Abstract
Primary ciliary dyskinesia (PCD) is a rare genetic condition that causes impaired mucociliary clearance due to poorly functioning cilia. PCD is one disease manifestation of the many recently recognized associations with ciliary malfunction, referred to as “ciliopathies.” Manifestations of PCD commonly begin in the neonatal period with cough, pneumonia, and chronic ear infections or effusions. Approximately half of the affected individuals have situs inversus totalis. The diagnosis is often made in later childhood or early adulthood, because symptoms mimic more common childhood illnesses and because the definitive diagnosis of PCD can be challenging. Treatment recommendations are largely based on therapies used for other conditions with impaired mucociliary clearance in the absence of evidence-based research specific for PCD. Early recognition and initiation of both otolaryngologic and pulmonary management might reduce potential long-term morbidities. The purpose of this article is to update primary care providers, allergists, and pediatric pulmonologists on recent advances in this interesting condition.
Introduction
Although the association of bronchiectasis, sinusitis, and dextrocardia with situs inversus had been recognized for over a century, that constellation of findings has been referred to as Kartagener's syndrome since Professor Kartagener's English language publication in 1962.1 During the evaluation of spermatozoa from infertile men by electron microscopy, Afzelius identified ultrastuctural abnormalities in the cilia, thereby accounting for poor motility; some of these men also had chronic bronchitis and bronchiectasis.2 Many of these men had similar ultrastructural abnormalities in their respiratory cilia.3 They coined the term “immotile cilia syndrome.”3 This provided the unifying concept that ciliary ultrastructural defects lead to dysfunctional or poorly motile cilia, which, in turn, result in abnormal mucociliary clearance, chronic sinopulmonary disease, and male infertility. They postulated that normal ciliary function is required to determine the laterality of the heart and abdominal contents during fetal development; in the absence of ciliary motility, the laterality occurs randomly. Poor function of the nodal cilia during embryonic development explains why dextrocardia and situs inversus occur in approximately half of the patients in whom primary ciliary dyskinesia (PCD) is identified.3 Subsequent evaluations documented that the cilia in these patients may not be immotile, but rather have both a reduced ciliary beat frequency and an abnormal wave form causing impaired mucociliary clearance.4 Therefore, a more encompassing label is PCD.4 PCD is estimated to occur in about 1 in every 20–60,000 individuals in the United States, although this may be an underestimate in the general population.5
Physiology and Cell Biology of Cilia
Flagella and cilia are present in a variety of species from simple single-cell organisms, such as Chlamydomonas, to very complex sensory organs, such as the retina and cochlea.6 The clinical manifestations of PCD can be understood with a basic knowledge of the normal distribution and function of cilia in humans. Ciliary ultrastructure is remarkably consistent throughout nature with the characteristic “9+2” microtubule pattern demonstrating 9 microtubular doublets in a ring surrounding 2 central microtubules7 (Fig. 1). The 9+2 microtubular architecture in healthy cilia is maintained by intertubular connections and accessory proteins. Nexin links connect the outer microtubular doubles, and radial spokes connect the outer doublets to the central microtubular pair. Dyneins are motor protein complexes that are attached to outer microtubules as distinct inner and outer dynein arms. The central pair extends from the basal body at the cell surface to just proximal to the tip of the cilium. Primary cilia, found in sensory organs, are usually nonmotile and lack the central pair and dynein arms.7
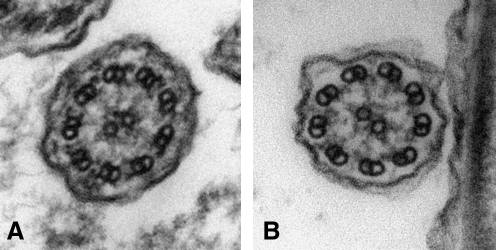
FIG. 1.
Cilia ultrastructure observed by transmission electron microscopy. (A) Normal cilium cross-section demonstrating the presence of all axonemal components including 9+2 microtubular arrangement, inner and outer dynein arms, and radial spokes. (B) abnormal cilium architecture with missing outer dynein arms (magnification 80,000×).
In humans, cilia can be motile or immotile depending on their location and intended function.6,7 Motile cilia and ciliated epithelia are found in the upper respiratory tract (middle ear mucosa, nasal passages, paranasal sinuses, Eustachian tubes, and nasopharynx), the lower respiratory tract (trachea, bronchi down to the respiratory bronchioles), the ependymal lining of the brain, and reproductive tract of both men (ductules emanating from the testis and epididymus) and women (Fallopian tubes, endometrial lining of the cervix).7 Cilia in the respiratory tract are a key component of effective mucociliary clearance. Each respiratory epithelial cell has approximately 200 cilia projecting from its surface. The cilia of a particular cell and the cilia from adjacent cells beat in a coordinated fashion to propel mucus toward the oropharynx. The normal beat frequency is 10–12 beats per second. The wave form is similar to the arm stroke of a swimmer doing the crawl stroke; during the effective phase, the cilia are fully extended, and during the recovery phase, they are bent and closer to the cell surface. Having an adequate number of cilia, the correct beat frequency, appropriate wave form, and intercellular coordination are all necessary for effective mucociliary clearance.8
More than 200 proteins make up the ciliary ultrastructure. The outer and inner dynein arms are composed of ATPase and provide the energy for motility. The inner doublets, radial spokes, and nexin arms are made of tubulin and provide the structural stability for motility in a specified direction.7 Similar to other chronic respiratory diseases where several different mutations lead to abnormal proteins producing a similar, but variable, phenotype (eg, cystic fibrosis, alpha-1-antitrypsin deficiency), mutations in several ciliary proteins have been associated with the clinical features of PCD. Dynein arm deficiency is the most commonly identified abnormality and the defect associated with the most severely disrupted motility, often being completely immotile7 (Table 1). The details of specific protein deficiencies are provided below.
Table 1.
Electron Microscopic Findings in Primary Ciliary Dyskinesia Versus Acquired Cilia Abnormality
| PCD | Acquired defects | |
|---|---|---|
| EM ultrastructure | Dynein arm deficiency | Compound cilia |
| Outer arms | Added peripheral tubules | |
| Inner arms | Deleted peripheral tubules | |
| Both | Added central pairs | |
| Translocation of central tubules | Translocation of central tubules | |
| Aplasia (generalized) | Aplasia (patchy) | |
| Beat Frequency | Slow or absent | May be normal or reduced |
| Wave form | Abnormal | May be normal or abnormal |
EM, electron microscopy; PCD, primary ciliary dyskinesia.
Clinical Manifestations of PCD
The symptoms of PCD emanate in organs where ciliary motility is an important component of normal function. The respiratory tract, where cilia are present from the middle ear to the conducting bronchioles, is universally affected. Upper and lower respiratory tract manifestations are cardinal features of PCD and often present shortly after birth.8,9 Many patients present in the neonatal period with respiratory distress characterized by chest congestion, coughing, tachypnea, and hypoxia; infrequently respiratory failure occurs that is generally attributed to “wet lungs,” neonatal pneumonia, or transient tachypnea of the newborn.9,10 This suggests that cilia play a critical role in clearing fetal lung fluid after birth. If a chest radiograph obtained in the newborn period reveals dextrocardia and/or situs inversus, then PCD should be considered as an explanation of these abnormalities.
Upper respiratory tract problems include chronic year-around nasal drainage that may begin in the first weeks of life, chronic sinusitis, and chronic serous otitis media. If myringotomy tubes are placed, then chronic otorrhea often ensues. These children commonly have associated conductive hearing loss with chronic middle ear effusion and recurrent otitis media.11 Chronic and recurrent sinusitis is a common feature in older children and adults.10 Lower respiratory tract features include chronic productive cough, chronic and recurrent bronchitis, and recurrent pneumonia. They are at a risk to develop bronchiectasis. Situs inversus totalis, a unique feature in Kartagener's triad, occurs in approximately 50% of patients with PCD, but not all patients with dextrocardia and situs inversus will have ciliary dyskinesia. A recent study reported that thoracic and abdominal heterotaxy (situs ambiguous) associated with complex congenital heart disease occurs in about 5% of cases with PCD.12 Unlike cystic fibrosis (CF), steatorrhea and growth failure are not characteristics of PCD.
Diagnosis of PCD is frequently delayed, because the presenting symptoms (rhinitis, otitis media, cough, and recurrent bronchitis) are common in otherwise healthy children without underlying medical problems. The distinguishing symptoms and clinical features of PCD by age groups, listed in Table 2, should prompt the clinician to suspect PCD and consider further investigation.
Table 2.
Distinguishing Symptoms and Features of Primary Ciliary Dyskinesia by Age Group
| Neonates and infants |
| Neonatal respiratory distress, especially in term infants, with no obvious cause (tachypnea, “wet lung,” atelectasis) |
| Rhinitis–neonatal onset, persistent (“born with a cold”) |
| Situs inversus totalis |
| Heterotaxy syndromes including congenital heart disease |
| Infants and older children |
| Chronic wet cough, usually with sputum production |
| Middle lobe atelectasis and bronchiectasis |
| Chronic secretory otitis media |
| Persistent otorrhea after tympanostomy tube insertion |
| Conductive hearing loss |
| Chronic pansinusitis |
| Nasal polyposis |
| Older children and adults |
| Non-CF or “idiopathic” bronchiectasis |
| Chronic mucopurulent sputum production |
| Presence of lower airway Pseudomonas aeruginosa or nontuberculous mycobacteria |
| Male infertility due to spermatozoa immotility |
| Ectopic pregnancy and decreased fertility in females |
| Chronic pansinusitis |
CF, cystic fibrosis.
Abnormal physical examination findings frequently encountered in the ears include middle ear fluid, otorrhea, and tympanic membrane scarring or perforation. Abnormalities in the nasal passages include mucosal congestion, edema, mucoid or mucopurulent drainage, and occasionally polyps. Abnormal chest auscultation findings can be variable and may not be present early in life. Some children will have intermittent wheezing, coarse rhonchi, and/or crackles. For those with situs inversus, the heart tones will be heard loudest over the right hemithorax, and the liver edge may be palpable on the left side instead of the right. Digital clubbing may be apparent in older individuals with bronchiectasis, but this occurs less frequently than in patients with CF.
Lung function tests may be normal during early childhood, but many children will have varying degrees of airflow obstruction later in life.10,13,14 Limited longitudinal data in PCD reveals that lung function remains relatively stable in a significant percentage of individuals but can certainly decline over time,13–16 although at a slower rate than it occurs with CF.10 Radiographic abnormalities of PCD include peribronchial thickening, atelectasis, and airtrapping that may eventually lead to bronchiectasis.14,17 These findings typically occur in the middle and lower lobes, which can be a distinguishing feature from CF, where abnormalities tend to be more evident in the upper lobes. Densely opacified segmental or lobar atelectasis with associated bronchiectasis in the middle lobe seems to be a common occurrence in childhood.10
Chronic airway infection begins in early childhood and is believed to be a leading cause of morbidity and mortality in PCD. The most common bacteria isolated in respiratory cultures from children and adolescents with PCD are oropharyngeal flora, nontypeable Haemophilus influenza, Staphylococcus aureus, and Streptococcus pneumoniae. Older patients with more advanced lung disease have a higher incidence of infection with Pseudomonas aeruginosa and nontuberculous mycobacteria.10
Diagnosing PCD
The diagnosis of PCD is based on the presence of a typical clinical phenotype combined with identification of ciliary dysmotility and/or specific ciliary ultrastructural defects.18,19 Since the specialized techniques and expertise required to make the diagnosis are neither standardized nor readily available, it has been suggested that patients suspected of having PCD be referred to tertiary diagnostic centers.19
Screening Tests
Nasal nitric oxide measurements
Several studies have shown that nasal nitric oxide (nNO) levels are extremely low in PCD,10,18,19 although not in all patients.20 There is little overlap between nNO in patients with PCD and other lung diseases, thus suggesting that nNO measurement may be a useful screening test for PCD in children older than 5 years of age.21 A normal or high nNO value can help exclude PCD, especially in patients whose history is not strongly suggestive. However, low nNO is not diagnostic of PCD, as low levels have been reported in other conditions with overlapping clinical features, including CF.10 As discussed in an editorial, the explanation for the low nNO in PCD is not yet clear, but it may be due, in part, to reduced nitric oxide synthase.21 Further, what role a low nNO may play in the pathobiology or infectious risk in PCD is speculative.21 Therefore, confirmation of the diagnosis of PCD by ciliary ultrastructure analysis and/or genetic mutation analysis is still required. Ongoing efforts are being made to standardize nNO measurements and define its utility in children less than 5 years of age. nNO measurements are currently being performed by using research quality procedures in specialty diagnostic and research centers.19
Other screening tests
Saccharin testing as a measurement of mucociliary clearance has been advocated as a diagnostic tool.22 This test can be difficult to perform and may be unreliable in younger children. It is no longer being performed at most PCD specialty diagnostic centers. Research is ongoing in to the utility of radioaerosol mucociliary clearance studies, which may eventually prove to be an adjunctive diagnostic test for PCD.16
Definitive Diagnostic Tests
Assessing the ultrastructure and function of ciliated epithelium has been the primary means to diagnose PCD. Ciliated epithelial cells can be obtained either from the nose or trachea, either by brushings or mucosal biopsies. A few studies have examined the diagnostic yields and success rates of different sampling techniques.23,24 Experience plays an important role in the success rates, as sites performing the highest volume of biopsies have the highest proportion of interpretable specimens and may reach a success rate as high as 88%.23
Ciliary ultrastructure
Examination of ciliary ultrastructure by electron microscopy remains the traditionally accepted diagnostic test for PCD. The most common ultrastructural defect is absent or shortened outer dynein arms. Other identified abnormalities include absent or shortened inner dynein arms, combined defects of both the outer and inner dynein arms, central apparatus abnormalities including loss of radial spokes or transpositions, and combined defects of the inner dynein arms and central apparatus.7,10,23 Ciliary aplasia, or complete absence of cilia, has also been described.25
It can be challenging to obtain an adequate biopsy specimen with a sufficient number of cilia that are technically satisfactory for accurate interpretation. Significant expertise is required to produce high-quality transmission electron micrographs, which are adequate to distinguish between primary (“genetic”) and secondary (“nonspecific”) defects in ciliary ultrastructure26 (Table 1). Inner dynein arms, in particular, are difficult to visualize, as they are less electron dense and occur less frequently along the ciliary axoneme. Further, dynein arm composition varies along the ciliary length, such that ultrastructural defects can be missed, depending on the site of the ciliary cross-section. Therefore, a precise diagnosis can be mistaken or uncertain. If the diagnosis remains in doubt, then repeat biopsies should be performed at another time after treatment of infection and inflammation.
Normal ultrastructure, in the absence of abnormal ciliary function studies, does not exclude PCD as a diagnostic consideration. Disease-causing mutations in the DNAH11 gene have been identified in individuals with a compatible clinical phenotype who have normal ultrastructure, but abnormal ciliary function.27 Therefore, it is possible that individuals with normal ciliary ultrastructure, who have a classic clinical phenotype and low nNO levels, may, in fact, have disease causing mutations in DNAH11 or in genes yet to be determined.18,19
Ciliary motility
Visualization of ciliary beat frequency by simple light microscopy has been used as a screening test for PCD. Although this approach may identify markedly diminished or absent beat frequency, ciliary beating may appear normal in some cases of PCD.19,27 Therefore, the use of conventional light microscopic assessment of ciliary motility should be discouraged as a screening technique. With recent advances in high-speed phase-contrast video imaging, analysis of ciliary wave motion and beat frequency by digital high-speed videomicroscopy is now being used as part of routine diagnostic testing at some European centers.19,28 Abnormalities in ciliary wave form have been related to specific ultrastructural defects.4 However, due to lack of standardization in measurements, potentially subjective interpretation, the need for specialized equipment, and the need for living epithelial cells, this specialized technique is not yet being routinely used at PCD diagnostic centers in the United States.
Genetic testing
Genetic testing of PCD is quite challenging due to the extensive genetic heterogeneity, the large number of possible causative genes involved, and the large size of known disease-causing genes.18 There are now 8 genes known to be associated with PCD5 (Table 3). Mutations in 2 dynein genes, DNAI1 and DNAH5, which encode for outer dynein arm proteins, account for approximately 30%–35% of confirmed PCD diagnoses.5 Mutation analysis of these 2 genes forms the basis of molecular genetic testing that is now commercially available.5 As previously mentioned, mutations in DNAH11 have been identified in patients presumed to be having PCD with no obvious ciliary ultrastructural abnormality.26 An international effort is underway to identify and define the genes and disease-causing mutations involved in the majority of patients with PCD. Currently, DNA testing may be helpful in establishing the diagnosis, but does not replace the need for confirmatory electron microscopic evaluation.
Table 3.
Recognized Gene Abnormalities Associated with Primary Ciliary Dyskinesia
| Gene identified | Chromosome | EM findings | Phenotypes |
|---|---|---|---|
| DNAH5 | 5 | ODA | PCD +/− SI |
| DNAI1 | 9 | ODA | PCD +/− SI |
| DNAH11 | 7 | normal | PCD +/− SI |
| TNXDC3 | 7 | ODA | KS |
| DNAI2 | 17 | ODA | PCD +/− SI |
| KTU (C14orf104) | 14 | ODA+IDA | PCD +/− SI |
| RSPH4A | 6 | CP | PCD |
| RSPH9 | 6 | CP | PCD |
Modified from Barbato et al.19
DNAH5, dynein, axonemal, heavy chain 5; DNAI1, dynein, axonemal, intermediate chain 1; DNAH11, dynein, axonemal, heavy chain 11; TNXDC3, thioredoxin domain containing 3; DNAI2, dynein, axonemal, intermediate chain 2; KTU, chromosome 14 open reading frame 104; RSPH4A, radial spoke head 4 homologue A; RSPH9, radial spoke head 9 homologue; ODA, outer dynein arms; IDA, inner dynein arms; CP, central pair; SI, situs inversus; KS, Kartagener's syndrome.
Treatment for PCD
At present, no specific therapies are available to correct the ciliary dysfunction in PCD at the cellular level. Recommendations for treatment are largely extrapolated from CF and other suppurative lung diseases in the absence of evidence-based studies. Respiratory management includes routine pulmonary monitoring (lung function testing, respiratory cultures, and chest imaging), regular airway clearance by combinations of physiotherapy and exercise, and aggressive treatment of upper and lower airways infections.19
Mucus clearance techniques are widely prescribed in patients with PCD with no evidence as to the efficacy of any particular modality. Recommendations for a specific patient should be individualized. Since cough may be an effective clearance mechanism, patients with PCD should be encouraged to actively cough. Exercise should be encouraged to mobilize mucus secretions that can be cleared by coughing. In a small study, exercise increased peak expiratory flow acutely, whereas bronchodilators did not.29 It is unknown whether mucus modifying aerosols such as recombinant human rhDNase, hypertonic saline, or mannitol are effective in PCD. Anecdotally, some patients in a small and uncontrolled study showed an improvement in respiratory symptoms with inhalation of rhDNase.19 Although aerosolized short-acting beta agonists may increase ciliary beat frequency in normal cilia in vitro, it is unclear whether they have the same effect on abnormal cilia. In one study, regular bronchodilator use was neither effective nor detrimental in terms of pulmonary function.30
With onset of worsening respiratory symptoms (increased cough and sputum production) or decline in lung function tests, antibiotics should be prescribed, preferably on the basis of respiratory culture results. Younger children with PCD will often receive frequent courses of antibiotics for recurrent and chronic otitis media and sinusitis. Although prophylactic oral antibiotics are not standard treatment in PCD, they can be considered if repeated courses of antibiotics are required. If P. aeruginosa is identified in respiratory cultures, then most PCD clinicians would prescribe an eradication regimen, akin to that recommended in patients with CF. As for chronic infection with this organism, inhaled anti-Pseudomonal antibiotics may be considered, despite lack of proved efficacy.19
Further lung protective strategies include avoidance of active and passive tobacco smoke exposure and other environmental pollutants, and minimization of exposure to respiratory pathogens. Patients with PCD should receive all appropriate childhood immunizations as well as the pneumococcal polysaccharide vaccine and annual influenza vaccinations.
Ear, nose, and throat management is particularly challenging, especially in younger children.11 Despite the recurrent and chronic middle ear effusions, myringotomy tube insertion has been discouraged by some European centers because of persistent otorrhea after tube insertion,19 although surgical intervention may benefit hearing.11 Routine nasal saline irrigations are generally recommended to those with chronic nasal congestion and recurrent sinusitis. Prolonged antibiotic treatment is frequently required for those with chronic sinusitis. The role of topical nasal steroids and antihistamines in PCD remains unclear.
Outcomes
The onset of lung disease in PCD occurs early in childhood, though the progression and severity of lung disease varies considerably among individuals with the condition. For the majority of individuals with PCD, the rate of lung function decline appears slower than in CF, especially with intensive therapy.15,16 Although some individuals die prematurely from respiratory failure as a result of PCD, many can live a near normal life span. Careful and comprehensive management of otolaryngologic and respiratory problems are recommended to minimize chronic organ damage and subsequent bronchiectasis.
Resources
A national consortium, the “Genetic Disorders of Mucociliary Clearance Consortium,” a member of the Rare Disease Clinical Research Network (http://rarediseasesnetwork.epi.usf.edu/gdmcc/index.htm), has been established to improve diagnosis and treatment of children with suspected or confirmed PCD. This consortium is comprised of 7 clinical research sites at the University of North Carolina at Chapel Hill, National Institute of Allergy and Infectious Diseases, The Children's Hospital and University of Colorado Denver School of Medicine, Washington University in St. Louis, Stanford University, University of Washington in Seattle, and University of Toronto (Hospital for Sick Children). Additionally, a PCD Foundation has been formed by the mother of a patient with PCD in an effort to raise awareness and to readily disperse information to patients and their families about PCD (www.pcdfoundation.org).
Conclusions
PCD is an uncommon genetic disease that often presents in the newborn period. Chronic otitis media and profuse rhinorrhea are nearly universal problems, particularly in early childhood. Chronic productive cough is also a dominant complaint. PCD is responsible for a considerable proportion of non-CF bronchiectasis in children. An estimated 50% of affected individuals have dextrocardia and situs inversus totalis (Kartagener's syndrome). The diagnosis can be challenging and until recently has depended on documentation of abnormal ciliary ultrastucture by electron microscopy. nNO measurement appears to be a useful screening test, particularly in children older than 5 years of age. Our knowledge about PCD disease-causing genetic mutations is still emerging, and genetic testing is an active area of investigation. Treatment of PCD is mainly extrapolated from CF and other suppurative lung diseases. The Genetic Diseases of Mucociliary Clearance Consortium is actively working to improve our diagnostic approach to those suspected of having PCD, studying the natural history of PCD-related lung disease, and initiating clinical trials to study treatment strategies in patients with PCD.
Acknowledgment
Dr. Sagel acknowledges support from the National Institutes of Health during the preparation of this article (U54 HL096458-07).
Author Disclosure Statement
Dr. Stillwell reports that no competing financial interests exist. Mr. Wartchow reports that no competing financial interests exist. Dr. Sagel reports receiving grant funding from the National Institutes of Health and the Cystic Fibrosis Foundation. He has no other competing financial interests.
References
- 1.Kartagener M. Stucki P. Bronchiectasis with situs inversus. Arch Pediatr. 1962;79:193–207. [PubMed] [Google Scholar]
- 2.Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–319. doi: 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- 3.Eliasson R. Mossberg B. Camner P. Afzelius BA. The immotile-cilia syndrome: a congenital ciliary abnormality as an etiologic factor in chronic airway infections and male sterility. N Engl J Med. 1977;297:1–6. doi: 10.1056/NEJM197707072970101. [DOI] [PubMed] [Google Scholar]
- 4.Rossman CM. Forrest JB. Lee RM. Newhouse AF. Newhouse MT. The dyskinetic cilia syndrome: abnormal ciliary motility in association with abnormal ciliary ultrastructure. Chest. 1981;80(Suppl):860–865. [PubMed] [Google Scholar]
- 5.Zariwala MA. Knowles MR. Omran H. Genetic defects in ciliary structure and function. Annu Rev Physiol. 2007;69:423–450. doi: 10.1146/annurev.physiol.69.040705.141301. [DOI] [PubMed] [Google Scholar]
- 6.Fliegauf M. Benzing T. Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 7.Afzelius BA. Cilia-related diseases. J Pathol. 2004;204:470–477. doi: 10.1002/path.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanner A. Salathe M. O'Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 9.Coren ME. Meeks M. Morrison I. Buchdahl RM. Bush A. Primary ciliary dyskinesia: age at diagnosis and symptom history. Acta Paediatr. 2002;91:667–669. doi: 10.1080/080352502760069089. [DOI] [PubMed] [Google Scholar]
- 10.Noone PG. Leigh MW. Sannuti A. Minnix SL. Carson JL. Hazucha M. Zariwala MA. Knowles MR. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;15:459–467. doi: 10.1164/rccm.200303-365OC. [DOI] [PubMed] [Google Scholar]
- 11.Campbell RG. Birman CS. Morgan L. Management of otitis media with effusion in children with primary ciliary dyskinesia: a literature review. Int J Pediatr Otorhinolaryngol. 2009;72:1630–1638. doi: 10.1016/j.ijporl.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy MP. Omran H. Leigh MW. Dell S. Morgan L. Molina PL. Robinson BV. Minnix SL. Olbrich H. Severin T. Ahrens P. Lange L. Morillas HN. Noone PG. Zariwala MA. Knowles MR. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation. 2007;115:2814–2821. doi: 10.1161/CIRCULATIONAHA.106.649038. [DOI] [PubMed] [Google Scholar]
- 13.Hellinckx J. Demedts M. De Boeck K. Primary ciliary dyskinesia: evolution of pulmonary function. Eur J Pediatr. 1998;157:422–426. doi: 10.1007/s004310050843. [DOI] [PubMed] [Google Scholar]
- 14.Santamaria F. Montella S. Tiddens HA. Guidi G. Casotti V. Maglione M. de Jong PA. Structural and functional lung disease in primary ciliary dyskinesia. Chest. 2008;134:351–357. doi: 10.1378/chest.07-2812. [DOI] [PubMed] [Google Scholar]
- 15.Ellerman A. Bisgaard H. Longitudinal study of lung function in a cohort of primary ciliary dyskinesia. Eur Respir J. 1997;10:2376–2379. doi: 10.1183/09031936.97.10102376. [DOI] [PubMed] [Google Scholar]
- 16.Marthin JK. Petersen N. Skovgaard LT. Nielsen KG. Lung function in patients with primary ciliary dyskinesia: a cross sectional and 3-decade longitudinal study. Am J Respir Crit Care Med. 2010;181:1262–1268. doi: 10.1164/rccm.200811-1731OC. [DOI] [PubMed] [Google Scholar]
- 17.Jain K. Padley SP. Goldstraw EJ. Kidd SJ. Hogg C. Biggart E. Bush A. Primary ciliary dyskinesia in the paediatric population: range and severity of radiological findings in a cohort of patients receiving tertiary care. Clin Radiol. 2007;62:986–993. doi: 10.1016/j.crad.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Leigh MW. Zariwala MA. Knowles MR. Primary ciliary dyskinesia: improving the diagnostic approach. Curr Opin Pediatr. 2009;21:320–325. doi: 10.1097/MOP.0b013e328329cddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbato A. Frischer T. Keuhni CE. Snijders D. Azebedo I. Baktai G. Bartoloni L. Eber E. Escribano A. Haarman E. Hesselmar B. Hogg C. Jorissen M. Lucas J. Neilsen KG. O'Callaghan C. Omran H. Pohunek P. Strippoli MP. Bush A. Primary ciliary dyskinesia: a consensus statement of diagnostic and treatment approaches in children. Eur Respir J. 2009;34:1264–1276. doi: 10.1183/09031936.00176608. [DOI] [PubMed] [Google Scholar]
- 20.Pifferi M. Bush A. Maggi F. Michelucci A. Ricci V. Conidi ME. Cangiotti AM. Bodini A. Simi P. Macchia P. Boner AL. Nasal nitric oxide and nitric oxide synthase expression in primary ciliary dyskinesia. Eur Respir J. 2011;37:572–577. doi: 10.1183/09031936.00044310. [DOI] [PubMed] [Google Scholar]
- 21.Sagel SD. Nasal nitric oxide: diagnostic value and physiologic significance in primary ciliary dyskinesia. J Pediatr. 2011;159:363–365. doi: 10.1016/j.jpeds.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Canciani M. Barlocco EG. Mastella G. de Santi MM. Gardi C. Lungarella G. The saccharin method for testing mucociliary function in patients suspected of having primary ciliary dyskinesia. Pediatr Pulmonol. 1988;5:210–214. doi: 10.1002/ppul.1950050406. [DOI] [PubMed] [Google Scholar]
- 23.Olin JT. Burns K. Carson JL. Metjian H. Atkinson JJ. Davis SD. Dell SD. Ferkol TW. Milla CE. Olivier KN. Rosenfeld M. Baker B. Leigh MW. Knowles MR. Sagel SD. Diagnostic yield of nasal scrape biopsies in primary ciliary dyskinesia: A multicenter experience. Pediatr Pulmonol. 2011;46:483–488. doi: 10.1002/ppul.21402. for the Genetic Disorders of Mucociliary Clearance Consortium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacCormick J. Robb I. Kovesi T. Carpenter B. Optimal biopsy techniques in the diagnosis of primary ciliary dyskinesia. J Otolaryngol. 2002;31:13–17. doi: 10.2310/7070.2002.19153. [DOI] [PubMed] [Google Scholar]
- 25.Wessels MW. Avital A. Failly Munoz A. Omran H. Blouin J-L. Willems PJ. Candidate gene analysis in three families with acilia syndrome. Am J Med Genet A. 2008;146A:1765–1767. doi: 10.1002/ajmg.a.32340. [DOI] [PubMed] [Google Scholar]
- 26.Carson JL. Collier AM. Hu SS. Acquired ciliary defects in nasal epithelium of children with acute viral upper respiratory infections. N Engl J Med. 1985;312:463–468. doi: 10.1056/NEJM198502213120802. [DOI] [PubMed] [Google Scholar]
- 27.Schwabe GC. Hoffman K. Loges NT, et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum Mutat. 2008;29:289–298. doi: 10.1002/humu.20656. [DOI] [PubMed] [Google Scholar]
- 28.Stannard WA. Chilvers MA. Rutman AR. Williams CD. O'Callaghan C. Diagnostic testing of patients suspected of primary ciliary dyskinesia. Am J Respir Crit Care Med. 2010;181:307–314. doi: 10.1164/rccm.200903-0459OC. [DOI] [PubMed] [Google Scholar]
- 29.Phillips GE. Thomas S. Heather S. Bush A. Airway response of children with primary ciliary dyskinesia to exercise and beta-2 agonist challenge. Eur Respir J. 1998;11:1389–1391. doi: 10.1183/09031936.98.11061389. [DOI] [PubMed] [Google Scholar]
- 30.Koh YY. Park Y. Jeong JH. Kim CK. Min Y-G. Chi JG. The effect of regular salbutamol on lung function and bronchial responsiveness in patients with primary ciliary dyskinesia. Chest. 2000;117:427–433. doi: 10.1378/chest.117.2.427. [DOI] [PubMed] [Google Scholar]