Abstract
Many of the cellular mechanisms underlying host responses to pathogens have been well conserved during evolution. As a result, Drosophila can be used to deconstruct many of the key events in host-pathogen interactions by using a wealth of well-developed molecular and genetic tools. In this review, we aim to emphasize the great leverage provided by the suite of genomic and classical genetic approaches available in flies for decoding details of host-pathogen interactions; these findings can then be applied to studies in higher organisms. We first briefly summarize the general strategies by which Drosophila resists and responds to pathogens. We then focus on how recently developed genome-wide RNA interference (RNAi) screens conducted in cells and flies, combined with classical genetic methods, have provided molecular insight into host-pathogen interactions, covering examples of bacteria, fungi and viruses. Finally, we discuss novel strategies for how flies can be used as a tool to examine how specific isolated virulence factors act on an intact host.
Introduction
Drosophila has emerged as an important model for examining the function of genes that are relevant to diverse human diseases affecting a broad range of cell types (for reviews, see Bier, 2005; Bier and McGinnis, 2008). Additionally, this model organism can serve as a host for a surprising variety of bacterial and viral pathogens. Seminal discoveries in the field of host-pathogen interactions have been made in Drosophila. For example, the Toll signaling pathway, which plays a central role in innate immunity, was first identified in Drosophila, and studies using this model organism have helped to identify and delineate fundamental conserved host genetic pathways involved in barrier formation and maintenance (Mace et al., 2005; Martin and Parkhurst, 2004; Pearson et al., 2009; Ting et al., 2005), innate immune signaling (Agaisse and Perrimon, 2004; Dionne and Schneider, 2008; Ferrandon et al., 2007; Igaki et al., 2010; Ryu et al., 2010), the RNA interference (RNAi) response (Sabin et al., 2010), pathogen engulfment (Meister, 2004), and the evolution of intracellular pathogens (Haselkorn, 2010; Serbus et al., 2008). As discussed in detail below, Drosophila has also been used to identify and analyze the function of pathogen-derived virulence factors (Avet-Rochex et al., 2005; Avet-Rochex et al., 2007; Botham et al., 2008; Guichard et al., 2010; Guichard et al., 2006; Shelly et al., 2009; Guichard et al., 2011).
Many genetic tools are available to address the mechanisms of pathogen action in Drosophila. These include comprehensive genetic screens, or genome-wide RNAi screens, in cell lines and intact flies that can identify host pathways required to defend against pathogens. Reciprocally, it is also possible to search for pathogen-encoded factors that are required for virulence in flies. In vivo studies in flies are greatly facilitated by the ability to direct expression of transgenes encoding host or pathogen proteins in specific cell types using the GAL4-UAS transactivation system (Brand and Perrimon, 1993) or the new independently acting LexA system, which allows for combinatorial expression of genes in distinct or overlapping patterns (Yagi et al., 2010; Pfeiffer et al., 2010). Moreover, it is possible to perform epistasis experiments using combinations of dominant and recessive mutations (or mutants with opposing phenotypes) in a given pathway to determine the sequence in which genes act in that pathway. These versatile tools, combined with the rapid Drosophila life cycle, allow detailed genetic analysis of virulence factors that act on tissues or organs; such experiments would be much more difficult to conduct in vivo in mammalian systems.
In this review, we first outline the basic host defense mechanisms used by Drosophila to resist and respond to invading pathogens to provide context for our discussion of how flies can be used to deconstruct key mechanisms of host-pathogen interactions. We then focus on three related topics: (1) genome-wide RNAi screens in Drosophila cell lines infected with pathogens to identify host pathways for defense or that are exploited by pathogens (e.g. bacteria, fungi, viruses); (2) classical genetic and RNAi screens conducted in intact flies to delineate host defense pathways that are active in specific tissues (e.g. the gut) or to identify important virulence factors produced by the pathogen; and (3) analysis of the function of specific pathogen virulence factors in an intact organism. The studies reviewed here highlight the speed and power of Drosophila genetics for uncovering new pathways and factors in host-pathogen interactions, as well as for characterizing unknown activities of specific virulence factors. Identification of such elements in the host-pathogen relationship should help to guide studies in vertebrate systems and contribute to defining new targets for potential therapeutic intervention.
Brief overview of Drosophila immunity
Two first-order defenses protect metazoans from invasion by pathogens: (1) the external and internal epithelial barriers between the organism and its environment (Fig. 1A), and (2) innate immune mechanisms that have moderate degrees of specificity for detecting and responding to distinct pathogens (Fig. 1B–D). We summarize what is known regarding these two broadly acting systems in Drosophila, which serves as an important framework for our later discussion on strategies used to probe specific host or pathogen factors.
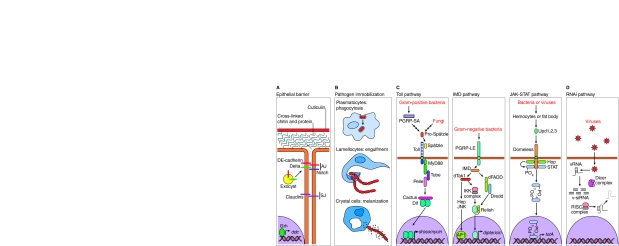
Fig. 1.
Overview of defensive pathways in Drosophila. (A) The epithelial barrier. The epithelial barrier consists of secreted proteins that form the hard outer cuticle, which is composed of an inner layer of cross-linked proteins and chitin (a polysaccharide), and an outer cuticulin layer. Additionally, a tight barrier between cells, consisting of an basolateral adhesive zone [involving adherens junctions (AJs)] and more basal sealing junctions [septate junctions (SJs)] prevents free passage of pathogens, macromolecules and solutes between cells in the paracellular space. In vertebrates, the equivalent of the SJ is the tight junction, which is located apically to the AJ (reviewed in Furuse and Tsukita, 2006). Proteins such as self-adhesive cadherins (DE-cadherin in Drosophila), catenins (not shown) and signaling molecules, such as the Notch receptor and its ligand Delta, are targeted to the AJ by the exocyst complex, the formation of which is initiated by an interaction between Rab11 and Sec15 (not shown; see section on bacterial toxins in the main text, and Fig. 3). AJ proteins link to the actin cytoskeleton to create cytoskeletal continuity between cells, and actin also links to the SJ (e.g. via the ZO1 protein Discs-large and Scribble; not shown). Claudins localized to SJs (in flies) or tight junctions (in vertebrates) play an important role in forming a band-like seal that prevents large molecules or objects from freely passing between cells. (B) Pathogen immobilization. There are three types of blood cells (hemocytes) in Drosophila: plasmatocytes, which bind to cellular pathogens and phagocytose them; lamellocytes, which wrap foreign bodies in sheets to engulf them; and crystal cells, which express the enzymes required to produce and secrete melanin to encase and immobilize pathogens. (C) Simplified schemes of the Toll, IMD and JAK-STAT immune signaling pathways are shown. The Toll signaling pathway mediates the response to many Gram-positive bacteria and fungal pathogens, which in many cases are recognized when secreted PGRPs initiate an extracellular proteolytic cascade culminating in the processing of pro-Spätzle into the mature Spätzle ligand for the Toll receptor. (This indirect mechanism of pathogen detection contrasts with that which occurs in mammals, in which Toll-family-member receptors directly bind to distinct pathogen-associated molecules.) Once activated by binding to Spätzle, Toll recruits a complex of DEATH-domain proteins (MyD88, Tube and Pelle), which results in dissociation of the inhibitory IκB-like protein Cactus from the NFκB-like transcription factor Dif, allowing Dif to translocate into the nucleus to activate expression of Toll-responsive genes, as typified by the AMP encoded by drosomycin. In the IMD pathway, Gram-negative bacteria are detected by a transmembrane PGRP (PGRP-LE), which signals via the cytoplasmic protein IMD. The pathway branches at IMD to activate the dFADD-Dredd complex and the MAPKKK Drosophila TAK1 (dTak1), where the pathway splits again. One branch acts via the IKK complex in concert with the dFADD-Dredd complex to activate the NFκB-like protein Relish by cleaving an inhibitory tail consisting of ankyrin repeats (circles). The DNA-binding domain of Relish then enters the nucleus and activates expression of IMD-responsive genes encoding AMPs, such as diptericin. The other branch emanating from dTak1 activates MAPKKs in the JNK and p38 pathways (at least in mammalian cells). In Drosophila, the MAPKK in the JNK pathway is Hemipterous (Hep). JNK (also known as Bsk in Drosophila) activation eventuates in activation of the AP1 transcription factor. The JAK-STAT pathway: infection of flies with bacteria or viruses leads to the production of signals such as the Unpaired (Upd) ligands, which bind and activate the Domeless receptor (related to vertebrate IL-6 receptor), leading to activation of the fly Janus kinase (JAK) Hopscotch (Hop). Activated Hop phosphorylates receptor-bound signal transducer and activator of transcription (STAT; STAT92E in flies), which then dimerizes, enters the nucleus and activates transcription of effector target genes such as totA (Agaisse and Perrimon, 2004; Folsch et al., 2003). (D) RNAi pathway. Once viruses enter the cell and shed their protective outer coat, viral RNA molecules are exposed to the cytoplasm and form double-stranded secondary structures or double-stranded reverse-transcribed RNA-DNA intermediates. These regions of double-stranded RNA are acted on by the Dicer complex to generate 21-base-pair double-stranded silencing oligonucleotides called viral siRNAs (v-siRNAs), which are then ‘melted’ to generate single strands that are complementary to the viral RNA; this, in combination with the RISC complex, leads to silencing of the viral RNA.
Barrier formation and maintenance
The castle-wall defense system in animals can be thought of as a fortified epithelial tube consisting of an external epidermal covering and an internal component comprising the gut (or endoderm). The formation of both the outer epithelial barrier and the inner intestinal barrier depends on the formation and maintenance of intercellular junctions, and many basic discoveries in this field have been made in Drosophila (Banerjee et al., 2006; Furuse and Tsukita, 2006; Wirtz-Peitz and Zallen, 2009). Such studies have delineated key mechanisms involved in establishing apical-basal polarity, including the assembly of distinct protein complexes at adherens junctions and septate junctions (claudin-dependent junctions that share important similarities with vertebrate tight junctions). One highly conserved feature of this process is the role of the exocyst protein complex in trafficking proteins such as cadherins and cell signaling components to adherens junctions (Andrews et al., 2002; Beronja et al., 2005; Blankenship et al., 2007; Jafar-Nejad et al., 2005; Langevin et al., 2005; Mehta et al., 2005; Murthy et al., 2003; Murthy et al., 2005; Murthy and Schwarz, 2004; Murthy et al., 2010).
The outer epithelial barrier
At first glance, the mammalian epidermis seems very different from that of flies (Fig. 1A), but there are striking parallels with respect to the formation and maintenance of epithelial barriers in the two species, illustrating a probable common ancestral origin. For example, claudin-family proteins forming the tight junctions between epithelial cells seem to have similar functions in both flies (Behr et al., 2003; Nelson et al., 2010; Wu et al., 2004) and mice (Furuse et al., 2002) [see the 2009 article by Furuse for a review on the role of claudins and other tight junction proteins in mammalian epithelia (Furuse, 2009)]. Similarly, the transcription factor Grainyhead (Grh) plays an important role in regulating the expression of genes that are required to form the cross-linked outer epidermal surface both in flies (Bray and Kafatos, 1991) and mice (Matsuki et al., 1998; Ting et al., 2005) (although the set of Grh target genes seems to be different in each species). Grh also regulates genes that are involved in wound repair both in flies (Mace et al., 2005) and mice (Ting et al., 2005).
The inner intestinal barrier
It is noteworthy that Drosophila and vertebrate intestinal epithelia are also similar in several respects. These parallels include: the fact that stem cells play an important role in replacing cells that have undergone pathogen-dependent apoptosis; the sequential deployment of Wnt and Hedgehog (Hh) signaling during the differentiation of intestinal epithelial cells (Pitsouli and Perrimon, 2008; Takashima et al., 2008); and the formation of the morphologically specialized brush border microvilli and the underlying cytoskeletal terminal web (Li et al., 2007; Morgan et al., 1995; Phillips and Thomas, 2006).
A challenge faced by intestinal cells is that they must tolerate commensal bacteria, with which they have a mutualistic relationship (Backhed et al., 2005; Dale and Moran, 2006; Sansonetti and Medzhitov, 2009), while also mounting a vigorous response to pathogens (for a review, see Ryu et al., 2010). One important pathway involved in this distinction controls the production of reactive oxygen species (ROS) by the dual-oxidase (Duox) transmembrane protein (Ha et al., 2009; Ha et al., 2005a; Ha et al., 2005b), which also plays a key role in the human gut (for a review, see Ryu et al., 2010). Genetic analysis in Drosophila has revealed bi-stable control of Duox activity in the gut. In the presence of commensal bacteria and absence of pathogenic species, low-level activation of the immune deficiency (IMD) pathway of the innate immune system (see later) induces negative feedback of the Duox pathway (at both the level of expression and activity), resulting in low basal levels of ROS production. By contrast, when invading pathogens are detected by host immune signaling, expression and activity of Duox components is greatly increased, leading to destruction of the pathogenic bacteria (Ha et al., 2009).
The inducible ROS-producing Duox system works in parallel with other immune pathways, such as the Jun N-terminal kinases (JNK) pathway. JNK signaling is activated in intestinal epithelial cells of adult flies following ingestion of pathogenic Pseudomonas aeruginosa (Apidianakis et al., 2009), which leads to proliferation of intestinal stem cells to compensate for apoptotic loss of mature infected cells (for a review, see Pitsouli et al., 2009). An interesting aspect of this pathogen in Drosophila is that, in combination with an activated oncogenic form of RAS, it can lead to overproliferation of stem cells to form tumors (Apidianakis et al., 2009). Whether the elevated incidence of human cancers of the intestinal tract as a result of associated bacterial infection (Bornschein et al., 2009; Selgrad et al., 2008) is similarly influenced by RAS activation remains to be determined. Another interesting emerging theme is the elucidation of host pathways involved in detecting cell damage in the intestine, which then regulate stem cell mediated repair of the damaged epithelium. These studies have revealed important contributions of the insulin (Amcheslavsky et al., 2009) and TSC-TORC1 (Amcheslavsky et al., 2011) pathways, as well as of Hippo (Hpo)-mediated activation of the JAK-STAT and Epidermal growth factor receptor (EGFR) pathways (Ren et al., 2010). Finally, experiments involving oral infection of flies with Erwinia carotovora, a natural Drosophila pathogen, suggest that gut homeostasis is maintained by active tissue repair of cell damage caused by bacteria (Buchon et al., 2009). The observation that ROS can trigger apoptosis followed by repair in the larval gut (Gupta et al., 2010) suggests that the Duox pathway provides compensatory feedback to pathways controlling apoptosis and stem cells to ensure that host cells damaged by ROS exposure are duly replaced. Overall, these studies provide an excellent foundation for further analysis of how the gut responds to pathogens by repairing damage and differentially responding to commensal versus pathogenic bacteria.
The innate immune response
Broadly speaking, the innate immune response consists of three parts: (1) pathogen immobilization (Fig. 1B), (2) core immune signaling pathways (Toll, IMD and JAK-STAT) (Fig. 1C) and (3) the RNAi pathway (Fig. 1D). Because there have been several excellent reviews describing these pathways, we only summarize here their key elements, as depicted in Fig. 1, and refer the reader to other sources for more in depth descriptions (Agaisse and Perrimon, 2004; Akira et al., 2006; Bhavsar et al., 2007; Brodsky and Medzhitov, 2009; Diacovich and Gorvel, 2010; Dionne and Schneider, 2008; Ferrandon et al., 2007; Folsch et al., 2003; Sansonetti, 2008).
Pathogen immobilization
The most basic innate response to bacterial or fungal infection is a cellular response (Jiravanichpaisal et al., 2006) that immobilizes the invading microbe by phagocytosis, engulfment or a melanization reaction that traps it (Fig. 1B). Pathogens can also be immobilized in flies and other insects by a clotting reaction (Dushay, 2009). Once immobilized, the pathogen can then be either destroyed extracellularly by antimicrobial peptides (AMPs) or eliminated intracellularly. Three basic types of Drosophila blood cell (known as hemocytes) perform these functions: plasmatocytes, which are professional phagocytic cells akin to mammalian macrophages; lamellocytes, which wrap themselves around invading microorganisms to form an enveloping capsule; and crystal cells, which contain the enzymes that catalyze melanization (Meister, 2004) (Fig. 1B). As discussed later in more detail, many host genes that are required for phagocytosis have been identified using Drosophila in a series of genome-scale cell-based screens. Similar studies in the future might shed light on genes that are essential for lamellocyte and crystal cell function. Autophagy is another general mechanism important for clearing bacteria (Yano et al., 2008) and viruses (Cherry, 2009; Shelly et al., 2009). It should be pointed out, however, that autophagy can also be hijacked for the benefit of the pathogen, as in the case of poliovirus, which derives its envelope membranes from autophagic vesicles (Suhy et al., 2000).
Core signaling pathways
The second part of the Drosophila innate immune response comprises a set of core signaling pathways (Fig. 1C): the Toll pathway, the IMD pathway and the JAK-STAT pathway. The activities of these pathways are modulated by other pathways, such as that mediated by target of rapamycin (TOR) or Eiger-Wengen [Drosophila homologs of human tumor necrosis factor (TNF) and TNF receptor]. When induced following pathogen infection, innate immune pathways result in the production of AMPs such as Drosomycin and Diptericin (Dionne and Schneider, 2008; Ferrandon et al., 2007; Agaisse and Perrimon, 2004; Dionne and Schneider, 2008; Ferrandon et al., 2007; Folsch et al., 2003).
The RNAi pathway
The third part of the Drosophila innate immune response is the double-stranded RNAi pathway that is involved in defending against many types of viral infections, and which also protects against viral infection in plants and animals (for a review, see Sabin et al., 2010) (Fig. 1D). The RNAi pathway is activated by viral nucleic acids and can be broken down into two main steps: (1) biogenesis of 21-base-pair double-stranded viral small interfering RNAs (siRNAs), which is accomplished by the Dicer protein complex, and (2) the silencing of viral RNAs by the host-induced viral siRNAs, which is accomplished by the RNA-induced silencing complex (RISC). This innate protective system has been highly amenable to analysis using genome-wide screening in Drosophila cells (see below).
Identifying host defense factors through genome-wide screens
RNAi screens involving infection of Drosophila cell lines
One of the great recent technical advances in the field of Drosophila cell biology has been the development of efficient whole genome RNAi screens to identify genes required for specific cellular processes (Mohr et al., 2010; Perrimon and Mathey-Prevot, 2007; Perrimon et al., 2010). In such assays, Drosophila cell lines such as hemocyte-derived S2 cells or Kc cells (which can be induced by hormone treatment to differentiate into neurons) are grown in 384-well plates and treated with a library of double-stranded RNAs that have been designed for highly selective RNAi-mediated knockdown of each of the predicted Drosophila coding messenger RNAs (mRNAs). These cells are then assayed for performance of a cellular process such as cell viability, cell shape changes or bacterial uptake by phagocytosis. By screening such libraries in replicate and then re-screening RNAi candidates that test positive for a specific effect, it is possible to approximate genome-wide coverage of all genes required in these cells for a given process [for an excellent, comprehensive review of such RNAi screens, see Cherry (Cherry, 2008)]. Such screens have been used to identify many host response factors that are crucial during infection by bacteria, fungi and viruses.
Identifying host response factors to bacteria
Several straightforward RNAi screens have been conducted to identify genes that are required for phagocytosis of various species of bacteria by S2 cells. For these experiments, ingestion of bacteria expressing green fluorescent protein (GFP) is monitored and host genes involved in phagocytosis are revealed on the basis of the identity of specific RNAi molecules that inhibit uptake of fluorescence. These screens have revealed that distinct sets of host genes are essential during infection by various pathogens. For example, different pathogens are recognized by distinct cell surface receptors, such as peptidoglycan recognition proteins (PGRPs) (Ramet et al., 2002), SR-C1 (Ramet et al., 2001), Eater (Kocks et al., 2005), Nimrod (Kurucz et al., 2007) or DSCAM (Watson et al., 2005). However, these screens also defined a core set of intracellular uptake components that are regulated in all types of bacterial infection tested: these included genes required for actin remodeling (e.g. genes encoding proteins of the Arp2/3 complex) and endocytosis (e.g. COPI and COPII), as well as genes encoding factors that are required to recycle endosomes to the cell surface, such as proteins in the exocyst complex (Agaisse et al., 2005; Cheng et al., 2005; Philips et al., 2005; Ramet et al., 2002; Stroschein-Stevenson et al., 2006; Stuart et al., 2007).
Other genes involved in the response to bacterial infection that have been identified in RNAi screens are required for host cells to clear ingested bacteria. Again, these screens defined a set of generally required genes that limit bacterial survival or replication, such as genes encoding endosomal sorting complex required for transport (ESCRT) proteins (Philips et al., 2008), as well as genes preventing the growth of specific pathogens, such as lysosomal β-hexosaminidase, which restricts growth of Mycobacterium marinum but not Listeria monocytogenes or Salmonella typhimurium (Koo et al., 2008). In other standard genetic studies, intracellular microorganisms such as Wolbachia were found to also engage in mutualistic symbiotic relationships with the host, such as protecting the host against viral infection (Hedges et al., 2008; Teixeira et al., 2008) and nutritional supplementation (Brownlie et al., 2009), which presumably arose during co-evolution of the endosymbiont and host.
RNAi technology can also be used in a combinatorial fashion to knock down the activity of two or more genes at a time, which permits detection of genes acting in parallel in a given process or pathogenic infection. In one study, Dorer and colleagues performed a series of single and double gene knockdown experiments of 73 genes in Kc cells to test the hypothesis that Legionella pneumophila, the agent of Legionnaires’ disease, recruits membrane material from endoplasmic reticulum (ER)-to-Golgi trafficking (Dorer et al., 2006). Although few single knockdowns had much of an effect, the authors found evidence supporting their hypothesis in several double knockdown experiments. For example, double knockdown of the intermediate compartment and Golgi-tethering factor transport protein particle (TRAPP) together with the ER SNARE protein Sec22 resulted in reduced pathogen replication efficiency. They also showed a requirement in bacterial replication for the Cdc48-p97 complex that is involved in ER-associated degradation, and demonstrated that this complex is also important for Legionella pneumophila replication in mouse bone-marrow-derived macrophages. These studies underscore the role of endocytosis in phagocytic host cells and, owing to the combinatorial power of the system used, revealed a role for endocytic steps carried out by parallel mechanisms.
Identifying host response factors to fungi
Fungi generally activate the Toll signaling pathway of the Drosophila innate immune system via a specific set of PGRP detection peptides (Fig. 1C). RNAi screens similar to those performed to identify host genes required for phagocytosis of bacteria have also been carried out to identify host factors involved in response to fungi such as Candida albicans (Stroschein-Stevenson et al., 2006; Stroschein-Stevenson et al., 2009). Beyond identifying genes with broad expected functions, such as regulators of the actin cytoskeleton and vesicular trafficking, these studies also identified genes required for the uptake of specific fungal pathogens. One of these proteins, Macroglobulin complement related (Mcr), is a secreted protein that binds directly to C. albicans and promotes its internalization. Interestingly, Mcr is related to four other Drosophila thioester proteins (Teps), two of which are selectively required for phagocytosis of specific bacterial species (TepII for Escherichia coli and TepIII for Staphylococcus aureus), but not for phagocytosis of C. albicans (Stroschein-Stevenson et al., 2006).
Identifying host response factors to viruses
In addition to being susceptible to infection by bacterial and fungal pathogens, Drosophila is also a natural host for viruses such as Drosophila C virus (DCV), Drosophila X virus (DCX) and Flock House virus, and, perhaps surprisingly, by a broad variety of viruses causing disease in humans such as Sindbis virus, vesicular stomatitis virus (VSV; a virus of the Rhabdoviridae family, which includes the well-known rabies virus), Rift Valley fever virus, dengue virus and West Nile virus (Cherry et al., 2005; Cherry et al., 2006; Cherry and Perrimon, 2004; Galiana-Arnoux et al., 2006; van Rij et al., 2006; Wang et al., 2006). Genome-wide RNAi screens have identified several important host factors that are exploited by viruses, such as factors required selectively for replication of influenza virus (Hao et al., 2008) or propagation of dengue virus (Sessions et al., 2009). Similarly, viruses such as DCV that have transcripts with internal ribosome-binding sites depend on several host translation factors that are not required for other types of viruses lacking these sites (Cherry et al., 2005). DCV also requires the host factor COPI to generate a vesicular compartment, which is necessary for viral replication, and COPI is also required for the replication of the related poliovirus in human cells (Cherry et al., 2006). As another example, infection by vaccinia virus (the prototypical poxvirus) was found to depend on the AMP-activated kinase (AMPK) complex, the master energy sensor of the cell, for endocytic entry and actin remodeling (Moser et al., 2010). The authors found a similar requirement for AMPK in facilitating vaccinia infection of mouse embryonic fibroblasts and showed that this kinase was also involved in viral entry via the process of macropinocytosis.
As mentioned above, the RNAi pathway plays a key role in defending against viral infection. Genome-wide and targeted RNAi screens have contributed to the elucidation of this pathway (Galiana-Arnoux et al., 2006; Nayak et al., 2010; Otsuka et al., 2007; Sabin et al., 2009; van Rij et al., 2006; Wang et al., 2006) (for a review, see Sabin et al., 2010) and the importance of the systemic spread of an RNAi activating signal (probably some large viral double-stranded RNA) for stimulating RNAi-dependent immunity throughout the organism (Saleh et al., 2009). Interestingly, siRNAs do not spread from cell to cell in Drosophila (Roignant et al., 2003), in contrast to the mechanism by which RNAi molecules are directly distributed in plants (Palauqui et al., 1997; Winston et al., 2002) and nematodes (Fire et al., 1998; Voinnet et al., 1998) to mediate systemic immunity.
Screens involving infection of intact adult flies
As a complement to cell-based screening methods, it is also possible to screen for host genes that are required to combat pathogen infection using intact flies. Although these screens are more laborious than screens in Drosophila cell lines, or whole-genome RNAi screens in worms (i.e. Caenorhabditis elegans screens can be done on plates), screens using intact flies can be accomplished either by classic mutagenesis or by screening high quality collections of stable UAS-RNAi stocks. A great advantage of the latter approach is that one can use the GAL4-UAS expression system (Brand and Perrimon, 1993) to drive expression of UAS-RNAi constructs throughout the organism or in specific subsets of cells or stages of development (Fig. 2B). For such experiments, a strain of flies carrying a transgene under the control of the yeast upstream activating sequence (UAS) is crossed to a strain of flies expressing the GAL4 transcription factor (which binds to the UAS sequence and activates transcription in a particular pattern, e.g. in the gut). The progeny then express the UAS transgene of interest in the pattern determined by the GAL4 ‘driver’ stock, permitting expression of genes in specific cell types at specific stages of development. This level of control permits investigators to identify the cells or organs in which gene functions are required [e.g. epidermis, fat body (the main source of systemic AMPs, and an approximate model of the mammalian liver), hemocytes or gut].
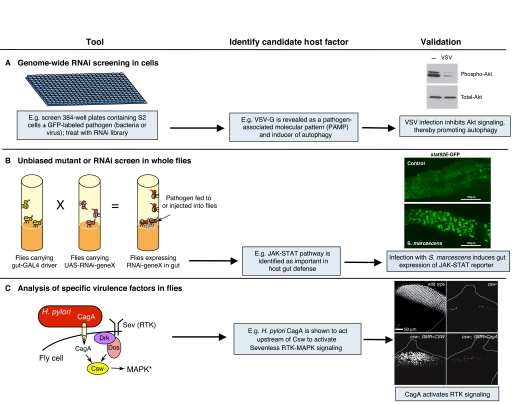
Fig. 2.
Tools for studying host-pathogen interactions in Drosophila. (A) Genome-wide RNAi screens in S2 or Kc cells infected with pathogens are among the most effective tools available in Drosophila for studying host-pathogen interactions. For example, one screen indicated that VSV-G activates host immunity, reduces Akt signaling and induces autophagy. Right panel adapted from Shelly et al. (Shelly et al., 2009), with permission. (B) Unbiased mutant or RNAi screens in whole flies can also be used to identify host or pathogen factors involved in virulence. Such screens demonstrated the importance of the JAK-STAT pathway for host immunity in the gut. ‘Validation’ panel adapted from Cronin et al. (Cronin et al., 2009), with permission. (C) Analysis of specific virulence factors in flies using epistasis and other genetic experiments can identify specific steps in a pathway that are altered by the virulence factor. One example of this general approach is shown in the right panel, in which it was found that the CagA protein from H. pylori functions upstream of the phosphatase Corkscrew (Csw) to activate signaling by the Sevenless receptor tyrosine kinase (RTK). Asterisk indicates activation. Right panel from Botham et al. (Botham et al., 2008), with permission.
Screening for defense pathways in the gut
In one screen using adult flies, host defense factors that are required to protect against intestinal infection with the opportunistic broad-host-spectrum pathogen Serratia marcescens were first identified by using a large collection of fly lines in which ∼13,000 individual RNAi molecules were used to knock down target gene expression throughout the organism (Cronin et al., 2009). RNAi molecules that caused increased lethality following infection were then tested further for their role in defending against S. marcescens infection of the gut by expressing the relevant UAS-RNAi constructs with gut-specific and hemocyte-specific drivers. These studies first confirmed the dependence on the IMD (but not Toll) innate immune pathway for responding to infection by S. marcescens (as would be expected for a Gram-negative bacterium), and also revealed an important role for the JAK-STAT pathway in responding to infection in the gut (Fig. 2C). Further analysis of JAK-STAT signaling showed that this pathway regulates stem cell proliferation and thereby intestinal epithelial homeostasis during infection. These results obtained in intact flies provide an important complement to screens performed in C. elegans, which also identified several signaling systems important for innate immunity (Irazoqui et al., 2010). Studies of damage and repair by gut pathogens can be conducted in Drosophila because flies, but not worms, have intestinal stem cells that replenish epithelial cells after they undergo programmed cell death during infection (see above).
Flies as a genetic model for cholera infection
Systematic screens such as that mentioned above (Cronin et al., 2009) can also be used to identify host factors co-opted by a pathogen that, when mutated, render the host resistant to the pathogen. An example using a traditional genetic approach is the case of Vibrio cholerae, in which investigators showed that feeding V. cholerae bacteria to flies caused rapid death (i.e. in 2–3 days) that required the function of the primary virulence factor cholera toxin (CTX) (Blow et al., 2005). CTX is an ADP ribosyl transferase that specifically ribosylates the Gαs subunit of a host trimeric Gs protein, resulting in constitutive activation of adenylate cyclase (Middlebrook and Dorland, 1984). The dependence on CTX was unexpected because flies lack the enzymes required to synthesize the GM1 ganglioside that serves as the CTX receptor and that is present in most vertebrates and a few invertebrates. Accordingly, feeding flies purified CTX holotoxin had no effect (Blow et al., 2005). Paradoxically, however, full virulence of ctx-mutant bacteria could be restored by feeding infected flies purified CTX, suggesting that, in the presence of the bacteria, a novel alternative route of CTX delivery to host cells in the gut might be employed.
Further analysis showed that several host target factors known from mammalian studies were required by V. cholera to infect flies, such as proteins mediating the dehydrating effects of CTX-dependent cAMP production – including a Gαs subunit, adenylate cyclase and an SK-type potassium ion channel (Blow et al., 2005). Having established flies as a model for V. cholerae infection, the authors then screened a large collection of stocks with mapped transposon insertions into the fly genome and identified mutations that either enhanced or reduced severity of infection. This strategy identified several host genes important for the response to V. cholerae infection, including those conferring resistance when mutated and that presumably are exploited by bacteria (e.g. components of the TNF and IMD pathways) as well as those used in host defense (e.g. the apoptotic pathway) (Berkey et al., 2009).
Identifying pathogen virulence factors in Drosophila
Adult flies and cells have also been used to screen for pathogen-encoded factors that contribute to virulence. One particularly elegant screen for bacterial virulence factors was carried out for P. aeruginosa, an opportunistic human pathogen that can cause serious disease. Over 4000 transposon insertion mutants of the bacterium were screened by injecting them into the adult fly hemolymph and measuring percent lethality. This resulted in the identification of 15 different bacterial loci that contributed significantly to virulence (Kim et al., 2008). The authors examined the basis of virulence for one of these genes, hudR. hudR encodes a transcription factor that represses expression of the neighboring gene hudA, which is involved in ubiquinone biosynthesis. On the basis of their genetic analysis, the authors hypothesized that the decreased virulence of hudR mutants resulted from overexpression of hudA. They confirmed this hypothesis by showing that overexpression of hudA in a hudR-mutant background resulted in attenuated virulence of P. aeruginosa in flies and that hudA hudR double mutants had normal virulence.
Flies have also been used to differentiate virulence of P. aeruginosa strains, such as those isolated from the sputum of cystic fibrosis patients (who are particularly sensitive to infection by this pathogen) (Lutter et al., 2008; Salunkhe et al., 2005; Sibley et al., 2008). For these assays, flies are either fed different strains of bacteria obtained from burn wounds or from cystic fibrosis patients, or bacteria are inoculated by wounding flies. Similar infection experiments can be performed to identify interactions between P. aeruginosa and other microbes present in sputum that could contribute to the virulence of this pathogen (Sibley et al., 2008).
Virulence factors of human fungal pathogens can also be identified in Drosophila (Ben-Ami et al., 2010; Chamilos et al., 2010; Chamilos et al., 2008; Lamaris et al., 2007). For example, gliotoxin produced by the filamentous fungus Aspergillus fumigatus is required for virulence of this pathogen in both flies and mice (Spikes et al., 2008), and Cas5 has been shown to be a transcription factor regulating a set of genes required for integrity of the cell wall of C. albicans (Chamilos et al., 2009). Adult flies have also been used as an intact organism to screen for drugs that block fungal infection (Chamilos et al., 2006a; Chamilos et al., 2006b; Lamaris et al., 2009; Lamaris et al., 2008; Lionakis et al., 2005).
Investigating functions and targets of virulence factors
In the previous section, we discussed strategies by which flies can be used to screen for pathogen virulence factors. In this final section, we consider the advantages of Drosophila as a model for analyzing virulence factor function, and for identifying the host proteins and pathways that they target (e.g. Fig. 2C). Although cell-based expression systems and biochemical experiments performed with purified virulence factors can be invaluable for establishing mechanism of action, they do not necessarily predict how such factors will act in an infected organism – either systemically or in selected tissues, in which cell-autonomous and non-cell-autonomous processes might be important. Model systems such as flies and worms are ideal for this level of analysis owing to the great variety of genetic tools available to tease apart the effects that such factors might have on specific host pathways and biological processes. Although flies and worms are only distantly related to humans, many virulence factors target host proteins and pathways that are among the most conserved in eukaryotes – thus, studying the effect of pathogens in these organisms is often highly relevant to human disease. In addition, as discussed below, studies in model organisms also enable examination of the combinatorial effect of two or more virulence factors, which is more challenging in intact mammals. Finally, we highlight in Box 1 how studying the effect of toxins can shed light on basic cellular processes.
Box 1. Toxins can be used to probe cellular processes.
One of the first uses of toxins in flies was to genetically ablate specific cells with cell-lethal toxins such as diphtheria toxin (Kunes and Steller, 1991) or ricin (Moffat et al., 1992). It is also possible to block the neuronal activity of cells without killing them, as with tetanus toxin (TTX), which was used to block synaptic transmission in the nervous system (Allen et al., 1999; Baines et al., 1999; Reddy et al., 1997; Sweeney et al., 1995) and activity-dependent regulation of synaptic size and function (Nakayama et al., 2006). TTX has been used in a myriad of Drosophila studies to inhibit neurotransmission in various processes, including learning and memory, locomotion and courtship (for a review, see Martin et al., 2002), circadian rhythms (Johard et al., 2009; Kaneko et al., 2000), and the serotonin-dependent response to light (Rodriguez Moncalvo and Campos, 2009). Similarly, application of cholera toxin (CTX), an ADP-ribosylation factor, was used to study the function of the Gα protein Concertina, which is involved in initiating embryonic gastrulation (Morize et al., 1998). Similarly, transgenic expression of a UAS–CTX-A construct helped to distinguish which heteromeric G proteins contribute to wing maturation (Katanayeva et al., 2010). Indeed, these and other toxins, which neutralize or alter the activities of multiple host proteins, can be used to perform a variety of in vivo pharmacological studies to complement classical genetic loss-of-function studies.
Analyzing the function of bacterial toxins in intact flies
Drosophila is an excellent in vivo genetic system for analyzing toxin activities in a multicellular and organ context given the highly conserved nature of many host targets of these virulence factors. For example, flies have been used to study the activity of the virulence factor ExoS from P. aeruginosa, which encodes a factor containing a domain with Rho-GAP activity (which can inactivate host small GTPases of the Rho/Rac subfamily). During P. aeruginosa infection, ExoS is injected into host cells by a type-II secretion system (TTSS), and infection of flies with P. aeruginosa leads to rapid death that depends on TTSS function (Fauvarque et al., 2002). When the GAP domain of ExoS (ExoSGAP) is expressed in fly hemocytes, phagocytosis is inhibited (Avet-Rochex et al., 2005). In addition, expression of ExoSGAP in flies increases their sensitivity to infection by P. aeruginosa (Avet-Rochex et al., 2005), and this effect can be rescued by co-expressing host Rac2 with ExoSGAP (Avet-Rochex et al., 2007). These studies provide evidence that host Rac2 is inhibited by bacterial ExoSGAP during infection.
A second example illustrating the utility of Drosophila for investigating toxin activities in vivo is provided by studies of Helicobacter pylori (Fig. 2C), which is associated with the development of gastric ulcers and cancer in humans. Under normal circumstances, ligand-initiated receptor tyrosine kinase (RTK) signaling in both fly and mammalian cells is mediated by a receptor-associated protein complex including Grb2 (Drk), Gab (Dos) and Shp-2 [Corkscrew (Csw)] (Drosophila protein names are shown in parentheses) that then activates signaling via the downstream components of the Ras-MAPK pathway. Drosophila played a prominent role in discovering key components of this pathway and in establishing the order of molecular events that take place during signaling (Simon, 2000). In mammalian cells, the H. pylori virulence factor CagA activates RTK signaling at the level of SHP-2, a tyrosine phosphatase that is homologous to Drosophila Csw (Hatakeyama, 2008; Hatakeyama, 2009), which acts downstream of Gab (Dos in flies) (Herbst et al., 1996; Raabe et al., 1996). Studies in Drosophila confirmed the hypothesis that CagA can bypass the need for signal-dependent activation of Dos in an intact organism, because CagA expression in Drosophila embryos or in the adult eye was capable of rescuing dos-mutant phenotypes (Botham et al., 2008). Furthermore, the ability to activate effectors of the Sevenless RTK pathway in the eye was shown to be dependent on the downstream effector Csw, validating the hypothesized role of CagA in the RTK signaling pathway acting between Gab (Dos) and SHP-2 (Csw).
Identifying unknown activities of bacterial toxins
Beyond providing a multicellular model for assigning known biochemical activities to virulence factors, Drosophila can also be used as a tool to discover completely new activities of virulence factors. For example, Bacillus anthracis, the etiological agent of anthrax, produces two toxic factors required for systemic virulence (Lacy and Stevens, 1999; Mourez, 2004; Tournier et al., 2007; Guichard et al., 2011): lethal factor (LF), a zinc metalloprotease that cleaves MAPKKs (Duesbery et al., 1998; Vitale et al., 1998), and edema factor (EF), a highly active calmodulin-dependent adenylate cyclase (Leppla, 1982). Both LF and EF are essential for the lethal effects of anthrax (Pezard et al., 1991), which culminates in vascular failure and septic-shock-like death. An important unanswered question is, how do LF and EF, with such seemingly disparate enzymatic activities, collaborate during infection (particularly within vascular endothelial cells, which become leaky at advanced stages of disease, leading to death)?
In initial studies, we showed that anthrax toxins act on Drosophila homologs of their known targets in mammalian cells (Guichard et al., 2006). In addition to these known effects of LF and EF, we observed that both toxins also caused adult wing and bristle phenotypes similar to those caused by inhibition of the Notch signaling pathway, and blocked expression of Notch target genes in developing wing imaginal discs (Guichard et al., 2010). Moreover, these toxins interacted in a synergistic fashion to block Notch signaling (Fig. 3A). Further analysis of this Notch-like phenotype revealed that it resulted from failure to recycle the Notch ligand Delta to the cell surface (Guichard et al., 2010). EF was found to reduce the levels and activity of the small GTPase Rab11, whereas LF reduced cell surface levels of the Rab11 binding partner, Sec15 (Fig. 3B). Sec15 is part of an octameric protein complex known as the exocyst, which targets proteins, including Delta and the cell adhesion molecule DE-cadherin, to adherens junctions (accordingly, DE-cadherin trafficking to adherens junctions was also reduced by EF and LF). These results from flies were validated in human vascular and lung endothelial cells by our collaborators in Victor Nizet’s laboratory (Fig. 3C), who also showed that EF reduced epithelial barrier integrity in a cell culture assay and in vivo in mice (Guichard et al., 2010).
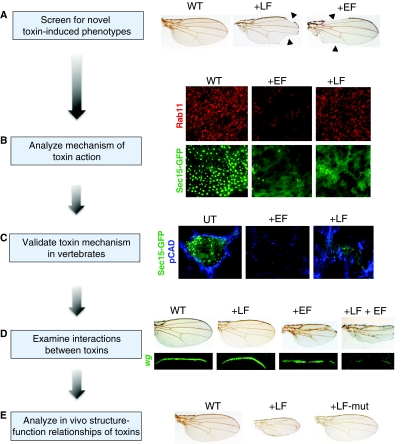
Fig. 3.
An example of genetic analysis of toxin activity in Drosophila. (A) Screen for novel toxin-induced phenotypes. Expression of the anthrax toxins lethal factor (LF) or edema factor (EF) in the wing margin primordium results in notching along the edge of the wing, defects that are typical of mutations in components of the Notch signaling pathway (Guichard et al., 2010). WT, wild type. (B) Analyze mechanisms of toxin action. The Notch-like phenotypes caused by expression of LF or EF in the wing both result from inhibition of endocytic recycling of membrane cargo to the AJ by the exocyst complex. EF acts by reducing the levels and activity of the Rab11 GTPase, which indirectly results in a loss of large vesicles containing its binding partner Sec15-GFP, a component of the exocyst complex. LF does not seem to alter Rab11 levels or function, but inhibits the formation of large Sec15 vesicles (Guichard et al., 2010). (C) Validate toxin mechanism in vertebrates. Human brain microvascular endothelial cells were treated with purified EF toxin or LF toxin. As in fly cells, both toxins greatly reduce the number of Sec15-GFP vesicles in these cells and reduce cadherin expression (Guichard et al., 2010). (D) Examine interactions between toxins. Cooperative interactions between toxins or other virulence factors can be assessed by co-expressing them in specific cells and comparing the effects of both toxins to that of the action of either toxin alone. In the example shown, anthrax toxins were expressed alone or in combination using a weak GAL4 driver to express low levels of the toxins. Each panel consists of an adult wing (top) and a larval wing imaginal disc showing expression of the Notch target gene wingless (wg) along the future edge of wing in third instar larvae (bottom). Expression of LF or EF alone (+LF or +EF, respectively) has little or no effect on formation of the wing margin (compared with WT). When LF and EF are co-expressed, the wing margin virtually disappears, as does expression of wg along the primordium of the wing margin. (E) In vivo structure-function analysis of toxins. The systemic activities of mutant forms of toxins or other virulence factors can be assessed in Drosophila. Such activities include cell-non-autonomous effects mediated by intercellular signaling systems, which are difficult to screen for in cell culture. In the simple case shown in this panel, high levels of LF expression lead to reduced wing size (middle panel) and a single point mutation in the LF catalytic domain renders it inactive (right panel). Panels A–D adapted from Guichard et al. (Guichard et al., 2010) with permission. Panel E adapted from Guichard et al. (Guichard et al., 2006), with permission.
Maintenance of vascular integrity depends on cell-cell adhesion (Dejana et al., 2009), and cell-cell communication mediated by Notch signaling plays a role in promoting the formation of primary (or patent) vessels over more permeable microvessels (Hellstrom et al., 2007; Leslie et al., 2007; Lobov et al., 2007; Roca and Adams, 2007; Siekmann and Lawson, 2007; Suchting et al., 2007). By inhibiting these two interrelated processes, and possibly interactions between endothelial cells and other vascular cell types such as mural cells, anthrax toxins might contribute to the late-stage effects of anthrax infection when disruption of endothelial barrier function leads to lethal vascular collapse. Once sufficient levels of anthrax toxins are produced, they can be fatal even if the bacterial infection is eliminated with antibiotic treatment. Thus, these studies of anthrax toxins initiated in flies and validated in mammalian models might ultimately have therapeutic implication for treating humans infected with anthrax or for other conditions compromising vascular integrity.
Identifying unknown activities of viral factors
Given the compact sizes of viral genomes, only few viral proteins fall into the category of bona fide virulence factors, similar to the potent bacterial toxins discussed above. By contrast, most viral proteins are dedicated to basic processes essential to the virus life cycle, such as entry or exit, replication, or manipulation of host processes such as transcription or translation. Model organisms are useful for examining specific interactions between viral and host proteins to gain insights into their mechanisms of action.
An excellent example of using the full complement of Drosophila tools to study a viral pathogen was carried out by Cherry and colleagues, who showed that fly cells can be infected with VSV. VSV can replicate in these cells to generate mature viral particles that can infect mammalian cells. They showed that infection of adult flies with VSV induces autophagy (Shelly et al., 2009) and that autophagy was mediated by VSV-G, a pathogen surface protein that is recognized by Drosophila cells. The authors found that induction of autophagy plays an important role in protecting against VSV infection and then asked what host pathways might mediate the autophagy response to VSV. A variety of elegant genetic epistasis experiments demonstrated that the PI3K-Akt pathway was attenuated by VSV infection, thereby relieving its constitutive inhibition of autophagy (Fig. 2A). Akt activation is also attenuated by expression of the SARS-Coronovirus Membrane protein in flies, which in this case results in increased apoptosis (Chan et al., 2007).
In another study, host factors required for the HIV accessory protein Nef to downregulate expression of the human CD4 protein were identified by RNAi screening in Drosophila S2 cells expressing human CD4. These factors included components of the clathrin-associated AP2 complex, which was then validated as an essential cellular component mediating a similar Nef-CD4 interaction in human cells (Chaudhuri et al., 2007).
In vivo structure-function analysis of toxins
Classic genetic approaches in Drosophila can also be applied to probing structure-function relationships of toxins or other virulence factors. One straightforward approach to define domains of a toxin that are important for producing the phenotype of interest is to mutagenize flies carrying a UAS-toxin construct and to screen for loss of the phenotype that results from expression of the wild-type toxin (Fig. 3E). One can then PCR amplify the mutated UAS-transgenes and sequence the putative mutant allele to determine the molecular nature of the loss-of-function mutation. It is also possible to screen for mutations in the transgene that results in altered phenotypes caused by dominant gain-of-function mutations (Guichard et al., 2002).
Conclusions and outlook
An important goal of this review has been to convince readers from other fields that flies provide a broad range of advantages for studying host-pathogen interactions at the level of the cell, tissue, organ and intact organism. As discussed, genome-wide RNAi screens in Drosophila cell culture have generated a wealth of new information regarding the genes involved in mediating basic host cellular responses to pathogens, such as those involved in innate immunity, phagocytosis and restriction of intracellular pathogen survival. These cellular studies can be complemented by studies that aim to identify host resistance factors and pathogen virulence factors using intact flies as infection models. Studies in flies also provide the potential to explore mutualistic interactions with intracellular endosymbionts, and to conduct mechanistic analysis of specific virulence factors, and combinations of these factors, using the state-of-the-art genetic tools available in Drosophila.
A particular advantage of model systems such as yeast, C. elegans and Drosophila is the potential to examine arrays of genetic combinations to identify factors produced by the host or pathogen that act redundantly (host) or that genetically interact (host or pathogen). These types of studies are inherently number intensive (known as ‘the n problem’), because many combinations must be analyzed in a comprehensive fashion. However, there are excellent examples in which combinatorial genetic analysis has been used to investigate cellular processes involved in other types of human disease. For example, the interacting components of the DNA mismatch repair machinery were first identified in yeast, and the same components were found to interact in humans in a dominant manner and to contribute to cancer (Kolodner, 1995). Drosophila cells and intact fly mis-expression systems (e.g. combined RNAi expression) are also well suited for such analyses, which would be prohibitively expensive and labor intensive in vertebrate models. It is of course important to validate results obtained in single cells or invertebrate model systems in vertebrates, a process previously referred to as ‘closing-the-loop’ (Bier, 2005). It is possible to envision a tiered system of analysis in which initial discoveries that are made using powerful model genetic systems, including yeast, worms and flies (in cells and in whole organisms), are then validated in vertebrate models, including zebrafish, mice and human cells, and finally are linked via human genetics to specific disease processes. For example, in a recent study of genes on human chromosome 21 causing congenital heart defects when overexpressed in individuals with Down syndrome, a combined genetic analysis in flies, mice and humans pointed to two interacting genes, DSCAM and COL6A2, as contributing to formation of atrial septal defects (Grossman et al., 2011).
In addition to assessing combinatorial contributions of host factors, Drosophila is well suited for examining cooperative interactions between pathogen virulence factors, as presented in the examples above. Many pathogens produce a complex cocktail of virulence factors, subsets of which are often co-expressed from neighboring genes in so-called pathogenicity islands. These co-regulated virulence factors are typically delivered by a dedicated injection system and often act by unknown means in various combinations in different host cell types. Such virulence factors from a given pathogenicity island can be expressed in various combinations in specific cell types to identify specific cellular contexts in which they interact. Given the great success of the fly for analyzing the activities of single host or pathogenic factors in disease processes, it will interesting to see whether it also serves as a robust system to study more complex networks of interactions between host pathways or pathogen virulence factors. With the advent of whole genome RNAi tools and comprehensive mutant collections, flies should also provide an important intact model system for identifying unknown activities of virulence factors that act in a multicellular context to inhibit specific signaling systems or to alter contact between neighboring cells in structured tissues and organs. Combined use of Drosophila cells and intact flies in moderate- to high-throughput drug screens is also emerging as an effective strategy to identify compounds, or combinations of existing compounds, that alter the activity of host pathways to counter the effect of pathogens. Clearly, flies have a bright future as tools for further deconstructing human host-pathogen interactions.
Acknowledgments
We thank Bill McGinnis, Victor Nizet, Steve Wasserman, Emily Troemel, Raffi Aroian, Margery Smelkinson and Beatriz Cruz-Moreno for helpful discussions and comments on the manuscript.
Footnotes
FUNDING
This work was supported by the National Institutes of Health [AI070654 to E.B.]; and the National Science Foundation [IOS-0744662 to E.B.].
COMPETING INTERESTS
The authors declare that they do not have and competing or financial interests.
REFERENCES
- Agaisse H., Perrimon N. (2004). The roles of JAK/STAT signaling in Drosophila immune responses. Immunol. Rev. 198, 72–82 [DOI] [PubMed] [Google Scholar]
- Agaisse H., Burrack L. S., Philips J. A., Rubin E. J., Perrimon N., Higgins D. E. (2005). Genome-wide RNAi screen for host factors required for intracellular bacterial infection. Science 309, 1248–1251 [DOI] [PubMed] [Google Scholar]
- Akira S., Uematsu S., Takeuchi O. (2006). Pathogen recognition and innate immunity. Cell 124, 783–801 [DOI] [PubMed] [Google Scholar]
- Allen M. J., Shan X., Caruccio P., Froggett S. J., Moffat K. G., Murphey R. K. (1999). Targeted expression of truncated glued disrupts giant fiber synapse formation in Drosophila. J. Neurosci. 19, 9374–9384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amcheslavsky A., Jiang J., Ip Y. T. (2009). Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 4, 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amcheslavsky A., Ito N., Jiang J., Ip Y. T. (2011). Tuberous sclerosis complex and Myc coordinate the growth and division of Drosophila intestinal stem cells. J. Cell Biol. 193, 695–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews H. K., Zhang Y. Q., Trotta N., Broadie K. (2002). Drosophila sec10 is required for hormone secretion but not general exocytosis or neurotransmission. Traffic 3, 906–921 [DOI] [PubMed] [Google Scholar]
- Apidianakis Y., Pitsouli C., Perrimon N., Rahme L. (2009). Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc. Natl. Acad. Sci. USA 106, 20883–20888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avet-Rochex A., Bergeret E., Attree I., Meister M., Fauvarque M. O. (2005). Suppression of Drosophila cellular immunity by directed expression of the ExoS toxin GAP domain of Pseudomonas aeruginosa. Cell. Microbiol. 7, 799–810 [DOI] [PubMed] [Google Scholar]
- Avet-Rochex A., Perrin J., Bergeret E., Fauvarque M. O. (2007). Rac2 is a major actor of Drosophila resistance to Pseudomonas aeruginosa acting in phagocytic cells. Genes Cells 12, 1193–1204 [DOI] [PubMed] [Google Scholar]
- Backhed F., Ley R. E., Sonnenburg J. L., Peterson D. A., Gordon J. I. (2005). Host-bacterial mutualism in the human intestine. Science 307, 1915–1920 [DOI] [PubMed] [Google Scholar]
- Baines R. A., Robinson S. G., Fujioka M., Jaynes J. B., Bate M. (1999). Postsynaptic expression of tetanus toxin light chain blocks synaptogenesis in Drosophila. Curr. Biol. 9, 1267–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Sousa A. D., Bhat M. A. (2006). Organization and function of septate junctions: an evolutionary perspective. Cell Biochem. Biophys. 46, 65–77 [DOI] [PubMed] [Google Scholar]
- Behr M., Riedel D., Schuh R. (2003). The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev. Cell 5, 611–620 [DOI] [PubMed] [Google Scholar]
- Ben-Ami R., Lamaris G. A., Lewis R. E., Kontoyiannis D. P. (2010). Interstrain variability in the virulence of Aspergillus fumigatus and Aspergillus terreus in a Toll-deficient Drosophila fly model of invasive aspergillosis. Med. Mycol. 48, 310–317 [DOI] [PubMed] [Google Scholar]
- Berkey C. D., Blow N., Watnick P. I. (2009). Genetic analysis of Drosophila melanogaster susceptibility to intestinal Vibrio cholerae infection. Cell. Microbiol. 11, 461–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beronja S., Laprise P., Papoulas O., Pellikka M., Sisson J., Tepass U. (2005). Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J. Cell Biol. 169, 635–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavsar A. P., Guttman J. A., Finlay B. B. (2007). Manipulation of host-cell pathways by bacterial pathogens. Nature 449, 827–834 [DOI] [PubMed] [Google Scholar]
- Bier E. (2005). Drosophila, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 6, 9–23 [DOI] [PubMed] [Google Scholar]
- Bier E., McGinnis W. (2008). Chapter 3, model organisms in the study of development and disease. In Molecular Basis of Inborn Errors of Development (ed. Epstein C. J., Erickson R. P., Wynshaw-Boris A.), pp. 25–48 New York: Oxford University Press [Google Scholar]
- Blankenship J. T., Fuller M. T., Zallen J. A. (2007). The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. J. Cell Sci. 120, 3099–3110 [DOI] [PubMed] [Google Scholar]
- Blow N. S., Salomon R. N., Garrity K., Reveillaud I., Kopin A., Jackson F. R., Watnick P. I. (2005). Vibrio cholerae infection of Drosophila melanogaster mimics the human disease cholera. PLoS Pathog. 1, e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornschein J., Rokkas T., Selgrad M., Malfertheiner P. (2009). Helicobacter pylori and clinical aspects of gastric cancer. Helicobacter 14 Suppl. 1, 41–45 [DOI] [PubMed] [Google Scholar]
- Botham C. M., Wandler A. M., Guillemin K. (2008). A transgenic Drosophila model demonstrates that the Helicobacter pylori CagA protein functions as a eukaryotic Gab adaptor. PLoS Pathog. 4, e1000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 [DOI] [PubMed] [Google Scholar]
- Bray S. J., Kafatos F. C. (1991). Developmental function of Elf-1: an essential transcription factor during embryogenesis in Drosophila. Genes Dev. 5, 1672–1683 [DOI] [PubMed] [Google Scholar]
- Brodsky I. E., Medzhitov R. (2009). Targeting of immune signalling networks by bacterial pathogens. Nat. Cell Biol. 11, 521–526 [DOI] [PubMed] [Google Scholar]
- Brownlie J. C., Cass B. N., Riegler M., Witsenburg J. J., Iturbe-Ormaetxe I., McGraw E. A., O’Neill S. L. (2009). Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 5, e1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N., Broderick N. A., Poidevin M., Pradervand S., Lemaitre B. (2009). Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5, 200–211 [DOI] [PubMed] [Google Scholar]
- Chamilos G., Lewis R. E., Kontoyiannis D. P. (2006a). Lovastatin has significant activity against zygomycetes and interacts synergistically with voriconazole. Antimicrob. Agents Chemother. 50, 96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamilos G., Lionakis M. S., Lewis R. E., Lopez-Ribot J. L., Saville S. P., Albert N. D., Halder G., Kontoyiannis D. P. (2006b). Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J. Infect. Dis. 193, 1014–1022 [DOI] [PubMed] [Google Scholar]
- Chamilos G., Lewis R. E., Hu J., Xiao L., Zal T., Gilliet M., Halder G., Kontoyiannis D. P. (2008). Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc. Natl. Acad. Sci. USA 105, 9367–9372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamilos G., Nobile C. J., Bruno V. M., Lewis R. E., Mitchell A. P., Kontoyiannis D. P. (2009). Candida albicans Cas5, a regulator of cell wall integrity, is required for virulence in murine and toll mutant fly models. J. Infect. Dis. 200, 152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamilos G., Bignell E. M., Schrettl M., Lewis R. E., Leventakos K., May G. S., Haas H., Kontoyiannis D. P. (2010). Exploring the concordance of Aspergillus fumigatus pathogenicity in mice and Toll-deficient flies. Med. Mycol. 48, 506–510 [DOI] [PubMed] [Google Scholar]
- Chan C. M., Ma C. W., Chan W. Y., Chan H. Y. (2007). The SARS-Coronavirus Membrane protein induces apoptosis through modulating the Akt survival pathway. Arch. Biochem. Biophys. 459, 197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri R., Lindwasser O. W., Smith W. J., Hurley J. H., Bonifacino J. S. (2007). Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor. J. Virol. 81, 3877–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L. W., Viala J. P., Stuurman N., Wiedemann U., Vale R. D., Portnoy D. A. (2005). Use of RNA interference in Drosophila S2 cells to identify host pathways controlling compartmentalization of an intracellular pathogen. Proc. Natl. Acad. Sci. USA 102, 13646–13651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry S. (2008). Genomic RNAi screening in Drosophila S2 cells: what have we learned about host-pathogen interactions? Curr. Opin. Microbiol. 11, 262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry S. (2009). VSV infection is sensed by Drosophila, attenuates nutrient signaling, and thereby activates antiviral autophagy. Autophagy 5, 1062–1063 [DOI] [PubMed] [Google Scholar]
- Cherry S., Perrimon N. (2004). Entry is a rate-limiting step for viral infection in a Drosophila melanogaster model of pathogenesis. Nat. Immunol. 5, 81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry S., Doukas T., Armknecht S., Whelan S., Wang H., Sarnow P., Perrimon N. (2005). Genome-wide RNAi screen reveals a specific sensitivity of IRES-containing RNA viruses to host translation inhibition. Genes Dev. 19, 445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry S., Kunte A., Wang H., Coyne C., Rawson R. B., Perrimon N. (2006). COPI activity coupled with fatty acid biosynthesis is required for viral replication. PLoS Pathog. 2, e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin S. J., Nehme N. T., Limmer S., Liegeois S., Pospisilik J. A., Schramek D., Leibbrandt A., Simoes R., de M., Gruber S., Puc U., et al. (2009). Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science 325, 340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale C., Moran N. A. (2006). Molecular interactions between bacterial symbionts and their hosts. Cell 126, 453–465 [DOI] [PubMed] [Google Scholar]
- Dejana E., Tournier-Lasserve E., Weinstein B. M. (2009). The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev. Cell 16, 209–221 [DOI] [PubMed] [Google Scholar]
- Diacovich L., Gorvel J. P. (2010). Bacterial manipulation of innate immunity to promote infection. Nat. Rev. Microbiol. 8, 117–128 [DOI] [PubMed] [Google Scholar]
- Dionne M. S., Schneider D. S. (2008). Models of infectious diseases in the fruit fly Drosophila melanogaster. Dis. Model. Mech. 1, 43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorer M. S., Kirton D., Bader J. S., Isberg R. R. (2006). RNA interference analysis of Legionella in Drosophila cells: exploitation of early secretory apparatus dynamics. PLoS Pathog. 2, e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesbery N. S., Webb C. P., Leppla S. H., Gordon V. M., Klimpel K. R., Copeland T. D., Ahn N. G., Oskarsson M. K., Fukasawa K., Paull K. D., et al. (1998). Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280, 734–737 [DOI] [PubMed] [Google Scholar]
- Dushay M. S. (2009). Insect hemolymph clotting. Cell. Mol. Life Sci. 66, 2643–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvarque M. O., Bergeret E., Chabert J., Dacheux D., Satre M., Attree I. (2002). Role and activation of type III secretion system genes in Pseudomonas aeruginosa-induced Drosophila killing. Microb. Pathog. 32, 287–295 [DOI] [PubMed] [Google Scholar]
- Ferrandon D., Imler J. L., Hetru C., Hoffmann J. A. (2007). The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 7, 862–874 [DOI] [PubMed] [Google Scholar]
- Fire A., Xu S., Montgomery M. K., Kostas S. A., Driver S. E., Mello C. C. (1998). Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 [DOI] [PubMed] [Google Scholar]
- Folsch H., Pypaert M., Maday S., Pelletier L., Mellman I. (2003). The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol. 163, 351–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M. (2009). Knockout animals and natural mutations as experimental and diagnostic tool for studying tight junction functions in vivo. Biochim. Biophys. Acta 1788, 813–819 [DOI] [PubMed] [Google Scholar]
- Furuse M., Tsukita S. (2006). Claudins in occluding junctions of humans and flies. Trends Cell Biol. 16, 181–188 [DOI] [PubMed] [Google Scholar]
- Furuse M., Hata M., Furuse K., Yoshida Y., Haratake A., Sugitani Y., Noda T., Kubo A., Tsukita S. (2002). Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J. Cell Biol. 156, 1099–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiana-Arnoux D., Dostert C., Schneemann A., Hoffmann J. A., Imler J. L. (2006). Essential function in vivo for Dicer-2 in host defense against RNA viruses in Drosophila. Nat. Immunol. 7, 590–597 [DOI] [PubMed] [Google Scholar]
- Grossman T. R., Gamliel A., Wessells R. J., Taghli-Lamallem O., Jepsen K., Ocorr K., Korenberg J. R., Peterson K. L., Rosenfeld M. G., Bodmer R., et al. (2011). Over-expression of DSCAM and COL6A2 cooperatively generates congenital heart defects. PLoS Genet .(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard A., Srinivasan S., Zimm G., Bier E. (2002). A screen for dominant mutations applied to components in the Drosophila EGF-R pathway. Proc. Natl. Acad. Sci. USA 99, 3752–3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard A., Park J. M., Cruz-Moreno B., Karin M., Bier E. (2006). Anthrax lethal factor and edema factor act on conserved targets in Drosophila. Proc. Natl. Acad. Sci. USA 103, 3244–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard A., McGillivray S. M., Cruz-Moreno B., van Sorge N. M., Nizet V., Bier E. (2010). Anthrax toxins cooperatively inhibit endocytic recycling by the Rab11/Sec15 exocyst. Nature 467, 854–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard A., Nizet V., Bier E. (2011). New insights into the biological effects of anthrax toxins: linking cellular to organismal responses. Microbes Infect .[Epub ahead of print] doi: 10.1016/j.micinf.2011.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. C., Mishra M., Sharma A., Deepak Balaji T. G., Kumar R., Mishra R. K., Chowdhuri D. K. (2010). Chlorpyrifos induces apoptosis and DNA damage in Drosophila through generation of reactive oxygen species. Ecotoxicol. Environ. Saf. 73, 1415–1423 [DOI] [PubMed] [Google Scholar]
- Ha E. M., Oh C. T., Bae Y. S., Lee W. J. (2005a). A direct role for dual oxidase in Drosophila gut immunity. Science 310, 847–850 [DOI] [PubMed] [Google Scholar]
- Ha E. M., Oh C. T., Ryu J. H., Bae Y. S., Kang S. W., Jang I. H., Brey P. T., Lee W. J. (2005b). An antioxidant system required for host protection against gut infection in Drosophila. Dev. Cell 8, 125–132 [DOI] [PubMed] [Google Scholar]
- Ha E. M., Lee K. A., Seo Y. Y., Kim S. H., Lim J. H., Oh B. H., Kim J., Lee W. J. (2009). Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in drosophila gut. Nat. Immunol. 10, 949–957 [DOI] [PubMed] [Google Scholar]
- Hao L., Sakurai A., Watanabe T., Sorensen E., Nidom C. A., Newton M. A., Ahlquist P., Kawaoka Y. (2008). Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature 454, 890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselkorn T. S. (2010). The Spiroplasma heritable bacterial endosymbiont of Drosophila. Fly 4, 80–87 [DOI] [PubMed] [Google Scholar]
- Hatakeyama M. (2008). Linking epithelial polarity and carcinogenesis by multitasking Helicobacter pylori virulence factor CagA. Oncogene 27, 7047–7054 [DOI] [PubMed] [Google Scholar]
- Hatakeyama M. (2009). Helicobacter pylori and gastric carcinogenesis. J. Gastroenterol. 44, 239–248 [DOI] [PubMed] [Google Scholar]
- Hedges L. M., Brownlie J. C., O’Neill S. L., Johnson K. N. (2008). Wolbachia and virus protection in insects. Science 322, 702. [DOI] [PubMed] [Google Scholar]
- Hellstrom M., Phng L. K., Hofmann J. J., Wallgard E., Coultas L., Lindblom P., Alva J., Nilsson A. K., Karlsson L., Gaiano N., et al. (2007). Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780 [DOI] [PubMed] [Google Scholar]
- Herbst R., Carroll P. M., Allard J. D., Schilling J., Raabe T., Simon M. A. (1996). Daughter of sevenless is a substrate of the phosphotyrosine phosphatase Corkscrew and functions during sevenless signaling. Cell 85, 899–909 [DOI] [PubMed] [Google Scholar]
- Igaki T., Kanda H., Okano H., Xu T., Miura M. (2010). Eiger and Wengen: the Drosophila orthologs of TNF/TNFR. Adv. Exp. Med. Biol. 691, 45–50 [DOI] [PubMed] [Google Scholar]
- Irazoqui J. E., Urbach J. M., Ausubel F. M. (2010). Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nat. Rev. Immunol. 10, 47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafar-Nejad H., Andrews H. K., Acar M., Bayat V., Wirtz-Peitz F., Mehta S. Q., Knoblich J. A., Bellen H. J. (2005). Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev. Cell 9, 351–363 [DOI] [PubMed] [Google Scholar]
- Jiravanichpaisal P., Lee B. L., Soderhall K. (2006). Cell-mediated immunity in arthropods: hematopoiesis, coagulation, melanization and opsonization. Immunobiology 211, 213–236 [DOI] [PubMed] [Google Scholar]
- Johard H. A., Yoishii T., Dircksen H., Cusumano P., Rouyer F., Helfrich-Forster C., Nassel D. R. (2009). Peptidergic clock neurons in Drosophila: ion transport peptide and short neuropeptide F in subsets of dorsal and ventral lateral neurons. J. Comp. Neurol. 516, 59–73 [DOI] [PubMed] [Google Scholar]
- Kaneko M., Park J. H., Cheng Y., Hardin P. E., Hall J. C. (2000). Disruption of synaptic transmission or clock-gene-product oscillations in circadian pacemaker cells of Drosophila cause abnormal behavioral rhythms. J. Neurobiol. 43, 207–233 [DOI] [PubMed] [Google Scholar]
- Katanayeva N., Kopein D., Portmann R., Hess D., Katanaev V. L. (2010). Competing activities of heterotrimeric G proteins in Drosophila wing maturation. PLoS ONE 5, e12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Park S. Y., Heo Y. J., Cho Y. H. (2008). Drosophila melanogaster-based screening for multihost virulence factors of Pseudomonas aeruginosa PA14 and identification of a virulence-attenuating factor, HudA. Infect. Immun. 76, 4152–4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C., Cho J. H., Nehme N., Ulvila J., Pearson A. M., Meister M., Strom C., Conto S. L., Hetru C., Stuart L. M., et al. (2005). Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell 123, 335–346 [DOI] [PubMed] [Google Scholar]
- Kolodner R. D. (1995). Mismatch repair: mechanisms and relationship to cancer susceptibility. Trends Biochem. Sci. 20, 397–401 [DOI] [PubMed] [Google Scholar]
- Koo I. C., Ohol Y. M., Wu P., Morisaki J. H., Cox J. S., Brown E. J. (2008). Role for lysosomal enzyme beta-hexosaminidase in the control of mycobacteria infection. Proc. Natl. Acad. Sci. USA 105, 710–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunes S., Steller H. (1991). Ablation of Drosophila photoreceptor cells by conditional expression of a toxin gene. Genes Dev. 5, 970–983 [DOI] [PubMed] [Google Scholar]
- Kurucz E., Markus R., Zsamboki J., Folkl-Medzihradszky K., Darula Z., Vilmos P., Udvardy A., Krausz I., Lukacsovich T., Gateff E., et al. (2007). Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr. Biol. 17, 649–654 [DOI] [PubMed] [Google Scholar]
- Lacy D. B., Stevens R. C. (1999). Sequence homology and structural analysis of the clostridial neurotoxins. J. Mol. Biol. 291, 1091–1104 [DOI] [PubMed] [Google Scholar]
- Lamaris G. A., Chamilos G., Lewis R. E., Kontoyiannis D. P. (2007). Virulence studies of Scedosporium and Fusarium species in Drosophila melanogaster. J. Infect. Dis. 196, 1860–1864 [DOI] [PubMed] [Google Scholar]
- Lamaris G. A., Ben-Ami R., Lewis R. E., Kontoyiannis D. P. (2008). Does pre-exposure of Aspergillus fumigatus to voriconazole or posaconazole in vitro affect its virulence and the in vivo activity of subsequent posaconazole or voriconazole, respectively? A study in a fly model of aspergillosis. J. Antimicrob. Chemother. 62, 539–542 [DOI] [PubMed] [Google Scholar]
- Lamaris G. A., Ben-Ami R., Lewis R. E., Chamilos G., Samonis G., Kontoyiannis D. P. (2009). Increased virulence of Zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J. Infect. Dis. 199, 1399–1406 [DOI] [PubMed] [Google Scholar]
- Langevin J., Morgan M. J., Sibarita J. B., Aresta S., Murthy M., Schwarz T., Camonis J., Bellaiche Y. (2005). Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev. Cell 9, 365–376 [DOI] [PubMed] [Google Scholar]
- Leppla S. H. (1982). Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 79, 3162–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie J. D., Ariza-McNaughton L., Bermange A. L., McAdow R., Johnson S. L., Lewis J. (2007). Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development 134, 839–844 [DOI] [PubMed] [Google Scholar]
- Li B. X., Satoh A. K., Ready D. F. (2007). Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J. Cell Biol. 177, 659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis M. S., Lewis R. E., May G. S., Wiederhold N. P., Albert N. D., Halder G., Kontoyiannis D. P. (2005). Toll-deficient Drosophila flies as a fast, high-throughput model for the study of antifungal drug efficacy against invasive aspergillosis and Aspergillus virulence. J. Infect. Dis. 191, 1188–1195 [DOI] [PubMed] [Google Scholar]
- Lobov I. B., Renard R. A., Papadopoulos N., Gale N. W., Thurston G., Yancopoulos G. D., Wiegand S. J. (2007). Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl. Acad. Sci. USA 104, 3219–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter E. I., Faria M. M., Rabin H. R., Storey D. G. (2008). Pseudomonas aeruginosa cystic fibrosis isolates from individual patients demonstrate a range of levels of lethality in two Drosophila melanogaster infection models. Infect. Immun. 76, 1877–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace K. A., Pearson J. C., McGinnis W. (2005). An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science 308, 381–385 [DOI] [PubMed] [Google Scholar]
- Martin J. R., Keller A., Sweeney S. T. (2002). Targeted expression of tetanus toxin: a new tool to study the neurobiology of behavior. Adv. Genet. 47, 1–47 [DOI] [PubMed] [Google Scholar]
- Martin P., Parkhurst S. M. (2004). Parallels between tissue repair and embryo morphogenesis. Development 131, 3021–3034 [DOI] [PubMed] [Google Scholar]
- Matsuki M., Yamashita F., Ishida-Yamamoto A., Yamada K., Kinoshita C., Fushiki S., Ueda E., Morishima Y., Tabata K., Yasuno H., et al. (1998). Defective stratum corneum and early neonatal death in mice lacking the gene for transglutaminase 1 (keratinocyte transglutaminase). Proc. Natl. Acad. Sci. USA 95, 1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S. Q., Hiesinger P. R., Beronja S., Zhai R. G., Schulze K. L., Verstreken P., Cao Y., Zhou Y., Tepass U., Crair M. C., et al. (2005). Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron 46, 219–232 [DOI] [PubMed] [Google Scholar]
- Meister M. (2004). Blood cells of Drosophila: cell lineages and role in host defence. Curr. Opin. Immunol. 16, 10–15 [DOI] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. (1984). Bacterial toxins: cellular mechanisms of action. Microbiol. Rev. 48, 199–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat K. G., Gould J. H., Smith H. K., O’Kane C. J. (1992). Inducible cell ablation in Drosophila by cold-sensitive ricin A chain. Development 114, 681–687 [DOI] [PubMed] [Google Scholar]
- Mohr S., Bakal C., Perrimon N. (2010). Genomic screening with RNAi: results and challenges. Annu. Rev. Biochem. 79, 37–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N. S., Heintzelman M. B., Mooseker M. S. (1995). Characterization of myosin-IA and myosin-IB, two unconventional myosins associated with the Drosophila brush border cytoskeleton. Dev. Biol. 172, 51–71 [DOI] [PubMed] [Google Scholar]
- Morize P., Christiansen A. E., Costa M., Parks S., Wieschaus E. (1998). Hyperactivation of the folded gastrulation pathway induces specific cell shape changes. Development 125, 589–597 [DOI] [PubMed] [Google Scholar]
- Moser T. S., Jones R. G., Thompson C. B., Coyne C. B., Cherry S. (2010). A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog. 6, e1000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourez M. (2004). Anthrax toxins. Rev. Physiol. Biochem. Pharmacol. 152, 135–164 [DOI] [PubMed] [Google Scholar]
- Murthy M., Schwarz T. L. (2004). The exocyst component Sec5 is required for membrane traffic and polarity in the Drosophila ovary. Development 131, 377–388 [DOI] [PubMed] [Google Scholar]
- Murthy M., Garza D., Scheller R. H., Schwarz T. L. (2003). Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron 37, 433–447 [DOI] [PubMed] [Google Scholar]
- Murthy M., Ranjan R., Denef N., Higashi M. E., Schupbach T., Schwarz T. L. (2005). Sec6 mutations and the Drosophila exocyst complex. J. Cell Sci. 118, 1139–1150 [DOI] [PubMed] [Google Scholar]
- Murthy M., Teodoro R. O., Miller T. P., Schwarz T. L. (2010). Sec5, a member of the exocyst complex, mediates Drosophila embryo cellularization. Development 137, 2773–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Kazama H., Nose A., Morimoto-Tanifuji T. (2006). Activity-dependent regulation of synaptic size in Drosophila neuromuscular junctions. J. Neurobiol. 66, 929–939 [DOI] [PubMed] [Google Scholar]
- Nayak A., Berry B., Tassetto M., Kunitomi M., Acevedo A., Deng C., Krutchinsky A., Gross J., Antoniewski C., Andino R. (2010). Cricket paralysis virus antagonizes Argonaute 2 to modulate antiviral defense in Drosophila. Nat. Struct. Mol. Biol. 17, 547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K. S., Furuse M., Beitel G. J. (2010). The Drosophila claudin Kune-kune is required for septate junction organization and tracheal tube size control. Genetics 185, 831–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Jing Q., Georgel P., New L., Chen J., Mols J., Kang Y. J., Jiang Z., Du X., Cook R., et al. (2007). Hypersusceptibility to vesicular stomatitis virus infection in Dicer1-deficient mice is due to impaired miR24 and miR93 expression. Immunity 27, 123–134 [DOI] [PubMed] [Google Scholar]
- Palauqui J. C., Elmayan T., Pollien J. M., Vaucheret H. (1997). Systemic acquired silencing: transgene-specific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J. 16, 4738–4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. C., Juarez M. T., Kim M., Drivenes O., McGinnis W. (2009). Multiple transcription factor codes activate epidermal wound-response genes in Drosophila. Proc. Natl. Acad. Sci. USA 106, 2224–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N., Mathey-Prevot B. (2007). Applications of high-throughput RNA interference screens to problems in cell and developmental biology. Genetics 175, 7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N., Ni J. Q., Perkins L. (2010). In vivo RNAi: today and tomorrow. Cold Spring Harb. Perspect. Biol. 2, a003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezard C., Berche P., Mock M. (1991). Contribution of individual toxin components to virulence of Bacillus anthracis. Infect. Immun. 59, 3472–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips J. A., Rubin E. J., Perrimon N. (2005). Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science 309, 1251–1253 [DOI] [PubMed] [Google Scholar]
- Philips J. A., Porto M. C., Wang H., Rubin E. J., Perrimon N. (2008). ESCRT factors restrict mycobacterial growth. Proc. Natl. Acad. Sci. USA 105, 3070–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. D., Thomas G. H. (2006). Brush border spectrin is required for early endosome recycling in Drosophila. J. Cell Sci. 119, 1361–1370 [DOI] [PubMed] [Google Scholar]
- Pitsouli C., Perrimon N. (2008). Developmental biology: our fly cousins’ gut. Nature 454, 592–593 [DOI] [PubMed] [Google Scholar]
- Pitsouli C., Apidianakis Y., Perrimon N. (2009). Homeostasis in infected epithelia: stem cells take the lead. Cell Host Microbe 6, 301–307 [DOI] [PubMed] [Google Scholar]
- Pfeiffer B. D., Ngo T. T., Hibbard K. L., Murphy C., Jenett A., Truman J. W., Rubin G. M. (2010). Refinement of tools for targeted gene expression in Drosophila. Genetics 186, 735–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabe T., Riesgo-Escovar J., Liu X., Bausenwein B. S., Deak P., Maroy P., Hafen E. (1996). DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between sevenless and Ras1 in Drosophila. Cell 85, 911–920 [DOI] [PubMed] [Google Scholar]
- Ramet M., Pearson A., Manfruelli P., Li X., Koziel H., Gobel V., Chung E., Krieger M., Ezekowitz R. A. (2001). Drosophila scavenger receptor CI is a pattern recognition receptor for bacteria. Immunity 15, 1027–1038 [DOI] [PubMed] [Google Scholar]
- Ramet M., Manfruelli P., Pearson A., Mathey-Prevot B., Ezekowitz R. A. (2002). Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 416, 644–648 [DOI] [PubMed] [Google Scholar]
- Reddy S., Jin P., Trimarchi J., Caruccio P., Phillis R., Murphey R. K. (1997). Mutant molecular motors disrupt neural circuits in Drosophila. J. Neurobiol. 33, 711–723 [DOI] [PubMed] [Google Scholar]
- Ren F., Wang B., Yue T., Yun E. Y., Ip Y. T., Jiang J. (2010). Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc. Natl. Acad. Sci. USA 107, 21064–21069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca C., Adams R. H. (2007). Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 21, 2511–2524 [DOI] [PubMed] [Google Scholar]
- Rodriguez Moncalvo V. G., Campos A. R. (2009). Role of serotonergic neurons in the Drosophila larval response to light. BMC Neurosci. 10, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roignant J. Y., Carre C., Mugat B., Szymczak D., Lepesant J. A., Antoniewski C. (2003). Absence of transitive and systemic pathways allows cell-specific and isoform-specific RNAi in Drosophila. RNA 9, 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J. H., Ha E. M., Lee W. J. (2010). Innate immunity and gut-microbe mutualism in Drosophila. Dev. Comp. Immunol. 34, 369–376 [DOI] [PubMed] [Google Scholar]
- Sabin L. R., Zhou R., Gruber J. J., Lukinova N., Bambina S., Berman A., Lau C. K., Thompson C. B., Cherry S. (2009). Ars2 regulates both miRNA- and siRNA-dependent silencing and suppresses RNA virus infection in Drosophila. Cell 138, 340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin L. R., Hanna S. L., Cherry S. (2010). Innate antiviral immunity in Drosophila. Curr. Opin. Immunol. 22, 4–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M. C., Tassetto M., van Rij R. P., Goic B., Gausson V., Berry B., Jacquier C., Antoniewski C., Andino R. (2009). Antiviral immunity in Drosophila requires systemic RNA interference spread. Nature 458, 346–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salunkhe P., Smart C. H., Morgan J. A., Panagea S., Walshaw M. J., Hart C. A., Geffers R., Tummler B., Winstanley C. (2005). A cystic fibrosis epidemic strain of Pseudomonas aeruginosa displays enhanced virulence and antimicrobial resistance. J. Bacteriol. 187, 4908–4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J. (2008). Host-bacteria homeostasis in the healthy and inflamed gut. Curr. Opin. Gastroenterol. 24, 435–439 [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Medzhitov R. (2009). Learning tolerance while fighting ignorance. Cell 138, 416–420 [DOI] [PubMed] [Google Scholar]
- Selgrad M., Koornstra J. J., Fini L., Blom M., Huang R., Devol E. B., Boersma-van Ek W., Dijkstra G., Verdonk R. C., de Jong S., et al. (2008). JC virus infection in colorectal neoplasia that develops after liver transplantation. Clin. Cancer Res. 14, 6717–6721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbus L. R., Casper-Lindley C., Landmann F., Sullivan W. (2008). The genetics and cell biology of Wolbachia-host interactions. Annu. Rev. Genet. 42, 683–707 [DOI] [PubMed] [Google Scholar]
- Sessions O. M., Barrows N. J., Souza-Neto J. A., Robinson T. J., Hershey C. L., Rodgers M. A., Ramirez J. L., Dimopoulos G., Yang P. L., Pearson J. L., Garcia-Blanco M. A. (2009). Discovery of insect and human dengue virus host factors. Nature 458, 1047–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly S., Lukinova N., Bambina S., Berman A., Cherry S. (2009). Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity 30, 588–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C. D., Duan K., Fischer C., Parkins M. D., Storey D. G., Rabin H. R., Surette M. G. (2008). Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog. 4, e1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmann A. F., Lawson N. D. (2007). Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445, 781–784 [DOI] [PubMed] [Google Scholar]
- Simon M. A. (2000). Receptor tyrosine kinases: specific outcomes from general signals. Cell 103, 13–15 [DOI] [PubMed] [Google Scholar]
- Spikes S., Xu R., Nguyen C. K., Chamilos G., Kontoyiannis D. P., Jacobson R. H., Ejzykowicz D. E., Chiang L. Y., Filler S. G., May G. S. (2008). Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J. Infect. Dis. 197, 479–486 [DOI] [PubMed] [Google Scholar]
- Stroschein-Stevenson S. L., Foley E., O’Farrell P. H., Johnson A. D. (2006). Identification of Drosophila gene products required for phagocytosis of Candida albicans. PLoS Biol. 4, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroschein-Stevenson S. L., Foley E., O’Farrell P. H., Johnson A. D. (2009). Phagocytosis of Candida albicans by RNAi-treated Drosophila S2 cells. Methods Mol. Biol. 470, 347–358 [DOI] [PubMed] [Google Scholar]
- Stuart L. M., Boulais J., Charriere G. M., Hennessy E. J., Brunet S., Jutras I., Goyette G., Rondeau C., Letarte S., Huang H., et al. (2007). A systems biology analysis of the Drosophila phagosome. Nature 445, 95–101 [DOI] [PubMed] [Google Scholar]
- Suchting S., Freitas C., le Noble F., Benedito R., Breant C., Duarte A., Eichmann A. (2007). The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl. Acad. Sci. USA 104, 3225–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhy D. A., Giddings T. H., Jr, Kirkegaard K. (2000). Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles. J. Virol. 74, 8953–8965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney S. T., Broadie K., Keane J., Niemann H., O’Kane C. J. (1995). Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14, 341–351 [DOI] [PubMed] [Google Scholar]
- Takashima S., Mkrtchyan M., Younossi-Hartenstein A., Merriam J. R., Hartenstein V. (2008). The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature 454, 651–655 [DOI] [PubMed] [Google Scholar]
- Teixeira L., Ferreira A., Ashburner M. (2008). The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting S. B., Caddy J., Hislop N., Wilanowski T., Auden A., Zhao L. L., Ellis S., Kaur P., Uchida Y., Holleran W. M., et al. (2005). A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science 308, 411–413 [DOI] [PubMed] [Google Scholar]
- Tournier J. N., Quesnel-Hellmann A., Cleret A., Vidal D. R. (2007). Contribution of toxins to the pathogenesis of inhalational anthrax. Cell. Microbiol. 9, 555–565 [DOI] [PubMed] [Google Scholar]
- van Rij R. P., Saleh M. C., Berry B., Foo C., Houk A., Antoniewski C., Andino R. (2006). The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 20, 2985–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale G., Pellizzari R., Recchi C., Napolitani G., Mock M., Montecucco C. (1998). Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem. Biophys. Res. Commun. 248, 706–711 [DOI] [PubMed] [Google Scholar]
- Voinnet O., Vain P., Angell S., Baulcombe D. C. (1998). Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell 95, 177–187 [DOI] [PubMed] [Google Scholar]
- Wang X. H., Aliyari R., Li W. X., Li H. W., Kim K., Carthew R., Atkinson P., Ding S. W. (2006). RNA interference directs innate immunity against viruses in adult Drosophila. Science 312, 452–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson F. L., Puttmann-Holgado R., Thomas F., Lamar D. L., Hughes M., Kondo M., Rebel V. I., Schmucker D. (2005). Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science 309, 1874–1878 [DOI] [PubMed] [Google Scholar]
- Winston W. M., Molodowitch C., Hunter C. P. (2002). Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 295, 2456–2459 [DOI] [PubMed] [Google Scholar]
- Wirtz-Peitz F., Zallen J. A. (2009). Junctional trafficking and epithelial morphogenesis. Curr. Opin. Genet. Dev. 19, 350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu V. M., Schulte J., Hirschi A., Tepass U., Beitel G. J. (2004). Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J. Cell Biol. 164, 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi R., Mayer F., Basler K. (2010). Refined LexA transactivators and their use in combination with the Drosophila Gal4 system. Proc. Natl. Acad. Sci. USA 107, 16166–16171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T., Mita S., Ohmori H., Oshima Y., Fujimoto Y., Ueda R., Takada H., Goldman W. E., Fukase K., Silverman N., et al. (2008). Autophagic control of listeria through intracellular innate immune recognition in Drosophila. Nat. Immunol. 9, 908–916 [DOI] [PMC free article] [PubMed] [Google Scholar]