Abstract
Aging affects many aspects of the cellular function of macrophages. Macrophages play a critical role in innate immunity, acting as sentinels to fight pathogens, promoting wound healing, and orchestrating the development of the specific acquired immune response. However, little is known about how age influences the ability of macrophage to change phenotypes in response to environmental factors. This study examined the age-associated defects on macrophage polarization toward a pro-inflammatory (M1) or an anti-inflammatory (M2) phenotype. Adherent splenocytes enriched for macrophages were cultured with or without lipopolysaccharide (LPS), a combination of interferon (IFN)-γ and tumor necrosis factor (TNF)-α or interleukin (IL)-4. A panel of M1 markers, inducible nitric oxide synthase (iNOS), IL-6, IL-1β, and TNF-α, and M2 markers, including arginase-1 (Arg1), Ym1, and Found In Inflammatory Zone 1 (FIZZ1), were analyzed. IL-6 mRNA in cells from aged mice was decreased by 78% and 58% compared with young after stimulation with LPS or IFN-γ and TNF-α (P<0.05), respectively. Also, there was a marked reduction in the induced levels of iNOS, IL-1β, and TNF-α in cells from aged mice relative to young controls. Similarly, IL-4 exposure resulted in a reduction of M2 markers in adherent splenocytes from aged mice compared with younger animals. This was consistent with a 28% decrease in splenic F4/80+IL-4R+ cells in aged mice relative to controls, although IL-4R expression on these cells did not vary between age groups. In contrast, levels of M1 and most M2 markers, save for FIZZ1, in bone marrow-derived macrophages were similar between the age groups, irrespective of stimuli. These data imply that impaired macrophage polarization in the elderly may dysregulate the development of the host response, making them more susceptible to infectious diseases and that the aging microenvironment may be a key modulator of these macrophage-elicited responses.
Introduction
The relationship between advanced age and immunologic deficits is becoming an area of rapidly advancing research. This dysregulation of the immune system translates into the inability of the elderly to effectively combat infection and contributes to the increased incidence of chronic disease states and autoimmune conditions with age (Chung and others 2009). Increasing age leads to numerous changes in the immune system, which results in refractory responses to vaccination and a significant decline in protective immunity (Kumar and Burns 2008; Grubeck-Loebenstein and others 2009). For example, the yearly influenza vaccine is only 40%–60% efficacious in older subjects (eg, ≥65 years old) (Vu and others 2002). Additionally, it has been reported that the quality of life and disability of patients with advanced schistosomiasis as quantified by their clinical symptoms deteriorate with age (Jia and others 2011). Hence, the elderly are more susceptible to viral, bacterial, and parasitic infections and reactivation of latent viruses, resulting in increased morbidity and mortality. The deficits in immunologic responses to invading pathogens that develop with age are referred to as immunosenescence. Even in the absence of an immune challenge, healthy, aged individuals have a significantly higher basal inflammatory state where circulating levels of cytokines, including interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α, are elevated (Franceschi and others 2000). This progressive pro-inflammatory state, termed “inflamm-aging,” renders the older subjects more susceptible to a poor prognosis after systemic insults.
While it is well documented that both B and T lymphocyte compartments of the adaptive immune system deteriorate with advancing age (Cancro and others 2009; Maue and others 2009), the impact of aging on many aspects of the innate immune response has been underinvestigated. One such unexplored area that has not been examined is the effect that the aging microenvironment has on macrophage polarization. Macrophages from aged mice and humans display impairments in their functional activity ranging from a defective response in early immune defense to a decreased capacity to promote development of specific immune responses (Lloberas and Celada 2002; Plowden and others 2004; Stout and Suttles 2005; Mahbub and others 2011). Based on their polarization status, macrophages can be broadly categorized into classically activated or M1 macrophages, induced by the bacterial cell wall component lipopolysaccharide (LPS) or a combination of Th1 cytokines, interferon-γ (IFN-γ) and TNF-α, or alternatively activated or M2 macrophages, which are induced by Th2 cytokines such as IL-4 and IL-13 (Gordon 2003). M1 macrophages upregulate pro-inflammatory mediators, including inducible nitric oxide synthase (iNOS), TNF-α, IL-6, and IL-12 and increase their production of reactive oxygen species and nitrogen intermediates (Stout and others 2005; Gordon 2007). On the contrary, anti-inflammatory M2 macrophages upregulate their expression of arginase-1 (Arg1), scavenger and mannose receptors, as well as their levels of intracellular proteins Found In Inflammatory Zone 1 (FIZZ1) and Ym1. Arg1 is responsible for metabolism of arginine to ornithine and polyamines in M2 macrophages, thereby diminishing substrate availability for nitric oxide (NO) production by M1 macrophages. FIZZ-1 and Ym1 are produced in copious amounts in allergic inflammation and other pathological states where a highly polarized Th2 response is prevalent (Nair and others 2003, 2005; Murray and Wynn 2011).
Based on our previous study (Boehmer and others 2004), we hypothesized that the aging microenvironment should suppress the ability of macrophages to mount an appropriate immune response irrespective of an M1 or M2-inducing stimulus and that this suppression is an effect of the aging milieu as opposed to an intrinsic defect in macrophages.
Materials and Method
Animals
Young (10–12 weeks old) and aged (18–20 months old) WT female BALB/c mice were purchased from the National Institute of Aging colony at Charles River Laboratories. Animals were housed under similar conditions at their respective facilities. They were free of potential endemic viral pathogens that could influence their inflammatory response. All animals were maintained in an environmentally controlled facility at Loyola University Medical Center for at least 1 week before experimentation. At the time of sacrifice, all mice were dissected and the organs were screened for visible tumors and/or gross abnormalities. If any abnormalities, including tumors, were found, these animals were removed from the study. The experimental protocols described here followed the guidelines established by the publication, Principles of Laboratory Animal Care (NIH publication No. 86–23, revised 1985), and were approved by the Loyola University Chicago Institutional Animal Care and Use Committee. To avoid confounding factors related to circadian rhythms, all animal studies were performed between 8 and 9 a.m.
Isolation and culture of primary splenic cells
For cell culture, splenic macrophages were enriched by plastic adherence as previously described (Boehmer and others 2005). Briefly, spleens were aseptically removed and disrupted to yield a cell suspension in RPMI 1640 medium, supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), streptomycin (100 μg/mL), and 2 mM glutamine (culture medium) (GIBCO-BRL, Grand Island, NY). After red blood cell lysis with ACK Lysis Buffer (Invitrogen Corp., Carlsbad, CA), white blood cells were counted in a hemocytometer and viability was determined by Trypan Blue exclusion. Two ×106 cells were seeded in 96-well plates in 200 μL culture medium. After incubation for 2 h at 37°C and 5% CO2, nonadherent cells were discarded by medium aspiration and washed twice with warm phosphate-buffered saline (PBS). This method resulted in an adherent population enriched in macrophages. Adherent cells were treated with culture medium alone or medium containing 100 ng/mL LPS from Escherichia coli 0111:B4 (Sigma Biosciences, St. Louis, MO), 100 U/mL recombinant IL-4 (R&D systems, Minneapolis, MN) or a combination of 100 U/mL IFN-γ (BD Biosciences, , San Jose, CA) and 20 ng/mL TNF-α (BD Biosciences). Supernatants were collected after 20 h and adherent cells were lysed using Trizol Reagent (Invitrogen) and stored at −80°C.
Growth and stimulation of bone marrow-derived macrophages
Bone marrow-derived macrophages (BMMs) were grown as previously described (Zhang and others 2008). Briefly, femurs were isolated and flushed using DMEM/F12-10 media supplemented with 10% FBS, penicillin (100 U/mL), streptomycin (100 μg/mL), and 2 mM glutamine (GIBCO-BRL, Grand Island, NY). Cells were passed through a cell strainer, counted using a hemacytometer, resuspended in media containing 1,000 U/mL M-CSF (R&D systems), and adjusted to a concentration of 2×106 cells/mL before plating them in 100 mm Petri dishes. They were incubated in a humidified chamber with 5% CO2 at 37°C for 7 days. Cells were washed every 2–3 days with warm PBS and media was replaced. Fully differentiated BMMs at day 7 were characterized by their expression of the cell surface markers, F4/80 and CD11b (>90% cells express both markers), as measured by flow cytometry. BMMs were cultured with medium alone or medium containing LPS, IL-4, and/or IFN-γ and TNF-α at the concentrations described above. Supernatants were collected after 20 h and cells were lysed using Trizol Reagent and stored at −80°C.
RNA isolation and real-time reverse transcription-polymerase chain reaction
RNA was isolated from splenic adherent cells or BMMs using Trizol Reagent according to manufacturer's instructions. cDNA was produced using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) was performed on certain M1 and M2 markers (iNOS, IL-6, Arg1, FIZZ1, and Ym1) using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an endogenous control gene. Each cDNA (20 ng) sample was amplified in duplicate using inventoried, M1/M2 gene-specific and control (GAPDH) TaqMan Gene Expression Assay primer sets. Assays were performed using the 7500 Fast Real-Time RT-PCR Gene Expression System (Applied BioSystems, Carlsbad, CA) and data analyzed by SDS software 1.4 by the 2−ΔΔCT relative quantification method (Livak and Schmittgen 2001; Zhang and others 2008).
Measurement of proinflammatory cytokines
The concentrations of TNF-α, IL-1β, and IL-6 in macrophage supernatants were measured by commercially available OptEIA ELISA kits (BD Pharmingen, San Diego, CA) according to the manufacturer's instructions. The lowest detectable limit of these kits is 15.6 pg/mL.
Flow cytometry
Analyses utilizing flow cytometry were performed as previously described (Boehmer and others 2005). Total splenocytes were washed with fluorescence-activated cell sorting (FACS) buffer (PBS containing 0.5% bovine serum albumin, 2 mM ethylenediaminetetraacetic acid, and 0.01% sodium azide), blocked with 1 μg/mL rat IgG (Jackson Laboratories, Bar Harbor, Maine) and anti-CD16/32 antibody for 15 min (eBioscience, San Diego, CA). After this, cells were stained using rat anti-mouse antibodies of saturating concentrations at 4°C. After incubating the cells for 30 min, they were washed twice and resuspended in 0.5 mL FACS buffer. APC-conjugated F4/80 and PE-conjugated CD11b (eBioscience) were used for macrophage characterization and IL-4 receptors with biotinylated antibodies against the receptor (Leinco Technologies, St Louis, MO) followed by subsequent incubation with streptavidin-PerCP-Cy5.5 (eBioscience). Fluorescence was measured by flow cytometry (FACSCanto; BD Biosciences) and analyzed with FlowJo Software (Tree Star, Inc., Ashland, OR).
Statistical analysis
Data are expressed as mean+standard error of the mean. For comparisons of 2 groups, an unpaired student's t-test was used on GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA). Groups were considered significantly different at P values less than 0.05.
Results
M1 responses in adherent splenocytes from aged mice
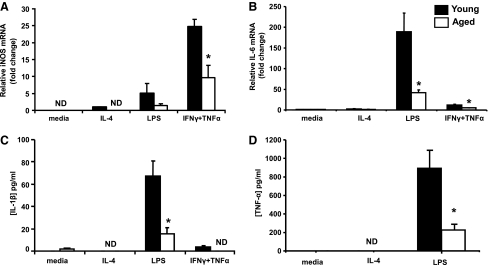
iNOS expression and production of pro-inflammatory cytokines were used as markers of M1 responses. Adherent splenocytes from young and aged mice were cultured in the presence of LPS or a combination of Th1 cytokines, IFN-γ, and TNF-α for 20 h. iNOS was undetectable in cultures of cells incubated with medium only (Fig. 1A). Stimulation with LPS led to an increase in the iNOS message where the induction was 5-fold higher in the cells from young mice compared with aged. Treatment with IFN-γ and TNF-α induced high levels of iNOS in cells from both young and aged mice. iNOS levels were 60% lower in cells from aged mice relative to cells from young, highlighting the inability of adherent splenocytes from aged mice to appropriately upregulate expression of iNOS, the key enzyme involved in NO production (Fig. 1A, P<0.05). LPS treatment triggered a pronounced 190-fold increase in IL-6 mRNA by adherent splenocytes from young mice compared with media-treated controls. Cells from aged mice, however, only expressed 22% the level of IL-6 mRNA generated by cells from young counterparts (Fig. 1B, P<0.05). This observation was consistent with IL-6 production as reported previously (Boehmer and others 2005). Similarly, a 58% less IL-6 mRNA was seen in cells harvested from aged mice relative to young after exposure to IFN-γ and TNF-α stimulation. There was a marked upregulation in the production of IL-1β and TNF-α after LPS stimulation of cells from young mice (70-fold and 900-fold, respectively, P<0.05). On the contrary, cells from aged mice displayed a blunted response whereby the levels of production of IL-1β and TNF-α were approximately one-fourth that of cells from young mice (P<0.05) (Fig. 1C, D). Furthermore, M1 markers, p40 subunit, the common subunit shared by IL-12 and IL-23 as well as the chemokine monocyte chemoattractant protein-1 were also produced in lower levels by adherent cells from aged mice compared with young after stimulation with LPS or a combination of IFN-γ and TNF-α (data not shown). Treatment of cells with the Th2 cytokine IL-4 did not lead to a significant induction of any of the M1 markers, as expected. After stimulation, adherent splenocytes from aged mice failed to upregulate M1 markers compared with cells from young mice, suggesting that macrophages from aged mice are less able to mount an appropriate pro-inflammatory response after encountering biologic stimuli.
FIG. 1.
M1 responses in adherent splenocytes from young and aged mice. (A) Relative iNOS and (B) IL-6 expression was measured via real-time PCR using cDNA from adherent cells obtained from the spleens of young and aged mice. The cells were stimulated for 20 h in the presence of IL-4, LPS, or a combination of IFNγ and TNFα. Protein levels of (C) IL-1β and (D) TNF-α in cell supernatants harvested 20 h after stimulation were quantified by multiplex bead array. N=4–8 mice, *P<0.05 compared with young of the same treatment group. All data are normalized to values from media-treated samples from young mice to highlight the baseline differences between the age groups, if any. iNOS, inducible nitric oxide synthase; interferon-γ, IFN-γ; TNF-α, tumor necrosis factor-α; IL, interleukin; LPS, lipopolysaccharide; PCR, polymerase chain reaction; ND, not detectable.
M2 responses in aged mice after IL-4 stimulation in vitro
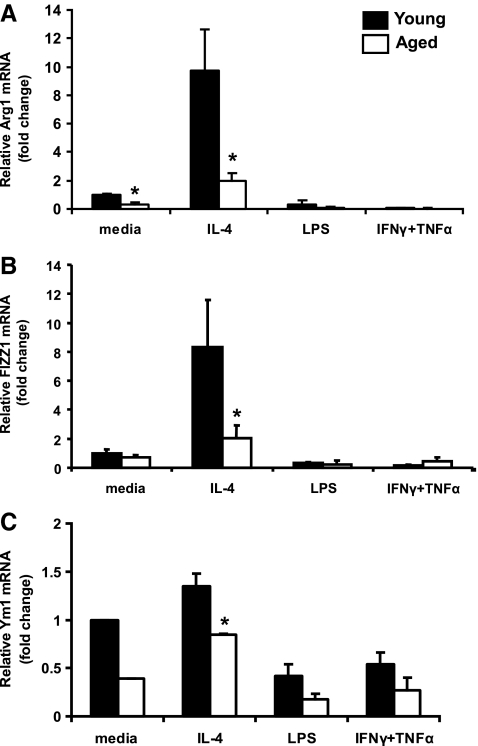
The Th2 cytokine IL-4 is the immunological counterpart of IFN-γ and is a classical inducer of M2 genes, including Arg1, FIZZ1, and Ym1 (Gordon 2003; Martinez and others 2009). To investigate the effect of aging on M2 responses, adherent splenocytes from young and aged mice were treated with IL-4 for 20 h. Basal levels of Arg1 message was 68% lower in adherent splenocytes from aged mice as compared with young. This deficit was not overcome after treatment with IL-4, with Arg1 transcript levels elevated by 10- and 6-fold in cells from young and aged mice, respectively. Together, this resulted in a 5-fold increase in Arg1 mRNA in adherent cells in young mice as compared with aged (Fig. 2A, P<0.05). Similarly, IL-4 treatment led to an 8-fold induction of FIZZ-1 above baseline levels in cells from young mice, whereas the fold induction over basal levels in the aged was only 3-fold (Fig. 2B, P<0.05). IL-4 stimulated cells from aged mice displayed a marked 75% and 35% reduction in FIZZ-1 and Ym1 expression when compared with young, respectively (Fig. 2B and C, P<0.05). Treating cells with LPS or a combination of IFN-γ and TNF-α did not trigger induction of expression of any of the M2 genes but instead led to suppression in basal transcript levels. In summary, there was a significantly lower level of expression of Arg1, FIZZ1, and Ym1 mRNAs after incubation of splenic cells with Th2 cytokines, suggesting a defective elicitation of an M2 response by splenic macrophages.
FIG. 2.
M2 responses in adherent splenocytes from young and aged mice. Relative mRNA expression of (A) Arg1, (B) FIZZ1, and (C) Ym1 was quantified by real-time PCR using cDNA from adherent cells obtained from the spleens of young and aged mice. Cells were stimulated in vitro for 20 h in the presence of IL-4, LPS, or a combination of IFNγ and TNF-α. N=3–7 mice *P<0.05 compared with young of the same treatment group. All data are normalized to values from media-treated samples from young mice to highlight the baseline differences between the age groups, if any. Arg1, arginase-1; FIZZ1, Found In Inflammatory Zone 1.
IL-4 receptor levels on total splenocytes in young and aged mice
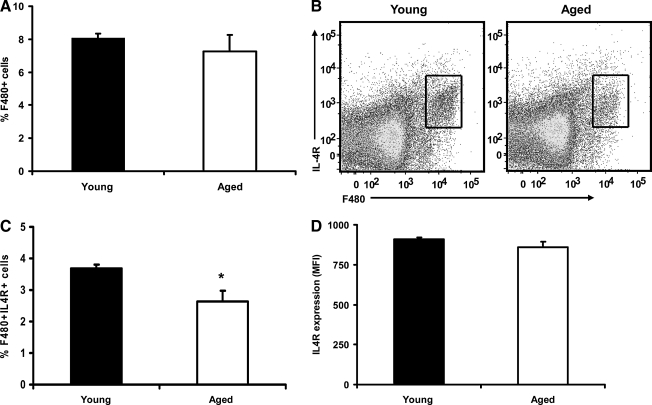
As mentioned earlier, IL-4 signaling is responsible for the polarization of macrophages into an M2 phenotype and consequently upregulating relevant M2 genes. Therefore, reduced expression of IL-4 receptor (IL-4R) on the cell surface of macrophages could be a mechanism by which advanced age reduces the M2 response. To determine if cells from aged mice expressed lower numbers of IL-4R than cells from young animals, total splenocytes were stained with antibodies against F4/80 and IL-4R and the percentages of cells co-expressing both markers as well as the frequency of IL-4R (mean fluorescence intensity, MFI) on F4/80+ cells were determined by flow cytometry. An initial comparison between the total percentages of F4/80+ cells in young and aged mice yielded that there was no difference between the age groups. Although there was a tendency in aged mice to display slightly lower numbers of F4/80+ cells, it was not statistically significant (Fig. 3A). However, when the percentages of F4/80+IL4R+ cells were compared between the groups, there were fewer double positive cells in the aged mice compared with their young counterparts (Fig. 3B, P<0.05). As shown in Fig. 3, gates were drawn surrounding the F4/80+IL4R+ cells and MFI was used as a measure of surface expression of IL-4R on F4/80+ cells (Fig. 3B, lower right). No difference in receptor expression was observed between the age groups. These data suggest that the age-related defect in M2 response may be due, in part, to the reduced percentages of F4/80+ cells expressing IL-4R in aged mice and not because of frequency of IL-4R on the surface of these cells.
FIG. 3.
Percentage and expression of IL-4 receptors on F4/80+ cells in the spleen. Spleen cells from young and aged mice were stained with F4/80 (pan macrophage marker) and IL-4R to assess co-expression as well as MFI of IL-4R in F4/80+ cells. (A) Analysis showing total percentage of F4/80+ cells from the spleen of young and aged mice. (B) Representative dot plots showing total F4/80+ macrophage population in the spleen expressing IL-4R (inset). (C) Graphical representation of the total percentage of cells that co-expresses F4/80 and IL-4R in the spleen from young and aged mice. (D) Plot showing IL-4R expression as MFI on F4/80+IL-4R+ double-positive cells in the spleen from young and aged mice lower right. N=5 mice per group, P<0.05, when compared with young mice. Gating strategies were based on unstained and single color controls and the gates were kept constant among all age and treatment groups. MFI, mean fluorescence intensity.
M1 and M2 responses by BMMs
To determine whether the age-associated defect in eliciting the appropriate M1/M2 responses is a result of intrinsic defects of the cell or the surrounding aging milieu, bone marrow progenitor cells were isolated and differentiated ex vivo into BMMs, thereby removing them from the aged microenvironment. BMMs were stimulated with LPS, a combination of IFN-γ and TNF-α, or IL-4. As above, at 20 h after stimulation, supernatants were collected for assessment of production of pro-inflammatory cytokines and cells were lysed for cDNA preparation and RT-PCR analysis.
Although iNOS message was upregulated ∼500-fold over media-treated control after culture with LPS in both age groups there was no difference in transcripts levels in young and aged mice (Table 1). Similarly, copious amounts of IL-6 were produced after LPS stimulation, and the levels were comparable between cells from young and aged animals (7,479±937 versus 6,916±331 pg/mL). Furthermore, there were no differences observed in IL-1β or TNF-α production between the age groups irrespective of the stimuli. IL-4 stimulation did not produce detectable levels of M1 cytokines. Cell supernatants stimulated with a combination of the Th1 cytokines were not tested for TNF-α production to avoid false-positives due to the exogenous addition of recombinant TNF-α (Table 1).
Table 1.
Levels of M1 Markers in Bone Marrow-Derived Macrophages
| |
|
|
LPS |
IFNγ+TNFα |
||
|---|---|---|---|---|---|---|
| M1 markers | Young | Aged | Young | Aged | Young | Aged |
| iNOS mRNA (fold change relative to control) | 0.37±0.07 | 0.42±0.21 | 457±106 | 571±137 | 149±12.0 | 155±24.7 |
| IL-6 (pg/mL) | ND | ND | 7,479±937 | 6,916±331 | 4.3±1.7 | 4.2±0.7 |
| IL-1β (pg/mL) | ND | ND | 249±41 | 277±8 | 320±66 | 284±61 |
| TNF-α (pg/mL) | ND | ND | 40,261±5096 | 54,223±4659 | NV | NV |
iNOS mRNA is expressed as fold change over control group, which are unstimulated samples from young mice. Relative mRNA expression was determined via real-time PCR using cDNA from bone marrow-derived macrophages grown from bone-marrow progenitor cells from young and aged mice stimulated for 20 h in the presence of LPS or a combination of IFN-γ and TNF-α. Cytokine values from cell supernatants determined 20 h after stimulation by BioRad multiplex bead array and expressed as pg/mL±standard error of the mean. N=4–5 mice. No significant differences were observed between groups.
iNOS, inducible nitric oxide synthase; interferon-γ, IFN-γ; TNF-α, tumor necrosis factor-α; IL, interleukin; LPS, lipopolysaccharide; PCR, polymerase chain reaction; ND=not detectable, NV=not verified.
M2 markers Arg1 and Ym1 were significantly upregulated ∼470- and 370-fold above baseline after IL-4 treatment of cells from young and aged mice, respectively, but, like M1 markers, there was no difference between age groups (Table 2). Moreover, LPS stimulation led to comparable levels of Arg1 induction in both age groups. This is supported by recent literature, which reported upregulation of Arg1 in a TLR-4-MyD88-dependent manner during mycobacteria infection (El Kasmi and others 2008; Qualls and others 2010). However, there was a ∼2-fold increase in FIZZ1 message in aged mice compared with their young counterparts (15,169±2,640 versus 7,204±1,235 pg/mL) after IL-4 stimulation, which could be a protein-specific defect in BMMs from aged mice. Overall, the data show that expression or production of M1 and M2 markers, save for FIZZ1 are similar in BMMs from young and aged mice, which suggest that the inadequate M1/M2 response displayed by adherent splenocytes may not be due to intrinsic defects in macrophages.
Table 2.
Expression of M2 Markers in Bone Marrow-Derived Macrophages
| |
|
|
LPS |
IFNγ+TNFα |
||
|---|---|---|---|---|---|---|
| M2 marker (fold change relative to control) | Young | Aged | Young | Aged | Young | Aged |
| Arg1 | 468±39 | 371±31 | 13.7±3.9 | 19.7±3.9 | 0.2±0.1 | 0.2±0.1 |
| FIZZ1 | 7,204±1,235 | 15,169±2640a | ND | ND | ND | 1.0±0.1 |
| Ym1 | 3±0.3 | 2±0.2 | 0.2±0.09 | 0.2±0.05 | 0.38±0.05 | 0.17±0.03 |
Relative mRNA expression levels are presented as fold change over control group, which are unstimulated samples from young mice. Arg1, FIZZ1, and Ym1 mRNA expression levels were quantified by real-time PCR using cDNA from bone marrow macrophages grown from bone marrow progenitor cells from young and aged mice and were stimulated for 20 h in the presence of IL-4, LPS, and a combination of IFN-γ and TNF-α. N=4–5 mice.
p<0.05 compared with young of the same treatment group.
Arg1, arginase-1; FIZZ1, Found In Inflammatory Zone 1.
Discussion
The data presented in this study show that there is an aberrant M1 and M2 response by primary adherent splenocytes obtained from aged mice relative to young. This is exemplified by decreased expression or production of M1 markers iNOS, IL-6, IL-1β, and TNF-α and M2 markers Arg1, FIZZ1, and Ym1. While there was no difference in the level of expression of IL-4R per F4/80+ cell, there was a reduction in the numbers of splenic F4/80+ cells expressing IL-4R, which may play a role in the decreased M2 response in vivo. Further experimentation using BMMs isolated from the aging microenvironment and grown ex vivo showed that M1/M2 polarization is comparable in young and aged mice. This suggests that the global decrease in M1 and M2 markers in aged mice may not be an intrinsic defect in macrophages per se. As the aberrant M1/M2 phenotype observed in primary adherent splenocytes in aged mice was restored upon isolation of bone marrow macrophage precursors, it may imply an effect of the surrounding aging microenvironment in macrophage development. This concept of the in vivo aged milieu altering macrophage function is consistent with the observations of Gomez and others (2006a), where they found that serum from aged rats altered the capacity of macrophages from young rats to induce TNF-α and other proinflammatory mediators (for a review, see Gomez and others 2005).
Our observation showing reduced iNOS expression in cells from aged versus young mice supports earlier studies that document an age-dependent decline in iNOS expression and NO production in different strains of mice from splenic and peritoneal macrophages, irrespective of bacterial or cytokine stimuli (Kissin and others 1997; Lu and others 1999). Reactive nitrogen species, together with oxygen radicals, are the hallmarks of macrophage cytotoxic activity toward certain pathogens (Nathan and Hibbs 1991; Karupiah and others 1993; Lowenstein and others 1994). Hence, decreased NO production by macrophages from the elderly could alter the balance between host defense mechanisms and pathogen virulence, thereby rendering the host susceptible to infection.
LPS binding to toll-like receptor 4 (TLR4) triggers nuclear factor kappa B (NF-κB) activation and pro-inflammatory cytokine secretion by both MyD88-dependent and -independent pathways during bacterial infection (Takeda and Akira 2005; Kumar and others 2009). In contrast, exposure to Th1 cytokines, IFN-γ and TNF-α, mimics a non-bacterial scenario whereby M1 markers are induced by cytokines in the inflammatory environment as a result of viral or fungal infection. Consistent with previous studies from our laboratory (Boehmer and others 2004, 2005) and others (Renshaw and others 2002; Plowden and others 2004; Chelvarajan and others 2005, 2006), the present study shows that relative to cells from young counterparts, cells from aged mice have decreased expression and production of IL-6, IL-1β, and TNF-α, which are well-known M1 markers. IL-6, IL-1β, and TNF-α regulate myriad factors in the inflammatory microenvironment, which are crucial for host defense (Kopf and others 1994; Tracey and Cerami 1994; Gabay and others 2010). Reduction in these cytokine levels can affect innate immune effector functions, including phagocytic activity and NO production, as well as the induction of T cell responses (Balkwill 1992; Tracey and Cerami 1994; Michlewska and others 2009).
To elucidate the defects in pro-inflammatory cytokine production in the aged, TLR4 expression and intracellular signaling in macrophages have been studied. While research conducted in our laboratory showed comparable levels in TLR4 expression in splenic and peritoneal macrophages from young and aged mice (Boehmer and others 2004, 2005). Renshaw and others (2002) showed reduced TLR4 expression in splenic cells from aged mice compared with young. Along with experimenting with a different strain of mice, the gating strategy to characterize macrophages in the aforementioned study also differed. We used F4/80 as a pan macrophage marker, whereas they chose CD11b as their preferred molecule, which is a pan-leuckocyte marker not exclusive to macrophages. Assessment of signaling molecules downstream of TLR4 revealed that macrophages from aged mice showed decreased levels of phosporylated and total p38, c-Jun N terminal kinase (JNK), and NF-κB (Boehmer and others 2005).
Alternative activation of macrophages is mediated by IL-4 and IL-13, in a signal transducer and activator of transcription (STAT)-6-dependent manner, and is induced predominantly in Th2-driven responses, particularly in allergic, cellular, and humoral responses to parasitic and extracellular pathogens (Martinez and others 2009; Varin and Gordon 2009). For the first time, herein we report effects of aging on alternatively activated macrophages. Using established markers, our results show that adherent splenocytes from aged mice stimulated with IL-4 in vitro displayed a suppressed M2 response when compared with young. Reduced expression of Arg1 and FIZZ1 by stimulated cells from aged mice might indicate a defect that is 2-fold: inability to resolve existing inflammation driven by Th1 cytokines, subsequently leading to collateral tissue damage, as well as inappropriate elicitation of an M2 response, such as during a helminth invasion. Moreover, M2 macrophage expression of Arg1 and FIZZ1 have been show to play a critical role in the control of parasitic infection and dampening of Th2-driven pathologies (Hesse and others 2001; Loke and others 2007; Nair and others 2009; Pesce and others 2009). Studies using conditional IL-4R knockouts, where macrophages lack the receptor, highlight the importance of alternatively activated macrophages and their associated proteins. These knockout mice were extremely susceptible to Schistosoma mansoni infection where the mortality was attributed to increased Th1 cytokines and elevated iNOS activity among other factors (Herbert and others 2004). However, there seems to be a dual-regulatory role of M2 macrophages in response to parasitic infections by acting to down regulate the fibrogenic Th2 response. Arg1 and FIZZ1 have been shown to be critical in modulating fibrosis in pulmonary and wound healing models (Hesse and others 2001; Liu and others 2004; Loke and others 2007) as well as in control of the fibrogenic response after parasitic infection. Ym1 emerged as a marker of M2 macrophages in allergic airway inflammation due to the abundant expression of the protein during the development of the disease and other Th2-mediated pathologies (Raes and others 2002a, 2002b; Nair and others 2003; Cai and others 2009). Thus, the decreased expression of M2 markers by alternatively activated macrophages in aged mice may render these subjects susceptible to various Th2-dominated pathologies.
As discussed before, IL-4 signals via IL-4Rα on the surface of macrophages to induce alternative macrophage activation. Ligand binding causes receptor dimerization, which activates the JAK-STAT pathway. Janus kinase (JAK1) activation leads to phosphorylation of STAT6, which, in turn, dimerizes, migrates to the nucleus, and binds to promoters of M2 genes to induce transcription (Martinez and others 2009). One possibility that may account for the age-related decrease in M2 responses is the level of IL-4R expression on macrophages from young and aged mice. We found that although there was no difference in expression (MFI) of IL-4R on F4/80+ cells in the spleen of young and aged mice, total percentages of F4/80+IL-4R+ cells in the spleen were significantly lower in aged mice. This suggests that fewer receptors are available for ligand binding and subsequent downstream signaling. This decrease may account, in part, for the reduced transcription of M2 genes in cells from aged mice. However, the possibility still remains that this marked suppression of M2 response in aged mice may be, in large, due to defective signaling downstream of IL-4 receptors. It will be interesting to determine whether there are differences in total and/or phosphorylated levels of STAT-6 in the splenic macrophages, which will further corroborate our findings.
To assess the intrinsic defects in macrophages from aged mice, we harvested bone marrow progenitor cells and differentiated them into BMMs ex vivo, thus removing the cells from the aging environmental cues received during myeloid differentiation. Regarding the expression of specific M2 markers, similar levels of Arg1 and Ym1 mRNA were found in BMMs from young and aged mice; however, FIZZ1 expression was higher in cells from aged mice. This observation may be exclusive to FIZZ-1-specific functions in contrast to overall differences in M2 phenotypes between the age groups. Currently, it is unclear why there is a difference in FIZZ1 expression between BMMs from the 2 age groups and further definitive studies will be required to address this phenomenon. Further, the failure to demonstrate age-dependent differences in M1 and most M2 markers in BMMs from young and aged mice may suggest a specific role for the aging bone marrow microenvironment during macrophage differentiation. Specifically, the bone marrow milieu in aged mice may promote aberrant macrophage differentiation that result in inherent cellular defects, as seen in our splenic adherent cell cultures. It may be of interest to determine if longer term culture of primary splenic adherent cells would result in a loss of the aged phenotype similar to what was observed in the 7-day incubation of the BMMs, further implicating the aged microenvironment in cellular senescence with advanced age.
Multiple factors affect the age-specific microenvironment. Wu and others (2007) reported that higher expression of IL-1β, IL-6, and TNF-α, and lower levels of the anti-inflammatory peroxisome proliferator-activated receptor-γ were found in adipose tissue from aged mice, relative to those of young mice. Additionally, aged IL-6 knockout mice had better survival when compared with aged wild types after endotoxin challenge, identifying IL-6 as a key modulator contributing to the outcomes of infection or injury in the aged (Gomez and others 2006b). Moreover, a recent report identified myeloid-derived suppressor cells as a novel source of the elevated levels of Th1 cytokines associated with aging (Enioutina and others 2011). The constant pro-inflammatory environment possibly set up by adipocytes and MDSCs may be responsible for “anergy” observed in splenic macrophages from aged mice. Once cells are removed from this exaggerated cytokine milieu and cultured under optimum conditions, they displayed a young-like M1 and M2 phenotype as exemplified by our BMM studies.
In summary, our studies show age-associated defects in macrophage polarization, as adherent splenocytes from aged mice showed suppressed M1 and M2 responses. The reduced expression of M2 markers may be, in part, due to lower numbers of splenic F4/80+IL-4R+ cells in aged mice compared with young. We propose that the aging microenvironment, not macrophages per se, is responsible for immune suppression displayed by primary adherent splenocytes from young and aged mice, whereas BMMs from aged mice, cultured for prolonged periods of time in complete media fail to show differences in M1/M2 phenotype relative to their young counterparts. Together, these data suggest that depressed macrophage-mediated immune responses in the elderly may hinder their ability to fight invading pathogens and render them incapable of mounting a full-scale adaptive immune response.
Acknowledgments
The authors would like to thank Aleah L. Brubaker, Jessica L. Palmer, and Pamela L. Witte, Ph.D. (Director of the Immunology and Aging Program at Loyola University Medical Center) for critical review of this article and for thoughtful discussions. This work was supported by grants from the National Institutes of Health, National Institute of Aging (AG018859 E.J.K.), and the Ralph and Marian C. Falk Medical Research Trust (E.J.K.)
Author Disclosure Statement
No competing financial interests exist.
References
- Balkwill FR. Tumour necrosis factor and cancer. Prog Growth Factor Res. 1992;4(2):121–137. doi: 10.1016/0955-2235(92)90027-f. [DOI] [PubMed] [Google Scholar]
- Boehmer ED. Goral J. Faunce DE. Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75(2):342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- Boehmer ED. Meehan MJ. Cutro BT. Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126(12):1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Cai Y. Kumar RK. Zhou J. Foster PS. Webb DC. Ym1/2 promotes Th2 cytokine expression by inhibiting 12/15(S)-lipoxygenase: identification of a novel pathway for regulating allergic inflammation. J Immunol. 2009;182(9):5393–5399. doi: 10.4049/jimmunol.0803874. [DOI] [PubMed] [Google Scholar]
- Cancro MP. Hao Y. Scholz JL. Riley RL. Frasca D. Dunn-Walters DK. Blomberg BB. B cells and aging: molecules and mechanisms. Trends Immunol. 2009;30(7):313–318. doi: 10.1016/j.it.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelvarajan RL. Collins SM. Van Willigen JM. Bondada S. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J Leukoc Biol. 2005;77(4):503–512. doi: 10.1189/jlb.0804449. [DOI] [PubMed] [Google Scholar]
- Chelvarajan RL. Liu Y. Popa D. Getchell ML. Getchell TV. Stromberg AJ. Bondada S. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006;79(6):1314–1327. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- Chung HY. Cesari M. Anton S. Marzetti E. Giovannini S. Seo AY. Carter C. Yu BP. Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8(1):18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kasmi KC. Qualls JE. Pesce JT. Smith AM. Thompson RW. Henao-Tamayo M. Basaraba RJ. Konig T. Schleicher U. Koo MS. Kaplan G. Fitzgerald KA. Tuomanen EI. Orme IM. Kanneganti TD. Bogdan C. Wynn TA. Murray PJ. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol. 2008;9(12):1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enioutina EY. Bareyan D. Daynes RA. A role for immature myeloid cells in immune senescence. J Immunol. 2011;186(2):697–707. doi: 10.4049/jimmunol.1002987. [DOI] [PubMed] [Google Scholar]
- Franceschi C. Bonafe M. Valensin S. Olivieri F. De Luca M. Ottaviani E. De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Gabay C. Lamacchia C. Palmer G. IL-1 pathways in inflammation and human diseases. Nat. 2010;6(4):232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- Gomez CR. Acuna-Castillo C. Nishimura S. Perez V. Escobar A. Salazar-Onfray F. Sabaj V. Torres C. Walter R. Sierra F. Serum from aged F344 rats conditions the activation of young macrophages. Mech Ageing Dev. 2006a;127(3):257–263. doi: 10.1016/j.mad.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Gomez CR. Boehmer ED. Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17(5):457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Gomez CR. Goral J. Ramirez L. Kopf M. Kovacs EJ. Aberrant acute-phase response in aged interleukin-6 knockout mice. Shock. 2006b;25(6):581–585. doi: 10.1097/01.shk.000029553.39081.ec. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gordon S. The macrophage: past, present and future. Eur J Immunol. 2007;37(Suppl. 1):S9–S17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B. Della Bella S. Iorio AM. Michel JP. Pawelec G. Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res. 2009;21(3):201–209. doi: 10.1007/BF03324904. [DOI] [PubMed] [Google Scholar]
- Herbert DR. Holscher C. Mohrs M. Arendse B. Schwegmann A. Radwanska M. Leeto M. Kirsch R. Hall P. Mossmann H. Claussen B. Forster I. Brombacher F. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20(5):623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- Hesse M. Modolell M. La Flamme AC. Schito M. Fuentes JM. Cheever AW. Pearce EJ. Wynn TA. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167(11):6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- Jia TW. Utzinger J. Deng Y. Yang K. Li YY. Zhu JH. King CH. Zhou XN. Quantifying quality of life and disability of patients with advanced schistosomiasis japonica. PLoS Negl Trop Dis. 2011;5(2):e966. doi: 10.1371/journal.pntd.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karupiah G. Xie QW. Buller RM. Nathan C. Duarte C. MacMicking JD. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261(5127):1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- Kissin E. Tomasi M. McCartney-Francis N. Gibbs CL. Smith PD. Age-related decline in murine macrophage production of nitric oxide. J Infect Dis. 1997;175(4):1004–1007. doi: 10.1086/513959. [DOI] [PubMed] [Google Scholar]
- Kopf M. Baumann H. Freer G. Freudenberg M. Lamers M. Kishimoto T. Zinkernagel R. Bluethmann H. Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368(6469):339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Kumar H. Kawai T. Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388(4):621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Kumar R. Burns EA. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev Vaccines. 2008;7(4):467–479. doi: 10.1586/14760584.7.4.467. [DOI] [PubMed] [Google Scholar]
- Liu T. Dhanasekaran SM. Jin H. Hu B. Tomlins SA. Chinnaiyan AM. Phan SH. FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol. 2004;164(4):1315–1326. doi: 10.1016/S0002-9440(10)63218-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lloberas J. Celada A. Effect of aging on macrophage function. Exp Gerontol. 2002;37(12):1325–1331. doi: 10.1016/s0531-5565(02)00125-0. [DOI] [PubMed] [Google Scholar]
- Loke P. Gallagher I. Nair MG. Zang X. Brombacher F. Mohrs M. Allison JP. Allen JE. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179(6):3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- Lowenstein CJ. Dinerman JL. Snyder SH. Nitric oxide: a physiologic messenger. Ann Intern Med. 1994;120(3):227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- Lu Q. Ceddia MA. Price EA. Ye SM. Woods JA. Chronic exercise increases macrophage-mediated tumor cytolysis in young and old mice. Am J Physiol. 1999;276(2 Pt 2):R482–R489. doi: 10.1152/ajpregu.1999.276.2.R482. [DOI] [PubMed] [Google Scholar]
- Mahbub S. Brubaker AL. Kovacs EJ. Aging of the Innate Immune System: an Update. Curr Immunol Rev. 2011;7(1):104–115. doi: 10.2174/157339511794474181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO. Helming L. Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Maue AC. Yager EJ. Swain SL. Woodland DL. Blackman MA. Haynes L. T-cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol. 2009;30(7):301–305. doi: 10.1016/j.it.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michlewska S. Dransfield I. Megson IL. Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. Faseb J. 2009;23(3):844–854. doi: 10.1096/fj.08-121228. [DOI] [PubMed] [Google Scholar]
- Murray PJ. Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol. 2011;89(4):557–563. doi: 10.1189/jlb.0710409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MG. Cochrane DW. Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett. 2003;85(2):173–180. doi: 10.1016/s0165-2478(02)00225-0. [DOI] [PubMed] [Google Scholar]
- Nair MG. Du Y. Perrigoue JG. Zaph C. Taylor JJ. Goldschmidt M. Swain GP. Yancopoulos GD. Valenzuela DM. Murphy A. Karow M. Stevens S. Pearce EJ. Artis D. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206(4):937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MG. Gallagher IJ. Taylor MD. Loke P. Coulson PS. Wilson RA. Maizels RM. Allen JE. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect Immun. 2005;73(1):385–394. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan CF. Hibbs JB., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Pesce JT. Ramalingam TR. Mentink-Kane MM. Wilson MS. El Kasmi KC. Smith AM. Thompson RW. Cheever AW. Murray PJ. Wynn TA. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5(4):e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowden J. Renshaw-Hoelscher M. Engleman C. Katz J. Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3(4):161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- Qualls JE. Neale G. Smith AM. Koo MS. DeFreitas AA. Zhang H. Kaplan G. Watowich SS. Murray PJ. Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling. Sci Signal. 2010;3(135):ra62. doi: 10.1126/scisignal.2000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes G. De Baetselier P. Noel W. Beschin A. Brombacher F. Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002a;71(4):597–602. [PubMed] [Google Scholar]
- Raes G. Noel W. Beschin A. Brys L. de Baetselier P. Hassanzadeh GH. FIZZ1 and Ym as tools to discriminate between differentially activated macrophages. Dev Immunol. 2002b;9(3):151–159. doi: 10.1080/1044667031000137629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw M. Rockwell J. Engleman C. Gewirtz A. Katz J. Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169(9):4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- Stout RD. Jiang C. Matta B. Tietzel I. Watkins SK. Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175(1):342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- Stout RD. Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005;205:60–71. doi: 10.1111/j.0105-2896.2005.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K. Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17(1):1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- Varin A. Gordon S. Alternative activation of macrophages: immune function and cellular biology. Immunobiology. 2009;214(7):630–641. doi: 10.1016/j.imbio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Vu T. Farish S. Jenkins M. Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20(13–14):1831–1836. doi: 10.1016/s0264-410x(02)00041-5. [DOI] [PubMed] [Google Scholar]
- Wu D. Ren Z. Pae M. Guo W. Cui X. Merrill AH. Meydani SN. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179(7):4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- Zhang X. Goncalves R. Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol Chapter. 2008;14(Unit 14.1) doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]