Abstract
Purpose
Our objective was to describe the incidence of nonmalignant late complications and their association with health and functional status in a recent cohort of hematopoietic cell transplantation (HCT) survivors.
Patients and Methods
We determined the incidence of 14 nonmalignant late effects in adults who underwent transplantation from January 2004 through June 2009 at Fred Hutchinson Cancer Research Center who survived at least 1 year after HCT. Data were derived from review of medical records and annual self-reported questionnaires.
Results
The 1,087 survivors in the study had a median age at HCT of 53 years (range, 21 to 78 years) and were followed for a median of 37 months (range, 12 to 77 months) after HCT. The prevalence of pre-existing conditions ranged from 0% to 9.8%. The cumulative incidence of any nonmalignant late effect at 5 years after HCT was 44.8% among autologous and 79% among allogeneic recipients; 2.5% of autologous and 25.5% of allogeneic recipients had three or more late effects. Survivors with three or more late effects had lower physical functioning and Karnofsky score, lower likelihood of full-time work or study, and a higher likelihood of having limitations in usual activities. Predictors of at least one late effect were age ≥ 50 years, female sex, and unrelated donor, but not the intensity of the conditioning regimen.
Conclusion
The burden of nonmalignant late effects after HCT is high, even with modern treatments and relatively short follow-up. These late effects are associated with poor health and functional status, underscoring the need for close follow-up of this group and additional research to address these complications.
INTRODUCTION
Hematopoietic cell transplantation (HCT) is now a well-established treatment for many malignant and nonmalignant disorders. Improvements in transplantation techniques during the past three decades and the increasing number of transplantation procedures have resulted in a growing number of HCT survivors.1 Martin et al2 reported 80% survival at 20 years for patients who survived the first 5 years without recurrent disease, but their life expectancy remains lower than that observed in the general population. A recent Center for International Blood and Marrow Transplant Research (CIBMTR) study3 that examined long-term survival in 10,632 2-year survivors reported similar results, with an 85% probability of being alive at 10 years after transplantation. Survivors also have frequent treatment-related complications that continue to occur long after the procedure itself. Other observational studies4–10 have reported high rates of delayed nonmalignant complications after HCT, but most of them have focused on specific sequelae in patients who underwent HCT before 1990. Only a few studies11–14 have examined the overall burden of these health conditions in a more contemporary cohort. To the best of our knowledge, no studies have examined the pre-existing prevalence of these medical diagnoses before HCT and compared them with post-transplantation observations to better understand the direct contribution of the HCT procedure to their development.
The purpose of this study was to determine the prevalence of pretransplantation comorbidities and post-transplantation incidence of nonmalignant late effects that contribute to long-term morbidity after HCT in a contemporary cohort of patients. This cohort is reflective of transplantation practices introduced during the past decade, including the increased use of unrelated donors and mobilized blood cell grafts and the more frequent use of nonmyeloablative or reduced intensity conditioning regimens to enable HCT for older patients and those with comorbid medical conditions.
PATIENTS AND METHODS
This single-center retrospective descriptive study was designed to estimate the cumulative incidence of certain late effects in HCT survivors. Patients gave consent allowing their medical records to be used for research, and the institutional review board at the Fred Hutchinson Cancer Research Center (FHCRC) approved the study.
All consecutive adult patients who had a single HCT at FHCRC from January 1, 2004, through June 30, 2009, and survived for at least 1 year after their HCT were included in the study. One percent of the patients (n = 15) who underwent HCT during the study period was excluded because of lack of any contact with the patient during the year after HCT. The study patients had to have at least one follow-up at FHCRC or a documented note or correspondence in the medical records at or beyond 1 year. All incoming records and annual questionnaires from these patients and their physicians were screened for a predefined set of nonmalignant conditions. The self-reported outcomes retrieved from the patient questionnaires were not validated by using medical records. However, investigators have reported a good agreement between self-reported complications and medical records in cancer survivors.15 Fourteen conditions in five categories were captured: (1) musculoskeletal: avascular necrosis, joint replacement, and osteoporosis; (2) endocrine: diabetes mellitus (DM), thyroid disease, and adrenal insufficiency; (3) cardiovascular: coronary artery disease, cerebrovascular accident, and deep vein thrombosis; (4) organ specific: obstructive or restrictive lung disease, dialysis, organ transplantation; and (5) miscellaneous: iron overload and suicide or suicide attempt. Chronic graft-versus-host disease (GVHD) was excluded as a late effect in this report, since it is a syndrome with evolving diagnostic criteria and not a discrete entity like other conditions described in the report. Its incidence and characteristics have been reported elsewhere in more detail.16 Reliable information on medication usage by survivors was not readily available.
Information about demographic variables, disease and transplantation characteristics, and GVHD was available from the institutional database. We classified chronic myelogenous leukemia (chronic phase), myelodysplastic syndrome (refractory anemia/refractory anemia with ringed sideroblasts), and acute leukemia in first remission as low-risk disease; leukemia/lymphoma/myeloma not in remission, chronic myelogenous leukemia (accelerated phase/blast crisis), and myelodysplastic syndrome/refractory anemia with excess blasts in transformation as high-risk disease, and all others as intermediate-risk disease. Medical records of all study patients were reviewed to determine whether the late effects of interest were present as pre-existing medical conditions before HCT to examine the baseline prevalence of these conditions.
The association of these late effects with health and functional status was explored for a subset of the study cohort who completed at least one annual long term follow-up questionnaire sent out from July 7, 2008, through January 5, 2010. In this subset, physical and mental functioning were assessed by the Medical Outcomes Study Short Form 12 (SF-12).17 The scoring on the SF-12 health survey is norm-based, with a general population mean score of 50 and a standard deviation of 10. Higher scores indicate better functioning. The questionnaire also included information about self-reported performance status, activity level, work limitations, and current employment status. The survey was mailed once to each survivor; no reminders were sent to nonrespondents, as is the routine policy followed by the long term follow-up for these annual questionnaires.
Statistical Analysis
The cumulative incidence and median time to diagnosis of each late effect was calculated, excluding cases in which the medical condition existed before HCT.18 Cox regression was used to compare the rate of each late effect between autologous and allogeneic transplantations, treating death without the late effect as a competing risk. Time-dependent covariates were used to model the impact of particular late effects (present before or after HCT) on subsequent other late effects present after HCT. A multivariable logistic regression model was constructed for the binary outcome of occurrence of late effects after HCT that considered age, sex, race, diagnosis, disease stage, conditioning, donor type, and stem-cell source as candidate covariates.
A late effect was considered for the Quality-of-Life (QOL) analysis if it was present before or within 4 weeks after the questionnaire completion date. The association of the number of late effects with self-reported Karnofsky score was assessed by linear regression. Proportional odds models were fit by using logistic regression to evaluate associations between the number of late effects and ordinal measures of QOL such as work limitations and mental and physical functioning from the SF-12. The association between number of late effects and usual work status was evaluated by using the χ2 test. All reported P values are two-sided.
RESULTS
Clinical Characteristics of the Study Cohort
Table 1 summarizes the demographic and disease-related characteristics of the 1,087 survivors in the study cohort and the subgroup for which health and functional status information was available. Median age at HCT was 53 years (range, 21 to 78 years), and the median follow-up period from HCT was 37 months (range, 12 to 77 months). Sixty percent of the participants were males, 84% were white, and 54% had related or unrelated allogeneic donors. Eighty-five percent received myeloablative conditioning, of which 22% received total body irradiation (TBI). The cumulative incidence of chronic GVHD (both classic chronic and overlap syndrome, according to National Institutes of Health consensus criteria) was approximately 60% at 5 years after allogeneic HCT. Survival was 85% for autologous and 83% for allogeneic recipients at 3 years. Demographic and disease-related characteristics were similar for the overall cohort and the subgroup with patient-reported outcomes.
Table 1.
Clinical Characteristics of 1-Year Survivors Included in the Study and in the Subgroup for Quality-of-Life Analysis
| Characteristic | Entire Study Cohort(N = 1,087) |
Subgroup With Patient-Reported Outcomes(n = 544) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age at transplantation, years | ||||
| Median | 53 | 56 | ||
| Range | 21-78 | 21-78 | ||
| Follow-up of patients still alive, months | ||||
| Median | 37 | 41 | ||
| Range | 12-77 | 12-77 | ||
| Race* | ||||
| White | 857 | 84 | 441 | 86 |
| Nonwhite | 167 | 16 | 72 | 14 |
| Sex | ||||
| Male | 654 | 60 | 326 | 60 |
| Female | 433 | 40 | 218 | 40 |
| Diagnosis | ||||
| Chronic leukemia | 87 | 8 | 37 | 7 |
| Acute leukemia | 304 | 28 | 147 | 27 |
| Lymphoma | 299 | 28 | 155 | 28 |
| Multiple myeloma | 186 | 17 | 98 | 18 |
| Myelodysplastic syndrome | 144 | 14 | 74 | 14 |
| Other cancer | 15 | 1 | 3 | 1 |
| Nonmalignant disease | 52 | 5 | 30 | 6 |
| Disease risk | ||||
| Early | 236 | 22 | 117 | 22 |
| Intermediate | 314 | 29 | 158 | 29 |
| Advanced | 485 | 45 | 239 | 44 |
| Nonmalignant | 52 | 5 | 30 | 6 |
| Transplantation type | ||||
| Autologous/syngeneic | 493 | 45 | 259 | 48 |
| Related donor | 264 | 24 | 118 | 22 |
| Unrelated donor | 330 | 30 | 167 | 31 |
| Sex match* | ||||
| Female to male | 129 | 12 | 58 | 11 |
| Other sex combinations | 461 | 43 | 226 | 42 |
| Autologous/syngeneic | 493 | 46 | 259 | 48 |
| Graft source | ||||
| Peripheral blood | 977 | 90 | 496 | 91 |
| Bone marrow | 92 | 8 | 42 | 8 |
| Umbilical cord blood | 18 | 2 | 6 | 1 |
| Conditioning | ||||
| High-dose therapy with total body irradiation† | 236 | 22 | 111 | 20 |
| High-dose therapy without total body irradiation† | 688 | 63 | 350 | 64 |
| Reduced intensity | 163 | 15 | 83 | 15 |
| Graft-versus-host disease (allogeneic patients) | ||||
| Acute grade 2 | 364 | 61 | 176 | 62 |
| Acute grade 3 | 46 | 8 | 21 | 7 |
| NIH chronic treated with systemic treatment‡ | 344 | 60 | 179 | 64 |
Abbreviation: NIH, National Institutes of Health.
Race unknown for 63 patients in overall study cohort and for 31 patients in quality-of-life subgroup; sex match unknown for four patients in overall study cohort and one patient in quality-of-life subgroup.
Antithymocyte globulin included in conditioning regimen for 57 patients in overall study cohort and for 32 patients in quality-of-life subgroup.
Cumulative incidence at 5 years.
Prevalence of Medical Conditions Before HCT
The pretransplantation prevalence of each medical condition was examined to demonstrate the effect of age, prior treatments, and other factors not directly related to the HCT responsible for the occurrence of these conditions. Pretransplantation prevalence rates of the different conditions ranged from 0% to 7.5% in autologous survivors and 0.2% to 9.8% in allogeneic survivors. Thyroid disease, DM, and deep vein thrombosis (not catheter associated) were the three most prevalent conditions in both groups (Table 2).
Table 2.
Prevalence of Pretransplantation Comorbidities and Cumulative Incidence of New Post-HCT Late Effects at 5 Years After HCT, With Median and IQR of Time to Diagnosis
| Pretransplantation Comorbidities and/or New Post-HCT Late Effects | Autologous |
Allogeneic |
P for Autologous vAllogeneic Transplantation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretransplantation Prevalence (%) | Cumulative Incidence Since Transplantation |
Days to Diagnosis |
Pretransplantation Prevalence (%) | Cumulative Incidence Since Transplantation |
Days To Diagnosis Median (IQR) |
||||||
| % | SE | Median | IQR | % | SE | Median | IQR | ||||
| Avascular necrosis | 0.2 | 4.3 | 1.3 | 411 | 86-811 | 0.5 | 4.6 | 0.9 | 514 | 340-635 | .32 |
| Joint replacement | 1.8 | 4.2 | 1.3 | 1,098 | 463-1,199 | 1.4 | 3.0 | 0.8 | 645 | 362-766 | .92 |
| Osteoporosis | 2.4 | 9.7 | 1.7 | 420 | 229-500 | 1.4 | 23.0 | 1.9 | 364 | 223-464 | < .001 |
| Thyroid disease | 7.5 | 14.2 | 1.8 | 353 | 204-679 | 9.8 | 13.3 | 1.6 | 385 | 286-612 | .66 |
| Diabetes mellitus | 6.1 | 3.0 | 1.0 | 580 | 212-1,104 | 8.3 | 22.9 | 1.8 | 216 | 153-353 | < .001 |
| Adrenal insufficiency | 0.2 | 1.3 | 0.8 | 1,236 | 140-1,437 | 0.2 | 13.4 | 1.5 | 340 | 166-413 | < .001 |
| Cerebrovascular disease | 0.6 | 1.1 | 0.5 | 137 | 137-177 | 1.2 | 1.8 | 0.6 | 476 | 143-716 | .51 |
| Coronary artery disease | 3.5 | 1.9 | 1.0 | 1,444 | 778-1,447 | 3.0 | 2.7 | 0.8 | 453 | 329-1,059 | .14 |
| Iron overload | 0.2 | 0.7 | 0.4 | 392 | 334-572 | 2.2 | 25.4 | 2.0 | 363 | 227-432 | < .001 |
| Obstructive/restrictive lung disease | 1.6 | 8.2 | 1.3 | 369 | 330-392 | 1.5 | 36.9 | 2.1 | 363 | 235-504 | < .001 |
| Organ transplantation | 0 | 0.3 | 0.3 | 803 | 0.2 | — | — | .19 | |||
| Suicide or suicide attempt | 0.2 | 0.3 | 0.3 | 737 | 0.7 | 0.9 | 0.5 | 671 | 393-1,045 | .27 | |
| Deep venous thrombosis(not catheter associated) | 7.5 | 5.6 | 1.1 | 137 | 67-371 | 3.7 | 10.9 | 1.4 | 343 | 210-572 | .01 |
| Dialysis | 1.4 | 2.3 | 0.8 | 204 | 77-776 | 0 | 2.0 | 0.6 | 460 | 362-662 | .94 |
Abbreviations: HCT, hematopoietic cell transplantation; IQR, interquartile range.
Late Effects in HCT Survivors
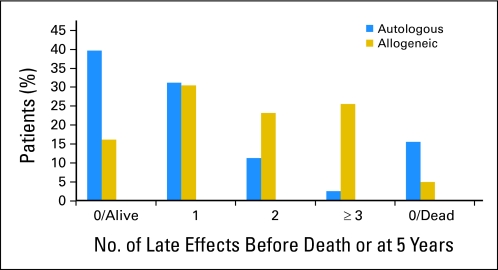
The cumulative incidence of one or more late effects at 5 years after HCT was 44.8% in autologous recipients and 79% in allogeneic recipients. The burden of late effects in autologous and allogeneic survivors is shown in Figure 1. Twenty-six percent of allogeneic survivors had three or more late effects compared with 2.5% of autologous survivors.
Fig 1.
Burden of new post-HCT late effects in autologous and allogeneic survivors.
Table 2 lists the cumulative incidence and median time to diagnosis of selected late effects in autologous and allogeneic recipients. There were no significant differences between the baseline prevalence and post-transplantation cumulative incidence of late effects in the subgroups with and without patient-reported outcomes except for dialysis, which had a higher cumulative incidence in the subgroup for which QOL data were not available (3.0% v 1.3%; P = .02; Appendix Table A1, online only). Association between the different common late effects is detailed in Table 3. Although chronic GVHD was excluded from the enumeration of late effects, its association with subsequent late effects was analyzed. As expected, chronic GVHD was significantly associated with development of certain subsequent late effects, including DM, osteoporosis, avascular necrosis/joint replacement, and lung disease. Most of these late effects were also present in allogeneic recipients without chronic GVHD, albeit at a lower rate, but still at a higher rate than in autologous recipients (Appendix Table A2, online only). A multivariate analysis showed that the risk of having any late effect in allogeneic survivors was associated with age ≥ 50 years, female sex, and unrelated donors but not with other factors like conditioning regimen intensity or source of stems cells. Age more than 50 years, underlying diagnosis other than multiple myeloma, and no TBI in the conditioning regimen increased the risk of having any late effects in the autologous group (Table 4).
Table 3.
HR (95% CI) for New Post-HCT Late Effect After Another Late Effect Is Already Present
| Prior Late Effect(prevalent pre-HCT or new post-HCT) |
Osteoporosis(161 events) |
Avascular Necrosis/Joint Replacement(53 events) |
Coronary Artery Disease/Cerebrovascular Disease(30 events) |
Dialysis(21 events) |
Diabetes Mellitus(139 events) |
Thyroid Disease(126 events) |
Lung Disease(237 events) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Osteoporosis | — | 1.0 | 0.5 to 2.3 | .97 | 0.5 | 0.1 to 2.0 | .27 | 1.3 | 0.4 to 4.5 | .70 | 1.6 | 0.9 to 2.8 | .14 | 0.5 | 0.3 to 1.1 | .06 | 1.6 | 1.1 to 2.3 | .03 | ||
| Avascular necrosis/joint replacement | 2.3 | 1.3 to 4.1 | .02 | — | 1.4 | 0.3 to 6.1 | .64 | 1.1 | 0.2 to 8.6 | .90 | 1.5 | 0.6 to 3.3 | .40 | 2.3 | 1.2 to 4.5 | .02 | 1.8 | 1.0 to 3.1 | .07 | ||
| Coronary artery disease/cerebrovascular disease | 1.5 | 0.9 to 2.8 | .18 | 1.1 | 0.3 to 3.4 | .93 | — | 1.9 | 0.4 to 8.3 | .42 | 1.4 | 0.7 to 2.7 | .39 | 1.1 | 0.5 to 2.3 | .86 | 1.0 | 0.6 to 1.8 | .92 | ||
| Dialysis | 0.4 | 0.1 to 2.8 | .26 | 0 | Undefined | .18 | 4.8 | 1.1 to 20 | .08 | — | 0 | Undefined | .06 | 1.0 | 0.2 to 3.9 | .96 | 0 | Undefined | .007 | ||
| Diabetes mellitus | 2.1 | 1.5 to 3.0 | < .001 | 2.1 | 1.2 to 3.8 | .02 | 2.6 | 1.2 to 5.5 | .02 | 1.2 | 0.4 to 3.5 | .78 | — | 1.3 | 0.8 to 2.0 | .28 | 1.5 | 1.1 to 2.1 | .01 | ||
| Thyroid disease | 1.6 | 1.1 to 2.4 | .02 | 1.1 | 0.5 to 2.2 | .86 | 1.9 | 0.9 to 4.4 | .13 | 0.6 | 0.1 to 2.5 | .43 | 1.2 | 0.7 to 1.9 | .45 | — | 1.2 | 0.8 to 1.7 | .42 | ||
| Lung disease | 2.1 | 1.4 to 3.0 | < .001 | 1.2 | 0.6 to 2.4 | .62 | 2.7 | 1.2 to 6.3 | .02 | 2.3 | 0.8 to 6.4 | .12 | 2.3 | 1.4 to 3.7 | .002 | 1.3 | 0.8 to 2.1 | .25 | — | ||
| Chronic graft-versus-host disease | 1.8 | 1.3 to 2.6 | < .001 | 1.8 | 1.0 to 3.3 | .04 | 1.8 | 0.8 to 4.1 | .14 | 1.6 | 0.6 to 4.3 | .35 | 4.7 | 3.3 to 6.8 | < .001 | 1.3 | 0.9 to 2.0 | .16 | 2.9 | 2.3 to 3.8 | < .001 |
Abbreviations: HCT, hematopoietic cell transplantation; HR, hazard ratio.
Table 4.
Multivariate Analysis for Experiencing at Least One New Post-HCT Late Effect
| Variable | Autologous Transplantation(n = 493; 171 events) |
Allogeneic Transplantation(n = 594; 400 events) |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age ≥ 50 years | 1.5 | 1.1 to 2.3 | .03 | 1.3 | 1.0 to 1.6 | .03 |
| Female | 1.1 | 0.8 to 1.5 | .61 | 1.5 | 1.2 to 1.9 | .001 |
| Nonwhite* | 0.8 | 0.5 to 1.1 | .16 | 0.9 | 0.7 to 1.1 | .30 |
| Lymphoma† | — | — | 0.9 | 0.6 to 1.4 | .69 | |
| Multiple myeloma‡ | 0.7 | 0.5 to 1.0 | .05 | — | — | |
| Myelodysplastic syndrome§ | — | — | 1.1 | 0.8 to 1.4 | .75 | |
| Other¶ | 1.2 | 0.6 to 2.3 | .67 | 0.7 | 0.4 to 1.2 | .19 |
| Early disease | 0.7 | 0.1 to 5.0 | .68 | 0.9 | 0.7 to 1.2 | .51 |
| Advanced disease | 1.1 | 0.7 to 1.8 | .65 | 1.0 | 0.8 to 1.4 | .78 |
| Total body irradiation in regimen∥ | 0.4 | 0.2 to 0.7 | < .001 | 1.1 | 0.8 to 1.4 | .47 |
| Bone marrow | — | — | 0.9 | 0.6 to 1.2 | .46 | |
| Unrelated donor | — | — | 1.3 | 1.1 to 1.6 | .006 | |
| Female donor/male patient | — | — | 1.2 | 0.9 to 1.6 | .12 | |
| Nonmyeloablative conditioning | — | — | 1.1 | 0.8 to 1.5 | .42 | |
Abbreviations: HCT, hematopoietic cell transplantation; HR, hazard ratio.
Known nonwhite; unknowns are included with reference group.
For autologous transplantation, lymphoma is the reference group; for allogeneic transplantation, leukemia is the reference group.
For allogeneic transplantation, two patients with multiple myeloma are included with the “other” category.
For autologous transplantation, two patients with myelodysplastic syndrome are included with the “other” category.
For autologous transplantation, all except lymphoma and multiple myeloma; for allogeneic transplantation, all except leukemia, lymphoma, and myelodysplastic syndrome.
In patients receiving myeloablative conditioning.
Distribution of Late Effects and Association With QOL
The four most frequent late effects in the groups with one, two, or three or more late effects were similar and included thyroid disease, pulmonary disease, osteoporosis, and diabetes. Survivors with three or more late effects reported worse physical functioning, higher likelihood of moderate to severe limitation of usual activities, and lower likelihood of full-time work or study than those with none, one, or two late effects. We examined the association between age and QOL to confirm that age was not confounding our results, especially since we found more retired individuals in the subgroup with three or more late effects. This analysis showed weak associations between age and QOL. Mental functioning was not significantly associated with the number of late effects. Table 5 details the relationship of number of late effects with health and functional status of survivors, irrespective of whether the medical conditions were pre-existing or they developed after HCT. They were given equal weight since the late effect occurred prior to or contemporaneous with QOL assessment in either case, and we assumed they would have produced similar clinical impact. In a multivariate analysis, poor physical functioning was significantly associated with joint replacement, DM, adrenal insufficiency, and pulmonary late effects (Appendix Table A3, online only).
Table 5.
Relationship of Quality-of-Life Measures With Late Effects in 544 Survivors
| Variable | No. of Prior/Contemporaneous Late Effects |
P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 (n = 182) |
1 (n = 152) |
2 (n = 107) |
3+ (n = 103) |
||||||
| No. | % | No. | % | No. | % | No. | % | ||
| Karnofsky score | < .001* | ||||||||
| Mean | 88.4 | 85.8 | 87.4 | 80.9 | |||||
| SD | 12.4 | 12.9 | 12.0 | 13.6 | |||||
| Work limitations | .003† | ||||||||
| No limits | 70 | 41 | 49 | 34 | 33 | 34 | 23 | 23 | |
| Limited a little | 59 | 34 | 58 | 40 | 40 | 41 | 38 | 38 | |
| Limited a lot | 25 | 15 | 20 | 14 | 15 | 15 | 18 | 18 | |
| Limited completely | 18 | 10 | 19 | 13 | 10 | 10 | 20 | 20 | |
| Current work status | .03‡ | ||||||||
| Full-time work/student | 69 | 39 | 48 | 32 | 25 | 23 | 22 | 21 | |
| Part-time work/student | 23 | 13 | 25 | 17 | 14 | 13 | 12 | 12 | |
| Work at home | 19 | 11 | 11 | 7 | 14 | 13 | 7 | 7 | |
| Retired | 48 | 27 | 53 | 35 | 44 | 41 | 46 | 45 | |
| Other | 20 | 11 | 14 | 9 | 10 | 9 | 16 | 16 | |
| PCS-12 | < .001† | ||||||||
| > 50 | 83 | 47 | 48 | 34 | 36 | 37 | 22 | 23 | |
| 40-50 | 31 | 18 | 39 | 28 | 21 | 21 | 19 | 20 | |
| < 40 | 61 | 35 | 53 | 38 | 41 | 42 | 54 | 57 | |
| MCS-12 | .24† | ||||||||
| > 50 | 129 | 74 | 115 | 82 | 80 | 82 | 62 | 65 | |
| 40-50 | 26 | 15 | 18 | 13 | 10 | 10 | 15 | 16 | |
| < 40 | 20 | 11 | 7 | 5 | 8 | 8 | 18 | 19 | |
Abbreviations: MCS, mental component score; PCS, physical component score; SD, standard deviation.
Ptrend from linear regression model.
Ptrend from proportional odds (logistic regression) model.
χ2 test.
DISCUSSION
Advances in transplantation techniques and supportive care have lowered early mortality associated with HCT, but recent studies1–3,6,19 have shown that mortality in 5- or 10-year survivors remains four- to nine-fold higher than in the general population for a long time after HCT. Our results, which adjust for any pre-existing medical conditions before HCT, show that the burden of late complications after HCT is high, and these late effects are associated with impaired functional status.
Sun et al13 evaluated risk factors and severity of chronic health conditions in 1,022 survivors who had HCT between 1974 and 1998 and found a cumulative incidence of 59% for all chronic health conditions and 35% for severe or life-threatening complications at 10 years. They noted a shift of cumulative incidence curves to the left (ie, occurring earlier) when comparing their results to those of the Childhood Cancer Survivor Study,20 and they proposed that older age, various toxicities of pretransplantation conditioning, and chronic GVHD could explain this phenomenon. Rovo et al21 showed a high prevalence of abnormal organ function, including decreased glomerular filtration rate, higher liver function tests, and decreased thyroid function in 44 survivors at a median of 17.5 years after HCT compared with sibling donors. Bresters et al11 evaluated a recent cohort of 162 pediatric HCT survivors who had allogeneic HCT between 2001 and 2008 and found a 93.2% cumulative incidence of late effects after a median follow-up of 7.2 years. In another pediatric study, Leung et al12 found that 87% of 155 patients had at least one late complication after a median follow-up of 9 years. In our study, the cumulative incidence of at least one late effect at 5 years was 44.8% in autologous HCT recipients and 79% in allogeneic HCT recipients. Although it is difficult to compare our study with prior studies because of different methodologies and different types of late effects examined, it appears that the higher magnitude and the earlier occurrence of some of the late effects we observed may be due to a higher proportion of unrelated donors and older recipients in our study, since these factors were associated with late effects in the multivariable analysis.
Differences in the profile of late effects between the autologous and allogeneic groups is not surprising, despite the fact that some late effects may be caused by factors common to both autologous and allogeneic recipients such as TBI, cytotoxic agents, and infections. A surprising observation for which we do not have any good explanation was that TBI was associated with a decreased incidence of late effects in autologous survivors. However, the spectrum of late effects in the TBI and non-TBI groups was not significantly different.
We also evaluated the association between development of one late effect and the risk of developing another subsequent late effect. This analysis was based on the premise that many of the late effects share common risk factors and may be pathophysiologically related. For example, dialysis was strongly associated with subsequent development of cerebrovascular accident or coronary artery disease (hazard ratio, 7.5; P = .01), perhaps reflecting the effects of underlying risk factors such as atherosclerosis, which is active in the causal pathways leading to both renal impairment and vascular disease. These results may be clinically relevant, since development of one late effect might indicate the need to screen for subsequent late effects and might identify high-risk patients who could benefit from intensive prevention strategies.
The similarities in the distribution of late effects in groups with one, two, or three or more late effects suggests that the cumulative burden of these effects might have caused the observed association with QOL. Many studies have evaluated QOL and physical and psychosocial functioning in HCT survivors with some conflicting results. Majhail et al22 reported a higher likelihood of functional impairment and poorer overall health in recipients of autologous HCT for lymphoma compared with sibling controls at a median follow-up of 6 years after transplantation. A study of 137 survivors (88% allogeneic recipients) at 10 years indicated persistent mental and physical limitations as measured by SF-36 scores, although no differences were seen in anxiety, depression, and work status compared with nontransplantation controls.14 More favorable results were reported by Duell et al23 who found that 93% of 798 recipients surviving for at least 5 years had Karnofsky scores of more than 90, and 89% had resumed full-time work or school. Similarly, in a prospective, longitudinal study of 320 patients, Lee et al24 found that 60% of the survivors reported recovery by 1 to 2 years after HCT, and less than 24% of patients reported specific bothersome symptoms.
Previous studies have identified older age, advanced disease at the time of HCT, and development of chronic GVHD as risk factors for poor QOL after HCT. Our results show that the presence of multiple late effects also predicts poor health and functional status of some survivors. We demonstrated that the degree of functional impairment increases with increasing number of late effects, likely due to physical limitations. The late effects that emerged as significantly associated with poor physical functioning were joint replacement, adrenal insufficiency, and DM. It is plausible that these complications serve as surrogate markers for severe chronic GVHD requiring long-term steroid treatment, both of which affect QOL adversely.
Lack of normal controls and a relatively short follow-up period are limitations of our study. The SF-12 is a generic instrument for functional assessment and is not HCT-specific. We have self-reported QOL data from only half the survivors, although analysis suggests that these patients are demographically and medically similar to the entire cohort with similar baseline prevalence and post-transplantation incidence of late effects. We could not confirm that specific late effects had persisted to the time of QOL assessment, but most frequent late effects assessed in our study are chronic medical problems or have sequelae that would be expected to affect QOL. We also acknowledge that there may be an element of participant bias in QOL assessment, since patients with physical problems may have an extra stimulus for answering the questionnaire. Conversely, it may also be that participants who return health surveys are more driven to maintain optimal health by use of health services and adoption of a healthy lifestyle. Another limitation of our study is that reporting bias may also cause some underestimation in autologous survivors because of differences in monitoring frequencies for some of these late effects.
Notwithstanding the limitations, our study demonstrates that the changes in transplantation practices over the past decade have had a variable impact on the risk of complications after HCT. Despite their positive impact on early transplantation-related mortality, the reduced intensity/nonmyeloablative conditioning regimens have not significantly decreased the incidence of late effects, perhaps because the median age of the transplantation population has increased.
Our results also highlight the need for more research to prevent or mitigate long-term morbidity after HCT, since these late effects are associated with poor health and impaired functional status and likely translate into high cost burdens. Possible interventions could be tested at the time of transplantation or during the post-transplantation period, including modifications in transplantation techniques, better supportive care, better patient education, closer monitoring in high-risk patients, or better treatments when late effects emerge. A shared care approach that involves close coordination between the transplantation center and primary care physicians and encourages self-management support for patients has the potential to improve survival, QOL, and patient satisfaction.
Acknowledgment
We thank H. Joachim Deeg, MD, for reviewing the manuscript and providing helpful comments.
Appendix
Table A1.
Baseline Prevalence and Post-Transplantation Cumulative Incidence of Late Effects in the Subgroup With or Without Patient-Reported Outcomes
| Variable | Subgroup With No Patient-Reported Outcomes |
Subgroup With Patient-Reported Outcomes |
||||||
|---|---|---|---|---|---|---|---|---|
| Pretransplantation Prevalence (%) | Cumulative Incidence Since Transplantation |
Pretransplantation Prevalence (%) | P (comparing pretransplantation prevalence) | Cumulative Incidence Since Transplantation |
P (comparing post-transplantation incidence) | |||
| % | SE | % | SE | |||||
| Avascular necrosis | 0.6 | 3.6 | 0.9 | 0.2 | .32 | 5.0 | 1.1 | .68 |
| Joint replacement | 1.5 | 1.8 | 0.7 | 1.7 | .81 | 4.7 | 1.2 | .34 |
| Osteoporosis | 1.7 | 13.6 | 1.7 | 2.0 | .65 | 20.3 | 2.0 | .04 |
| Thyroid disease | 7.9 | 10.6 | 1.6 | 9.6 | .34 | 16.6 | 1.8 | .02 |
| Diabetes mellitus | 8.7 | 14.3 | 1.7 | 5.9 | .08 | 13.5 | 1.6 | .43 |
| Adrenal insufficiency | 0.4 | 8.3 | 1.3 | 0.0 | .16 | 7.6 | 1.3 | .28 |
| Cerebrovascular disease | 1.1 | 1.8 | 0.6 | 0.7 | .52 | 1.2 | 0.5 | .38 |
| Coronary artery disease | 2.2 | 1.5 | 0.7 | 4.2 | .06 | 3.1 | 1.0 | .39 |
| Iron overload | 1.5 | 13.8 | 1.7 | 1.1 | .59 | 15.3 | 1.7 | .49 |
| Obstructive/restrictive lung disease | 1.9 | 24.5 | 2.1 | 1.3 | .46 | 24.5 | 1.9 | .65 |
| Organ transplantation | 0.2 | 0.0 | 0.0 | .32 | 0.2 | 0.2 | .31 | |
| Suicide or suicide attempt | 0.7 | 0.2 | 0.2 | 0.2 | .18 | 1.0 | 0.5 | .36 |
| Deep venous thrombosis(not catheter associated) | 6.3 | 9.4 | 1.4 | 4.6 | .23 | 7.9 | 1.2 | .34 |
| Dialysis | 0.2 | 3.0 | 0.8 | 1.1 | .06 | 1.3 | 0.5 | .02 |
Table A2.
Cumulative Incidence of Late Effects in Autologous Recipients and Allogeneic Recipients Without and With Chronic GVHD
| Late Effect | Autologous |
Allogeneic Without Chronic GVHD |
Allogeneic With Chronic GVHD |
|||||
|---|---|---|---|---|---|---|---|---|
| Cumulative Incidence (%) | SE | Cumulative Incidence (%) | SE | P (v autologous) | Cumulative Incidence (%) | SE | P (v autologous) | |
| Avascular necrosis | 4.3 | 1.3 | 3.8 | 1.4 | .78 | 5.2 | 1.3 | .23 |
| Joint replacement | 4.2 | 1.3 | 1.1 | 0.8 | .18 | 4.1 | 1.2 | .48 |
| Osteoporosis | 9.7 | 1.7 | 18.7 | 3.0 | .006 | 26.0 | 2.5 | < .001 |
| Thyroid disease | 14.2 | 1.8 | 8.7 | 2.1 | .06 | 16.3 | 2.2 | .49 |
| Diabetes mellitus | 3.0 | 1.0 | 14.9 | 2.3 | < .001 | 28.2 | 2.6 | < .001 |
| Adrenal insufficiency | 1.3 | 0.8 | 10.7 | 2.0 | < .001 | 15.2 | 2.1 | < .001 |
| Cerebrovascular disease | 1.1 | 0.5 | 0.9 | 0.6 | .79 | 2.3 | 0.9 | .29 |
| Coronary artery disease | 1.9 | 1.0 | 2.6 | 12 | .19 | 2.8 | 1.1 | .23 |
| Iron overload | 0.7 | 0.4 | 16.9 | 2.7 | < .001 | 31.0 | 2.7 | < .001 |
| Obstructive/restrictive lung disease | 8.2 | 1.3 | 22.0 | 2.9 | < .001 | 46.7 | 2.9 | < .001 |
| Organ transplantation | 0.3 | 0.3 | 0.0 | — | 0.0 | — | ||
| Suicide or suicide attempt | 0.3 | 0.3 | 0.9 | 0.7 | .25 | 0.9 | 0.7 | .46 |
| Deep venous thrombosis(not catheter associated) | 5.6 | 1.1 | 7.1 | 1.7 | .61 | 13.3 | 2.0 | .002 |
| Dialysis | 2.3 | 0.8 | 1.4 | 0.8 | .54 | 2.5 | 0.9 | .60 |
Abbreviation: GVHD, graft-versus-host disease.
Table A3.
Association of Individual Prior/Contemporaneous Late Effects With PCS-12 < 40
| Late Effect | OR | 95% CI | P |
|---|---|---|---|
| Avascular necrosis | 2.1 | 0.9 to 4.8 | .08 |
| Joint replacement | 3.7 | 1.5 to 9.1 | .003 |
| Osteoporosis | 1.2 | 0.7 to 1.9 | .48 |
| Thyroid disease | 1.2 | 0.8 to 1.9 | .38 |
| Diabetes | 1.6 | 1.0 to 2.6 | .05 |
| Adrenal insufficiency | 2.9 | 1.4 to 6.2 | .004 |
| Cerebrovascular accident | 0.9 | 0.2 to 3.6 | .83 |
| Coronary artery disease | 1.9 | 0.8 to 4.5 | .13 |
| Iron overload | 1.2 | 0.7 to 2.0 | .47 |
| Pulmonary | 1.6 | 1.0 to 2.4 | .04 |
| Organ transplantation | — | — | |
| Suicide | 2.2 | 0.4 to 13 | .39 |
| Deep venous thrombosis | 1.6 | 0.9 to 2.8 | .14 |
| Dialysis | 2.4 | 0.6 to 10 | .22 |
Abbreviations: OR, odds ratio; PCS, physical component score.
Footnotes
Supported by Grants No. CA18029, HL36444, and CA78902 from the National Institutes of Health. Y.I. is a recipient of the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad.
Presented in part at the American Society for Blood and Marrow Transplantation Tandem Meetings, Honolulu, HI, February 17-21, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Nandita Khera, Paul J. Martin, Stephanie J. Lee
Financial support: Paul J. Martin
Administrative support: Paul J. Martin
Provision of study materials or patients: Brenda M. Sandmaier
Collection and assembly of data: Nandita Khera, Yoshihiro Inamoto, Brenda M. Sandmaier, Paul J. Martin, Stephanie J. Lee
Data analysis and interpretation: Nandita Khera, Barry Storer, Mary E.D. Flowers, Paul A. Carpenter, Yoshihiro Inamoto, Brenda M. Sandmaier, Paul J. Martin, Stephanie J. Lee
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin PJ, Counts GW, Jr, Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28:1011–1016. doi: 10.1200/JCO.2009.25.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29:2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker KS, Armenian S, Bhatia S. Long-term consequences of hematopoietic stem cell transplantation: Current state of the science. Biol Blood Marrow Transplant. 2010;16:S90–S96. doi: 10.1016/j.bbmt.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: A report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: Report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmeister PA, Hingorani SR, Storer BE, et al. Hypertension in long-term survivors of pediatric hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:515–524. doi: 10.1016/j.bbmt.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Socié G, Cahn JY, Carmelo J, et al. Avascular necrosis of bone after allogeneic bone marrow transplantation: Analysis of risk factors for 4388 patients by the Société Française de Greffe de Moëlle (SFGM) Br J Haematol. 1997;97:865–870. doi: 10.1046/j.1365-2141.1997.1262940.x. [DOI] [PubMed] [Google Scholar]
- 9.Socié G, Salooja N, Cohen A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101:3373–3385. doi: 10.1182/blood-2002-07-2231. [DOI] [PubMed] [Google Scholar]
- 10.Tichelli A, Bucher C, Rovó A, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110:3463–3471. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 11.Bresters D, van Gils IC, Kollen WJ, et al. High burden of late effects after haematopoietic stem cell transplantation in childhood: A single-centre study. Bone Marrow Transplant. 2010;45:79–85. doi: 10.1038/bmt.2009.92. [DOI] [PubMed] [Google Scholar]
- 12.Leung W, Ahn H, Rose SR, et al. A prospective cohort study of late sequelae of pediatric allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore) 2007;86:215–224. doi: 10.1097/MD.0b013e31812f864d. [DOI] [PubMed] [Google Scholar]
- 13.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: A report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116:3129–3139. doi: 10.1182/blood-2009-06-229369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syrjala KL, Langer SL, Abrams JR, et al. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol. 2005;23:6596–6606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- 15.Louie AD, Robison LL, Bogue M, et al. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25:1191–1196. doi: 10.1038/sj.bmt.1702419. [DOI] [PubMed] [Google Scholar]
- 16.Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Choudhury JB. Non-parametric confidence interval estimation for competing risks analysis: Application to contraceptive data. Stat Med. 2002;21:1129–1144. doi: 10.1002/sim.1070. [DOI] [PubMed] [Google Scholar]
- 19.Pond GR, Lipton JH, Messner HA. Long-term survival after blood and marrow transplantation: Comparison with an age- and gender-matched normative population. Biol Blood Marrow Transplant. 2006;12:422–429. doi: 10.1016/j.bbmt.2005.11.518. [DOI] [PubMed] [Google Scholar]
- 20.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 21.Rovó A, Daikeler T, Halter J, et al. Late altered organ function in very long-term survivors after allogeneic hematopoietic stem cell transplantation: A paired comparison with their HLA-identical sibling donor. Haematologica. 2011;96:150–155. doi: 10.3324/haematol.2010.030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majhail NS, Ness KK, Burns LJ, et al. Late effects in survivors of Hodgkin and non-Hodgkin lymphoma treated with autologous hematopoietic cell transplantation: A report from the bone marrow transplant survivor study. Biol Blood Marrow Transplant. 2007;13:1153–1159. doi: 10.1016/j.bbmt.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duell T, van Lint MT, Ljungman P, et al. Health and functional status of long-term survivors of bone marrow transplantation: EBMT Working Party on Late Effects and EULEP Study Group on Late Effects—European Group for Blood and Marrow Transplantation. Ann Intern Med. 1997;126:184–192. doi: 10.7326/0003-4819-126-3-199702010-00002. [DOI] [PubMed] [Google Scholar]
- 24.Lee SJ, Fairclough D, Parsons SK, et al. Recovery after stem-cell transplantation for hematologic diseases. J Clin Oncol. 2001;19:242–252. doi: 10.1200/JCO.2001.19.1.242. [DOI] [PubMed] [Google Scholar]