Abstract
Calcineurin (CN) is a calcium- and calmodulin-dependent serine/threonine phosphatase. In immune cells, CN controls the activity of a wide range of transcription factors, including nuclear factor of activated T, nuclear factor-kappa B, c-fos, and Elk-1. CN plays an important role in synoviocyte activation and arthritis progression in vivo and this function is tightly linked to dysregulated intracellular Ca2+ store and Ca2+ response triggered by proinflammatory cytokines. In the present study, transgenic mice expressing human calcineurin-binding protein 1 (hCabin1) were generated, driven by type II collagen promoter, and the efficiency of these mice was investigated by experimental arthritis. These transgenic mice successfully expressed hCabin1 in joint tissue as well as other organs such as liver, heart, and brain. The overexpression of hCabin1 reduced the disease severity during collagen-induced arthritis. In fibroblast-like synoviocytes (FLSs) from hCabin1 transgenic mice, the productions of these cytokines, including interleukin (IL)-2, IL-4, and IFN-γ, were decreased and matrix metalloproteinases were also depressed in transgenic mice FLS. In addition, these effects were only found in the joint tissue, which is a major inflammation site. These findings will provide a better knowledge of the pathogenic mechanisms of rheumatoid arthritis and a potential animal model of the chronic inflammatory conditions, including atherosclerosis and transplantation.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that is characterized by chronic inflammation within the joint tissue, infiltration of activated immune cells, and synovial hyperplasia, leading to cartilage and bone destruction (Feldmann and others 1996). In the synovium, synoviocytes actively participate in chronic inflammatory responses as a major cell population of the invasive pannus (Firestein 1996). Synovial fibroblasts from RA patients have the potential to produce matrix-degrading enzymes and several cytokines such as interleukin (IL)-1β, IL-6, and IL-8 (Bucalca and others 1991). Moreover, synovial fibroblasts proliferate abnormally and invade the local environment and exhibit the characteristics of tumor cells including somatic mutation in HRAS and TP53 (Firestein and others 1997; Roivainen and others 1997).
Calcineurin (CN) is a calcium- and calmodulin-dependent serine/threonine phosphatase (Rusnak and others 2001; Klee and others 1998). CN plays a critical role in various biological processes such as cell proliferation, cardiovascular, and skeletal muscle development and apoptosis (Kahl and Means 2003; Groenendyk and others 2004; Schulz and Yutzey 2004). CN is best known for its role in the calcium-dependent regulation of the nuclear factor of activated T (NFAT) cells pathway (Crabtree 2001; Crabtree and Olson 2002; Groenendyk and others 2004). An increase in protein tyrosine phosphorylation and the cytoplasmic-free Ca2+ responses trigger CN phosphatase activity. This process has been suggested to be associated with lymphocyte abnormalities in autoimmune diseases such as lupus erythematosus (Liossis and others 1996, 1998). In immune cells, CN controls the activity of a wide range of transcription factors, including NFAT, nuclear factor-kappa B, FOS, and ELK1 (Baksh and Burakoff 2000). As a result, CN plays a crucial role in T-cell activation, cell growth, apoptosis, neuron depotentiation, and angiogenesis. Further, CN had been proposed to be a pathogen of cardiomyopathy and stroke (Crabtree and Olson 2002).
In recent studies, CN has played an important role in synoviocyte activation and arthritis progression in vivo and such a function is tightly linked to dysregulated intracellular Ca2+ store and Ca2+ response triggered by proinflammatory cytokine. Moreover, the selective inhibition of CN by the overexpression of CN-binding protein 1 (Cabin1), a natural CN antagonist, hampered synoviocyte activation (Yoo and others 2006). This study indicated that inactivation of CN by overexpression of Cabin would be a novel therapeutic strategy for RA. However, this result was limited to in vitro testing and not in vivo. For further study of the effect of Cabin1 in chronic inflammation such as RA, the establishment of the animal model, which can overexpress Cabin1 in a specific tissue, is very important.
In the present study, human Cabin1 (hCabin1) transgenic mice were generated; expressing the hCabin1, driven by the type II collagen promoter, the efficiency of these mice was investigated by experimental arthritis.
Methods and Materials
hCabin1 overexpression in transgenic mice
A 974-bp cDNA fragment encoding the CN binding domain of the hCabin1 was amplified from the placenta tissue by a polymerase chain reaction (PCR). The resulting PCR product was cloned into the mouse type II collagen promoter expression vector. The DNA construct was microinjected into fertilized embryos from BDF1 hybrid mice and transferred to the oviducts of the pseudopregnant recipient. The transgenic mice were confirmed by PCR. Transgenic mice were back-crossed with normal DBA/1 mice for at least 6 generations.
The overexpression of hCabin1 in transgenic mice
The total RNA was extracted from joint tissues and several other tissues of transgenic mice using TRIzol reagents, according to the manufacturer's protocol (M.R.C.). The cDNA synthesis was performed using a reverse transcription system (Promega). Synthesized cDNA was amplified with hCabin1-specific primer. For western blotting, tissues were homogenized in a nuclear extract lysis buffer [10 mM Tris HCl, 500 mM NaCl, 0.1% Nonidet p-40, 5 mM EDTA (pH 8.0)]. The resulting lysates were centrifuged at 53,000 rpm for 10 min. All procedures were carried out at 0°C–4°C. Forty micrograms of proteins was resolved in 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to nitrocellulose. The membranes were incubated with rabbit-anti human Cabin 1 antibody (abCam) followed by HRP-conjugated anti-human IgG (Santa Cruz).
Isolation and culture of fibroblast-like synovial cells
Fibroblast-like synoviocytes (FLSs) were prepared from the synovial tissues of hCabin1 transgenic mice. Fore and hind paws were removed at the ankle joint, the skin was removed, and the remaining tissue was carefully recovered with a scalpel in 1,000 μL volume of phosphate-buffered saline. The synovial tissue was digested with 2.5% collagenase (Sigma) for 4 h at 37°C to obtain a single-cell suspension. The FLS from passages 3 through 7 were seeded in 24-well plates at 2×104 cells/well in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and cultivated at 37°C for 24 h. The culture plates were incubated for 48 h in the presence of PMA and ionomycin. After 48 h, the cell-free culture supernatants were collected and stored at 4°C before use.
Western blotting for NFATc1 and NFATc2
For analysis of nuclear localization of NFATcs, the FLSs from each mouse were homogenized in cytoplasmic lysis buffer [0.5% Nonidet p-40, 25 mM KCl, 5 mM MgCl2, 10 mM Tris-HCl (pH 8.0)] and nuclear extract lysis buffer [10 mM Tris-HCl, 500 mM NaCl, 0.1% Nonidet p-40, 5 mM EDTA (pH 8.0)]. Each extract fraction was centrifuged at 13,000 rpm for 10 min. Forty micrograms of proteins from each fraction was dissolved in 8% SDS-PAGE and then transferred to nitrocellulose. The membranes were incubated with mouse monoclonal NFATc1 and NFATc2 antibodies, followed by HRP-conjugated anti-mouse IgG (Santa Cruz).
Collagen-induced arthritis
Age-matched male DBA/1 (Charles River) and Cabin1 overexpression mice were immunized with bovine type II collagen (CII; Chondrex) at 8–12 weeks of age, as previously described (Yu and others 2008). Briefly, CII was dissolved as 0.25% in 0.01 N acetic acids. Mice were immunized on day 0 using an intradermal injection of 100 μg CII emulsified in complete Freund's adjuvant. On day 14, the mice were boosted in the left footpad with the same amount of CII in an incomplete Freund's adjuvant. Three weeks after primary immunization, the mice were examined 2 to 3 times a week for the onset and severity of arthritis. The severity of arthritis was scored in a double-blind manner, with each paw assigned a separate clinical score as follows: 0=normal; 1=erythema and mild swelling confined to the ankle joint and toes; 2=erythema and mild swelling extending from the ankle to the midfoot; 3=erythema and severe swelling extending from the ankle to the metatarsal joints; and 4=ankylosing deformity with joint swelling.
Cytokines and matrix metalloproteinases levels in peripheral blood and FLSs
Cytokines levels in the culture supernatants and sera were measured by ELISA and reverse transcriptase-polymerase chain reaction (RT-PCR). Cell-free supernatants of FLSs and blood samples were used for ELISA. Blood samples were collected from the eye on day 42. Sera were isolated by centrifugation and kept at −20°C before use. IL-1β, tumor necrosis factor α (TNFα), IL-6, and IL-17 were measured using commercially available ELISA kits (Quantikine Mouse Immunoassay), according to the manufacturer's instructions. FLSs from the synovial tissue of mice with collagen-induced arthritis (CIA) were prepared for RT-PCR and total RNA was extracted. The expression of IL-2, IL-4, IL-10, and IFN-γ were measured by RT-PCR. Matrix metalloproteinase-2 (MMP-2), MMP-3, MMP-9, and MMP-12 were also measured. After loading on 1% agarose gel, relative expression levels of each cytokine were measured by the Image J software 1.38.
Histological analysis
For a histological examination of the joints, the right hindlimb from each mouse was fixed in 10% buffered formalin on day 42. The limbs were decalcified in a histological decalcifying agent (Calci-Clear Rapid; National Diagnostics) embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The fixed and stained slides of the joint sections were read by a trained observer.
Statistical analysis
Data were analyzed by chi-square analysis to determine the differences between groups. A value of P<0.05 was considered to be statistically significant.
Results
hCabin1 overexpression in transgenic mice
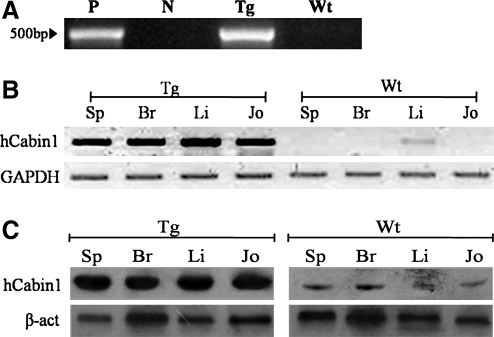
To generate hCabin1 overexpression in transgenic mice, a type II collagen promoter vector was constructed, known as mouse type II collagen specific. This construct was microinjected into mouse embryos and the progeny was tested as the founder of transgenic lines. To confirm transgenic status, total genomic DNA was extracted from the tail biopsies of candidate pups and then applied to PCR screening with the transgene-specific primers. As a result of screening, one mouse line was confirmed as a germ line transgenic line (Fig. 1A).
FIG. 1.
The generation of hCabin1 transgenic mouse. To confirm transgenic founder mouse, Total genomic DNA extracted from tail biopsies of 2-week-old pups was screened using PCR analysis (A). P, positive control; N, negative control; Tg, transgenic mouse; Wt, wild-type litter mate. Transgenic mice express hCabin1 in synovial and various tissues. RT-PCR was performed using total RNA isolated from joint tissue and several other tissues of transgenic mice (B). The expression of the hCabin1 protein was confirmed by western blotting (C). The hCabin1 protein was detected in a manner similar to mRNA. Sp, spleen; Br, brain; Li, liver; Jo, joint tissue; hCabin1, human Cabin1; PCR, polymerase chain reaction; RT, reverse transcriptase.
Expression of hCabin1 in transgenic mice
First, the expression of hCabin1 was tested by RT-PCR. The hCabin1 was only detected in the joint tissue of transgenic mice, but not in wild-type mice. However, this expression pattern was also observed in other tissues of transgenic mice such as spleen, brain, and liver (Fig. 1B). To further confirm the tissue on expression of hCabin1 in transgenic mice, western blotting analysis was performed on the same tissues. The hCabin1 proteins were also detected in a manner similar to mRNA (Fig. 1C).
Functional analysis of hCabin1 in transgenic mice
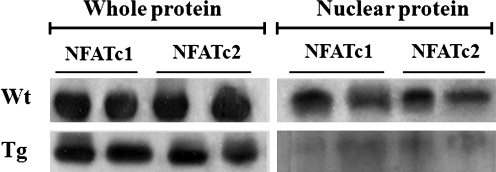
Because of overexpressed human-derived Cabin1 gene in mouse, the functional activity of hCabin1 was tested in the synovial tissues of these transgenic mice. The levels of the nuclear-localized NFATc1 and NFATc2 were compared by western blotting analysis. Figure 2 shows that both the nuclear-localized NFATc1 and NFATc2 were decreased in transgenic mice, but there was no difference in the total protein extract. These patterns demonstrated that overexpressed hCabin1 has functional activity in the synovial tissue of transgenic mice and blocks the mobilization of NFATc1 and NFATc2 into the nucleus.
FIG. 2.
NFATc nuclear localization was inhibited by the expression of hCabin1 in transgenic mice FLS. FLS was obtained from each mouse and then stimulated with PMA (50 ng/mL) and ionomycin (5 μg/mL) for 48 h. To analyze NFATc nuclear localization, proteins were separated into total proteins and nuclear proteins. Nuclear-localized NFATc was determined by western blotting. FLS, fibroblast-like synoviocyte; NFAT, nuclear factor of activated T.
The effects of hCabin1 on progression of inflammatory arthritis in mice
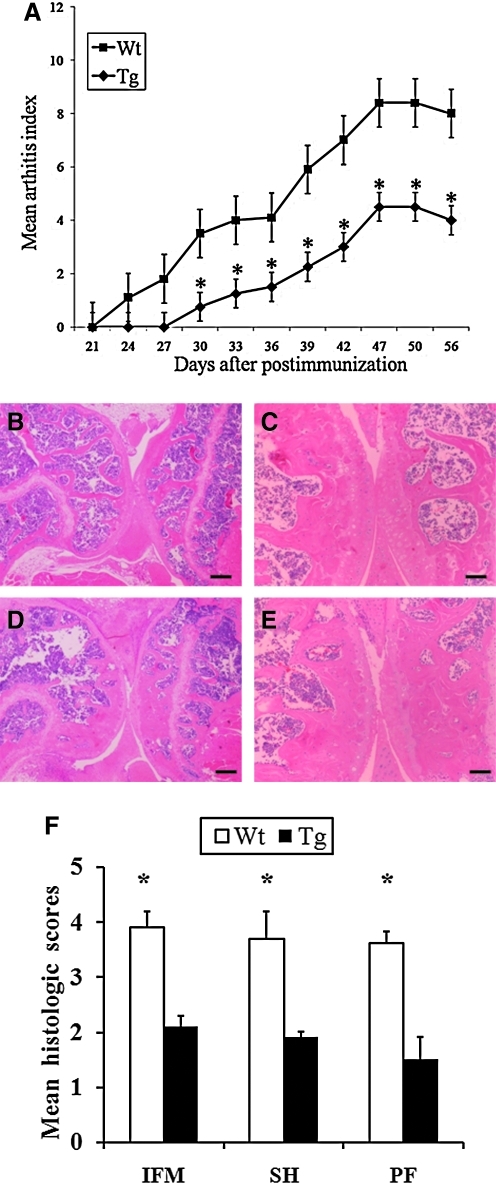
For further analysis of the effect of the hCabin1 overexpression in in vivo arthritic conditions, the CIA was generated in transgenic mice (Fig. 3A). Clinical severity was monitored after the first immunization. All animals developed some detectable level of disease activity. In the transgenic mice group, however, the clinical score curve was significantly suppressed (P<0.05) when compared with wild-type controls. Data indicate that the overexpressed hCabin1 significantly reduced the severity of inflammation, which is compatible with the clinical scores (P<0.05). A histological examination of joints from hCabin1 transgenic mice on day 42 after immunization also showed a lower degree of inflammation, destruction of cartilage and bone, infiltration of mononuclear cells, and proliferation of synovial cells (Fig. 3B, C). Also, histopathologic evaluation showed a marked reduction in synovial proliferation, cartilage damage, pannus formation, and bone erosion in hCabin1 transgenic mice when compared with wild-type controls (Fig. 3F). These results indicate that the administration of hCabin1 suppresses the clinical and pathological severity of arthritis.
FIG. 3.
The progression and severity of arthritis was reduced in transgenic mice (A). From 1 week after boosting, the clinical score was determined by visual inspection, as described in the Methods and Materials section. The total number of mice was 5 to 6 in each group (*P<0.05 versus arthritic wild-type mice). The histological examinations of the joints from collagen-induced arthritis mice were performed by hematoxylin and eosin staining. Mice were euthanized on day 42 after the primary immunization. The left hind foot, which received collagen boosting, was excluded. Upper panels (B and C, magnification, ×20 and ×40, respectively) represent the arthritic joints from transgenic mice and lower panels (D and E, magnification, ×20 and ×40, respectively) represent those from wild-type mice. Mean histology score for the degree of inflammation (IFM), synovial hyperplasia (SH), and pannus formation (PF) in transgenic mice and wild-type mice (F). Scale bars: (B and C) 100 μm; (D and E) 200 μm.
The differential regulation of productions of cytokine and MMPs by hCabin1
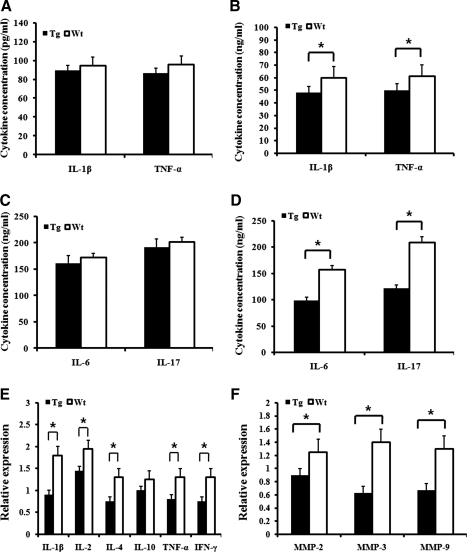
The production of several proinflammatory cytokines and MMPs in peripheral blood and FLSs from inflamed mice was also analyzed. The concentrations of IL-1β, TNFα, IL-6, and IL-17 in peripheral blood were not different in transgenic and wild-type mice (Fig. 4A, C). In FLS from hCabin1 transgenic mice, the levels of these cytokines including IL-2, IL-4, and IFN-γ were decreased (Fig. 4B, D, and E). In addition, MMPs were also depressed in transgenic mice FLS (Fig. 4F). These results demonstrate that the hCabin1 transgenic mice could suppress the activation of synoviocytes by targeted inhibition of CN and these responses occurred in the targeted tissue.
FIG. 4.
IL-1β and TNFα were repressed in the synovium of transgenic mice but not in peripheral blood. Proinflammatory cytokines levels were tested in peripheral blood serum (A) and (C) and FLS-free culture medium (B) and (D) from the arthritic mice by ELISA. Optical absorbance was measured using an ELISA reader at 540 and 450 nm (*P<0.05 versus arthritic wild-type mice). The expression of cytokines (E) and MMPs (F) was profiled by RT-PCR. FLSs were prepared from arthritic transgenic mice on day 42 after first immunization. Relative expression levels of each cytokine were measured by the Image J software 1.38 (*P<0.05 versus arthritic wild-type mice). MMP, matrix metalloproteinase; IL, interleukin; TNFα, tumor necrosis factor α.
Discussion
Cabin1/Cain, a CN-binding protein 1, was initially identified as one of 2 hits from a mouse T-cell cDNA library in a yeast 2-hybrid screen for CN-binding proteins and was named as such for its CN-binding activity (Sun and others 1998). Similar to CN, Cabin1 is widely expressed in all tissues and cell types examined, including the spleen, leukocytes, and T cells (Sun and others 1998). The targeted inhibition of CN by Cabin1 has been already reported in many kinds of disease. The mouse homolog of hCabin1 regulates the synaptic endocytosis of neurotransmitter vesicles (Lai and others 2000). Moreover, adenoviral gene transfer of Cain prevents the agonist-induced cardiomyocyte hypertrophy (Taigen and others 2000). Especially, in RA, the overexpression of cabin1 could suppress the production of IL-6 and MMp-2 in rheumatoid synoviocytes (Yoo and others 2006), suggesting that inhibition of CN by cabin1 would be a good target for treatment of RA. To establish the hCabin1 transgenic model, the CN-binding domain (5641–6614) in the C-terminus of hCabin1 was overexpressed, driven by a collagen-specific promoter. Overexpression of full-length Cabin1 or its C-terminal fragment in Jurkat T cells represses transcriptional activation of CN-responsive elements in the IL-2 promoter and blocks dephosphorylation of NFAT upon T-cell activation (Sun and others 1998). Further, overexpression of hCAbin1 peptide in arthritic FLSs reduced the production of IL-6 and MMP-2 (Yoo and others 2006).
These transgenic mice successfully expressed hCabin1 in joint tissue as well as other organs such as liver, heart, and brain. The functional activity of the overexpressed hCabin1 was tested by the localization of NFATc1 and NFATc2 in FLSs from each mouse. After treatment with PMA and ionomycin, nuclear NFATc1 and NFATc2 were significantly decreased. The expression of IL-2 was also downregulated in the FLS of hCabin1 transgenic mice. Dephosphorylation of NFAT by CN can induce nuclear localization of NFAT and increase CN-mediated IL-2 expression (Lee and Park 2006). These changes have been mainly found in FLS from hCabin1 transgenic mice. In RA, FLS is a key player in propagating both inflammation and joint destruction. In contrast to normal FLS, the RA FLS is hyperplastic and invasive (Huber and others 2006) and helps to perpetuate the RA inflammatory microenvironment by both elaborating inflammatory mediators and stimulating other localized inflammation (Abeles and Pillinger 2006). This observation suggests that the strategy employed in this study could efficiently suppress the activation of CN in FLS and the hCabin1 transgenic mouse would be a useful model for treatment of RA.
As aspects of pharmacological application, the most potent and well-known inhibitors of CN are a cyclic lipophilic undecapeptide (CsA) and a tacrolimus FK-506 (Dumont 2000). Both drugs exhibit a potent immunosuppressive activity and had been used in the treatment of many chronic inflammatory diseases, including RA (Tugwell and others 1995). Systemic administration of these drugs is associated with significant side effects. With prolonged therapeutic use of tacrolimus, blood levels are associated with hypertension, nephrotoxicity, psychiatric disturbances, and hyperlipidemia (Paul and Labisch 2002). The natural selective inhibitor, Cabin1, would minimize these side effects.
In this study, we generated for the first time transgenic mice that overexpress hCabin1 in joint tissue by transgenic engineering technique. The joint-specific overexpression of hCabin1 reduced the disease severity during CIA and the levels of the various cytokines and MMPs were also depressed. In addition, these effects were exhibited only in joint tissue, which is a major, individual inflammation site. These findings provide better knowledge of the pathogenic mechanisms of RA and the potential animal model of chronic inflammatory condition, including atherosclerosis and transplantation.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST; No. 2011-0000916), Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2010-12420000), and a grant from the Korean Ministry of Education, Science, and Technology (The Regional Core Research Program/Anti-aging and Well-being Research Center).
Author Disclosure Statement
No competing financial interests exist.
References
- Abeles AM. Pillinger MH. The role of the synovial fibroblast in rheumatoid arthritis: cartilage destruction and the regulation of matrix metalloproteinases. Bull NYU Hosp Jt Dis. 2006;64:20–24. [PubMed] [Google Scholar]
- Baksh S. Burakoff SJ. The role of calcineurin in lymphocyte activation. Semin Immunol. 2000;12:405–415. doi: 10.1006/smim.2000.0221. [DOI] [PubMed] [Google Scholar]
- Bucala R. Ritchlin C. Winchester R. Cerami A. Constitutive production of inflammatory and mitogenic cytokines by rheumatoid synovial fibroblasts. J Exp Med. 1991;173:569–574. doi: 10.1084/jem.173.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree GR. Calcium, calcineurin, and the control of transcription. J Biol Chem. 2001;276:2313–2316. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]
- Crabtree GR. Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Dumont FJ. FK506, an immunosuppressant targeting calcineurin function. Curr Med Chem. 2000;7:731–748. doi: 10.2174/0929867003374723. [DOI] [PubMed] [Google Scholar]
- Feldmann M. Brennan FM. Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39:1781–1790. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- Firestein GS. Echeverri F. Yeo M. Zvaifler NJ. Green DR. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci USA. 1997;94:10895–10900. doi: 10.1073/pnas.94.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenendyk J. Lynch J. Michalak M. Calreticulin, Ca2+, and calcineurin—signaling from the endoplasmic reticulum. Mol Cells. 2004;17:383–389. [PubMed] [Google Scholar]
- Huber LC. Distler O. Tarner I. Gay RE. Gay S. Pap T. Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology. 2006;45:669–675. doi: 10.1093/rheumatology/kel065. [DOI] [PubMed] [Google Scholar]
- Kahl CR. Means AR. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr Rev. 2003;24:719–736. doi: 10.1210/er.2003-0008. [DOI] [PubMed] [Google Scholar]
- Klee CB. Ren H. Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- Lai MM. Luo HR. Burnett PE. Hong JJ. Snyder SH. The calcineurin-binding protein cain is a negative regulator of synaptic vesicle endocytosis. J Biol Chem. 2000;275:34017–34020. doi: 10.1074/jbc.C000429200. [DOI] [PubMed] [Google Scholar]
- Lee M. Park J. Regulation of NFAT activation: a potential therapeutic target for immunosuppression. Mol Cells. 2006;22:1–7. [PubMed] [Google Scholar]
- Liossis SN. Ding XZ. Dennis GJ. Tsokos GC. Altered pattern of TCR/CD3-mediated protein-tyrosyl phosphorylation in T cells from patients with systemic lupus erythematosus. Deficient expression of the T cell receptor zeta chain. J Clin Invest. 1998;101:1448–1457. doi: 10.1172/JCI1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liossis SN. Kovacs B. Dennis B. Kammer GM. Tsokos GC. B cells from patients with systemic lupus erythematosus display abnormal antigen receptor-mediated early signal transduction events. J Clin Invest. 1996;98:2549–2557. doi: 10.1172/JCI119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul N. Labisch A. Health is a Crossroad, Nature and society, individual and community, in safeguarding public health. Gesundheitswesen. 2002;64:614–622. doi: 10.1055/s-2002-35539. [DOI] [PubMed] [Google Scholar]
- Roivainen A. Jalava A. Pirila L. Yli-Jama T. Tiusanen H. Toivanen P. H-ras oncogene point mutations in arthritic synovium. Arthritis Rheum. 1997;40:1636–1643. doi: 10.1002/art.1780400913. [DOI] [PubMed] [Google Scholar]
- Rusnak F. Mertz P. Calcineurin: form and function. Physiol Rev. 2001;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Schulz RA. Yutzey KE. Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol. 2004;266:1–16. doi: 10.1016/j.ydbio.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Sun L. Youn HD. Loh C. Stolow M. He W. Liu JO. Cabin 1, a negative regulator for calcineurin signaling in T lymphocytes. Immunity. 1998;8:703–711. doi: 10.1016/s1074-7613(00)80575-0. [DOI] [PubMed] [Google Scholar]
- Taigen T. De Windt LJ. Lim HW. Molkentin JD. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci USA. 2000;97:1196–1201. doi: 10.1073/pnas.97.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugwell P. Pincus T. Yocum D. Stein M. Gluck O. Kraag G. McKendry R. Tesser J. Baker P. Wells G. Combination therapy with cyclosporine and methotrexate in severe rheumatoid arthritis. N Engl J Med. 1995;333:137–141. doi: 10.1056/NEJM199507203330301. [DOI] [PubMed] [Google Scholar]
- Yoo SA. Park BH. Park GS. Koh HS. Lee MS. Ryu SH. Miyazawa K. Park SH. Cho CS. Kim WU. Calcineurin is expressed and plays a critical role in inflammatory arthritis. J Immunol. 2006;177:2681–2690. doi: 10.4049/jimmunol.177.4.2681. [DOI] [PubMed] [Google Scholar]
- Yu DH. Kim MO. Kim SH. Shin MJ. Kim BS. Kim HJ. Lee SR. Lee SG. Yoo SA. Kim WU. Hyun BH. Park YS. Kim TY. Ryoo ZY. The therapeutic effect of extracellular superoxide dismutase (EC-SOD) mouse embryonic fibroblast (MEF) on collagen-induced arthritis (CIA) mice. Cell Transplant. 2008;17:1371–1380. doi: 10.3727/096368908787648029. [DOI] [PubMed] [Google Scholar]