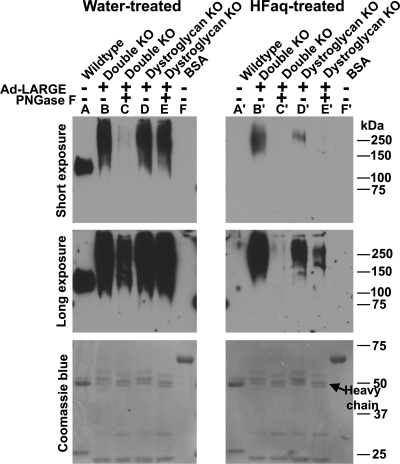
Fig. 5.
IIH6C4 immunoreactivity generated by overexpressing LARGE in POMT2/DG double-KO cells was sensitive to treatment with PNGase F and HFaq. POMT2/DG double-KO neural stem cells were infected with Ad-LARGE. VIA4-1 immunoprecipitate were isolated from the lysates and some were then treated with PNGase F. The samples were divided into equal halves and run in duplicate on SDS–PAGE. After transfer to the PVDF membrane, one set of the samples on PVDF membrane were treated with HFaq. Western blot analysis with IIH6C4 antibody was then carried out. Top panel, short exposure (45 s); middle panel, long exposure (5 min); bottom panel, Coomassie blue staining. (A and A′) Anti-β-DG immunoprecipitates from wild-type neural stem cells. IIH6C4 immunoreactivity on co-immunoprecipitated α-DG was completely abolished by HFaq treatment. (B and B′) VIA4-1 immunoprecipitates from double-KO cells. HFaq treatment dramatically reduced IIH6C4 immunoreactivity. (C and C′) VIA4-1 immunoprecipitates from double-KO cells treated with PNGase F. PNGase F treatment dramatically reduced IIH6C4 immunoreactivity (compare C with B). HFaq treatment further reduced IIH6C4 immunoreactivity (compare C′ with C). (D and D′) VIA4-1 immunoprecipitates from DG KO neural stem cells. HFaq treatment dramatically reduced IIH6C4 immunoreactivity. (E and E′) VIA4-1 immunoprecipitates from DG KO neural stem cells treated with PNGase F. PNGase F treatment reduced IIH6C4 immunoreactivity slightly (compare E with D). However, further HFaq treatment dramatically reduced IIH6C4 immunoreactivity (compare E′ with D′). (F and F′) BSA loading control. HFaq treatment did not change band intensities of BSA and other blotted proteins as revealed by Coomassie blue staining.
At least some LARGE modification on non-α-DG glycoproteins belongs to phosphoryl glycosylation
LARGE participates in the formation of an unidentified phosphoryl glycan onto O-linked mannose of α-DG, which is specifically removed by aqueous hydrofluoric acid (HFaq) treatment (Yoshida-Moriguchi et al. 2010). To determine whether LARGE-mediated glycosylation on non-α-DG glycoproteins also contained phosphoryl glycosylation, we treated VIA4-1 immunoprecipitates isolated from LARGE-overexpressing cells that were blotted onto polyvinylidene fluoride (PVDF) membrane (duplicate of Figure 5 lanes A–F) with HFaq that hydrolyzes a phosphoester bond (Yoshida-Moriguchi et al. 2010; Figure 5, lanes A′–F′). HFaq treatment did not affect the amount of proteins blotted onto the membrane as indicated by the apparent equal intensity of bovine serum albumin (BSA) and heavy and light chains of the antibody by Coomassie blue staining. As a control, HFaq treatment of β-DG immunoprecipitate isolated from wild-type neural stem cells completely abolished IIH6C4 immunoreactivity (compare Figure 5 lanes A with A′) consistent with previous finding that the phosphoryl glycosylation of α-DG was sensitive to HFaq (Yoshida-Moriguchi et al. 2010). VIA4-1 immunoprecipitate isolated from LARGE-overexpressing double-KO cells showed significantly reduced IIH6C4 immunoreactivity when treated with HFaq (77.8% reduction, 95% confidence interval 67.8–87.7%; compare Figure 5 lanes B with B′). However, residual IIH6C4 immunoreactivity remained (Figure 5, lane B′). PNGase F treatment followed by HFaq treatment further reduced IIH6C4 immunoreactivity (compare Figure 5 lanes C′ with C and B′). The IIH6C4 immunoreactivity was barely detectible even after overexposure (5 min of exposure; Figure 5, middle panel). These results suggested that there were N-linked glycans modified by LARGE, which were recognized by IIH6C4 immunoreactivity, some of which were sensitive and some of which were insensitive to HFaq treatment.
HFaq treatment of VIA4-1 immunoprecipitate from LARGE-overexpressing DG-deficient cells significantly reduced IIH6C4 immunoreactivity (91.0% reduction, 95% confidence interval 86.2–95.8%; compare Figure 5 lanes D′ with D). The PNGase F removal of N-linked glycans followed by HFaq treatment further reduced IIH6C4 immunoreactivity (compare Figure 5 lanes E′ with E and D′). The reduction in IIH6C4 immunoreactivity from HFaq treatment on DG-deficient cells was more pronounced than HFaq treatment of double-KO cells (compare Figure 5 lanes D and D′ with B and B′). Further, HFaq treatment reduced IIH6C4 immunoreactivity more strongly than PNGase F treatment (compare Figure 5 lanes D and D′ with D and E). These results indicate that when POMT2 is present, non-α-DG glycoproteins are modified by LARGE acting mostly on O-mannosyl phosphorylated glycans.
N-linked glycans are not required for LARGE overexpression to generate more IIH6C4 immunoreactivity on α-DG
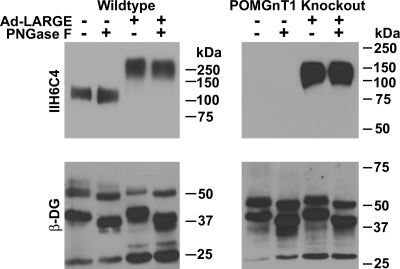
N-linked glycans do not contribute to the laminin-binding activity of α-DG (Ervasti and Campbell 1993). However, PNGase F treatment reduced laminin-binding activity in Chinese hamster ovary (CHO) cells overexpressing LARGE (Patnaik and Stanley 2005), raising the possibility that overexpressing LARGE may generate laminin-binding motifs on N-linked glycans of α-DG. Results shown in Figure 3 indicated that IIH6C4 immunoreactivity and laminin binding were not restored to α-DG in POMT2-KO cells. To further determine whether N-linked glycans of α-DG contribute to its glycosylation by LARGE, we overexpressed LARGE in wild-type and POMGnT1 KO cells and immunoprecipitated DGs with the anti-β-DG antibody. The immunoprecipitates were treated with PNGase F to remove N-linked glycans and immunoblotted with the IIH6C4 antibody (Figure 6). After PNGase F treatment, the molecular weight of β-DG and the heavy chain of mouse IgG were reduced, indicating the effectiveness of N-glycan removal by PNGase F. There is no N-linked glycosylation on the light chain of mouse IgG and thus no change in its migration. In the wild-type, equal IIH6C4 immunoreactivity was observed at ∼120 kDa for both untreated and PNGase F treated. IIH6C4 immunoreactivity was not readily detected in POMGnT1 KO as expected. LARGE overexpression increased IIH6C4 immunoreactivity in both the wild-type and the POMGnT1 KO samples. Similarly, PNGase F treatment did not show noticeable reduction in their IIH6C4 immunoreactivity. These results indicate that IIH6C4 immunoreactivity on α-DG generated by LARGE overexpression is not on N-linked glycans.
Fig. 6.
IIH6C4 immunoreactivity of α-DG generated by LARGE overexpression was not sensitive to PNGase F treatment. Wild-type and POMGnT1 KO neural stem cells were infected with Ad-LARGE virus. Anti-β-DG immunoprecipitates were isolated and immunoblotted with IIH6C4 and anti-β-DG. PNGase F treatment caused a shift in the migration of β-DG and the heavy chain of precipitating antibody, indicating effectiveness of treatment. PNGase treatment did not reduce the IIH6C4 immunoreactivity of α-DG of wild-type cells with or without LARGE overexpression nor α-DG of POMGnT1 KO cells with LARGE overexpression.
HFaq treatment completely removed IIH6C4 immunoreactivity from α-DG in LARGE-overexpressing cells
To examine whether all IIH6C4 immunoreactivity on α-DG caused by LARGE overexpression was from phosphoryl glycans, β-DG immunoprecipitates isolated from LARGE-overexpressing wild-type neural stem cells were treated by HFaq (Figure 7). Without LARGE overexpression, IIH6C4 immunoreactivity was completely removed by HFaq treatment (compare Figure 7 lanes A with A′). LARGE overexpression increased α-DG glycosylation as evidenced by increased apparent molecular mass. After HFaq treatment, IIH6C4 immunoreactivity became undetectable (Figure 7, lanes B and B′). However, HFaq treatment did not completely remove all IIH6C4 immunoreactivity from VIA4-1 immunoprecipitate isolated from LARGE-overexpressing wild-type cells. Again, some residual IIH6C4 immunoreactivity remained in VIA4-1 immunoprecipitates from lysates of LARGE-overexpressing double-KO or DG KO cells after HFaq treatment. Together with the results shown in Figures 3 and 6, it indicates that while LARGE does modify O-linked mannosyl glycans of α-DG by phosphoryl glycosylation, LARGE does not modify the N-linked glycans of α-DG.
Fig. 7.
HFaq treatment completely removed IIH6C4 immunoreactivity on LARGE-hyperglycosylated α-DG. Anti-β-DG or VIA4-1 immunoprecipitates were isolated from the lysates of neural stem cells that were infected with Ad-LARGE and separated by SDS–PAGE. After transfer to the PVDF membrane, the samples were treated with water (A–F) or HFaq (A′–F′). Western blot analyses with IIH6C4 and anti-β-DG were then carried out. (A and A′) β-DG immunoprecipitates from wild-type neural stem cells. (B and B′) β-DG immunoprecipitates from LARGE-overexpressing wild-type neural stem cells. IIH6C4 immunoreactivity on co-immunoprecipitated α-DG was completely abolished by HFaq treatment regarding of the overexpression of LARGE. (C and C′) VIA4-1 immunoprecipitates from LARGE-overexpressing wild-type neural stem cells. (D and D′) VIA4-1 immunoprecipitates from double-KO cells with LARGE overexpression. (E and E′) VIA4-1 immunoprecipitates from DG KO neural stem cells with LARGE overexpression. HFaq treatment dramatically reduced IIH6C4 immunoreactivity but with some residue signals (compare lanes C–E with C′–E′). (F and F′) BSA loading control. In the western blotting with anti-β-DG, signals were detected from wild-type cells (lanes A–C and A′–C′) but not from KO cells (lanes D and E and D′ and E′). HFaq treatment did not change the immunoreactivity of β-DG (compare lanes A–C with A′–C′). HFaq treatment did not change the band intensities of BSA and other blotted proteins as revealed by Coomassie blue staining.
Discussion
To determine whether LARGE differentially glycosylates α-DG and proteins other than α-DG, we overexpressed LARGE in POMT2 KO cells that were deficient in O-mannosyl glycosylation. Our results (summarized in Table I) showed that LARGE expression did not cause functional glycosylation of α-DG to confer laminin binding in the absence of O-mannose. However, IIH6C4 immunoreactivity and laminin-binding activity were readily observed on proteins other than α-DG, suggesting that LARGE-modified glycans other than O-mannosyl glycans on non-α-DG protein species. Additionally, overexpressing LARGE in POMT2 and DG double-deficient neural stem cells resulted in readily detectable IIH6C4 immunoreactivity and laminin-binding activity. These results indicate that LARGE mediates the glycosylation of α-DG and proteins other than α-DG to confer IIH6C4 immunoreactivity and laminin binding by different mechanisms. Although O-mannose is required for α-DG glycosylation by LARGE, it is not required for at least some non-α-DG glycoproteins to be glycosylated by LARGE.
Table I.
IIH6C4 immunoreactivity of anti-β-DG and VIA4-1 immunoprecipitates upon PNGase F or HFaq treatments
| Cell lines | Untreated | PNGase F | HFaq |
|---|---|---|---|
| β-DG IP | |||
| Wild type | Detected | No change | Undetected |
| Wild type + LARGE | Strong | No change | Undetected |
| POMGnT1 KO | Undetected | Undetected | ND |
| POMGnT1 KO + LARGE | Strong | No change | ND |
| VIA4-1 IP | |||
| Wild type + LARGE | Strong | ND | Significant reduction |
| DG KO + LARGE | Strong | Mild reduction | Significant reduction |
| Double KO + LARGE | Detected | Significant reduction | Significant reduction |
KO, knockout; ND, not done.
LARGE has two glycosyltransferase domains, one similar to β-1,3-N-acetylglucosaminyltransferase and the other similar to UDP-glucose:glycoprotein glucosyltransferase (Grewal et al. 2001; Patnaik and Stanley 2005; Aguilan et al. 2009). The precise biochemical activity of LARGE, however, is unknown. Interestingly, overexpressing LARGE generated hyperglycosylated IIH6C4 immunoreactive protein species that binds laminin (Barresi et al. 2004) in cells isolated from not only Largemyd mice but also patients with WWS, MEB and FCMD bearing mutations in POMT1, POMGnT1 and FKTN, respectively. It has been assumed that all the hyperglycosylated protein species in LARGE-overexpressing cells are α-DG (Barresi et al. 2004; Brockington et al. 2005; Fujimura et al. 2005; Patnaik and Stanley 2005; Bao et al. 2009; Hu, Li, Wu, et al. 2011). Since LARGE overexpression in α-DG-deficient cells generates IIH6C4 immunoreactivity with laminin-binding activity (Zhang et al. 2011), the IIH6C4 immunoreactivity detected in LARGE-overexpressing patient cells likely includes glycoproteins other than α-DG.
Three types of glycans have been found on α-DG, O-linked mannosyl glycans, mucin-type O-linked N-acetylgalactosaminyl glycans and N-linked glycans. O-linked mannose can have several different branches: GlcNAc linked through β1,2 linkage catalyzed by POMGnT1 (Yoshida et al. 2001), GlcNAc linked through β1,6 linkages catalyzed by β1,6-N-acetylglucosaminyltransferase IX (Inamori et al. 2003, 2004) and an unidentified phosphoryl glycan linked to carbon-6 via a phosphodiester bond (Yoshida-Moriguchi et al. 2010). The extension of the phosphoryl glycan branch of O-linked mannose on α-DG requires the activity of LARGE (Yoshida-Moriguchi et al. 2010). In the present study, IIH6C4 immunoblot analysis did not reveal any signal in β-DG immunoprecipitates from POMT2 KO cells with or without LARGE overexpression (Figure 3B). Meanwhile, β-DG could not be detected from VIA4-1 immunoprecipitates from POMT2 KO cells with or without LARGE overexpression (Figure 3A). These results indicate that LARGE overexpression in POMT2-deficient neural stem cells did not glycosylate α-DG, supporting that α-DG glycosylation by LARGE requires O-linked mannose. This finding implies that N-linked and mucin O-linked glycans are not sufficient for LARGE to glycosylate α-DG to generate IIH6C4 immunoreactivity. Our finding that PNGase F treatment did not affect the IIH6C4 immunoreactivity and laminin-binding activity of α-DG in LARGE-overexpressing wild-type cells supports the notion that N-linked glycans of α-DG are not involved in LARGE-mediated modification.
Overexpressing LARGE in CHO cells with specific glycosylation mutations suggested that LARGE can modify complex N- and mucin-type O-linked glycans of α-DG, using IIH6C4 immunoreactivity as evidence (Patnaik and Stanley 2005; Aguilan et al. 2009; Hu, Li, Wu, et al. 2011). Given the caveat of IIH6C4 immunoreactivity as a marker of glycosylated α-DG, it is possible that IIH6C4 immunoreactive species upon LARGE overexpression in CHO cells include non-α-DG glycoproteins as well. It is also possible that PNGase F sensitive IIH6C4 immunoreactive species may be of non-α-DG. In this study, IIH6C4 immunoreactivities from LARGE-overexpressing cells deficient in both α-DG and POMT2 were markedly reduced by PNGase F treatment (compare Figure 5 lanes C with B), indicating that at least some of the LARGE-mediated glycosylation on non-α-DG proteins are N-linked. Similarly, since HFaq treatment significantly reduced IIH6C4 immunoreactivity (compare Figure 5 lanes B′ with B and D′ with D), at least some of the LARGE-mediated glycosylation on non-α-DG proteins are phosphoryl glycan. However, since residual IIH6C4 immunoreactivity remains after HFaq treatment of LARGE-overexpressing double-KO and DG KO cells (Figure 5, lanes B′ and D′), it is possible that some LARGE-mediated modifications are not phosphoryl glycosylations on non-α-DG glycoproteins. Interestingly, we found that combined treatments of HFaq and PNGase F further reduced IIH6C4 immunoreactivity when compared with single treatments suggesting that some non-phosphoryl glycans may be on N-glycans of non-α-DG glycoproteins (compare C′ with C and B′ and E′ with E and D′). The presence of the residual IIH6C4 immunoreactivity after double treatment suggest that some LARGE modifications could be on other glycans such as mucin-type O-glycans (Patnaik and Stanley 2005; Aguilan et al. 2009; Hu, Li, Wu, et al. 2011).
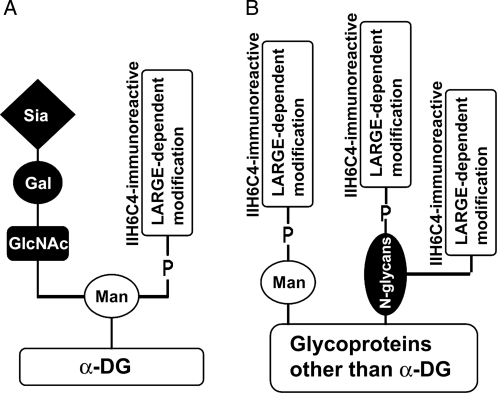
Our results are consistent with a model that hyperglycosylation of α-DG and non-α-DG by LARGE overexpression to generate ligand-binding functional glycan (LARGE-dependent modification) is via different mechanisms (Figure 8). For α-DG, LARGE expression causes the functional phosphoryl glycosylation of only O-linked mannose but not other types of sugar substitutions. With respect to proteins other than α-DG, LARGE expression causes the functional phosphoryl glycosylation of not only O-linked mannose but also N-glycans. Additionally, LARGE may also cause a modification on N-linked glycans of non-α-DG target that is not phosphoester linked. Our study does not address the LARGE glycosylation of mucin-type GalNAc glycosylation which likely occurs on proteins other than α-DG since IIH6C4 immunoreactivity is not recovered on α-DG in LARGE-overexpressing POMT2 KO cells.
Fig. 8.
Summary of differential glycosylation of α-DG and proteins other than α-DG by LARGE. (A) Of α-DG, LARGE-dependent glycosylation only occurs on O-linked mannose and the glycosylation is linked by phosphoester bonds. (B) Of proteins other than α-DG, LARGE-dependent glycosylation occurs on both O-linked mannose and N-linked glycans. Not all LARGE-dependent glycosylation on N-glycans is phosphoester linked. LARGE-dependent glycosylation on mucin-type glycans (Patnaik and Stanley 2005; Aguilan et al. 2009) likely occur on proteins other than α-DG.
In addition to being associated with CMDs, epigenetic silencing of LARGE expression causes loss of laminin-α-DG binding in epithelium-derived cancers (de Bernabe et al. 2009). LARGE forms a complex with β3-N-acetylglucosaminyltransferase 1 and its glycosylation has tumor suppression activity (Bao et al. 2009). Thus, hyperglycosylation by LARGE may have therapeutic potential. It is not known whether the non-α-DG targets of LARGE are of a single protein species or of several proteins. Future studies should be directed at the identification of non-α-DG targets, carbohydrate structure and physiological effects of LARGE-mediated glycosylation.
Materials and methods
Animals
Dag1-floxed mice (Moore et al. 2002; Jackson Laboratories, Bar Harbor, ME) were crossed with Nestin-Cre transgenic mice (Tronche et al. 1999; Jackson Laboratories) to obtain central nervous-specific KO mice of DG (Dag1f/f;Nestin-Cre+). POMT2-floxed mice (Hu, Li, Gagen, et al. 2011) were crossed with Emx1-Cre knock-in mice (Gorski et al. 2002) to obtain brain-specific KO of POMT2 (POMT2f/f;Emx1-Cre+). Triple crosses of Dagl-floxed, POMT2-floxed and Emx1-Cre knock-in mice were carried out to obtain brain-specific double KO of DG and POMT2 (Dag1f/fPOMT2f/f;Emx1-Cre+) mice. Protocols for animal usage were approved by the Institutional Animal Care and Use Committee of Upstate Medical University and adhered to the National Institutes of Health guidelines.
Neural stem cell cultures
To establish the neural stem cell cultures, the neocortical wall of fetuses at embryonic day 13.5 were excised, meninges-removed, cut into small pieces, treated with 0.5% trypsin for 2 min at 37°C and triturated with fire-polished Pasteur pipette. After filtration with a cell strainer, the cells were then grown as neural spheres in neural basal medium (Invitrogen, Carlsbad, CA) supplemented with B27 (minus vitamin A), 20 ng/mL fibroblast growth factor (FGF)-2, 20 ng/mL epidermal growth factor (EGF) and 2 ng/mL heparin.
To isolate clones, the neural stem cell cultures were trypsinized and triturated into individual cell suspensions and diluted to 6 cells/mL in the neural stem cell growth medium. Ten milliliters were seeded into 100 mm fibronectin-coated tissue culture dishes. Fresh FGF-2 and EGF were added once every 3 days. Colonies appeared in about a week and individual colonies were picked with a pipettor and subcultured in a 12-well plate with 1 mL of culture medium for an additional week. The cloned cells were trypsinized and expanded as neural spheres.
To overexpress LARGE in neural stem cells, an adenoviral vector for c-Myc-tagged human LARGE (Ad-LARGE) was constructed at Vector Biolabs (Philadelphia, PA). Twenty microliters (5 × 1012 viral particles/mL) of Ad-LARGE was added to neural stem cells cultured in 150 mm dishes. The cells were harvested 2 days after adenoviral infection for western blot analysis.
Genotyping of clonally expanded neural stem cells
DNA was extracted from each clone. PCR was carried out to confirm the genotypes of the clones. For Dag1 locus, the wild-type primers were GCCTTCCTCTTGACACTGA (forward) and GGACAGTCACTGGCTCATCA (reverse), whereas the KO primers were CGAACACTGAGTTCATCC and CAACTGCTGCATCTCTAC (50). The expected PCR fragment for the wild-type allele was a 217 bp amplicon and for KO a 585 bp amplicon. Primers for POMT2 genotype were CCTCAGATGCTGATCGGTCT and TCATTCCCAT GTAGCTGTGG, which generate a 106 bp amplicon only from wild type but not the truncated POMT2 allele.
RT–PCR analysis
RT–PCR was carried out to determine the expression of DG and POMT2 mRNA in the cloned neural progenitor cells. Total RNA was isolated from 5 × 105 neural stem cells with an RNeasy Micro kit (Qiagen, Valencia, CA). A total of 500 ng of total RNA from each sample was reverse transcribed into cDNA with Quantitech RT kit (Qiagen). RT–PCRs for POMGnT1 (Liu et al. 2006), DG (Moore et al. 2002) or POMT2 KO (Hu, Li, Gagen, et al. 2011) neural stem cells were described elsewhere.
Antibodies
Antibodies were obtained as follows: IIH6C4 and VIA4-1 (Ervasti and Campbell 1993) from Millipore Corporation (Billerica, MA); anti-β-DG (MANDAG2-7D11) from Developmental Studies Hybridoma Bank (Department of Biology, University of Iowa, Iowa City, IO); Rabbit polyclonal antibody against laminin-1 and monoclonal antibodies against c-Myc from Sigma-Aldrich (St Louis, MO).
Western blot analysis and laminin overlay
Cells were washed three times with ice-cold Hank's balanced salt solution and lysed with pre-chilled cell lysis buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl and 1% Triton X-100) with proteinase inhibitor cocktail (Roche). The lysates were centrifuged at 16,000 × g at 4°C for 20 min. The supernatant was collected.
For enrichment of glycoproteins by WGA-agarose, 3 mg of proteins of the total lysate was mixed with 50 μL of WGA-agarose (EY Laboratories, San Mateo, CA). After binding for 16 h, the WGA-gel was precipitated and washed three times with cold washing buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl and 0.1% Triton X-100) containing proteinase inhibitor cocktail. Bound glycoproteins were eluted by SDS–PAGE loading dye and separated on SDS–PAGE and electrotransferred onto PVDF membranes.
For immunoprecipitation with anti-β-DG or VIA4-1, 1.5 mg of total supernatant protein was mixed with affinity purified anti-β-DG (8 μg) or VIA4-1 (5 μg) and incubated for 2 h by rotation at 4°C. Protein G beads (10 μL, Pierce, Rockford, IL) were added and the tube rotated overnight at 4°C. Beads were collected by centrifugation at 500 × g for 4 min at 4°C and washed three times with ice-cold cell washing buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl and 0.1% Triton X-100) containing proteinase inhibitor cocktail. The beads were further mixed with 5× gel loading dye and incubated at 95°C for 5 min. The soluble proteins were separated on 8% SDS–PAGE gels and transferred onto PVDF membranes.
For western blot with IIH6C-4 and anti-β-DG antibodies, standard western blot procedures were carried out. Briefly, the PVDF membrane was incubated with 3% BSA in Tris-buffered saline with Tween-20 (TBST; 50 mM Tris, pH 7.4, 150 mM NaCl, 0.05% Tween-20) for 1 h to block non-specific binding. The membranes were incubated with the primary antibodies in TBST containing 3% BSA overnight. After washing three times with TBST, the membranes were incubated with Goat anti-mouse IgM or IgG conjugated with horseradish peroxidase (1:3000) for 45 min. After extensive washing with TBST, the signal was visualized with SuperSignal west pico chemiluminescent substrate (Thermo Scientific, Rockford, IL).
For laminin overlay assay, the PVDF membrane was incubated with TBS (50 mM Tris, pH 7.4, 150 mM NaCl) containing 3% BSA, 1 mM CaCl2 and 1 mM MgCl2 for an hour to block non-specific binding. The membrane was then incubated with 1.25 µg/mL of laminin-1 (Invitrogen) in TBST containing 1 mM CaCl2 and 1 mM MgCl2 overnight at 4°C. After washing, the detection of bound laminin was by standard western blot procedures in buffers containing 1 mM CaCl2 and 1 mM MgCl2.
Semi-quantitative analysis of western blot
X-ray films from western blot analysis that were not overexposed were scanned with a HP Scanjet 8300 scanner (Hewlett-Packard Company, Palo Alto, CA). A rectangular frame of same size was selected for all samples. The intensity within the selected areas was measured with ImageJ 1.42q (rsb.info.nih.gov/ij/download/). For every experiment, 3–5 repeats were analyzed. Statistical comparison of different samples was carried out by Student's t-test.
Treatment with cold HFaq
To hydrolyze phosphoester linkages, proteins blotted onto PVDF membranes were incubated with ice-cold 48% HFaq (Sigma-Aldrich) at 0°C for 20 h. The membranes were then washed with ice-cold water three times to remove residual HF. Controls were the same glycoprotein preparations that were separated by the same SDS–PAGE gel, electro-transferred to the same piece of PVDF membrane, cut into the middle of the membrane and were then treated with ice cold water instead. Both halves of membrane were then stapled together, stained with Coomassie blue and processed for IIH6C4 immunoblot analysis.
Removal of N-glycans
Removal of N-glycans on glycoproteins bound on WGA- or protein G-agarose beads was carried out by PNGase F treatment (New England Biolabs, Inc., Ipswich, MA) according to the manufacturer's suggestions. Briefly, proteins bound on the agarose beads were denatured with 1× glycoprotein denature buffer by incubation at 95°C for 10 min and quickly chilled on ice. Reaction buffer containing proteinase inhibitor cocktail (Roche) was added. PNGase F (50 units) was added to the mixture and incubated at 37°C for 16 h. Heat-inactivated PNGase F was added to the control samples and incubated likewise.
Mannitol analysis with HPAEC-PAD
Cell lysates were resolved by 10% SDS–PAGE. Gels were then placed in a dialysis bag with a minimum amount of elution buffer (25 mM Tris base, 250 mM glycine, pH 8.3). Proteins were electroeluted from the gel into the elution buffer by at 13.3 V/cm for 90 min. Eluted proteins were concentrated in an Amicon Ultra-15 Centrifugal Filter unit (Millipore Corporation) and buffer-changed to TE (10 mM Tris, pH 8.0, 1 mM ethylenediaminetetraacetic acid). O-linked mannose was analyzed at the Glycobiotechnology Core Resource Center at University of California at San Diego. O-glycans from the proteins were released by incubating the sample with 0.1 M NaOH/1 M NaBH4 at 45°C overnight. To remove peptide, the sample was passed through a Dowex 50 (H+ form) cartridge. The sample was then lyophilized and treated by several rounds of methanol/acetic acid addition followed by drying under a stream of nitrogen. Mannitol was analyzed by HPAEC-PAD with an MA-1 column.
Funding
This work was supported by National Institutes of Health (HD060458 and NS066582) to H.H.
Conflict of interest
None declared.
Abbreviations
BSA, bovine serum albumin; CHO, Chinese hamster ovary; CMD, congenital muscular dystrophy; DG, dystroglycan; EGF, epidermal growth factor; FCMD, Fukuyama congenital muscular dystrophy; FGF, fibroblast growth factor; FKRP, fukutin-related protein; Gal, galactose; GlcNAc, N-acetylglucosamine; HFaq, aqueous hydrofluoric acid; HPAEC-PAD, high-performance anion-exchange chromatography with pulsed amperometric detection; KO, knockout; LARGE, like-glycosyltransferase; MEB, muscle–eye–brain disease; PCR, polymerase chain reaction; PNGase F, peptide N-glycosidase F; POMGnT1, protein O-mannose β1,2 N-acetylglucosaminyltransferase1; POMT, protein O-mannosyltransferase; PVDF, polyvinylidene fluoride; RT, reverse transcription; SDS–PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; Sia, sialic acid; TBST, Tris-buffered saline with Tween-20; WGA, wheat germ agglutinin; WWS, Walker–Warburg syndrome.
Acknowledgements
We thank Drs. Brian Howell, Eric Olson and Mary Lou Vallano for critical reading of the manuscript. β-DG antibody was obtained from the Developmental Studies Hybridoma Bank at the University of Iowa. Mannitol analysis was carried out at the Glycobiotechnology Core Resource at University of California, San Diego.
References
- Aguilan JT, Sundaram S, Nieves E, Stanley P. Mutational and functional analysis of Large in a novel CHO glycosylation mutant. Glycobiology. 2009;19:971–986. doi: 10.1093/glycob/cwp074. doi:10.1093/glycob/cwp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasaka-Manya K, Manya H, Nakajima A, Kawakita M, Endo T. Physical and functional association of human protein O-mannosyltransferases 1 and 2. J Biol Chem. 2006;281:19339–19345. doi: 10.1074/jbc.M601091200. doi:10.1074/jbc.M601091200. [DOI] [PubMed] [Google Scholar]
- Bao X, Kobayashi M, Hatakeyama S, Angata K, Gullberg D, Nakayama J, Fukuda MN, Fukuda M. Tumor suppressor function of laminin-binding α-dystroglycan requires a distinct β3-N-acetylglucosaminyltransferase. Proc Natl Acad Sci USA. 2009;106:12109–12114. doi: 10.1073/pnas.0904515106. doi:10.1073/pnas.0904515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi R, Michele DE, Kanagawa M, Harper HA, Dovico SA, Satz JS, Moore SA, Zhang W, Schachter H, Dumanski JP, et al. LARGE can functionally bypass α-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat Med. 2004;10:696–703. doi: 10.1038/nm1059. doi:10.1038/nm1059. [DOI] [PubMed] [Google Scholar]
- Brockington M, Torelli S, Prandini P, Boito C, Dolatshad NF, Longman C, Brown SC, Muntoni F. Localization and functional analysis of the LARGE family of glycosyltransferases: Significance for muscular dystrophy. Hum Mol Genet. 2005;14:657–665. doi: 10.1093/hmg/ddi062. doi:10.1093/hmg/ddi062. [DOI] [PubMed] [Google Scholar]
- Brockington M, Yuva Y, Prandini P, Brown SC, Torelli S, Benson MA, Herrmann R, Anderson LV, Bashir R, Burgunder JM, et al. Mutations in the fukutin-related protein gene. FKRP. identify limb girdle muscular dystrophy 2I as a milder allelic variant of congenital muscular dystrophy MDC1C. Hum Mol Genet. 2001;10:2851–2859. doi: 10.1093/hmg/10.25.2851. doi:10.1093/hmg/10.25.2851. [DOI] [PubMed] [Google Scholar]
- Chai W, Yuen CT, Kogelberg H, Carruthers RA, Margolis RU, Feizi T, Lawson AM. High prevalence of 2-mono- and 2,6-di-substituted manol-terminating sequences among O-glycans released from brain glycopeptides by reductive alkaline hydrolysis. Eur J Biochem. 1999;263:879–888. doi: 10.1046/j.1432-1327.1999.00572.x. doi:10.1046/j.1432-1327.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- Chiba A, Matsumura K, Yamada H, Inazu T, Shimizu T, Kusunoki S, Kanazawa I, Kobata A, Endo T. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve α-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of α-dystroglycan with laminin. J Biol Chem. 1997;272:2156–2162. doi: 10.1074/jbc.272.4.2156. doi:10.1074/jbc.272.4.2156. [DOI] [PubMed] [Google Scholar]
- Clarke NF, Maugenre S, Vandebrouck A, Urtizberea JA, Willer T, Peat RA, Gray F, Bouchet C, Manya H, Vuillaumier-Barrot S, et al. Congenital muscular dystrophy type 1D (MDC1D) due to a large intragenic insertion/deletion, involving intron 10 of the LARGE gene. Eur J Hum Genet. 2011;19:452–457. doi: 10.1038/ejhg.2010.212. doi:10.1038/ejhg.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier SC, Lee CK, Chang BS, Bodell AL, Pai GS, Job L, Lagae LG, Al-Gazali LI, Eyaid WM, Enns G, et al. Mutations in POMT1 are found in a minority of patients with Walker-Warburg syndrome. Am J Med Genet A. 2005;133:53–57. doi: 10.1002/ajmg.a.30487. [DOI] [PubMed] [Google Scholar]
- de Beltran-Valero BD, Currier S, Steinbrecher A, Celli J, van Beusekom E, van der Zwaag B, Kayserili H, Merlini L, Chitayat D, Dobyns WB, et al. Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet. 2002;71:1033–1043. doi: 10.1086/342975. doi:10.1086/342975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beltran-Valero BD, Voit T, Longman C, Steinbrecher A, Straub V, Yuva Y, Herrmann R, Sperner J, Korenke C, Diesen C, et al. Mutations in the FKRP gene can cause muscle-eye-brain disease and Walker-Warburg syndrome. J Med Genet. 2004;41:e61. doi: 10.1136/jmg.2003.013870. doi:10.1136/jmg.2003.013870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bernabe DB, Inamori K, Yoshida-Moriguchi T, Weydert CJ, Harper HA, Willer T, Henry MD, Campbell KP. Loss of α-dystroglycan laminin binding in epithelium-derived cancers is caused by silencing of LARGE. J Biol Chem. 2009;284:11279–11284. doi: 10.1074/jbc.C900007200. doi:10.1074/jbc.C900007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bernabe DB, van Bokhoven H, van Beusekom E, Van den Akker W, Kant S, Dobyns WB, Cormand B, Currier S, Hamel B, Talim B, et al. A homozygous nonsense mutation in the fukutin gene causes a Walker-Warburg syndrome phenotype. J Med Genet. 2003;40:845–848. doi: 10.1136/jmg.40.11.845. doi:10.1136/jmg.40.11.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. doi:10.1016/0092-8674(91)90035-W. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. doi:10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finne J, Krusius T, Margolis RK, Margolis RU. Novel mannitol-containing oligosaccharides obtained by mild alkaline borohydride treatment of a chondroitin sulfate proteoglycan from brain. J Biol Chem. 1979;254:10295–10300. [PubMed] [Google Scholar]
- Fujimura K, Sawaki H, Sakai T, Hiruma T, Nakanishi N, Sato T, Ohkura T, Narimatsu H. LARGE2 facilitates the maturation of α-dystroglycan more effectively than LARGE. Biochem Biophys Res Commun. 2005;329:1162–1171. doi: 10.1016/j.bbrc.2005.02.082. doi:10.1016/j.bbrc.2005.02.082. [DOI] [PubMed] [Google Scholar]
- Gee SH, Blacher RW, Douville PJ, Provost PR, Yurchenco PD, Carbonetto S. Laminin-binding protein 120 from brain is closely related to the dystrophin-associated glycoprotein, dystroglycan, and binds with high affinity to the major heparin binding domain of laminin. J Biol Chem. 1993;268:14972–14980. [PubMed] [Google Scholar]
- Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Dystroglycan-α, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994;77:675–686. doi: 10.1016/0092-8674(94)90052-3. doi:10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal PK, Holzfeind PJ, Bittner RE, Hewitt JE. Mutant glycosyltransferase and altered glycosylation of α-dystroglycan in the myodystrophy mouse. Nat Genet. 2001;28:151–154. doi: 10.1038/88865. doi:10.1038/88865. [DOI] [PubMed] [Google Scholar]
- Grewal PK, McLaughlan JM, Moore CJ, Browning CA, Hewitt JE. Characterization of the LARGE family of putative glycosyltransferases associated with dystroglycanopathies. Glycobiology. 2005;15:912–923. doi: 10.1093/glycob/cwi094. doi:10.1093/glycob/cwi094. [DOI] [PubMed] [Google Scholar]
- Holzfeind PJ, Grewal PK, Reitsamer HA, Kechvar J, Lassmann H, Hoeger H, Hewitt JE, Bittner RE. Skeletal, cardiac and tongue muscle pathology, defective retinal transmission, and neuronal migration defects in the Largemyd mouse defines a natural model for glycosylation-deficient muscle-eye-brain disorders. Hum Mol Genet. 2002;11:2673–2687. doi: 10.1093/hmg/11.21.2673. doi:10.1093/hmg/11.21.2673. [DOI] [PubMed] [Google Scholar]
- Hu H, Li J, Gagen CS, Gray NW, Zhang Z, Qi Y, Zhang P. Conditional knockout of protein O-mannosyltransferase 2 reveals tissue specific roles of O-mannosyl glycosylation in brain development. J Comp Neurol. 2011;519:1320–1337. doi: 10.1002/cne.22572. doi:10.1002/cne.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Li ZF, Wu X, Lu Q. Large induces functional glycans in an O-mannosylation dependent manner and targets GlcNAc terminals on alpha-dystroglycan. PLoS One. 2011;6:e16866. doi: 10.1371/journal.pone.0016866. doi:10.1371/journal.pone.0016866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Li J, Zhang Z, Yu M. Pikachurin interaction with dystroglycan is diminished by defective O-mannosyl glycosylation in congenital muscular dystrophy models and rescued by LARGE overexpression. Neuros Lett. 2011;489:10–15. doi: 10.1016/j.neulet.2010.11.056. doi:10.1016/j.neulet.2010.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. doi:10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Inamori K, Endo T, Gu J, Matsuo I, Ito Y, Fujii S, Iwasaki H, Narimatsu H, Miyoshi E, Honke K, et al. N-Acetylglucosaminyltransferase IX acts on the GlcNAcβ1,2-Manα1-Ser/Thr moiety, forming a 2,6-branched structure in brain O-mannosyl glycan. J Biol Chem. 2004;279:2337–2340. doi: 10.1074/jbc.C300480200. doi:10.1074/jbc.C300480200. [DOI] [PubMed] [Google Scholar]
- Inamori K, Endo T, Ide Y, Fujii S, Gu J, Honke K, Taniguchi N. Molecular cloning and characterization of human GnT-IX, a novel β1,6-N-acetylglucosaminyltransferase that is specifically expressed in the brain. J Biol Chem. 2003;278:43102–43109. doi: 10.1074/jbc.M308255200. doi:10.1074/jbc.M308255200. [DOI] [PubMed] [Google Scholar]
- Kanagawa M, Omori Y, Sato S, Kobayashi K, Miyagoe-Suzuki Y, Takeda S, Endo T, Furukawa T, Toda T. Post-translational maturation of dystroglycan is necessary for pikachurin binding and ribbon synaptic localization. J Biol Chem. 2010;285:31208–31216. doi: 10.1074/jbc.M110.116343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano H, Kobayashi K, Herrmann R, Tachikawa M, Manya H, Nishino I, Nonaka I, Straub V, Talim B, Voit T, et al. Deficiency of α-dystroglycan in muscle–eye–brain disease. Biochem Biophys Res Commun. 2002;291:1283–1286. doi: 10.1006/bbrc.2002.6608. doi:10.1006/bbrc.2002.6608. [DOI] [PubMed] [Google Scholar]
- Kim DS, Hayashi YK, Matsumoto H, Ogawa M, Noguchi S, Murakami N, Sakuta R, Mochizuki M, Michele DE, Campbell KP, et al. POMT1 mutation results in defective glycosylation and loss of laminin-binding activity in α-DG. Neurology. 2004;62:1009–1011. doi: 10.1212/01.wnl.0000115386.28769.65. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Nakahori Y, Miyake M, Matsumura K, Kondo-Iida E, Nomura Y, Segawa M, Yoshioka M, Saito K, Osawa M, et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 1998;394:388–392. doi: 10.1038/28653. doi:10.1038/28653. [DOI] [PubMed] [Google Scholar]
- Kogelberg H, Chai W, Feizi T, Lawson AM. NMR studies of mannitol-terminating oligosaccharides derived by reductive alkaline hydrolysis from brain glycoproteins. Carbohydr Res. 2001;331:393–401. doi: 10.1016/s0008-6215(01)00051-9. doi:10.1016/S0008-6215(01)00051-9. [DOI] [PubMed] [Google Scholar]
- Krusius T, Finne J, Margolis RK, Margolis RU. Identification of an O-glycosidic mannose-linked sialylated tetrasaccharide and keratan sulfate oligosaccharides in the chondroitin sulfate proteoglycan of brain. J Biol Chem. 1986;261:8237–8242. [PubMed] [Google Scholar]
- Liu J, Ball SL, Yang Y, Mei P, Zhang L, Shi H, Kaminski HJ, Lemmon VP, Hu H. A genetic model for muscle–eye–brain disease in mice lacking protein O-mannose β1,2-N-acetylglucosaminyltransferase (POMGnT1) Mech Dev. 2006;123:228–240. doi: 10.1016/j.mod.2005.12.003. doi:10.1016/j.mod.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Longman C, Brockington M, Torelli S, Jimenez-Mallebrera C, Kennedy C, Khalil N, Feng L, Saran RK, Voit T, Merlini L, et al. Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of α-dystroglycan. Hum Mol Genet. 2003;12:2853–2861. doi: 10.1093/hmg/ddg307. doi:10.1093/hmg/ddg307. [DOI] [PubMed] [Google Scholar]
- Manya H, Chiba A, Yoshida A, Wang X, Chiba Y, Jigami Y, Margolis RU, Endo T. Demonstration of mammalian protein O-mannosyltransferase activity: Coexpression of POMT1 and POMT2 required for enzymatic activity. Proc Natl Acad Sci USA. 2004;101:500–505. doi: 10.1073/pnas.0307228101. doi:10.1073/pnas.0307228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. doi:10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Montanaro F, Lindenbaum M, Carbonetto S. α-Dystroglycan is a laminin receptor involved in extracellular matrix assembly on myotubes and muscle cell viability. J Cell Biol. 1999;145:1325–1340. doi: 10.1083/jcb.145.6.1325. doi:10.1083/jcb.145.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, et al. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–425. doi: 10.1038/nature00838. doi:10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- Patnaik SK, Stanley P. Mouse large can modify complex N- and mucin O-glycans on α-dystroglycan to induce laminin binding. J Biol Chem. 2005;280:20851–20859. doi: 10.1074/jbc.M500069200. doi:10.1074/jbc.M500069200. [DOI] [PubMed] [Google Scholar]
- Peng HB, Ali AA, Daggett DF, Rauvala H, Hassell JR, Smalheiser NR. The relationship between perlecan and dystroglycan and its implication in the formation of the neuromuscular junction. Cell Adhes Commun. 1998;5:475–489. doi: 10.3109/15419069809005605. doi:10.3109/15419069809005605. [DOI] [PubMed] [Google Scholar]
- Peyrard M, Seroussi E, Sandberg-Nordqvist AC, Xie YG, Han FY, Fransson I, Collins J, Dunham I, Kost-Alimova M, Imreh S, et al. The human LARGE gene from 22q12.3-q13.1 is a new, distinct member of the glycosyltransferase gene family. Proc Natl Acad Sci USA. 1999;96:598–603. doi: 10.1073/pnas.96.2.598. doi:10.1073/pnas.96.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Yamada H, Matsumura K, Shimizu T, Kobata A, Endo T. Detection of O-mannosyl glycans in rabbit skeletal muscle α-dystroglycan. Biochim Biophys Acta. 1998;1425:599–606. doi: 10.1016/s0304-4165(98)00114-7. [DOI] [PubMed] [Google Scholar]
- Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, et al. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci. 2008;11:923–931. doi: 10.1038/nn.2160. doi:10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR, Haslam SM, Sutton-Smith M, Morris HR, Dell A. Structural analysis of sequences O-linked to mannose reveals a novel Lewis X structure in Cranin (dystroglycan) purified from sheep brain. J Biol Chem. 1998;273:23698–23703. doi: 10.1074/jbc.273.37.23698. doi:10.1074/jbc.273.37.23698. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR, Kim E. Purification of cranin, a laminin binding membrane protein. Identity with dystroglycan and reassessment of its carbohydrate moieties. J Biol Chem. 1995;270:15425–15433. doi: 10.1074/jbc.270.25.15425. doi:10.1074/jbc.270.25.15425. [DOI] [PubMed] [Google Scholar]
- Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. doi:10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Kondo M, Sasaki J, Kurahashi H, Kano H, Arai K, Misaki K, Fukui T, Kobayashi K, Tachikawa M, et al. Fukutin is required for maintenance of muscle integrity, cortical histiogenesis and normal eye development. Hum Mol Genet. 2003;12:1449–1459. doi: 10.1093/hmg/ddg153. doi:10.1093/hmg/ddg153. [DOI] [PubMed] [Google Scholar]
- Talts JF, Andac Z, Gohring W, Brancaccio A, Timpl R. Binding of the G domains of laminin α1 and α2 chains and perlecan to heparin, sulfatides, α-dystroglycan and several extracellular matrix proteins. EMBO J. 1999;18:863–870. doi: 10.1093/emboj/18.4.863. doi:10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. doi:10.1038/12703. [DOI] [PubMed] [Google Scholar]
- van Reeuwijk J, Grewal PK, Salih MA, de Beltran-Valero BD, McLaughlan JM, Michielse CB, Herrmann R, Hewitt JE, Steinbrecher A, Seidahmed MZ, et al. Intragenic deletion in the LARGE gene causes Walker-Warburg syndrome. Hum Genet. 2007;121:685–690. doi: 10.1007/s00439-007-0362-y. doi:10.1007/s00439-007-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reeuwijk J, Janssen M, van den Elzen C, de Beltran-Valero BD, Sabatelli P, Merlini L, Boon M, Scheffer H, Brockington M, Muntoni F, et al. POMT2 mutations cause α-dystroglycan hypoglycosylation and Walker-Warburg syndrome. J Med Genet. 2005;42:907–912. doi: 10.1136/jmg.2005.031963. doi:10.1136/jmg.2005.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillaumier-Barrot S, Bouchet-Seraphin C, Chelbi M, Eude-Caye A, Charluteau E, Besson C, Quentin S, Devisme L, Le BC, Landrieu P, et al. Intragenic rearrangements in LARGE and POMGNT1 genes in severe dystroglycanopathies. Neuromuscul Disord. 2011 doi: 10.1016/j.nmd.2011.06.001. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Winder SJ. The complexities of dystroglycan. Trends Biochem Sci. 2001;26:118–124. doi: 10.1016/s0968-0004(00)01731-x. doi:10.1016/S0968-0004(00)01731-X. [DOI] [PubMed] [Google Scholar]
- Yamada H, Denzer AJ, Hori H, Tanaka T, Anderson LV, Fujita S, Fukuta-Ohi H, Shimizu T, Ruegg MA, Matsumura K. Dystroglycan is a dual receptor for agrin and laminin-2 in Schwann cell membrane. J Biol Chem. 1996;271:23418–23423. doi: 10.1074/jbc.271.38.23418. doi:10.1074/jbc.271.38.23418. [DOI] [PubMed] [Google Scholar]
- Yamada H, Shimizu T, Tanaka T, Campbell KP, Matsumura K. Dystroglycan is a binding protein of laminin and merosin in peripheral nerve. FEBS Lett. 1994;352:49–53. doi: 10.1016/0014-5793(94)00917-1. doi:10.1016/0014-5793(94)00917-1. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, Inazu T, Mitsuhashi H, Takahashi S, Takeuchi M, et al. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell. 2001;1:717–724. doi: 10.1016/s1534-5807(01)00070-3. doi:10.1016/S1534-5807(01)00070-3. [DOI] [PubMed] [Google Scholar]
- Yoshida-Moriguchi T, Yu L, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MB, Schachter H, Wells L, Campbell KP. O-mannosyl phosphorylation of alpha-dystroglycan is required for laminin binding. Science. 2010;327:88–92. doi: 10.1126/science.1180512. doi:10.1126/science.1180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Betel D, Schachter H. Cloning and expression of a novel UDP-GlcNAc:α-d-mannoside β1,2-N-acetylglucosaminyltransferase homologous to UDP-GlcNAc:α-3-d-mannoside β1,2-N-acetylglucosaminyltransferase I. Biochem J. 2002;361:153–162. doi: 10.1042/0264-6021:3610153. doi:10.1042/0264-6021:3610153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang P, Hu H. LARGE expression augments the glycosylation of glycoproteins in addition to α-dystroglycan conferring laminin binding. PLoS One. 2011;6:e19080. doi: 10.1371/journal.pone.0019080. doi:10.1371/journal.pone.0019080. [DOI] [PMC free article] [PubMed] [Google Scholar]