Abstract
Pluripotent mesenchymal stem cells (MSCs) are considered ideal therapeutic targets in regenerative medicine, as they hold the capacity to differentiate into higher order connective tissues. The potential to harness MSCs for disease treatment and acceleration of repair will ultimately depend on an improved understanding of how physical and/or chemical signals regulate their activity, and the ability of exogenous stimuli to enhance MSC proliferation and define MSC fate. Recent appreciation that bone marrow osteoprogenitors are inversely proportional to adipocyte precursors suggests that their shared progenitor, the MSC, will commit to one lineage at the cost of the other. This interrelationship may contribute to the phenotype of sedentary subjects who have more fat and less bone, while conversely, to the outcome of exercise being less fat and more bone. Mechanical biasing of MSC lineage selection suggests that physical signals may influence the quantity of both fat and bone through developmental, as well as metabolic or adaptive pathways. Considered with the recent finding that low magnitude mechanical signals (LMMS) suppress the development of subcutaneous and visceral fat without elevating energy expenditure, this indicates that MSCs are ideally positioned as mechanosensitive elements central to musculoskeletal adaptation, but that the signals needn’t be large to be influential. The biasing of MSC differentiation by mechanical signals represents a unique means by which adiposity can be inhibited while simultaneously promoting a better skeleton, and may provide the basis for a safe, non-invasive, non-pharmacologic strategy to prevent both obesity and osteoporosis, yet uniquely – without targeting the resident fat or bone cell.
Introduction
Osteoporosis and obesity, two of the most dreaded diseases in the U.S., affect over 30% of the American population and result in close to 200 billion dollars in annual health service costs. (1) Osteoporosis, a disease characterized by diminished bone density, is one of the most common age-related disorders, with atraumatic fractures severely compromising an individual’s quality of life. The U.S. Surgeon General estimates that 50% of women over the age of 65 are at risk of bone fracture, and within 50 years, costs to prevent and treat this disease alone may exceed 250 billion dollars. (1) Turning from the elderly to the young, conservative estimates indicate that 25% of American children are overweight, while 11% are obese, (2) both percentages strikingly higher than just 10 years ago. This obese population is predisposed to type 2 diabetes (3) and an elevated lifetime risk of cardiovascular disease and cancer (4).
Prior to 1994, type 2 diabetes mellitus was unusual in children; yet now, some clinics report that up to one-third of adolescent children with diabetes are afflicted with type 2 disease (5–7). Visceral adiposity and elevated free fatty acid levels are strongly correlated with insulin resistance (8), a problem that becomes even more devastating as overweight children grow into obese adults (9). Lifestyle factors, specifically, physical inactivity and poor dietary intake, are important targets for primary prevention of obesity and diabetes. Studies in adults suggest that modification of lifestyle and weight loss can decrease insulin resistance, improve measures of glycemic control, and reduce lipemia (10;11). Unfortunately, controlled clinical trials to determine if lifestyle interventions can prevent type 2 diabetes in adults (12;13) are rarely successful or sustained in the general U.S. population, as weight gain, and its concomitant complications, return quickly. In children, even the most exhaustive federally funded intervention studies have failed to yield compelling positive results (14;15). The role of exercise in preventing obesity has concentrated on calorie expenditure, but has ignored any “non-metabolic” role. Teaching away from a “burn-it or carry-it” perspective, a recent commentary in Obesity (16) referred to the relative success of vigorous physical activity interventions, but suggested that the positive results were actually due, in part, to mechanical stimulation of tissues rather than metabolizing of calories.
While both osteoporosis and obesity have garnered great public attention, effective and safe pharmacologic interventions at any scale for either disease have proven elusive. Even control of either osteoporosis or obesity has proven difficult, with perhaps the most common etiologic factor being a “sedentary lifestyle” and the most common intervention being exercise (17), indicating a pivotal role for mechanical signals in defining bone and fat mass. But is this disease-responsivity to mechanical signals coincidence, or is there a biologic connection? Herein, the capacity of mechanical signals to influence the fat and bone phenotype is examined, not so much in terms of a direct mechanical impact on the resident fat or bone cell population, but by biasing decision-making of their common progenitor, the mesenchymal stem cell (MSC) (Fig. 1).
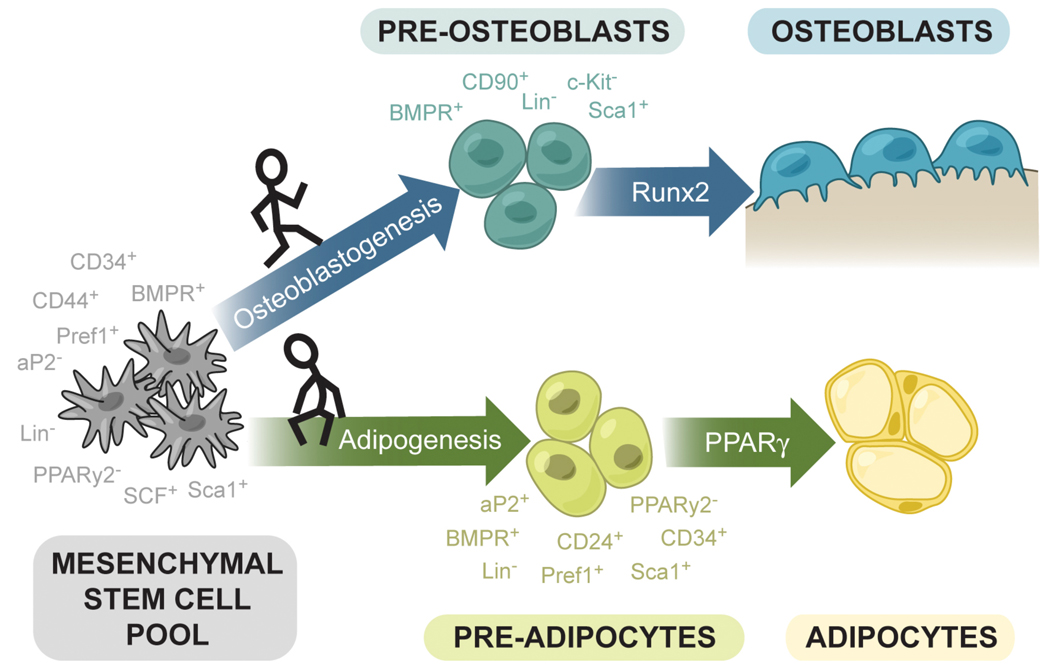
Fig. 1.
Schematic representation of the lineage potential of multipotent mesenchymal stem cells (MSCs) in the bone marrow. The development of mature cells such as adipocytes and osteoblasts proceeds through intermediate “progenitor” cells, preosteoblasts and preadipocytes. Although not thoroughly characterized, several combinations of surface markers have been utilized to enrich for MSCs, as well as committed but not yet fully differentiated precursor cells. The processes of commitment and differentiation are complex and also not well-characterized, but certain transcription factors such as Runx2 (bone) and PPARγ (fat) have been shown to promote the differentiation of one cell type and suppress the differentiation of the other.
Mesenchymal Stem Cells (MSCs)
The number of studies examining the therapeutic potential of MSCs has rapidly increased in recent years (18–20), yet the understanding of what drives decision-making in these precursors remains in the nascent stages. Contributing to this difficulty, both specific in situ and ex vivo identification of what actually constitutes the MSC population is a source of heated debate, as delineating stem cells from their neighbors has proven frustrating based on current histological and cytometric methods. To exemplify this point, a combination of markers that distinguish both MSCs and hematopoietic stem cells (HSCs) from 99.9% of the other cells residing in the bone marrow yields a population estimated, at best, at 3% purity (21).
For MSCs, the expression of specific, exclusive surface markers has yet to be well-characterized, and features that categorically define MSCs have not been reported. While cell populations obtained in the current isolation methods are essentially heterogeneous mixtures of several cell types, they are certainly enriched for MSCs (22). Not surprisingly, the question of what factors determine whether a MSC differentiates into either an adipocyte, osteoblast, or other cell type remains unknown, and is the focus of intense research. Of the various signals capable of inducing differentiation, various biochemical factors have been reported that typically drive the differentiation towards one cell type, with a parallel suppression of another pathway. Recent work on the differentiation of osteoblasts from MSCs highlights the finding that cell fate decisions can be markedly influenced by activating a very small subset of a particular signaling network, rather than requiring large shifts of gene expression (23). Thus, even subtle changes in the MSC milieu can have dramatic effects on the phenotype.
Mechanical Stimulation to Induce Cell Differentiation
In addition to the biochemical factors capable of altering stem cell fate, mechanical signals are becoming recognized as playing key and interacting roles in defining the differentiation pathway. Mechanotransduction is the process by which cells transduce physical force-induced signals into biochemical responses, resulting in altered gene expression, cell function and morphology, and extracellular matrix production. This adaptive process is critical for mediating appropriate responses to acclimate and accommodate functional loading in many tissues (24). The basis for mechanotransduction is that cells form networks, which are connected by intercellular adhesion complexes such as adherens junctions, gap junctions or by local paracrine signals (25). These networks are capable of acting as integrated units to transduce various stimuli, such as mechanical loading, into coordinated tissue responses. Not surprisingly, to transduce the mechanical signal requires the interaction of many signaling pathways (25), and due to the complexity, this is still not well-characterized. Various pathways such as cell-extracellular matrix interactions, cytokines, second messenger transmission through gap junctions and intercellular adhesive junctions enable cells to transmit mechanical signals to other cells (26).
As an example of cellular mechanotransduction in lineage determination, the role of mechanical forces in the control of adipogenesis has been linked to changes in extracellular matrix (ECM) proteins and matrix metalloproteinases (27). ECM components play an important role in regulating adipose tissue remodeling during adipocyte differentiation, by transducing cellular signals that can alter adipocyte gene transcription during adipogenesis (28). Examples of the importance of mechanical signals to bone and bone marrow are highlighted in the following section.
Mechanotransduction in Bone and Bone Marrow
Underlying the essence of mechanotransduction is the necessity that certain cells in the biological environment can act as receptors, which in turn can generate secondary, cytogenic signals that are aimed at target cells. How mechanical factors are sensed in the bone comprises a large body of active research, with many differing hypotheses regarding the mechanosensory element (29;30). The prevailing view is that osteocytes are responsible for detecting mechanical signals, and respond by signaling the effector cells, osteoblasts and osteoclasts, that modulate actual bone formation and resorption (31;32). Yet it is not “simply” the bone matrix, and the cells entombed within the material, that are subject to mechanical loading. Equally complex is the multitude of forces generated in the bone marrow cavity in response to mechanical loading, and includes strain, pressure, fluid flow, electric potentials and acceleration (32). Even as mechanotransduction by cells in the musculoskeletal system has long been a focus of research and technology development, the ability of mechanical signals to affect and alter the differentiation patterns of MSCs was only recently noted (33;34).
Enmeshed within the bone/bone marrow interaction is the concept of a stem cell “niche”, a specialized location where pluripotent cells reside and are regulated. The thought that the marrow cavity represents such a niche has gained traction in recent years, with the endosteal bone surface providing the primary location for marrow regeneration (35;36). Several models utilizing transgenic mice have shown that increases in hematopoiesis occur in conjunction with increases in both osteoblasts and trabecular bone, as an increased niche size is necessary to provide support for the increase in HSCs (37). Within this niche, it has been suggested that osteoblasts can play a direct role in stem cell function by providing support for HSCs (38). Thus, the ability of mechanical forces to effect changes in the bone and bone marrow are interlinked, and perhaps the responsiveness of bone to mechanical signals might provide insight into the ability of cells in the bone marrow to respond.
Absence of Mechanical Signals Promotes the Fat Phenotype
The site-specific bone wasting that occurs in aging, bed rest, and other sedentary lifestyles is paralleled by a reduction in the mechanical signals that reflect the dynamics of muscle contractibility (39). It is certainly possible that the absence of a mechanical signal to drive MSCs towards bone and/or muscle formation (40) is permissive to differentiating MSCs to preferentially commit, or default, to another lineage, such as adipocytes. Magnetic resonance imaging (MRI) provides evidence of this association showing that post-menopausal women have twice as much fat in the marrow as pre-menopausal women, and that women with low bone density have more bone marrow fat than women with normal bone density (41). The aging process is commonly associated with a redistribution of fat, away from the peripheral depots and into organs (i.e. bone marrow, liver, and muscle). The causality of the inverse bone/marrow fat connection is not clear, and certainly other factors such as age, activity level, and hormone status all contribute to the response. Importantly, application of mechanical signals to MSCs in culture can effectively suppress their differentiation towards adipogenesis, even in culture conditions that markedly prefer a “fat” pathway (42). Thus, while mechanical signals provide key anabolic signals to bone formation, it also appears that the absence of these signals encourages adipogenesis. Indeed, this is precisely the case with some isolated stem cells in vitro, where in the absence of an exogenously applied differentiation signal (i.e., chemical or biophysical stimuli), the default pathway is to form lipid-laden adipocytes. (43)
Mechanical Signals Driving the Bone Phenotype
Existing models have led to the hypothesis that amplitude-mediated parameters of exercise, such as strain magnitude (44) or strain energy density (45) are critical to defining the bone response and the resulting skeletal morphology (46). There is strong evidence that the resident bone cells recognize and respond to mechanically generated signals that stimulate the osteoblast/osteocyte network (47) and inhibit osteoclastogenesis (48), a process mediated by tissue strain (49), enhanced fluid flow (50), intramedullary pressure (51) and/or streaming potentials (52). Importantly, each of these physical parameters is more strongly correlated to the dynamics of the load environment (impact (53), strain rate (54) and strain gradients (55)) than the actual strain magnitude generated by the load.
It has been demonstrated that mechanically-mediated bone remodeling exhibits a strong interdependence of strain magnitude and cycle number, such that bone mass can be enhanced either with a few large strain events (56), or 100,000’s of extremely low magnitude strain signals (57), leading to a paradigm that bone structure depends as much on the persistent, low magnitude strains that arise during predominant activities (i.e., standing), as it does on the rarer strain events generated during strenuous activity (58). While the ability of large strain events to drive bone formation has been recognized for some time, more recent studies have indicated that very small mechanical signals, induced at relatively high frequency (cycles per second), can also promote bone formation (59).
Adult female sheep subject to 1 year of brief (20 min·d−1), low magnitude (0.3g, where 1g is earth’s gravitational field), high frequency (30Hz, where 3Hz is the stride frequency for an elite runner), mechanical signals realized a 34.2% increase in trabecular density in the proximal femur, as compared to control (CON) (p<0.01) (59). Significant increases were noted in both trabecular volume (+32%; p<0.04) and number (+45%; p<0.01), paralleled by decreased spacing (−36%; p=0.02) (60). µCT reconstructions of the distal femoral condyle (61) showed a 10.6% greater bone mineral content (BMC) in experimental animals (p<0.05) due to an 8.3% increase in trabeculae (p<0.01). Strength to failure was 26.7% greater (p<0.05), indicating an improvement in bone quality over controls. These results suggest an overall adaptation that increases bone stiffness and achieves a more uniform stress distribution (62), providing evidence that, similar to our findings with cortical bone (63;64), strain gradients may be critical in driving bone’s adaptation to mechanical signals. With this drive towards bone formation, and the recruitment of osteoblasts from the progenitor pool, it was realized that, if there is a finite number of mesenchymal precursors, there could be a parallel inhibition of adipose formation.
Mechanical Signals Suppress the Fat Phenotype
Considering the importance of exercise in stemming both osteoporosis and obesity, and the anabolic response of the skeletal system to low magnitude mechanical signals (LMMS), we hypothesized that mechanical signals anabolic to bone would, in parallel, curb the production of fat in the growing animal. Forty 7-week-old C57BL/6J (B6) male mice on a normal chow diet were randomized into LMMS or CON groups. For 15 weeks, LMMS mice were subject to 15min·d−1 of a 90Hz, 0.4gp-p signal. At 12 weeks (19 weeks of age) in vivo CT showed that torsal fat volume of LMMS mice was 27.4% lower than in CON (p=0.008; Fig. 2). In contrast, total lean volume of the torso (total volume minus fat and bone) was similar between LMMS and CON (p=0.7), while lean volume in ratio of body mass was 5.0% greater in LMMS than CON (p=0.01).
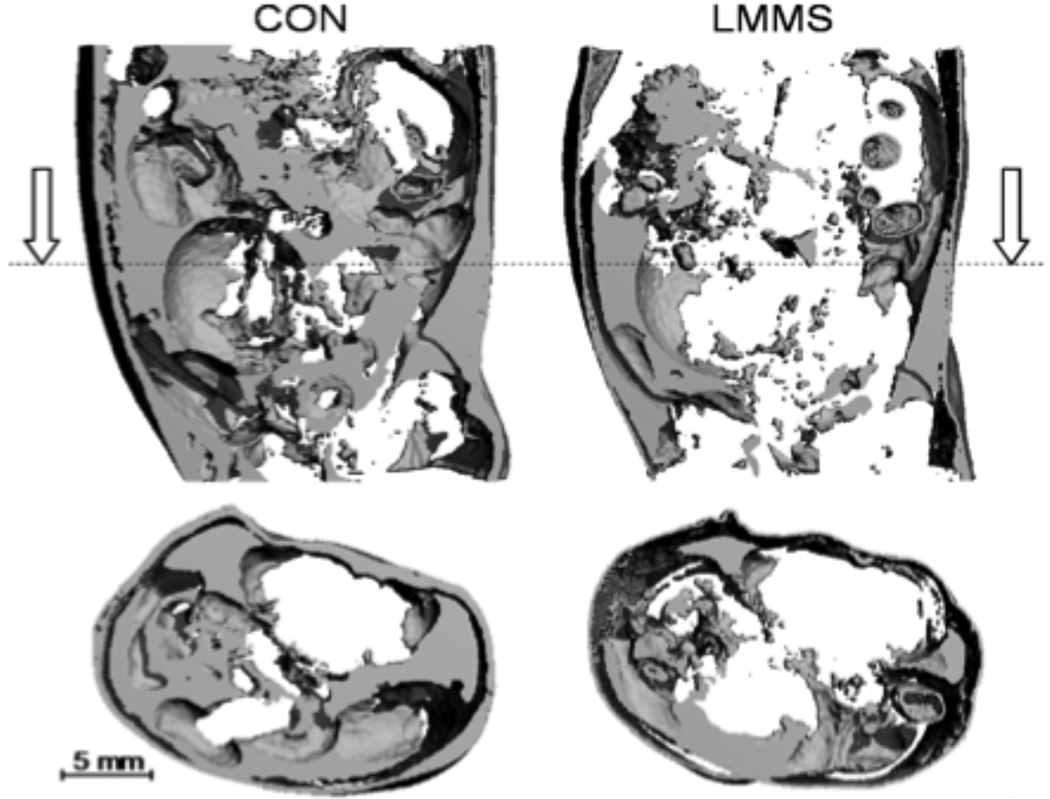
Fig. 2.
A longitudinal (top) and transverse (bottom) µCT reconstruction of abdominal fat content through the torso of a control (left) and LMMS (right) B6 mouse, performed in vivo at 12 weeks using CT signal parameters specifically sensitive to fat. Following 12 weeks of daily, 15 minute LMMS, fat within the torso was 27% lower than in controls. Reproduced from Proc Natl Acad Sci U S A. 2007 Nov 6;104(45):17879-84.
Fat volume data derived from in vivo CT calculated fat volume was validated against the weights of the dissected fat pads harvested post-sacrifice at 15 weeks (22 weeks of age), in which LMMS mice had 26.2% less epididymal (p=0.01) and 20.8% less subcutaneous (p=0.02) fat than CON mice. Weekly food intake of LMMS (26.4g·w−1 ± 2.1) and CON (27.0g·w−1 ± 2.1) were essentially identical. Lack of correlations between food intake and either total body mass (r2 = 0.15; p = 0.7) or fat volume (r2=0.008; p = 0.6) indicated that the lower adiposity in LMMS could not be explained by differences in food consumption.
Although there was a slight decrease in fasting glucose and insulin levels in the LMMS group (p=0.07), this result was not significant, suggesting that the applied LMMS did not affect liver or beta cell function. At sacrifice, triglycerides (TG, total mg in tissue) in adipose tissue of LMMS mice were 21.1% (p=0.3) lower than CON, and 39.1% lower in the liver (p=0.02). Total non-esterified free fatty acids (NEFA, total mmol in tissue) in adipose tissue were 37.2% lower in LMMS mice as compared to CON (p=0.01), while NEFA in the liver of LMMS mice was 42.6% lower (p=0.02). Numerous studies have demonstrated that dyslipidemia can have major negative impacts on metabolism, growth and development – in particular, intra-tissue lipid accumulation and intra-myocellular lipids have been closely linked to insulin resistance – and is considered the best predictor for future development of insulin resistance (65). The ability to suppress adipose tissue expansion by mechanical signals, as well as to limit NEFA and triglyceride production, suggests a mechanical approach to limit obesity may also improve dyslipidemia.
LMMS Suppresses Adiposity by Influencing Differentiation Pathways of MSCs
Bone marrow transplants studies where GFP-labeled bone marrow was injected into wild type recipient mice were used to determine if the suppression of adiposity in the growing animal by LMMS was achieved by redirecting bone marrow-derived MSCs away from an adipocytic fate (66). This was approached utilizing heterozygous B6 green fluorescent protein positive (GFP+) mice as bone marrow donors (67). Upon implantation into a wild type mouse (16 wild type male B6 mice, 8 weeks of age), the fate of the bone marrow stem cells can be monitored by the production of GFP. One week post-transplant, half the GFP+ recipient mice were subjected to LMMS (as above), and half served as sham-loaded CON. Sacrificed at 6 weeks, the epididymal fat pad and marrow from the tibia were harvested for examination. FACS analysis was performed on the epididymal fat pad and bone marrow isolated from GFP+ recipients using the GFP fluorescence signal. To help identify MSCs within the overall bone marrow cell population, cells were labeled with stem cell antigen-1 (Sca-1), an antigen typically associated with hematopoietic cells, but more recently shown to be expressed on cells with adipogenic, chondrogenic and osteogenic potential (68).
The ratio of GFP+ adipocytes in the epididymal fat pad to GFP+ MSCs was shown to be 19% lower (p<0.02) in animals subjected to LMMS relative to controls (CON: 101.2% ± 16.1%; LMMS 82.0% ± 11.1%). Data indicating reduced commitment to adipocytes were supported by the weight of the epididymal fat pad following 6 weeks of LMMS, which was 12.2% less than CON (p<0.03).
The brevity of the signal, and that loading inherent to LMMS is low relative even to normal weight bearing, suggests that the inhibition of adipogenesis was achieved by pathways other than an exercise-mediated increase in metabolic activity. Taken in consideration with the data from these GFP+ recipient mice, these results indicate that the reduced adiposity resulting from LMMS is achieved through influencing the differentiation of adipocyte precursors, MSCs, deterring them from the adipocytic lineage, and if paralleled by an increase in lean mass (see below), driving them to a musculoskeletal fate. Despite a similar diet, LMMS curbed fat gain by “simply” avoiding the creation of adipocytes.
If the processes of fat and bone formation are inversely coupled, it should be apparent by evaluating both systems simultaneously; improving musculoskeletal quality could also directly serve as an effective measure to prevent the onset of obesity (65;69). To assess the efficacy of the LMMS signal in response to a model of obesity (diet-induced), young adult (7-week C57/BL6) male mice were fed a high fat diet (45 kcal % fat) and randomized into either CON or LMMS-treated groups. At 12 weeks (19 weeks of age), neither body mass gains nor the average weekly food intake differed significantly between the LMMS or CON groups (CON weighed 32.9g ± 4.2g, while LMMS mice were 6.8% lighter at 30.7g ± 2.1g; p=0.15). TG and NEFA measured in plasma, epididymal adipose tissue, and liver were all lower in LMMS as compared to CON. Liver TG levels decreased by 25.6% (p=0.19) in LMMS animals, and were paralleled by a 33.0% (p=0.022) decrease in NEFA levels. Reflecting the decreased adipose burden, fasting serum levels of adipokines were decreased in LMMS. Compared to CON, circulating levels of leptin were decreased by 35.3% (p=0.05), adiponectin by 21.8% (p=0.009), and resistin by 15.8% (p=0.26). Circulating serum osteopontin (−7.5%, p=0.41) and osteocalcin (−14.6%, p=0.22) levels were not significantly affected by the mechanical signals. Thus, as the signal prevents the formation of adipocytes, the overall metabolic state of the animal appears improved as an outcome of LMMS stimulation.
Based on newly developed methodology allowing the spatial delineation of adipose tissue into subcutaneous and visceral fat depots in small animals using low-resolution micro-computed tomography (Fig. 3) (70;71), it was further seen that visceral adiposity was more sensitive than subcutaneous adiposity to the mechanical signal. (72) Total abdominal adiposity was segregated as either subcutaneous or visceral adipose tissue (SAT or VAT). LMMS animals had 28.5% (p=0.021) less VAT by volume, and 19.0% (p=0.016) less SAT by calculated volume. As obesity researchers have long since noted that the metabolic risk tends to be greater than, and show higher correlation to visceral adiposity than subcutaneous adiposity (73;74), the specific reduction of visceral fat in these animals is encouraging.
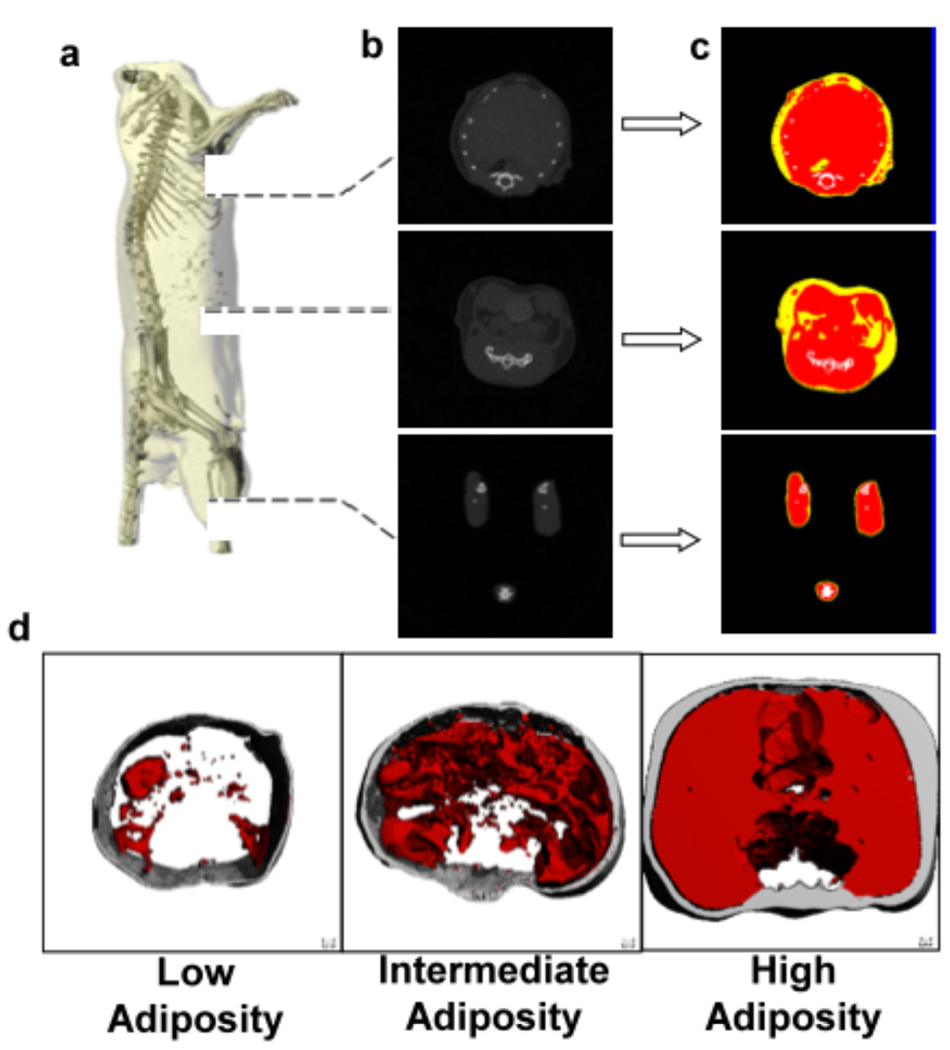
Fig. 3.
(a). Reconstructed µCT scan of a mouse in which the skeleton can be readily identified to define the region of interest. (b). The majority of the adipose tissue in the mouse is localized in the abdominal region, as the thoracic cavity and legs show lower prevalence of low density (fat) tissue. (c). To quantify fat volume in these different body compartments, tissues of different density were segregated and categorized as either fat (yellow), lean mass (red), or bone (white). (d). Representative images from three different animals with either low, intermediate, or high adiposity, with the threshold specific to fat applied. Subcutaneous fat is shown in gray, visceral fat in red. Reproduced from Med Eng Phys. 2009 Jan;31(1):34–41 with permission of Elsevier Limited.
It is important to note with these studies that the real power of this mechanical signal, particularly in regard to biasing MSC differentiation, will occur when there is the greatest population of cells “available” to be influenced. Thus, it could be readily argued that that the most influential time for mechanical loading strategies is while the subject is young, and thus the most relevant target should be in the prevention, rather than treatment, of bone loss and prevention of adiposity rather than treatment of obesity.
As evidenced by the suppressed influence of exercise to elicit large changes in BMD of older populations, we suspect that the real power of a method to affect MSC differentiation is during periods where there is a ready supply of undifferentiated cells in a “neutral” environment to be acted upon. Older individuals with significant amounts of marrow fat are caught in a positive feedback loop of sorts, where the fat that is present secretes factors into the local environment that inhibit bone, while promoting the development of additional fat. That said, it is also conceivable that “all” interventions, physically or chemically based, are more influential in the young than the old for this very reason: that there are more cells to impact during the early than the late years. Of course, if the mechanical (or other) interventions ultimately slow the progression of bone marrow towards fat, perhaps pharmacological interventions will also be more successful, as the potential cell populations available to respond will be much greater.
Mechanical Signals at the Cellular Level
While matrix strains two orders of magnitude below peak functional strains in bone are known to be anabolic (75;76), the means by which such LMMS cause bone formation is not clear. Matrix strains of less than 0.001% perturb cells by less than one angstrom, a deformation, per se, but one that is unlikely to be recognized by cells (77;78). Rather, byproducts of matrix deformation, (i.e., fluid flow-induced shear stresses, streaming potentials, fluid drag on pericellular processes) or perhaps enhanced nutrient transport, may contribute to a cell’s responsiveness to mechanical signals (32;79).
Instead of a matrix deformation-dependent pathway for mechanotransduction, the frequency sensitivity of bone’s adaptive system points towards a more fundamental pathway by which physical signals interact with cells in tissue. The physical acceleration of a cell may present a generic signal that can transmit physical challenges by altering intracellular cytoskeletal relationships (80–82). As such, it is possible that the mechanism by which MSCs sense mechanical signals is based on acceleration and deceleration of the cell, rather than distortion of the substrate. Therefore, cells may be responsive to LMMS despite the virtual absence of matrix strain (83–85) through accelerations/decelerations of the cell provoking out of phase motion with respect to the tethered nucleus, sufficient to activate a biologic response (85).
In addition, other physical signals such as pulsed electromagnetic fields or low intensity pulsed ultrasound (LIPUS) may exert similar effects on bone and fat tissue, based on similar mechanotransduction pathways. There is a great deal of preclinical and clinical evidence that ultrasound in general, and the signal used in the sonic accelerated fracture healing signal (SAFHS) device in particular, will accelerate fracture repair, catalyzing an array of hypotheses regarding the cascade of events and the effector cells. Potentially, the MSC population, which is recruited to the fracture site, could be further stimulated to proliferate and differentiate by LIPUS.
Transducing a Mechanical Signal into a Biologic Response
Evidence that chemical and physical signals influence MSC differentiation toward the osteoblastic over the adipocytic lineage is growing. For example, MSC entry into the adipocyte lineage is advanced by expression and action of PPARγ, which, when absent or present at single copy allows enhanced osteogenesis (86). PPARγ stimulation of MSCs towards adipogenic lineage selection is achieved, at least partly, through inhibition of canonical Wnt signaling (87), a pathway critically important to MSC entry into the osteogenic lineage and expansion of the osteoprogenitor pool (88). The reciprocal effect of β-catenin signaling on osteoblast/adipocyte lineage selection by MSCs has also been shown, where a high bone mass phenotype due to a constitutively activated Lrp5/Wnt signal is accompanied by decreased marrow fat (89). When considered in the context of bone’s sensitivity to mechanical signals, factors such as strain and shear are known to enhance bone cell function by accelerating differentiation along the osteogenic pathway (90), promoting osteoblast activity (91), and also by suppressing bone resorption (49). Indeed, activation and nuclear translocation of β-catenin (92;93) occurs within minutes of the introduction of mechanical signals. β-catenin activation is dependent upon strain’s ability to inactivate GSK3β, allowing for an increase in the β-catenin pool that can be activated (94), resulting in increased osteoblast differentiation and a greater commitment to the musculoskeletal system.
The critical role of β-catenin in early selection of the osteoprogenitor lineage, and the ability of mechanical signals to activate cellular β-catenin, begs the question of whether mechanical signals could influence osteoblast differentiation at very early stages. Primary marrow stem cells subjected to daily bouts of mechanical strain express bone lineage markers at double the rate as those devoid of deformation (42). A progressive decrease in both active and total β-catenin accompanied the adipogenic transformation of MSCs, but mechanical signals completely prevented such decreases in β-catenin and in doing so limited expression of both PPARγ and adiponectin (95). In agreement with the effects of strain on differentiated bone cells (94), mechanical preservation of the cellular β-catenin levels depends at least partially on the ability of strain to inactivate GSK3β. It has been proposed that in the “disuse” – or unstrained – condition, active GSK3β is permissive to adipogenesis by targeting β-catenin for degradation, and that mechanical factors, through different proximal effectors, primarily target β-catenin to regulate MSC lineage decisions biased towards osteoblastogenesis over adipogenesis.
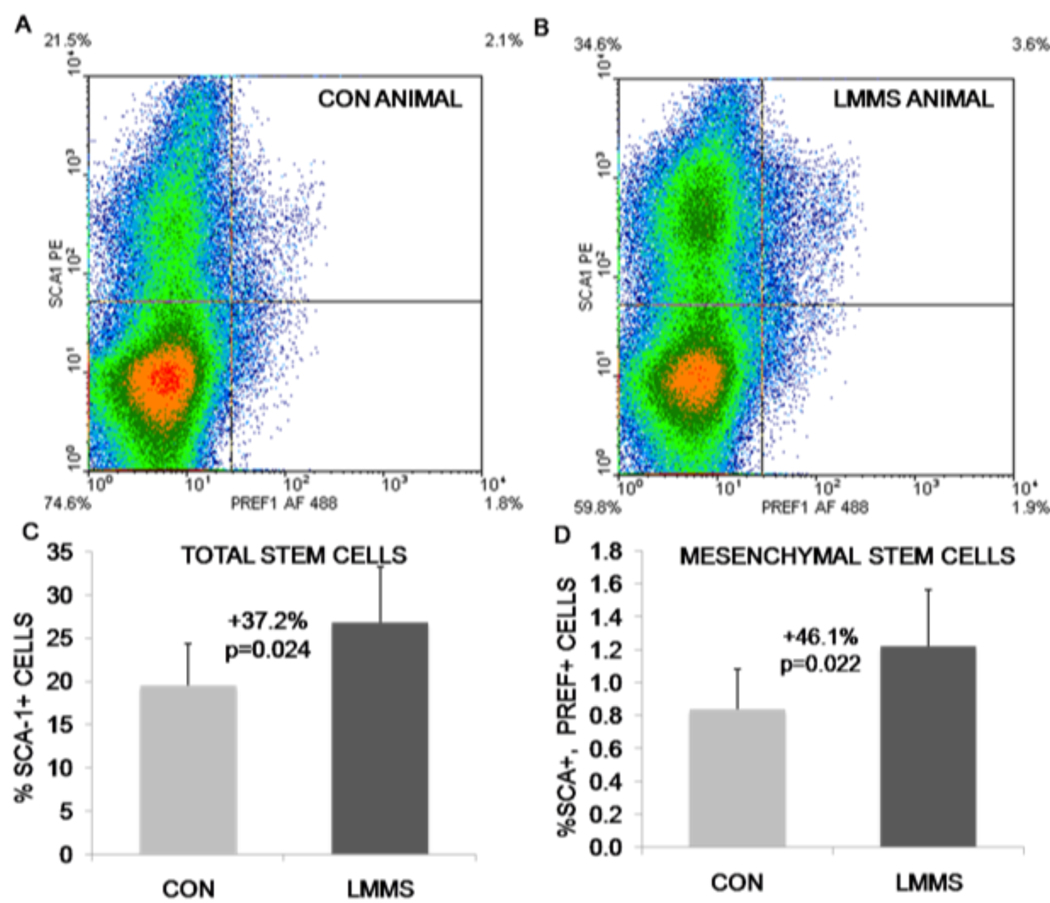
In addition to the effects of mechanical signals on cellular differentiation, we have shown that the LMMS signal also increases the number of stem cell progenitors in vivo. Flow cytometric measurements of total bone marrow from our animal studies (both for normal chow diet and high fat diet animals) yielded the surprising result that 6 weeks of LMMS treatment significantly increased the overall stem cell population (including HSCs and MSCs) relative to controls. LMMS-stimulated animals demonstrated a 37.2% (p=0.024) increase in stem cell numbers relative to sham CON animals based on expression of Sca-1. MSCs, as represented by cells positive for both Sca-1 and Preadipocyte factor-1 (Pref-1), represented a much smaller percentage of the total cells. Identified in this manner, LMMS-treated animals had a 46.1% (p=0.022) increase in specifically MSCs relative to CON (Fig. 4). Although the mechanism is still under study, this mechanical modulation of stem cell populations and potentially their proliferative capacity represents a unique therapeutic target for tissue regenerative therapy.
Fig. 4.
Representative density dot plots from flow cytometry experiments indicate the ability of LMMS to increase the number of stem cells in general (Sca-1 single positive, top quadrants), and MSCs specifically (both Sca-1 and Pref-1 positive, top right quadrant). Red, high cell density; blue, low cell density. Compared with control animals (A), LMMS increase the number of stem cells in the bone marrow of LMMS animals (B). The actual increase in total bone marrow-derived stem cell number (C) and MSC number (D) was calculated as percent positive cells/total cells for the cell fraction showing highest intensity staining. Reproduced from J Bone Miner Res 2009;24;50–61 with permission of the American Society for Bone and Mineral Research.
Summary
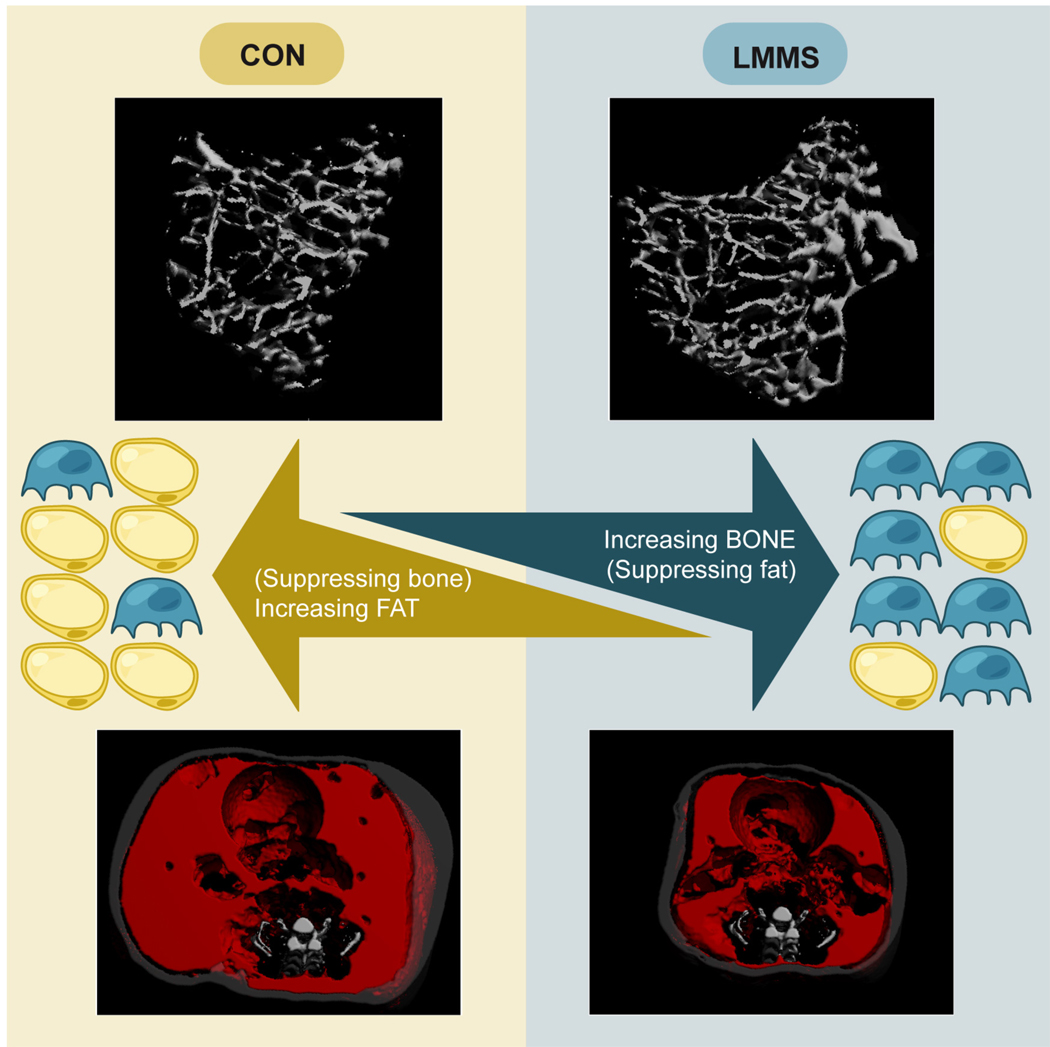
Clearly, the interaction between bone and fat is complex, and highly contingent on a multitude of factors including genetic, biochemical, and mechanical inputs that coordinate the overall interaction and resulting phenotype. The phenotypic response, as measured by amount of adipose and bone tissue, as well as the biochemical changes measured in the various organs including the liver, are surely resultant from more complex interactions than those currently explored. In particular, the role of signals originating from other cellular sources with specificity for MSCs, adipocytes and/or osteoblasts needs to be further assessed, both as contributors to the LMMS response and as outcomes to the stimulation. However, what is clear is that the increasing prevalence of, and costs associated with, osteoporosis and obesity represent major health concerns. Further, for obese individuals the excess adiposity actually puts them at risk for developing a multitude of additional diseases such as diabetes, cardiovascular disease and cancer. Pharmacological treatments for both have met with limited success, and carry several associated risks. Rather than simply exploring treatments for the compromised state once the disease has developed, it is generally accepted that primary prevention should be emphasized. With this, fundamental understanding of some of the interacting factors that drive the differential development of stem cells down either a bone or fat lineage holds promise for the discovery of new prevention and treatment strategies (Fig. 5). The ability of mechanical signals to influence MSC differentiation and to subvert MSC adipocyte formation towards the formation of more healthful tissues (i.e., bone or muscle) holds the promise to constrain overall adipose gain through developmental, rather than metabolic pathways.
Fig. 5.
The ability of LMMS to increase bone while decreasing fat is tied to the common progenitor stem cell from which osteoblasts and adipocytes differentiate. While an inverse relationship between bone and fat tissues has been observed previously, we now show that LMMS increase bone differentiation by decreasing fat differentiation, creating an inverse developmental link between obesity and osteoporosis. Shown in reconstructed µCT images, an LMMS-treated animal (right) is shown in comparison to an age-matched sham CON (left). Light gray represents bone, transparent gray represents subcutaneous fat, red represents visceral fat. Increasing fat in an animal tends to accumulate in the visceral (as opposed to subcutaneous) compartment and increases the circumference of the animal without significant impact to skeletal length.
Footnotes
Conflict of Interest: Dr. Luu, Dr. Pessin, Dr. Judex and Dr. Clinton Rubin report that they have submitted provisional patents to the USPTO on the ability of mechanical signals to control stem cell fate and modulate metabolic disorders; Dr. Clinton Rubin is also the founder of Juvent Medical, Inc. Dr. Janet Rubin: none reported.
References
- 1.Carmona RH. Surgeon General reports on bone health. J Calif Dent Assoc. 2005 Jan;33(1):9–11. [PubMed] [Google Scholar]
- 2.Dehghan M, Akhtar-Danesh N, Merchant AT. Childhood obesity, prevalence and prevention. Nutr J. 2005 Sep 2;4:24. doi: 10.1186/1475-2891-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cara JF, Chaiken RL. Type 2 diabetes and the metabolic syndrome in children and adolescents. Curr Diab Rep. 2006 Jun;6(3):241–250. doi: 10.1007/s11892-006-0041-8. [DOI] [PubMed] [Google Scholar]
- 4.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: The Bogalusa Heart Study. J Pediatr. 2007 Jan;150(1):12–17. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 5.Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr. 1996 May;128(5 Pt 1):608–615. doi: 10.1016/s0022-3476(96)80124-7. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999 Feb;22(2):345–354. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 7.Scott CR, Smith JM, Cradock MM, Pihoker C. Characteristics of youth-onset noninsulin-dependent diabetes mellitus and insulin-dependent diabetes mellitus at diagnosis. Pediatrics. 1997 Jul;100(1):84–91. doi: 10.1542/peds.100.1.84. [DOI] [PubMed] [Google Scholar]
- 8.Nijpels G. Determinants for the progression from impaired glucose tolerance to non-insulin-dependent diabetes mellitus. Eur J Clin Invest. 1998 Sep;28 Suppl 2:8–13. doi: 10.1046/j.1365-2362.1998.0280s2008.x. [DOI] [PubMed] [Google Scholar]
- 9.Harlan WR. Epidemiology of childhood obesity. A national perspective. Ann N Y Acad Sci. 1993 Oct 29;699:1–5. doi: 10.1111/j.1749-6632.1993.tb18831.x. [DOI] [PubMed] [Google Scholar]
- 10.Hughes TA, Gwynne JT, Switzer BR, Herbst C, White G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med. 1984 Jul;77(1):7–17. doi: 10.1016/0002-9343(84)90429-7. [DOI] [PubMed] [Google Scholar]
- 11.Henry RR, Wallace P, Olefsky JM. Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes. 1986 Sep;35(9):990–998. doi: 10.2337/diab.35.9.990. [DOI] [PubMed] [Google Scholar]
- 12.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001 May 3;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 13.Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002 Dec;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luepker RV, Perry CL, McKinlay SM, Nader PR, Parcel GS, Stone EJ, Webber LS, Elder JP, Feldman HA, Johnson CC, et al. CATCH collaborative group. Outcomes of a field trial to improve children's dietary patterns and physical activity. The Child and Adolescent Trial for Cardiovascular Health. JAMA. 1996 Mar 13;275(10):768–776. doi: 10.1001/jama.1996.03530340032026. [DOI] [PubMed] [Google Scholar]
- 15.Williamson DA, Copeland AL, Anton SD, Champagne C, Han H, Lewis L, Martin C, Newton RL, Jr, Sothern M, Stewart T, Ryan D. Wise Mind project: a school-based environmental approach for preventing weight gain in children. Obesity (Silver Spring) 2007 Apr;15(4):906–917. doi: 10.1038/oby.2007.597. [DOI] [PubMed] [Google Scholar]
- 16.Gutin B. Child obesity can be reduced with vigorous activity rather than restriction of energy intake. Obesity (Silver Spring) 2008 Oct;16(10):2193–2196. doi: 10.1038/oby.2008.348. [DOI] [PubMed] [Google Scholar]
- 17.Lakka TA, Bouchard C. Physical activity, obesity and cardiovascular diseases. Handb Exp Pharmacol. 2005;(170):137–163. doi: 10.1007/3-540-27661-0_4. [DOI] [PubMed] [Google Scholar]
- 18.Porada CD, Zanjani ED, Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006 Sep;1(3):365–369. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- 19.Liu M, Han ZC. Mesenchymal stem cells: biology and clinical potential in type 1 diabetes therapy. J Cell Mol Med. 2008 Aug;12(4):1155–1168. doi: 10.1111/j.1582-4934.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007 Apr;211(1):27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 21.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008 Feb 22;132(4):598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kassem M. Stem cells: potential therapy for age-related diseases. Ann N Y Acad Sci. 2006 May;1067:436–442. doi: 10.1196/annals.1354.062. [DOI] [PubMed] [Google Scholar]
- 23.Kratchmarova I, Blagoev B, Haack-Sorensen M, Kassem M, Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005 Jun 3;308(5727):1472–1477. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- 24.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006 Jan;10(1):11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Ko KS, McCulloch CA. Intercellular mechanotransduction: cellular circuits that coordinate tissue responses to mechanical loading. Biochem Biophys Res Commun. 2001 Aug 3;285(5):1077–1083. doi: 10.1006/bbrc.2001.5177. [DOI] [PubMed] [Google Scholar]
- 26.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006 May;20(7):811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 27.Avram MM, Avram AS, James WD. Subcutaneous fat in normal and diseased states 3. Adipogenesis: from stem cell to fat cell. J Am Acad Dermatol. 2007 Mar;56(3):472–492. doi: 10.1016/j.jaad.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Kubo Y, Kaidzu S, Nakajima I, Takenouchi K, Nakamura F. Organization of extracellular matrix components during differentiation of adipocytes in long-term culture. In Vitro Cell Dev Biol Anim. 2000 Jan;36(1):38–44. doi: 10.1290/1071-2690(2000)036<0038:OOEMCD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006 Feb 15;367:1–16. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Dallas SL, Maser R, Calvet JP, Bonewald L, Quarles LD. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J Biol Chem. 2006 Oct 13;281(41):30884–30895. doi: 10.1074/jbc.M604772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008 Jan;42(1):172–179. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin YX, Kaplan T, Saldanha A, Rubin C. Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J Biomech. 2003 Oct;36(10):1427–1437. doi: 10.1016/s0021-9290(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 33.Song G, Ju Y, Shen X, Luo Q, Shi Y, Qin J. Mechanical stretch promotes proliferation of rat bone marrow mesenchymal stem cells. Colloids Surf B Biointerfaces. 2007 Aug 1;58(2):271–277. doi: 10.1016/j.colsurfb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Terraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, Seliktar D, Elisseeff J. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells. 2007 Nov;25(11):2730–2738. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- 35.Haylock DN, Nilsson SK. Osteopontin: a bridge between bone and blood. Br J Haematol. 2006 Sep;134(5):467–474. doi: 10.1111/j.1365-2141.2006.06218.x. [DOI] [PubMed] [Google Scholar]
- 36.Papayannopoulou T, Scadden DT. Stem-cell ecology and stem cells in motion. Blood. 2008 Apr 15;111(8):3923–3930. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003 Oct 23;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 38.Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996 Jan 15;87(2):518–524. [PubMed] [Google Scholar]
- 39.Huang RP, Rubin CT, McLeod KJ. Changes in postural muscle dynamics as a function of age. J Gerontol A Biol Sci Med Sci. 1999 Aug;54(8):B352–B357. doi: 10.1093/gerona/54.8.b352. [DOI] [PubMed] [Google Scholar]
- 40.Mao JJ, Nah HD. Growth and development: hereditary and mechanical modulations. Am J Orthod Dentofacial Orthop. 2004 Jun;125(6):676–689. doi: 10.1016/j.ajodo.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 41.Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging. 2005 Aug;22(2):279–285. doi: 10.1002/jmri.20367. [DOI] [PubMed] [Google Scholar]
- 42.David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, Vico L, Guignandon A. Mechanical loading down-regulates peroxisome proliferators-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007 May;148(5):2553–2562. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- 43.Bennett CN, Hodge CL, MacDougald OA, Schwartz J. Role of Wnt10b and C/EBPalpha in spontaneous adipogenesis of 243 cells. Biochem Biophys Res Commun. 2003 Feb 28;302(1):12–16. doi: 10.1016/s0006-291x(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 44.Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985 Jul;37(4):411–417. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- 45.Fyhrie DP, Carter DR. A unifying principle relating stress to trabecular bone morphology. J Orthop Res. 1986;4(3):304–317. doi: 10.1002/jor.1100040307. [DOI] [PubMed] [Google Scholar]
- 46.van der Meulen MC, Huiskes R. Why mechanobiology? A survey article. J Biomech. 2002 Apr;35(4):401–414. doi: 10.1016/s0021-9290(01)00184-1. [DOI] [PubMed] [Google Scholar]
- 47.Burger EH, Klein-Nulen J. Responses of bone cells to biomechanical forces in vitro. Adv Dent Res. 1999 Jun;13:93–98. doi: 10.1177/08959374990130012201. [DOI] [PubMed] [Google Scholar]
- 48.Rubin J, Fan X, Biskobing DM, Taylor WR, Rubin CT. Osteoclastogenesis is repressed by mechanical strain in an in vitro model. J Orthop Res. 1999 Sep;17(5):639–645. doi: 10.1002/jor.1100170504. [DOI] [PubMed] [Google Scholar]
- 49.Rubin J, Murphy TC, Rahnert J, Song H, Nanes MS, Greenfield EM, Jo H, Fan X. Mechanical inhibition of RANKL expression is regulated by H-Ras-GTPase. J Biol Chem. 2006 Jan 20;281(3):1412–1418. doi: 10.1074/jbc.M508639200. [DOI] [PubMed] [Google Scholar]
- 50.Knothe Tate ML, Knothe U. An ex vivo model to study transport processes and fluid flow in loaded bone. J Biomech. 2000 Feb;33(2):247–254. doi: 10.1016/s0021-9290(99)00143-8. [DOI] [PubMed] [Google Scholar]
- 51.Qin YX, Lin W, Rubin C. The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. Ann Biomed Eng. 2002 May;30(5):693–702. doi: 10.1114/1.1483863. [DOI] [PubMed] [Google Scholar]
- 52.Mak AF, Zhang JD. Numerical simulation of streaming potentials due to deformation-induced hierarchical flows in cortical bone. J Biomech Eng. 2001 Feb;123(1):66–70. doi: 10.1115/1.1336796. [DOI] [PubMed] [Google Scholar]
- 53.Heinonen A, Kannus P, Sievänen H, Oja P, Pasanen M, Rinne M, Uusi-Rasi K, Vuori I. Randomised controlled trial of effect of high-impact exercise on selected risk factors for osteoporotic fractures. Lancet. 1996 Nov 16;348(9038):1343–1347. doi: 10.1016/S0140-6736(96)04214-6. [DOI] [PubMed] [Google Scholar]
- 54.O'Connor JA, Lanyon LE, MacFie H. The influence of strain rate on adaptive bone remodelling. J Biomech. 1982;15(10):767–781. doi: 10.1016/0021-9290(82)90092-6. [DOI] [PubMed] [Google Scholar]
- 55.Gross TS, Edwards JL, McLeod KJ, Rubin CT. Strain gradients correlate with sites of periosteal bone formation. J Bone Miner Res. 1997 Jun;12(6):982–988. doi: 10.1359/jbmr.1997.12.6.982. [DOI] [PubMed] [Google Scholar]
- 56.Rubin CT, Lanyon LE. Kappa Delta Award paper. Osteoregulatory nature of mechanical stimuli: function as a determinant for adaptive remodeling in bone. J Orthop Res. 1987;5(2):300–310. doi: 10.1002/jor.1100050217. [DOI] [PubMed] [Google Scholar]
- 57.Qin YX, Rubin CT, McLeod KJ. Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J Orthop Res. 1998 Jul;16(4):482–489. doi: 10.1002/jor.1100160414. [DOI] [PubMed] [Google Scholar]
- 58.Adams DJ, Spirt AA, Brown TD, Fritton SP, Rubin CT, Brand RA. Testing the daily stress stimulus theory of bone adaptation with natural and experimentally controlled strain histories. J Biomech. 1997 Jul;30(7):671–678. doi: 10.1016/s0021-9290(97)00004-3. [DOI] [PubMed] [Google Scholar]
- 59.Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001 Aug 9;412(6847):603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 60.Rubin C, Turner A, Mallinckrodt C, Jerome C, McLeod K, Bain S. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone. 2002 Mar;30(3):445–452. doi: 10.1016/s8756-3282(01)00689-5. [DOI] [PubMed] [Google Scholar]
- 61.Rubin C, Turner A, Müller R, Mittra E, McLeod K, Lin W, Qin YX. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002 Feb;17(2):349–357. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- 62.Judex S, Boyd S, Qin YX, Turner S, Ye K, Müller R, Rubin C. Adaptations of trabecular bone to low magnitude vibrations result in more uniform stress and strain under load. Ann Biomed Eng. 2003 Jan;31(1):12–20. doi: 10.1114/1.1535414. [DOI] [PubMed] [Google Scholar]
- 63.Gross TS, Edwards JL, McLeod KJ, Rubin CT. Strain gradients correlate with sites of periosteal bone formation. J Bone Miner Res. 1997 Jun;12(6):982–988. doi: 10.1359/jbmr.1997.12.6.982. [DOI] [PubMed] [Google Scholar]
- 64.Judex S, Gross TS, Zernicke RF. Strain gradients correlate with sites of exercise-induced bone-forming surfaces in the adult skeleton. J Bone Miner Res. 1997 Oct;12(10):1737–1745. doi: 10.1359/jbmr.1997.12.10.1737. [DOI] [PubMed] [Google Scholar]
- 65.Unger RH. Minireview: Weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003 Dec;144(12):5159–5165. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- 66.Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, Mittal V, Rosen CJ, Pessin JE, Judex S. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A. 2007 Nov 6;104(45):17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA, Benezra R, Mittal V. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007 Jun 15;21(12):1546–1558. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hachisuka H, Mochizuki Y, Yasunaga Y, Natsu K, Sharman P, Shinomiya R, Ochi M. Flow cytometric discrimination of mesenchymal progenitor cells from bone marrow-adherent cell populations using CD34/44/45(−) and Sca-1(+) markers. J Orthop Sci. 2007 Mar;12(2):161–169. doi: 10.1007/s00776-006-1098-6. [DOI] [PubMed] [Google Scholar]
- 69.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006 May;119(5 Suppl 1):S10–S16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lublinsky S, Luu YK, Rubin CT, Judex S. Automated separation of visceral and subcutaneous adiposity in in vivo microcomputed tomographies of mice. J Digit Imaging. 2008 Sep 3; doi: 10.1007/s10278-008-9152-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luu YK, Lublinsky S, Ozcivici E, Capilla E, Pessin JE, Rubin CT, Judex S. In vivo quantification of subcutaneous and visceral adiposity by micro-computed tomography in a small animal model. Med Eng Phys. 2009 Jan;31(1):34–41. doi: 10.1016/j.medengphy.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, Rubin CT. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009 Jan;24(1):50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003 Dec;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr, O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007 Jul 3;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 75.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004 Mar;19(3):343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 76.Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, Rubin CT, Judex S. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006 Nov;39(5):1059–1066. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 77.Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci U S A. 2004 Nov 23;101(47):16689–16694. doi: 10.1073/pnas.0407429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.You J, Yellowley CE, Donahue HJ, Zhang Y, Chen Q, Jacobs CR. Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J Biomech Eng. 2000 Aug;122(4):387–393. doi: 10.1115/1.1287161. [DOI] [PubMed] [Google Scholar]
- 79.Malone AM, Batra NN, Shivaram G, Kwon RY, You L, Kim CH, Rodriguez J, Jair K, Jacobs CR. The role of actin cytoskeleton in oscillatory fluid flow-induced signaling in MC3T3-E1 osteoblasts. Am J Physiol Cell Physiol. 2007 May;292(5):C1830–C1836. doi: 10.1152/ajpcell.00352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garman R, Gaudette G, Donahue LR, Rubin C, Judex S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res. 2007 Jun;25(6):732–740. doi: 10.1002/jor.20354. [DOI] [PubMed] [Google Scholar]
- 81.Garman R, Rubin C, Judex S. Small oscillatory accelerations, independent of matrix deformations, increase osteoblast activity and enhance bone morphology. PLoS ONE. 2007 Jul 25;2(7):e653. doi: 10.1371/journal.pone.0000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ozcivici E, Garman R, Judex S. High-frequency oscillatory motions enhance the simulated mechanical properties of non-weight bearing trabecular bone. J Biomech. 2007;40(15):3404–3411. doi: 10.1016/j.jbiomech.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka SM, Li J, Duncan RL, Yokota H, Burr DB, Turner CH. Effects of broad frequency vibration on cultured osteoblasts. J Biomech. 2003 Jan;36(1):73–80. doi: 10.1016/s0021-9290(02)00245-2. [DOI] [PubMed] [Google Scholar]
- 84.Tjandrawinata RR, Vincent VL, Hughes-Fulford M. Vibrational force alters mRNA expression in osteoblasts. FASEB J. 1997 May;11(6):493–497. doi: 10.1096/fasebj.11.6.9194530. [DOI] [PubMed] [Google Scholar]
- 85.Bacabac RG, Smit TH, Van Loon JJ, Doulabi BZ, Helder M, Klein-Nulend J. Bone cell responses to high-frequency vibration stress: does the nucleus oscillate within the cytoplasm? FASEB J. 2006 May;20(7):858–864. doi: 10.1096/fj.05-4966.com. [DOI] [PubMed] [Google Scholar]
- 86.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004 Mar;113(6):846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol Cell Biol. 2006 Aug;26(15):5827–5837. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006 May;116(5):1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qiu W, Andersen TE, Bollerslev J, Mandrup S, Abdallah BM, Kassem M. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res. 2007 Nov;22(11):1720–1731. doi: 10.1359/jbmr.070721. [DOI] [PubMed] [Google Scholar]
- 90.Fan X, Rahnert JA, Murphy TC, Nanes MS, Greenfield EM, Rubin J. Response to mechanical strain in an immortalized pre-osteoblast cell is dependent on ERK1/2. J Cell Physiol. 2006 May;207(2):454–460. doi: 10.1002/jcp.20581. [DOI] [PubMed] [Google Scholar]
- 91.You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem. 2001 Apr 20;276(16):13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- 92.Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS, Lanyon LE. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biol Chem. 2007 Jul 13;282(28):20715–20727. doi: 10.1074/jbc.M703224200. [DOI] [PubMed] [Google Scholar]
- 93.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006 Oct 20;281(42):31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 94.Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008 Oct 24;283(43):29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008 Dec;149(12):6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]