Abstract
Preeclampsia is a pregnancy-specific disorder characterized by hypertension and excess protein excretion in the urine. It is an important cause of maternal and fetal morbidity and mortality worldwide. The disease is almost exclusive to humans and delivery of the pregnancy continues to be the only effective treatment. The disorder is probably multifactorial, although most cases of preeclampsia are characterized by abnormal maternal uterine vascular remodeling by fetally derived placental trophoblast cells. Numerous in vitro and animal models have been used to study aspects of preeclampsia, the most common being models of placental oxygen dysregulation, abnormal trophoblast invasion, inappropriate maternal vascular damage and anomalous maternal-fetal immune interactions. Investigations into the pathophysiology and treatment of preeclampsia continue to move the field forward, albeit at a frustratingly slow pace. There remains a pressing need for novel approaches, new disease models and innovative investigators to effectively tackle this complex and devastating disorder.
Preeclampsia: the clinical syndrome
Preeclampsia is the most common hypertensive disease of pregnancy, affecting 5–8% of pregnancies (Saftlas et al., 1990) and accounting for nearly 18% of maternal deaths (ACOG, 2002) in the United States. Little change has been noted in the incidence of this disease in the United States during the national data-collection periods of 1993–1997 and 2001–2005 (Berg et al., 2009). Preeclampsia is also associated with adverse fetal outcomes, including intrauterine growth retardation (IUGR), placental abruption, oligohydramnios and non-reassuring fetal surveillance. It is clinically defined as hypertension and proteinuria with onset following the 20th week of pregnancy (Wagner, 2001). Preeclampsia can be further differentiated into mild and severe forms.
Mild preeclampsia is defined by a systolic blood pressure of >140 mmHg or a diastolic blood pressure >90 mmHg in combination with 300 mg of proteinuria over 24 hours. Blood pressure elevations must be confirmed via two separate measurements taken at least 6 hours apart. Severe preeclampsia is diagnosed if there are more severe elevations of blood pressure or evidence of other end-organ dysfunction. The specific criteria as defined by the American Congress of Obstetricians and Gynecologists (ACOG) are shown in Box 1. Patients with severe preeclampsia can also exhibit hemoconcentration due to intravascular volume depletion and elevated serum uric acid levels (Wagner, 2001). HELLP syndrome is a specific variant of severe preeclampsia. HELLP is an acronym for hemolysis, elevated liver enzymes and low platelets. It has been suggested that, to meet the criteria for HELLP syndrome, a patient’s test results must indicate: microangiopathic anemia on a peripheral smear; liver aspartate aminotransferase (AST) levels >70; lactate dehydrogenase (LDH) levels >600 or total bilirubin >1.2 (indicative of significant hemolysis); and a platelet count <100,000 (Sibai, 2004). Other recommendations are less stringent and recognize the diagnosis of partial HELLP syndrome when some of the above characteristics are absent.
Box 1. American Congress of Obstetrics and Gynecology (ACOG) criteria for diagnosis of severe preeclampsia.
Preeclampsia is considered severe if one or more of the following criteria is present (ACOG, 2002):
Blood pressure of 160 mm Hg systolic or higher, or 110 mm Hg diastolic or higher on two occasions at least 6 hours apart while the patient is on bed rest
Proteinuria of 5 g or higher in a 24-hour urine specimen, or 3+ or greater in two random urine samples collected at least 4 hours apart
Oliguria of less than 500 ml in 24 hours
Cerebral or visual disturbances
Pulmonary edema or cyanosis
Epigastric or right upper-quadrant pain
Impaired liver function
Thrombocytopenia
Fetal growth restriction.
The multiple criteria for the diagnosis of severe preeclampsia illustrate the multifocal nature of the disease. Elevated proteinuria and oliguria are indicative of renal dysfunction. Headache and visual changes are evidence of central nervous system involvement. Impaired liver dysfunction is typically defined as liver function tests [AST or alanine aminotransferase (ALT) levels] that exceed twice the upper limit of normal (ACOG, 2002). Fetal growth restriction is variously defined as an estimated fetal weight of less than the 10th, 5th or 3rd percentile (Figueras and Gardosi, 2011).
Management of preeclampsia consists of two options: delivery or observation. Management decisions depend on the gestational age at which preeclampsia is diagnosed. The only effective treatment for preeclampsia is delivery of the fetus and placenta, and the decision to deliver involves balancing the potential benefit to the fetus of further in utero development with fetal and maternal risk of progressive disease, including the development of eclampsia, which is preeclampsia complicated by maternal seizures. The decision can be difficult for the clinician because expectantly managed (actively surveyed) preeclampsia can progress and threaten the life of both the mother and the fetus. However, premature birth remains a leading cause of neonatal morbidity and mortality worldwide (Fonseca et al., 2007). Mild preeclampsia at 37 0/7 weeks gestation or greater should be treated with expeditious delivery (Wagner, 2001). Women diagnosed with mild preeclampsia prior to 37 weeks of gestation can be managed expectantly until they reach 37 weeks provided they undergo regular antenatal testing and maternal evaluation to monitor for fetal deterioration and/or progression to severe preeclampsia. It is generally recommended that patients with severe preeclampsia deliver once they reach 32–34 0/7 weeks of gestation. The onset of severe preeclampsia prior to fetal viability (23–25 weeks of gestation) is also generally treated by delivering the fetus. Management of severe preeclampsia when onset is detected between 24 and 34 weeks of gestation requires complex decision making and should optimally involve a practitioner with extensive experience, such as a Maternal-Fetal Medicine specialist. Expectant management over this gestational age range has been shown to have benefit to the fetus, but should only be done if the disease process can be managed to minimize the risk to the mother (Sibai, 2004).
Clinical terms.
- ALT (alanine aminotransferase)
measurement of ALT levels is a common blood test. Elevations in ALT are indicative of liver damage. Normal range in pregnancy is 3–32 U/l.
- AST (aspartate aminotransferase)
measurement of AST levels is another common blood test. Elevations in AST are indicative of liver damage. The normal range in pregnancy is 2–33 U/l.
- Cervical exam
a digital exam of the patient’s cervix that is commonly performed by the practitioner during pregnancy. Dilatation, effacement (thinning), position, consistency and station of the fetal-presenting part are assessed. These values are combined to give a Bishop score. A low Bishop score (<6) indicates a low likelihood of successful induction of labor. A high Bishop score (>8) is associated with a high likelihood of successful induction of labor.
- Eclampsia
seizures occurring in a pregnant woman that are not the result of a prior neurological condition. Occurs almost exclusively in women with preeclampsia.
- Epigastric pain
pain located just inferior to the sternum, in the upper and central portion of the abdomen. Can indicate liver involvement in a woman with preeclampsia.
- Fetal heart monitoring
continuous tracing of the fetal heart rate. The presence of accelerations and beat-to-beat variability are reassuring. The loss of variability, especially when accompanied by decelerations in rate, is suggestive of fetal distress.
- Hemoglobin and hematocrit
analysis provides measurements of the red blood cell count. Hemoglobin is reported in g/dl and hematocrit is reported as a percentage of the blood volume. Normal ranges vary across pregnancy, but are generally between 9.5 and 15 g/dl for hemoglobin and 28–40% for hematocrit.
- Oligohydramnios
decreased volume of amniotic fluid.
- Oliguria
low urine output (below 300–500 ml/day).
- Placental abruption
separation of the placental lining from the mother’s uterus. Can cause severe and abrupt late-pregnancy bleeding and is a cause of fetal and maternal mortality.
- Proteinuria
excess protein in the urine; indicates kidney damage.
The multifocal nature of preeclampsia is related to its pathogenesis. Although the exact pathway leading to preeclampsia continues to be poorly defined, many promising insights are discovered every year. Continued interest in this disease process, as evidenced by over 25,000 published articles on preeclampsia, has led to a variety of useful but imperfect in vitro and animal models for a disorder that is fairly restricted, albeit not exclusive, to humans. In this Clinical Puzzle article, we discuss the multiple pathological factors and processes that contribute to preeclampsia and the existing experimental models used to study them, and highlight outstanding research questions in the field.
Pathophysiology of preeclampsia
The clinical manifestations of hypertension and proteinuria that define preeclampsia probably represent the late stage of a disease that begins very early in pregnancy. There are multiple theories, and little agreement, about the ultimate cause of preeclampsia, and it is likely that many different initial insults converge on a common pathophysiology (or two common pathophysiologies, if considering early- and late-onset preeclampsia separately). However, what is clear is that all forms of the disease are characterized by a disruption of vascular remodeling and a systemic anti-angiogenic response. The underlying mechanisms contributing to these changes remain unclear and might overlap. Among the possible mechanisms that have been most studied are alterations in the maternal immune response to the allogenic fetus and placental oxygen dysregulation (including inappropriate placental hypoxia and hypoxia-reoxygenation injury).
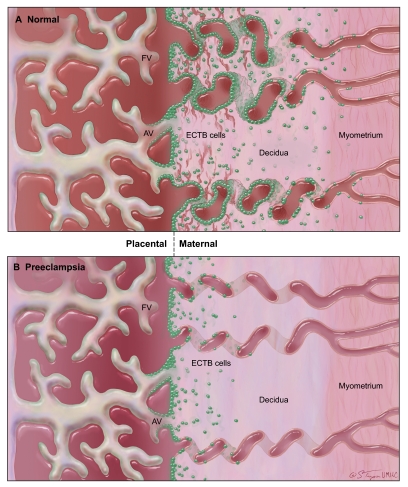
In normal pregnancy, cytotrophoblast cells originating in the anchoring villi of the fetal portion of the placenta attach to and invade the maternal endometrium (a process known as interstitial invasion). A subset of these extravillous trophoblast cells acquires endothelial characteristics and invades maternal spiral arteries (known as endovascular invasion). During early pregnancy, these trophoblast cells plug the spiral arteries, maintaining a hypoxic uterine environment. They ultimately replace some of the endothelial cells in the vessel wall and alter vessel compliance so that it becomes ‘leaky’ and allows maternal blood to fill the intervillous spaces of the placenta (Fig. 1) (Kaufmann et al., 2003; Hunkapiller and Fisher, 2008).
Fig. 1.
Invasion defects in preeclampsia. (A) In a normal placenta, extravillous cytotrophoblast (ECTB) cells (green) move into the decidua (endometrium) and myometrium via interstitial invasion. Some ECTB cells enter maternal spiral arteries and replace the endothelial cells of the vessel walls, becoming endovascular ECTB (eECTB) cells, increasing vessel compliance and maximizing blood flow into placental blood spaces. (B) In the placenta of a preeclamptic patient, interstitial invasion is shallow and limited, with many ECTB cells in the basal plate remaining attached to anchoring villi (AV). Endovascular invasion is nearly absent, and spiral arterioles remain ‘stiff’. FV, floating villi. Image courtesy of The Curators of the University of Missouri (2011), a public corporation.
It is clear from placental samples examined at term, as well as from Doppler ultrasound study of placental perfusion, that the remodeling of spiral arteries is incomplete in patients with preeclampsia. Fewer trophoblast cells are present within the spiral arterioles, and the vessel walls remain stiff (Khong et al., 1986; Aquilina and Harrington, 1996). Although, by definition, preeclampsia can be diagnosed only after the 20th week of pregnancy (and clinically typically presents even later), this vascular remodeling occurs during the first two trimesters. In normal pregnancies, endovascular extravillous cytotrophoblast cells have been identified by 9 weeks of gestation (Craven et al., 1998), and intervillous blood flow is not established until 10–12 weeks of gestation (Caniggia et al., 2000; Burton et al., 2009). Thus, poor trophoblast invasion is an early event in disease progression, although it has not been determined whether it is the cause of preeclampsia or a result of another underlying problem. It has been hypothesized that, without proper remodeling of maternal spiral arteries, the placenta is deprived of oxygen and that the resulting hypoxia triggers the symptoms of preeclampsia. However, poor trophoblast invasion is also observed in intrauterine growth restriction without hypertension, suggesting that this alone is not sufficient to cause preeclampsia (Khong et al., 1986; Roberts and Hubel, 2009).
There is strong evidence that changes in circulating levels of regulators of angiogenesis cause many of the clinically significant symptoms of preeclampsia. Members of the vascular endothelial growth factor family, VEGF-A, VEGF-B and placental growth factor (PLGF), act through a family of membrane receptors to regulate angiogenesis. Binding of VEGF-A to VEGFR2, or PLGF to VEGFR1 [also known as Fms-like tyrosine kinase-1 (FLT-1)] promotes angiogenesis, whereas the soluble form of FLT-1 (sFLT-1) inhibits angiogenesis (Wu et al., 2010). Clinical studies have demonstrated an increase in circulating levels of sFLT-1, and a significant increase in the ratio of sFLT-1 to PLGF, in both early- and late-onset preeclampsia (Levine et al., 2004). Similarly, increased placental expression and circulating concentrations of soluble endoglin, an inhibitor of capillary formation, are associated with preeclampsia and are positively correlated with disease severity (Venkatesha et al., 2006).
Models of preeclampsia
Several key aspects of preeclampsia can be studied in vitro (see below), but the nature of the disease limits the utility of cell culture models. Specifically, preeclampsia involves changes in the behavior of fetal trophoblast cells, their interactions with maternal endothelium and the reaction of the maternal system to these vascular changes. Therefore, whole-animal models are needed to recapitulate the complex interactions that underlie preeclampsia. An ideal animal model of this disease would exhibit all the symptoms seen in women with preeclampsia, including hypertension, proteinuria, endothelial dysfunction and an imbalance of angiogenic factors, all of which arise secondary to poor trophoblast invasion and resolve following delivery of the placenta (McCarthy et al., 2011a).
Differences in placentation among mammals make the search for a model that satisfies these criteria a challenge. Artiodactyla, or cloven-hoofed mammals, have a highly evolved, non-invasive placental type that reduces the usefulness of traditional large animal models such as sheep for the study of trophoblast invasion (Wildman et al., 2006). Although cost and availability are limiting, non-human primate placentas are most similar to those in humans. However, even among primates, spontaneous preeclampsia has only been reported in baboon twins (Hennessy et al., 1997) and in the patas monkey, and not all of the current criteria for preeclampsia were assessed in these animals (Gille et al., 1977; Palmer et al., 1979). Similar reports of preeclampsia in the guinea pig also predate the establishment of current criteria and might represent ketosis (or toxemia) rather than preeclampsia (Rogers et al., 1964; Ganaway and Allen, 1971). In veterinary medicine, ketosis of pregnancy, which is a well-defined condition in ruminants, is called pregnancy toxemia, a term that often was used in the past for preeclampsia (Brozos et al., 2011). Among laboratory models, the guinea pig and other caviomorph rodents (e.g. degus) have a placental morphology and degree of placental invasion that is most like those found in humans, including the presence of populations of cells that resemble cytotrophoblast cells and extravillous trophoblast cells (Mess et al., 2007).
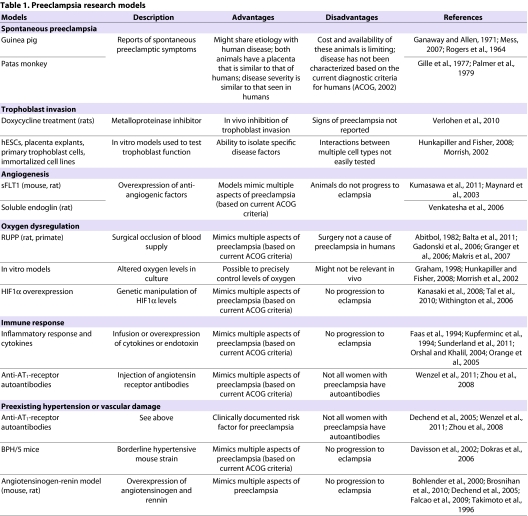
Nonetheless, the vast majority of work with animal models of preeclampsia has been performed in the rat and mouse. These rodents share a hemochorial placental type with humans, and their placentas display both interstitial and endovascular trophoblast invasion as well as remodeling of maternal arteries, albeit to a lesser extent than in humans. Rat placentas exhibit more invasion than mouse placentas. Although they do not progress to eclampsia, there are numerous rodent models that display some or all of the key features of preeclampsia. These have been extremely useful in elucidating the pathophysiology and potential causes of the disease. In the following sections, we describe the currently available in vitro and in vivo models that are used to study certain aspects of preeclampsia (summarized in Table 1).
Table 1.
Preeclampsia research models
Models of trophoblast invasion
As discussed, poor trophoblast invasion of maternal spiral arteries is a key feature of preeclampsia. There are multiple in vitro models of trophoblast invasion, including cultured placental explants, primary trophoblast cells, human embryonic stem cells and human choriocarcinoma cells (Fig. 2). These cells can be cultured under typical conditions on plastic dishes, or using invasion chambers coated with Matrigel or other extracellular matrix substitutes (Hunkapiller and Fisher, 2008). Co-culture of primary or transformed trophoblast cells with endothelial monolayers, explanted capillaries or vessels generated in vitro has also been used, and might be more relevant for the assessment of endovascular invasion (Hunkapiller and Fisher, 2008). An animal model that directly tests the ability of reduced trophoblast invasion to cause preeclamptic symptoms has not been reported. One potential model for this aspect of the disease involves administration of doxycycline to pregnant rats. In this model, doxycycline acts as a matrix metalloproteinase inhibitor, and reduces spiral artery remodeling and trophoblast invasion (Verlohren et al., 2010). The authors of this study used doxycycline administration in an existing transgenic model of preeclampsia (the renin-angiotensin model discussed below), but did not report whether doxycycline treatment alone causes preeclampsia symptoms.
Fig. 2.
Factors contributing to the pathophysiology of preeclampsia. The multiple factors that have been proposed to contribute to preeclampsia can be divided into four main categories. Biological models have been used to show that factors in each category contribute to the main symptoms of preeclampsia (hypertension and proteinuria). In addition, these factors influence each other. For example, placental oxygen disruption might impair trophoblast invasion and vice-versa; increased expression of inflammatory cytokines increases expression of anti-angiogenic sFLT-1; autoimmune responses increase placental HIF1α expression; and trophoblast death due to hypoxia might increase autoantibody production.
Modeling the anti-angiogenic response
Overexpression of anti-angiogenic factors in rodents represents one important class of animal models of preeclampsia (Fig. 2). Overexpression of circulating sFLT-1 in pregnant rats (Maynard et al., 2003) or in the placenta of pregnant mice (Kumasawa et al., 2011) is sufficient to cause the hypertension, proteinuria and renal damage characteristic of preeclampsia. Overexpression of soluble endoglin (sENG) in pregnant rats also increases blood pressure and proteinuria, although not to the same extent as overexpression of sFLT-1, and increases are most severe with simultaneous overexpression of sFLT-1 and sENG (Venkatesha et al., 2006). The effect of sFLT-1 is not dependent on pregnancy: hypertension is also observed in sFLT-1-injected non-pregnant rats (Maynard et al., 2003). Thus, these models might be most relevant for studying the downstream pathophysiology and treatment of preeclampsia, rather than its initial, pregnancy-specific cause.
Models of oxygen dysregulation
Several experimental models have been used to explore the role of oxygen deprivation and hypoxia-reperfusion injury in the pathophysiology of preeclampsia (Fig. 2). Particularly useful as a test of this hypothesis is the rat reduced uterine perfusion pressure (RUPP) model, in which the ovarian arteries that supply the uterus are surgically narrowed to reduce uterine blood (and oxygen) flow (Abitbol, 1982). Although similar approaches have been taken in several other species, including primates (Makris et al., 2007), this has been most commonly used in the rat, resulting in nearly 80 publications. The RUPP model exhibits proteinuria, hypertension, increased levels of sFLT-1 and the inflammatory cytokine interleukin-6 (IL-6), reduced levels of PLGF, and fetal growth restriction (Gadonski et al., 2006; Granger et al., 2006; Balta et al., 2011; George et al., 2011), making it an excellent potential model to test treatments that address any of these symptoms of preeclampsia. However, placentas in the RUPP model do not exhibit reduced trophoblast invasion, a key aspect of preeclampsia. Moreover, the fundamental drawback of this model is that surgical occlusion of arteries is not the cause of the human disease (McCarthy et al., 2011b).
A related hypothesis states that hypoxia is not only a result of insufficient trophoblast invasion of spiral arteries, but a cause of it. This has chiefly been tested through in vitro models that allow precise control of local oxygen concentrations. These models have been used to examine the effect of hypoxia and hypoxia-reperfusion injury on extravillous trophoblast differentiation and invasion. In both primary first trimester placental cells (Hunkapiller and Fisher, 2008) and embryonic-stem-cell-derived trophoblast (J.M.S., D.J.S. and L.C.S., unpublished observations), invasion through a Matrigel matrix is inhibited by hypoxic conditions. Conversely, in HTR-8/SVneo cells (immortalized cells derived from extravillous trophoblast), hypoxia enhances invasion in vitro (Graham et al., 1998). Similarly, exposure of whole pregnant rats to hypoxia via a hyperbaric chamber was found to stimulate trophoblast cell invasion in vivo (Rosario et al., 2008).
Hypoxia-inducible factor 1-α (HIF-1α) is a transcription factor that plays a key role in mediating cellular and systemic responses to hypoxia. Multiple methods of inducing excess HIF-1α have been used to assess whether hypoxia plays a causal role in preeclampsia. These include HIF-1α-overexpressing transgenic mice (Tal et al., 2010), knock down of the HIF-1α inhibitor CITED2 (Withington et al., 2006) and knockout of the COMT enzyme, which produces the HIF-1α inhibitor 2-methoxyestradiol (2-ME) (Kanasaki et al., 2008). These models show incomplete remodeling of maternal spiral arteries, fetal and placental growth restriction, hypertension, and proteinuria. By contrast, injection of 2-ME suppresses HIF-1α in the COMT model, and reverses symptoms. However, the ability of these models to reproduce many features of the human disease does not distinguish whether it is hypoxia per se or inappropriate activation of the hypoxia pathway that results in preeclampsia (Cannagia and Winter, 2002; Kanasaki et al., 2008; Rolfo et al., 2010). Regardless of which hypothesis is correct, models that induce a hypoxic response in the placenta seem to best recapitulate multiple aspects of preeclampsia, and could be useful to test potential treatments for the disorder.
Case study.
A 31-year-old female, pregnant with her first child, at 30 0/7 weeks of gestation presented to the obstetrics triage unit with a chief complaint of decreased fetal movement. Fetal heart monitoring showed a reactive fetal tracing (indicating a sign of fetal well-being). The mother’s blood pressure was found to be 170/90 on two separate occasions. Laboratory evaluation revealed a normal hemoglobin and hematocrit (Hgb and Hct) with no evidence of hemoconcentration, normal platelets, and mild elevations in uric acid and liver function tests (AST and ALT). The patient was admitted to labor and delivery with a diagnosis of severe preeclampsia for continuous monitoring, serial laboratory evaluations and a course of betamethasone to accelerate fetal lung maturity. She was started on magnesium sulfate for seizure prophylaxis. At 2 hours after admission, the patient complained of a sudden onset of severe (10/10) epigastric pain. Repeat laboratory tests demonstrated thrombocytopenia with a platelet count of 70,000, and AST and ALT each >ten times the upper limit of normal. Cervical exam was unfavorable, indicating a very low likelihood of successful induction of labor. The patient was diagnosed with HELLP syndrome and delivered by emergency Cesarean section under general anesthesia. Immediately after delivery, the patient’s blood pressure returned to normal. Serial laboratory evaluations showed an immediate reversal of all laboratory abnormalities: all values returned to within normal range within 24 hours. Magnesium sulfate was continued for 24 hours after delivery. The patient was discharged from the hospital on post-operative day number 3. The infant remained in the neonatal intensive care unit.
The idea that preeclampsia results from inappropriate activation of hypoxic factors, particularly HIF-1α, was proposed partly on the basis that multiple markers of oxidative stress are observed in preeclamptic placentas (Burton and Jauniaux, 2011). Such stress markers would not be predicted to be induced by hypoxia alone. An alternative explanation for this observation posits that hypoxia-reperfusion injury – rather than hypoxia alone – plays a causal role in preeclampsia. According to this theory, failed trophoblast-mediated remodeling of high-resistance maternal spiral arteries into non-vasoactive blood conduits creates alternating periods of hypoxia and normoxia as the vessels contract and relax (Hung et al., 2001). Using cultured term placental fragments as an in vitro model, it has been shown that reoxygenation of hypoxic tissue results in the production of pro-inflammatory cytokines and sFLT-1 (Hung et al., 2001), but no animal model has been developed to directly test whether hypoxia-reperfusion injury plays a role in preeclampsia. Perhaps the closest model available for testing this hypothesis is the peroxiredoxin-III-knockout mouse, a model of oxidative damage in which placental lipid peroxidation and tumor necrosis factor-α (TNFα) production are enhanced (Li et al., 2010). However, these animals do not exhibit hypertension or systemic endothelial changes.
Immune models
There are several lines of evidence supporting a role for maternal immune response in the development of preeclampsia (Fig. 2). First, several immune-associated risk factors increase the probability that a woman will develop preeclampsia, including preexisting autoimmune disease (Duckitt and Harrington, 2005; Trogstad et al., 2011). Second, primiparity, a change of partner and a short initial coitus-to-conception interval are all risk factors for preeclampsia, suggesting that the response to paternal antigens plays a role (Basso et al., 2001; Trogstad et al., 2011). This hypothesis is supported by the ability of seminal plasma to suppress the female recipient’s response to paternal antigens (Maitra et al., 2009; Robertson et al., 2009). Finally, concentrations of inflammatory cytokines are significantly increased, and placental production of the anti-inflammatory cytokine IL-10 is decreased, in women with preeclampsia (Kupferminc et al., 1994; Vince et al., 1995; Makris et al., 2006).
Multiple animal models have been developed to examine the role of the immune response, particularly inflammation, in the pathogenesis of preeclampsia. Administration of low-dose endotoxin (a bacterial derivative that stimulates the immune response) to pregnant rats results in hypertension and proteinuria, but increases in anti-angiogenic factors have not been reported in this model (Faas et al., 1994). Two inflammatory cytokines that are elevated in the serum of women with preeclampsia, TNFα (Kupferminc et al., 1994; Benyo et al., 2001) and IL-6 (Vince et al., 1995), have been used to create animal models that demonstrate the role of inflammation in preeclampsia. TNFα-infused pregnant rats and baboons exhibit elevated blood pressure, increased urinary protein and elevated circulating concentrations of sFLT-1 (LaMarca et al., 2005; Sunderland et al., 2011). In addition, IL-6 administration causes similar increases in blood pressure and proteinuria in pregnant rats, although sFLT-1 levels in these animals were not assessed (Orshal and Khalil, 2004). Conversely, exposure of an IL-10-knockout mouse to a hypoxic environment during pregnancy resulted in preeclampsia symptoms, whereas only fetal growth restriction occurred in wild-type mice exposed to hypoxia (Lai et al., 2011). Inhibition of IL-10 by passive immunization (i.e. with a monoclonal antibody to IL-10) during early gestation increases blood pressure in pregnant baboons (Orange et al., 2005).
Autoantibodies to angiotensin II type I (AT1) receptors and phospholipids have been seen in some women with preeclampsia, and these might increase disease risk (Redman and Sargent, 2010; Abou-Nassar et al., 2011). Thus, passive immunization against autoantigens has also been used to produce animal models of preeclampsia. In both rats and mice, injection of anti-AT1-receptor antibodies during pregnancy induces symptoms of preeclampsia, including hypertension, proteinuria and defects in vascular remodeling (Zhou et al., 2008; Wenzel et al., 2011). Interestingly, this treatment also increases HIF-1α expression, suggesting a potential pathophysiological link between hypoxic and immune factors (Wenzel et al., 2011). It has also been suggested that hypoxia results in trophoblast cell death and increased shedding of paternal antigens into the maternal circulatory system, thereby triggering maternal allogenic immune responses (Johansen et al., 1999).
Models of preexisting risk of hypertension and vascular damage
The severity and sudden consequences of preeclampsia distinguish it from chronic hypertension. However, because it is possible that some young women have undiagnosed hypertension and then do not present for prenatal care until after 20 weeks of gestation, it can be clinically difficult to distinguish between a patient with pregnancy-induced hypertension and a pregnant patient who had pre-existing hypertension. Preexisting hypertension prior to pregnancy, a family history of hypertension or previous vascular damage from diabetes all increase the risk of developing preeclampsia (Scazzocchio and Figueras, 2011; Trogstad et al., 2011). It is therefore important to study models that assess the role of underlying hypertension in the evolution of preeclampsia. The BPH/5 mouse strain, which is mildly hypertensive, has been used to study the link between preexisting hypertension and preeclampsia. These mice develop multiple preeclampsia-like symptoms, including late gestational hypertension, proteinuria, endothelial dysfunction, poor placental development and abnormal maternal uterine arteries (Davisson et al., 2002; Dokras et al., 2006). This important model has been used to test potential therapies for preeclampsia that could be used to treat patients with preexisting hypertension, including administration of the pro-angiogenic factor VEGF121, which prevents the development of preeclampsia-like symptoms in BPH/5 mice (Woods et al., 2010).
Many studies have explored the link between general hypertension and preeclampsia through alterations in the renin-angiotensin system, a major endocrine regulator of blood pressure. The model described above involving injection of anti-AT1-receptor antibodies exhibits preeclampsia symptoms not just because of an immune response, but because the antibodies activate the AT1 receptor, which mediates most of the blood-pressure-increasing activities of angiotensin II (Zhou et al., 2008). This has been shown by co-injection of an AT1 receptor antagonist and anti-AT1-receptor autoantibodies; the antagonist blocks the ability of the autoantibodies to trigger preeclampsia in pregnant mice (Zhou et al., 2008). Furthermore, human angiotensinogen-overexpressing female mice or rats mated with human renin-overexpressing males develop pregnancy-specific symptoms resembling preeclampsia, including hypertension and intrauterine growth restriction (Takimoto et al., 1996; Falcao et al., 2009; Brosnihan et al., 2010). Mouse offspring with both transgenes also exhibit hypertension outside of pregnancy (Falcao et al., 2009). The induction of preeclampsia signs is dependent on parent of origin such that renin and angiotensin are expressed in the placenta on the fetal and maternal sides, respectively, of the maternal-fetal interface (Takimoto-Ohnishi et al. 2005; Brosnihan et al., 2010). Finally, anti-AT1-receptor autoantibodies have been found in the angiotensinogen-renin model, suggesting yet another mode of action (Dechend et al., 2005).
Translation of preeclampsia models into clinical testing and treatments
The various models discussed above have provided many insights into the processes and factors that contribute to preeclampsia. In future, the use of in vitro models will continue to deepen our understanding of normal and pathological trophoblast differentiation and invasion. These models, however, are limited in their ability to explain a disease of complex interactions and effects on protean tissues. The development of in vivo animal models, particularly in the rat and mouse, has added to in vitro approaches by replicating many of the essential features of preeclampsia. However, no single model recapitulates all aspects of the clinical syndrome, and none of them accurately models progression of the disease from preeclampsia to its most dangerous endpoint, eclampsia. In fact, the only successful drug used in the treatment of preeclampsia is magnesium sulfate. Although there are recent data suggesting that magnesium sulfate has neuroprotective effects on the fetus (Reeves et al., 2011), it is classically used in the treatment of preeclamptic women exclusively for the prevention of convulsions associated with eclampsia. Delivery of the pregnancy remains the only standard and effective treatment of preeclampsia, and preterm birth as the result of this treatment is the major cause of perinatal morbidity and mortality.
Despite their drawbacks, in vitro and animal models of preeclampsia can still be used fruitfully to explore preeclampsia’s causes and potential treatments. For instance, models have been used to investigate the fairly controversial issue of low-dose aspirin treatment for the prevention of preeclampsia. The rationale for this treatment was based on the large amount of in vitro and animal data linking preeclampsia to impaired placental perfusion and ischemia, and, furthermore, on data linking ischemia to endothelial dysfunction and platelet activation (Janes et al., 1995) and consumption (Redman et al., 1978). Aspirin irreversibly inhibits the production of thromboxine A2 in platelets and thereby inhibits platelet aggregation, so the use of this relatively common and presumably safe medication seemed to offer a biologically plausible intervention. Additional in vitro and animal studies suggested that early abnormalities in trophoblast-mediated maternal spiral artery remodeling (Abitbol, 1982; Balta et al., 2011; Hung et al., 2001; Makris et al., 2007; Li et al., 2010) might result in hypoxia-reoxygenation injury in the placenta secondary to oxidative damage. These studies provide further rationale for use of low-dose aspirin for the prevention of preeclampsia, owing to a different mechanism of action: the known ability of aspirin to modulate thromboxane- but not prostacyclin-mediated vasoconstriction (Masotti et al., 1979; Thorp et al., 1988).
Although studies on the use of low-dose aspirin for the prevention of preeclampsia have flooded the literature since the 1980s (Bujold et al., 2010; Rossi and Mullin, 2011; Sibai et al., 1993; Subtil et al., 2003), there remains considerable controversy regarding its efficacy. Despite a large meta-analysis reporting a small benefit of aspirin in preventing preeclampsia [relative risk (RR), 0.9; confidence interval (CI), 0.83–0.97] (Askie et al., 2007), two other very recent meta-analyses on this topic came to opposing conclusions. In 2011, Rossi and Mullin used pooled data from approximately 5000 women at high risk and 5000 women at low risk for preeclampsia and reported no effect of low-dose aspirin in the prevention of the disease (Rossi and Mullin, 2011). In 2010, Bujold et al. pooled data from over 11,000 women enrolled in randomized controlled trials evaluating low-dose aspirin in the treatment of pregnant women at moderate or high risk for preeclampsia (Bujold et al., 2010). They concluded that women who initiated treatment at less than 16 weeks of gestation had an RR of 0.47 (CI, 0.34–0.65) for developing preeclampsia and a 0.09 RR (CI 0.02–0.37) for developing severe preeclampsia compared with controls. Aspirin treatment started after 16 weeks of gestation did not prevent disease. Such markedly different conclusions might be explained by differences in the chosen study populations. The Rossi study did not stratify patients by time of aspirin treatment initiation during gestation. Evidence that abnormalities in placental vascular flow characteristics and in serum biomarkers can be seen among women at high risk for preeclampsia as early as 7 weeks of gestation (Cnossen et al., 2008; Salomon et al., 2003) indicates that early initiation might be important in disease prevention. Other design differences between the studies conducted by Rossi and Mullin versus Bujold et al. point to the possibility that aspirin treatment is most effective in moderate- to high-risk patients.
The quest to identify disease biomarkers that would allow for accurate and early prediction of preeclampsia has intensified in the past decade. A combination of in vitro data, results from animal model studies and clinical investigations has been used to develop diagnostics based on maternal characteristics, ultrasonographic velocity measurements, and serum-, urine- and tissue-based assays to identify pregnant women who are most likely to develop preeclampsia (Carty et al., 2011; Goetzinger et al., 2010; Kenny et al., 2010; Leslie et al., 2011). In addition, a recent report used chorionic villus samples taken from pregnant women at 11 weeks of gestation to develop an mRNA profile for those destined to become preeclamptic (Farina et al., 2011). The authors of this study suggest that alterations in IL-8, matrix metal-loproteinase-9, human leukocyte antigen-G (HLA-G) and chemokine (CXC motif) ligand 10 are potentially useful for assessing risk, but that altered expressions of neurokinin B and HLA-C are the most predictive. Although none of the many biomarker profiles suggested to date optimally predicts the disease, the latter study demonstrates one of the novel approaches being taken to allow early detection and suggests that multiple markers will need to be included to develop a useful predictive test.
Basic and clinical research opportunities.
To develop an animal model that manifests all the signs of preeclampsia and progresses to eclampsia.
To develop an animal model that directly tests the ability of reduced trophoblast invasion to cause the features of preeclampsia.
To determine whether there is a single common etiology of preeclampsia, or whether it represents a class of related diseases with different etiologies.
To use relevant animal models to test potential drug therapies for the treatment of some or all forms of preeclampsia.
To identify key biomarkers of preeclampsia that will reliably enable early diagnosis of the disease.
The future
Despite the publication of over 25,000 articles on the etiology, prediction, diagnosis and treatment of preeclampsia, many basic questions remain. Is it one or many diseases? Can we accurately predict those women who will manifest the disease by using a single set of parameters? If diagnosed early enough, can the disorder be prevented? If so, what will an effective prevention strategy entail? Can we reverse a process that might begin with dysregulation of trophoblast invasion at its earliest stages? Many models have been developed to address these questions, but many others must be developed before we have the necessary tools to fully understand this complex disorder. As we can currently model the vascular pathology of preeclampsia with some success, it is most likely that targeting vascular abnormalities will be the focus of emerging therapies (e.g. statin therapy). By contrast, basic research will probably focus on identifying and modeling the initiation of the disease process, an area in which there is little consensus. The field remains in desperate need of bold investigators, innovative modeling approaches and new insights into pathophysiology.
Footnotes
COMPETING INTERESTS
The authors declare that they have no competing or financial interests.
FUNDING
This work was funded by the National Institutes of Health [grant number 5R01HD067759 (L.C.S. and D.J.S.)].
REFERENCES
- Abitbol M. M. (1982). Simplified technique to produce toxemia in the rat: considerations on cause of toxemia. Clin. Exp. Hypertens. B 1, 93–103 [DOI] [PubMed] [Google Scholar]
- Abou-Nassar K., Carrier M., Ramsay T., Rodger M. A. (2011). The association between antiphospholipid antibodies and placenta mediated complications: a systematic review and meta-analysis. Thromb. Res. 128, 77–85 [DOI] [PubMed] [Google Scholar]
- ACOG (2002). American College of Obstetricians and Gynecologists practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Int. J. Gynaecol. Obstet. 77, 67–75 [PubMed] [Google Scholar]
- Aquilina J., Harrington K. (1996). Pregnancy hypertension and uterine artery Doppler ultrasound. Curr. Opin. Obstet. Gynecol. 8, 435–440 [PubMed] [Google Scholar]
- Askie L. M., Duley L., Henderson-Smart D. J., Stewart L. A. (2007). Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 369, 1791–1798 [DOI] [PubMed] [Google Scholar]
- Balta O., Boztosun A., Deveci K., Gulturk S., Ekici F., Kaya A., Cetin A., Cetin M. (2011). Reduced uterine perfusion pressure model is not successful to mimic severe preeclampsia. Placenta 32, 675–680 [DOI] [PubMed] [Google Scholar]
- Basso O., Christensen K., Olsen J. (2001). Higher risk of pre-eclampsia after change of partner. An effect of longer interpregnancy intervals? Epidemiology 12, 624–629 [DOI] [PubMed] [Google Scholar]
- Benyo D. F., Smarason A., Redman C. W., Sims C., Conrad K. P. (2001). Expression of inflammatory cytokines in placentas from women with preeclampsia. J. Clin. Endocrinol. Metab. 86, 2505–2512 [DOI] [PubMed] [Google Scholar]
- Berg C. J., Mackay A. P., Qin C., Callaghan W. M. (2009). Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993–1997 and 2001–2005. Obstet. Gynecol. 113, 1075–1081 [DOI] [PubMed] [Google Scholar]
- Brosnihan K. B., Hering L., Dechend R., Chappell M. C., Herse F. (2010). Increased angiotensin II in the mesometrial triangle of a transgenic rat model of preeclampsia. Hypertension 55, 562–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozos C., Mavrogianni V. S., Fthenakis G. C. (2011). Treatment and control of peri-parturient metabolic diseases: pregnancy toxemia, hypocalcemia, hypomagnesemia. Vet. Clin. North Am. Food. Anim. Pract. 27, 105–113 [DOI] [PubMed] [Google Scholar]
- Bujold E., Roberge S., Lacasse Y., Bureau M., Audibert F., Marcoux S., Forest J. C., Giguere Y. (2010). Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet. Gynecol. 116, 402–414 [DOI] [PubMed] [Google Scholar]
- Burton G. J., Jauniaux E. (2011). Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 25, 287–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G. J., Charnock-Jones D. S., Jauniaux E. (2009). Regulation of vascular growth and function in the human placenta. Reproduction 138, 895–902 [DOI] [PubMed] [Google Scholar]
- Caniggia I., Winter J. L. (2002). Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies – a review. Placenta 23 Suppl. A, S47–S57 [DOI] [PubMed] [Google Scholar]
- Caniggia I., Winter J., Lye S. J., Post M. (2000). Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta 21 Suppl. A, S25–S30 [DOI] [PubMed] [Google Scholar]
- Carty D. M., Siwy J., Brennand J. E., Zurbig P., Mullen W., Franke J., McCulloch J. W., Roberts C. T., North R. A., Chappell L. C., et al. (2011). Urinary proteomics for prediction of preeclampsia. Hypertension 57, 561–569 [DOI] [PubMed] [Google Scholar]
- Cnossen J. S., Vollebregt K. C., de Vrieze N., ter Riet G., Mol B. W., Franx A., Khan K. S., van der Post J. A. (2008). Accuracy of mean arterial pressure and blood pressure measurements in predicting pre-eclampsia: systematic review and meta-analysis. BMJ 336, 1117–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven C. M., Morgan T., Ward K. (1998). Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta 19, 241–252 [DOI] [PubMed] [Google Scholar]
- Davisson R. L., Hoffmann D. S., Butz G. M., Aldape G., Schlager G., Merrill D. C., Sethi S., Weiss R. M., Bates J. N. (2002). Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension 39, 337–342 [DOI] [PubMed] [Google Scholar]
- Dechend R., Gratze P., Wallukat G., Shagdarsuren E., Plehm R., Brasen J. H., Fiebeler A., Schneider W., Caluwaerts S., Vercruysse L., et al. (2005). Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension 45, 742–746 [DOI] [PubMed] [Google Scholar]
- Dokras A., Hoffmann D. S., Eastvold J. S., Kienzle M. F., Gruman L. M., Kirby P. A., Weiss R. M., Davisson R. L. (2006). Severe feto-placental abnormalities precede the onset of hypertension and proteinuria in a mouse model of preeclampsia. Biol. Reprod. 75, 899–907 [DOI] [PubMed] [Google Scholar]
- Duckitt K., Harrington D. (2005). Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ 330, 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faas M. M., Schuiling G. A., Baller J. F., Visscher C. A., Bakker W. W. (1994). A new animal model for human preeclampsia: ultra-low-dose endotoxin infusion in pregnant rats. Am. J. Obstet. Gynecol. 171, 158–164 [DOI] [PubMed] [Google Scholar]
- Falcao S., Stoyanova E., Cloutier G., Maurice R. L., Gutkowska J., Lavoie J. L. (2009). Mice overexpressing both human angiotensinogen and human renin as a model of superimposed preeclampsia on chronic hypertension. Hypertension 54, 1401–1407 [DOI] [PubMed] [Google Scholar]
- Farina A., Zucchini C., De Sanctis P., Morano D., Sekizawa A., Purwosunu Y., Okai T., Rizzo N. (2011). Gene expression in chorionic villous samples at 11 weeks of gestation in women who develop pre-eclampsia later in pregnancy: implications for screening. Prenat. Diagn. 31, 181–185 [DOI] [PubMed] [Google Scholar]
- Figueras F., Gardosi J. (2011). Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am. J. Obstet. Gynecol. 204, 288–300 [DOI] [PubMed] [Google Scholar]
- Fonseca E. B., Celik E., Parra M., Nicolaides K. H., Fetal Medicine Foundation Second Trimester Screening Group (2007). Progesterone and the risk of preterm birth among women with a short cervix. N. Engl. J. Med. 357, 462–469 [DOI] [PubMed] [Google Scholar]
- Gadonski G., LaMarca B. B., Sullivan E., Bennett W., Chandler D., Granger J. P. (2006). Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension 48, 711–716 [DOI] [PubMed] [Google Scholar]
- Ganaway J. R., Allen A. M. (1971). Obesity predisposes to pregnancy toxemia (ketosis) of guinea pigs. Lab. Anim. Sci. 21, 40–44 [PubMed] [Google Scholar]
- George E. M., Cockrell K., Aranay M., Csongradi E., Stec D. E., Granger J. P. (2011). Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension 57, 941–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille J. H., Moore D. G., Sedgwick C. J. (1977). Placental infarction: a sign of pre-eclampsia in a patas monkey (Erythrocebus patas). Lab. Anim. Sci. 27, 119–121 [PubMed] [Google Scholar]
- Goetzinger K. R., Singla A., Gerkowicz S., Dicke J. M., Gray D. L., Odibo A. O. (2010). Predicting the risk of pre-eclampsia between 11 and 13 weeks’ gestation by combining maternal characteristics and serum analytes, PAPP-A and free beta-hCG. Prenat. Diagn. 30, 1138–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham C. H., Fitzpatrick T. E., McCrae K. R. (1998). Hypoxia stimulates urokinase receptor expression through a heme protein-dependent pathway. Blood 91, 3300–3307 [PubMed] [Google Scholar]
- Granger J. P., LaMarca B. B., Cockrell K., Sedeek M., Balzi C., Chandler D., Bennett W. (2006). Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol. Med. 122, 383–392 [DOI] [PubMed] [Google Scholar]
- Hennessy A., Gillin A. G., Painter D. M., Kirwan P. J., Thompson J. F., Horvath J. S. (1997). Evidence for preeclampsia in a baboon pregnancy with twins. Hypertens. Pregnancy 16, 223–228 [Google Scholar]
- Hung T. H., Skepper J. N., Burton G. J. (2001). In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am. J. Pathol. 159, 1031–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller N. M., Fisher S. J. (2008). Chapter 12. Placental remodeling of the uterine vasculature. Methods Enzymol. 445, 281–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes S. L., Kyle P. M., Redman C., Goodall A. H. (1995). Flow cytometric detection of activated platelets in pregnant women prior to the development of pre-eclampsia. Thromb. Haemost. 74, 1059–1063 [PubMed] [Google Scholar]
- Johansen M., Redman C. W., Wilkins T., Sargent I. L. (1999). Trophoblast deportation in human pregnancy – its relevance for pre-eclampsia. Placenta 20, 531–539 [DOI] [PubMed] [Google Scholar]
- Kanasaki K., Palmsten K., Sugimoto H., Ahmad S., Hamano Y., Xie L., Parry S., Augustin H. G., Gattone V. H., Folkman J., et al. (2008). Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 453, 1117–1121 [DOI] [PubMed] [Google Scholar]
- Kaufmann P., Black S., Huppertz B. (2003). Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol. Reprod. 69, 1–7 [DOI] [PubMed] [Google Scholar]
- Kenny L. C., Broadhurst D. I., Dunn W., Brown M., North R. A., McCowan L., Roberts C., Cooper G. J., Kell D. B., Baker P. N. (2010). Robust early pregnancy prediction of later preeclampsia using metabolomic biomarkers. Hypertension 56, 741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong T. Y., De Wolf F., Robertson W. B., Brosens I. (1986). Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br. J. Obstet. Gynaecol. 93, 1049–1059 [DOI] [PubMed] [Google Scholar]
- Kumasawa K., Ikawa M., Kidoya H., Hasuwa H., Saito-Fujita T., Morioka Y., Takakura N., Kimura T., Okabe M. (2011). Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc. Natl. Acad. Sci. USA 108, 1451–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferminc M. J., Peaceman A. M., Wigton T. R., Rehnberg K. A., Socol M. L. (1994). Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am. J. Obstet. Gynecol. 170, 1752–1757; discussion 1757–1759 [PubMed] [Google Scholar]
- Lai Z., Kalkunte S., Sharma S. (2011). A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension 57, 505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca B. B., Bennett W. A., Alexander B. T., Cockrell K., Granger J. P. (2005). Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 46, 1022–1025 [DOI] [PubMed] [Google Scholar]
- Leslie K., Thilaganathan B., Papageorghiou A. (2011). Early prediction and prevention of pre-eclampsia. Best Pract. Res. Clin. Obstet. Gynaecol. 25, 343–354 [DOI] [PubMed] [Google Scholar]
- Levine R. J., Maynard S. E., Qian C., Lim K. H., England L. J., Yu K. F., Schisterman E. F., Thadhani R., Sachs B. P., Epstein F. H., et al. (2004). Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 350, 672–683 [DOI] [PubMed] [Google Scholar]
- Li L., Obinata M., Hori K. (2010). Role of peroxiredoxin III in the pathogenesis of pre-eclampsia as evidenced in mice. Oxid. Med. Cell. Longev. 3, 71–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U., Davis S., Reilly C. M., Li L. (2009). Differential regulation of Foxp3 and IL-17 expression in CD4 T helper cells by IRAK-1. J. Immunol. 182, 5763–5769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris A., Xu B., Yu B., Thornton C., Hennessy A. (2006). Placental deficiency of interleukin-10 (IL-10) in preeclampsia and its relationship to an IL10 promoter polymorphism. Placenta 27, 445–451 [DOI] [PubMed] [Google Scholar]
- Makris A., Thornton C., Thompson J., Thomson S., Martin R., Ogle R., Waugh R., McKenzie P., Kirwan P., Hennessy A. (2007). Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 71, 977–984 [DOI] [PubMed] [Google Scholar]
- Masotti G., Galanti G., Poggesi L., Abbate R., Neri Serneri G. G. (1979). Differential inhibition of prostacyclin production and platelet aggregation by aspirin. Lancet 2, 1213–1217 [DOI] [PubMed] [Google Scholar]
- Maynard S. E., Min J. Y., Merchan J., Lim K. H., Li J., Mondal S., Libermann T. A., Morgan J. P., Sellke F. W., Stillman I. E., et al. (2003). Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 111, 649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy F. P., Kingdom J. C., Kenny L. C., Walsh S. K. (2011a). Animal models of preeclampsia; uses and limitations. Placenta 32, 413–419 [DOI] [PubMed] [Google Scholar]
- McCarthy F. P., Drewlo S., Kingdom J., Johns E. J., Walsh S. K., Kenny L. C. (2011b). Peroxisome proliferator-activated receptor-{gamma} as a potential therapeutic target in the treatment of preeclampsia. Hypertension. [DOI] [PubMed] [Google Scholar]
- Mess A., Zaki N., Kadyrov M., Korr H., Kaufmann P. (2007). Caviomorph placentation as a model for trophoblast invasion. Placenta 28, 1234–1238 [DOI] [PubMed] [Google Scholar]
- Orange S., Rasko J. E., Thompson J. F., Vaughan J., Olive E., Pedler M., Horvath J. S., Hennessy A. (2005). Interleukin-10 regulates arterial pressure in early primate pregnancy. Cytokine 29, 176–185 [DOI] [PubMed] [Google Scholar]
- Orshal J. M., Khalil R. A. (2004). Interleukin-6 impairs endothelium-dependent NO-cGMP-mediated relaxation and enhances contraction in systemic vessels of pregnant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R1013–R1023 [DOI] [PubMed] [Google Scholar]
- Palmer A. E., London W. T., Sly D. L., Rice J. M. (1979). Spontaneous preeclamptic toxemia of pregnancy in the patas monkey (Erythrocebus patas). Lab. Anim. Sci. 29, 102–106 [PubMed] [Google Scholar]
- Redman C. W., Sargent I. L. (2010). Immunology of pre-eclampsia. Am. J. Reprod. Immunol. 63, 534–543 [DOI] [PubMed] [Google Scholar]
- Redman C. W., Bonnar J., Beilin L. (1978). Early platelet consumption in pre-eclampsia. Br. Med. J. 1, 467–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves S. A., Gibbs R. S., Clark S. L. (2011). Magnesium for fetal neuroprotection. Am. J. Obstet. Gynecol. 204, 202.e1–202.e4 [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Hubel C. A. (2009). The two stage model of preeclampsia: variations on the theme. Placenta 30 Suppl. A, S32–S37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. A., Guerin L. R., Bromfield J. J., Branson K. M., Ahlstrom A. C., Care A. S. (2009). Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol. Reprod. 80, 1036–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. B., Klein R., Blumenthal H. T. (1964). Placental permeability in a toxemia-susceptible strain of guinea pigs. Am. J. Obstet. Gynecol. 88, 495–501 [DOI] [PubMed] [Google Scholar]
- Rolfo A., Many A., Racano A., Tal R., Tagliaferro A., Ietta F., Wang J., Post M., Caniggia I. (2010). Abnormalities in oxygen sensing define early and late onset preeclampsia as distinct pathologies. PLoS ONE 5, e13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario G. X., Konno T., Soares M. J. (2008). Maternal hypoxia activates endovascular trophoblast cell invasion. Dev. Biol. 314, 362–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A. C., Mullin P. M. (2011). Prevention of pre-eclampsia with low-dose aspirin or vitamins C and E in women at high or low risk: a systematic review with meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 158, 9–16 [DOI] [PubMed] [Google Scholar]
- Saftlas A. F., Olson D. R., Franks A. L., Atrash H. K., Pokras R. (1990). Epidemiology of preeclampsia and eclampsia in the United States, 1979–1986. Am. J. Obstet. Gynecol. 163, 460–465 [DOI] [PubMed] [Google Scholar]
- Salomon L. J., Benattar C., Audibert F., Fernandez H., Duyme M., Taieb J., Frydman R. (2003). Severe preeclampsia is associated with high inhibin A levels and normal leptin levels at 7 to 13 weeks into pregnancy. Am. J. Obstet. Gynecol. 189, 1517–1522 [DOI] [PubMed] [Google Scholar]
- Scazzocchio E., Figueras F. (2011). Contemporary prediction of preeclampsia. Curr. Opin. Obstet. Gynecol. 23, 65–71 [DOI] [PubMed] [Google Scholar]
- Sibai B. M. (2004). Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet. Gynecol. 103, 981–991 [DOI] [PubMed] [Google Scholar]
- Sibai B. M., Caritis S. N., Thom E., Klebanoff M., McNellis D., Rocco L., Paul R. H., Romero R., Witter F., Rosen M., et al. (1993). Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N. Engl. J. Med. 329, 1213–1218 [DOI] [PubMed] [Google Scholar]
- Subtil D., Goeusse P., Houfflin-Debarge V., Puech F., Lequien P., Breart G., Uzan S., Quandalle F., Delcourt Y. M., Malek Y. M. (2003). Randomised comparison of uterine artery Doppler and aspirin (100 mg) with placebo in nulliparous women: the Essai Regional Aspirine Mere-Enfant study (Part 2). BJOG 110, 485–491 [DOI] [PubMed] [Google Scholar]
- Sunderland N. S., Thomson S. E., Heffernan S. J., Lim S., Thompson J., Ogle R., McKenzie P., Kirwan P. J., Makris A., Hennessy A. (2011). Tumor necrosis factor alpha induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine 56, 192–199 [DOI] [PubMed] [Google Scholar]
- Takimoto E., Ishida J., Sugiyama F., Horiguchi H., Murakami K., Fukamizu A. (1996). Hypertension induced in pregnant mice by placental renin and maternal angiotensinogen. Science 274, 995–998 [DOI] [PubMed] [Google Scholar]
- Takimoto-Ohnishi E., Saito T., Ishida J., Ohnishi J., Sugiyama F., Yagami K., Fukamizu A. (2005). Differential roles of renin and angiotensinogen in the feto-maternal interface in the development of complications of pregnancy. Mol. Endocrinol. 19, 1361–1372 [DOI] [PubMed] [Google Scholar]
- Tal R., Shaish A., Barshack I., Polak-Charcon S., Afek A., Volkov A., Feldman B., Avivi C., Harats D. (2010). Effects of hypoxia-inducible factor-1alpha overexpression in pregnant mice: possible implications for preeclampsia and intrauterine growth restriction. Am. J. Pathol. 177, 2950–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp J. A., Walsh S. W., Brath P. C. (1988). Low-dose aspirin inhibits thromboxane, but not prostacyclin, production by human placental arteries. Am. J. Obstet. Gynecol. 159, 1381–1384 [DOI] [PubMed] [Google Scholar]
- Trogstad L., Magnus P., Stoltenberg C. (2011). Pre-eclampsia: risk factors and causal models. Best Pract. Res. Clin. Obstet. Gynaecol. 25, 329–342 [DOI] [PubMed] [Google Scholar]
- Venkatesha S., Toporsian M., Lam C., Hanai J., Mammoto T., Kim Y. M., Bdolah Y., Lim K. H., Yuan H. T., Libermann T. A., et al. (2006). Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 12, 642–649 [DOI] [PubMed] [Google Scholar]
- Verlohren S., Geusens N., Morton J., Verhaegen I., Hering L., Herse F., Dudenhausen J. W., Muller D. N., Luft F. C., Cartwright J. E., et al. (2010). Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy. Hypertension 56, 304–310 [DOI] [PubMed] [Google Scholar]
- Vince G. S., Starkey P. M., Austgulen R., Kwiatkowski D., Redman C. W. (1995). Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br. J. Obstet. Gynaecol. 102, 20–25 [DOI] [PubMed] [Google Scholar]
- Wagner M. (2001). What every midwife should know about ACOG and VBAC. Critique of ACOG Practice Bulletin #5, July 1999, Vaginal birth after previous cesarean section. Midwifery Today Int. Midwife Fall, 41–43 [PubMed] [Google Scholar]
- Wenzel K., Rajakumar A., Haase H., Geusens N., Hubner N., Schulz H., Brewer J., Roberts L., Hubel C. A., Herse F., et al. (2011). Angiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension 58, 77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman D. E., Chen C., Erez O., Grossman L. I., Goodman M., Romero R. (2006). Evolution of the mammalian placenta revealed by phylogenetic analysis. Proc. Natl. Acad. Sci. USA 103, 3203–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withington S. L., Scott A. N., Saunders D. N., Lopes Floro K., Preis J. I., Michalicek J., Maclean K., Sparrow D. B., Barbera J. P., Dunwoodie S. L. (2006). Loss of Cited2 affects trophoblast formation and vascularization of the mouse placenta. Dev. Biol. 294, 67–82 [DOI] [PubMed] [Google Scholar]
- Woods A. K., Hoffmann D. S., Weydert C. J., Butler S. D., Zhou Y., Sharma R. V., Davisson R. L. (2010). Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice. Hypertension 57, 94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. T., Stefanini M. O., Mac Gabhann F., Kontos C. D., Annex B. H., Popel A. S. (2010). A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. J. Cell. Mol. Med. 14, 528–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C. C., Zhang Y., Irani R. A., Zhang H., Mi T., Popek E. J., Hicks M. J., Ramin S. M., Kellems R. E., Xia Y. (2008). Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat. Med. 14, 855–862 [DOI] [PMC free article] [PubMed] [Google Scholar]