Abstract
Purpose
Adipokines are linked to obesity and insulin sensitivity and have recently been related to breast cancer risk and prognosis. We investigated the associations of plasma leptin and adiponectin with mammographic density and disease status and assessed their prognostic effect on recurrence-free survival in premenopausal women at risk for breast cancer.
Patients and Methods
We measured circulating lipids, insulin-like growth factor 1, glucose, insulin and insulin sensitivity (calculated by homeostasis model assessment [HOMA] index), leptin, adiponectin, and leptin-to-adiponectin ratio in 235 premenopausal women with pT1mic/pT1a breast cancer (n = 21), intraepithelial neoplasia (n = 160), or 5-year Gail risk of 1.3% or greater (n = 54) who participated in a 2 × 2 trial of low-dose tamoxifen, fenretinide, both agents, or placebo over a 2-year period.
Results
At baseline, adiponectin levels were directly associated with mammographic density and HDL cholesterol and negatively associated with leptin, leptin-to-adiponectin ratio, body mass index (BMI), and HOMA index. Median adiponectin levels were lower in affected than in unaffected women (P = .006). After a median of 7.2 years and total of 57 breast neoplastic events, there was a 12% reduction in the risk of breast neoplastic events per unit increase of adiponectin (adjusted hazard ratio, 0.88; 95% CI, 0.81 to 0.96; P = .03). There was no interaction between treatment and adiponectin levels.
Conclusion
Low adiponectin levels are associated with a history of prior intraepithelial neoplasia or pT1mic/pT1a breast cancer and higher risk of second breast neoplastic events in premenopausal women. The associations are independent of BMI, mammographic density, and treatment. Our findings support the role of adiponectin as a potential target for premenopausal breast cancer prevention and treatment.
INTRODUCTION
Obesity and disordered energy balance have been shown to play an important role in breast cancer risk and progression, although the association is modified by menopausal status. Several studies have shown a positive association between obesity and breast cancer among postmenopausal women,1–3 whereas an inverse association has been found among premenopausal women as regards general obesity, although central obesity has been reported to be possibly associated with an increased breast cancer risk before menopause.4,5 The higher breast cancer risk observed in obese women can partially be explained by the role of adipose tissue in controlling the levels and bioactivity of sex steroids. Moreover, the emerging role of adipocytes in the regulation of energy homeostasis through the secretion of adipokines6 is currently under investigation for its putative relationship with cancer.
Leptin and adiponectin are the two major adipokines secreted by adipocytes. Leptin levels increase in obesity and decrease during fasting,7,8 whereas adiponectin is reduced in obesity and insulin-resistant states and increases in response to severe weight loss.9–11 Recent evidence demonstrates that the leptin-to-adiponectin (L-A) ratio could be a useful index for insulin resistance in diabetic patients in clinical practice.12 Insulin levels have been found to be positively associated with breast cancer risk13 and with distant recurrence and specific mortality.14 The observation that insulin resistance in obese patients is also associated with breast cancer development15,16 has prompted studies designed to investigate whether decreased adiponectin levels might explain the association between breast cancer and obesity or insulin resistance.17 However, the association of adiponectin with premenopausal breast cancer is unclear, with a few studies finding no association18–21 and others finding only a trend for weak association.22,23
In the present study, we assessed plasma adiponectin and leptin levels in premenopausal women to determine their association with disease status and mammographic density, one of the strongest predictors of breast cancer risk, and also to evaluate their role as predictors of second breast neoplastic events. Ours was a 2 × 2 phase II trial of low-dose tamoxifen and fenretinide for breast cancer prevention.
PATIENTS AND METHODS
Participants
A total of 235 premenopausal women were enrolled onto a randomized, double-blind, placebo-controlled 2 × 2 trial of tamoxifen and fenretinide—a vitamin A derivative—for breast cancer prevention (Fig 1). Description of study design, participant characteristics, and main findings have been published previously.24,25 Briefly, 160 premenopausal women with an intraepithelial neoplasia (IEN; ductal intraepithelial neoplasia [DIN], 130; lobular intraepithelial neoplasia [LIN], 30) or microinvasive breast cancer (pT1mic or pT1a, N0; n = 21) and 54 healthy women at increased risk (≥ 1.3% according to Gail model) were randomly assigned to receive either tamoxifen 5 mg/d or fenretinide 200 mg/d, both agents, or placebo for 2 years. Women were stratified according to center and disease status (Gail v IEN/T1). All participants signed a consent form approved by the local institutional review board.
Fig 1.
CONSORT diagram.
Assay Methods
Fasting blood samples were collected at baseline and yearly for 2 years of treatment duration and 1 year of follow-up. Blood samples were centrifuged at 1,800 g, and serum and plasma were stored at −80°C. Serum glucose concentration and lipid profile were measured on fresh samples, whereas insulin-like growth factor I (IGF-I), insulin, and leptin were determined on frozen samples using methods previously described.26 We used the homeostasis model assessment (HOMA) as a surrogate index of insulin sensitivity, which was calculated as [fasting insulinemia (mU/L) × glycemia (mmol/L)]/22.5, in accordance with methods previously described.26,27
Plasma adiponectin was measured using a commercial enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN). In this assay, the minimum detectable dose was 0.25 ng/mL; intra-assay coefficients of variability were 2.5% (19.8 ng/mL), 3.4% (69.9 ng/mL), and 4.7% (143 ng/mL); interassay precisions from 40 different assays of three samples of known concentrations of 20.5, 74.4, and 157 ng/mL were 6.8%, 5.8%, and 6.9%, respectively. Plasma samples underwent at most one freeze-thaw cycle before leptin determination and at most two freeze-thaw cycles before adiponectin measurement during an average of 4.5 and 7 years of storage for leptin and adiponectin, respectively. Notably, leptin and adiponectin concentrations were reported not to be influenced by freeze-thaw cycles28,29 or prolonged storage.30–32 Mammographic percent density was measured at baseline and 12, 24, and 36 months using the computer-assisted method described by Boyd et al33 and validated by our group.25,34
Follow-Up and Survival Update
After study completion, participants underwent follow-up, with at least one annual visit. Women who were not seen in the clinic were contacted annually by telephone to ascertain disease and vital status. Disease-free survival was defined as time from random assignment to next breast event (ie, disease recurrence at local, regional, or distant site; new contralateral breast cancer; or death). All medical records of breast events were examined centrally to assess clinical confirmation of disease.
Statistical Analysis
Median values, range interquartiles, and results from nonparametric Wilcoxon tests were presented to investigate differences in adiponectin and leptin levels and L-A ratio according to risk strata and breast events. χ2 or Fisher's exact tests were used to analyze the association between categorical variables. We calculated Spearman correlation coefficients between adiponectin, leptin, L-A ratio, IGF-I, HDL cholesterol (HDL-C), body mass index (BMI), and HOMA index with mammographic percent density, a recognized prognostic factor of breast cancer.
Adiponectin and leptin levels and L-A ratio were investigated as continuous and categorical variables, considering median and quartile values. Analysis of covariance was used to evaluate the association between these biomarkers and mammographic percent density. Residual plots assessed the validity of model assumptions. Risk analyses were conducted using logistic regression, and odds ratios were calculated to assess the probability of having IEN/T1, as compared with unaffected high-risk women, or of having a breast event by biomarkers.
Time to death and time to recurrence were defined as time from surgery to event of interest. All patients alive or free of disease at last follow-up date were considered right censored. Disease-free survival was estimated by the Kaplan-Meier method. Log-rank and linear-rank tests were used to compare survival time between groups and evaluate linear trends. A Cox proportional hazards model was used to identify independent predictors of survival, with adjustment for relevant covariates. We evaluated the influence of mammographic percent density, HOMA index, BMI, waist-hip ratio (WHR), age, treatment group, risk stratum, family history, reproductive factors, and several biomarkers on the association of adiponectin and leptin levels with breast events. We further evaluated the influence of positive margins and radiotherapy on the association of adiponectin with breast events in the subgroup of affected women. Given the different breast cancer risks by disease status and the prognostic relevance of mammographic percent density, Cox models evaluating the influence of adiponectin were adjusted for these two potential confounding factors. All statistical tests were two sided, and P values less than .05 were considered statistically significant. Statistical analyses were performed with SAS, version 8.2 (SAS Institute, Cary, NC).
RESULTS
After a median follow-up of 7.2 years, a total of 57 breast neoplastic events (IEN/T1 stratum, 53; Gail stratum, four) were observed. All events but four (n = 53) were second breast neoplasms in women with prior IEN (n = 50) or pT1mic/T1a disease (n = 3): DIN, 19; LIN, two; sarcoma, one; stage I breast cancers, 31; ipsilateral neoplasms, 40; and contralateral neoplasms, 13. In the Gail stratum, one woman developed LIN, and three women developed invasive breast cancers.
Baseline patient and tumor characteristics by treatment allocation arm are reported in Table 1. Margin involvement was present in 12% of affected women, and 35% of women with DIN or microT1 received radiotherapy following institutional guidelines. Adiponectin, leptin, and L-A ratio at baseline and 24 months (end of treatment) were not different by treatment group, and changes in biomarker levels during treatment were not significant (data not shown).
Table 1.
Baseline Patient and Tumor Characteristics
| Characteristic | Tamoxifen Plus Placebo (n = 58) | Fenretinide Plus Placebo (n = 59) | Tamoxifen Plus Fenretinide (n = 60) | Placebo Plus Placebo (n = 58) |
|---|---|---|---|---|
| Disease status, No. | ||||
| Gail | 14 | 13 | 14 | 13 |
| LIN | 7 | 9 | 9 | 5 |
| DIN | 32 | 32 | 30 | 36 |
| T1a | 5 | 5 | 7 | 4 |
| Age at entry, years | ||||
| Median | 47 | 46 | 46 | 46 |
| IQ range | 42-49 | 43-50 | 41-49 | 43-49 |
| Body mass index, kg/m2 | ||||
| Median | 23 | 23 | 23 | 23 |
| IQ range | 22-25 | 21-25 | 21-25 | 21-25 |
| ER status, No. | ||||
| Positive | 24 | 24 | 26 | 29 |
| Negative | 5 | 5 | 5 | 7 |
| Unknown | 15 | 17 | 15 | 9 |
| PgR status, No. | ||||
| Positive | 24 | 21 | 19 | 30 |
| Negative | 5 | 8 | 12 | 6 |
| Unknown | 15 | 17 | 15 | 9 |
| Mammographic percent density | ||||
| Median | 48 | 47 | 47 | 45 |
| IQ range | 40-61 | 36-57 | 35-60 | 32-58 |
| IGF-I, ng/mL | ||||
| Median | 130 | 143 | 140 | 146 |
| IQ range | 111-165 | 116-182 | 118-182 | 114-181 |
| HDL-C, mg/dL | ||||
| Median | 65 | 71 | 69 | 68 |
| IQ range | 57-78 | 57-81 | 60-79 | 60-78 |
NOTE. No significant differences by treatment group.
Abbreviations: DIN, ductal intraepithelial neoplasia; ER, estrogen receptor; HDL-C, high-density lipoprotein cholesterol; IGF-I, insulin-like growth factor I; IQ, interquartile; LIN, lobular intraepithelial neoplasia; PgR, progesterone receptor.
Table 2 lists baseline median values and interquartile ranges of plasma adiponectin, leptin, and L-A ratio according to disease status at baseline and new breast events occurring during follow-up. We found a significantly lower median level of adiponectin (P = .006) and a higher L-A ratio (P = .035) in women in the IEN/T1 stratum compared with women in the Gail stratum, whereas no significant difference in leptin levels was observed. Similarly, adiponectin levels were significantly lower (P = .002) and L-A ratio significantly higher (P = .02) in women with a subsequent breast neoplastic event compared with women who had no new breast event during follow-up. The relationships of adiponectin, leptin, and L-A ratio with IGF-I, HDL-C, BMI, HOMA index, and mammographic percent density are summarized in Table 3. Adiponectin was negatively correlated with leptin, BMI, HOMA index, and L-A ratio, whereas a significant positive association with mammographic percent density and HDL-C was observed. Leptin and L-A ratio showed similar statistically significant correlations, although they occurred in the opposite direction. As expected, there was a strong negative correlation between baseline BMI and mammographic percent density (P < .001). BMI was similar in women with baseline adiponectin levels below and above the median value of 10 μg/mL (22.7 v 22.9 kg/m2). Furthermore, BMI was similar by risk group (22.5 and 23.0 kg/m2 for Gail and IEN/T1, respectively).
Table 2.
Baseline Adiponectin, Leptin, and L-A Ratio by Disease Stratum and New Breast Cancer Events During Follow-Up
| Biomarker | No. of Patients | Median | IQ Range |
|---|---|---|---|
| Risk stratum | |||
| Adiponectin, μg/mL* | |||
| Gail | 54 | 12 | 8.6-16.1 |
| IEN/T1 | 181 | 9.8 | 6.9-13.5 |
| Leptin, ng/mL | |||
| Gail | 54 | 10 | 6.2-16 |
| IEN/T1 | 181 | 10.8 | 7.3-15.6 |
| L-A ratio† | |||
| Gail | 54 | 0.87 | 0.54-1.35 |
| IEN/T1 | 181 | 1.13 | 0.63-1.85 |
| New breast cancer events | |||
| Adiponectin, μg/mL‡ | |||
| No | 178 | 10.9 | 7.8-14.4 |
| Yes | 57 | 8.1 | 6.5-12.2 |
| Leptin, ng/mL | |||
| No | 178 | 10.6 | 6.8-15.4 |
| Yes | 57 | 11.7 | 7.3-16.2 |
| L-A ratio§ | |||
| No | 178 | 0.98 | 0.55-1.6 |
| Yes | 57 | 1.32 | 0.8-2.26 |
Abbreviations: IEN, intraepithelial neoplasia; IQ, interquartile; L-A, leptin- to-adiponectin.
Wilcoxon test P = .006.
Wilcoxon test P = .035.
Wilcoxon test P = .002.
Wilcoxon test P = .02.
Table 3.
Baseline Spearman Correlations (ρ) and No. of Observations
| Variable | Adiponectin |
Leptin |
L-A Ratio |
BMI |
MX % Density |
HOMA Index |
IGF-I |
HDL-C |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ρ | No. | ρ | No. | ρ | No. | ρ | No. | ρ | No. | ρ | No. | ρ | No. | ρ | No. | |
| Adiponectin | 1 | 235 | −0.16* | 235 | −0.65† | 235 | −0.18‡ | 235 | 0.23‡ | 195 | −0.20‡ | 235 | 0.003 | 234 | 0.29† | 235 |
| Leptin | 1 | 235 | 0.83† | 235 | 0.66† | 235 | −0.30† | 195 | 0.34† | 235 | −0.06 | 234 | −0.14* | 235 | ||
| L-A ratio | 1 | 235 | 0.58† | 235 | −0.35† | 195 | 0.36† | 235 | −0.06 | 234 | −0.27† | 235 | ||||
| BMI | 1 | 235 | −0.39† | 195 | 0.27† | 235 | −0.09 | 234 | −0.26† | 235 | ||||||
| MX % density | 1 | 195 | −0.20‡ | 195 | −0.11 | 194 | 0.11 | 195 | ||||||||
| HOMA index | 1 | 235 | 0.19‡ | 234 | −0.16* | 235 | ||||||||||
| IGF-I | 1 | 234 | 0.03 | 234 | ||||||||||||
| HDL-C | 1 | 235 | ||||||||||||||
NOTE. Pairwise comparisons were not significant unless otherwise indicated.
Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; HOMA, homeostasis model assessment; IGF-I, insulin-like growth factor I; L-A, leptin-to-adiponectin; MX, mammographic.
P < .05.
P < .001.
P < .01.
When we evaluated the association between mammographic percent density and adiponectin levels in an analysis of covariance model adjusting for BMI, we found a significant positive association (P = .03), indicating that adiponectin may be independently associated with mammographic density. We then compared women with prior IEN/T1 with unaffected high-risk women and found that adiponectin was associated with disease status; risk of having baseline IEN/T1 decreased by 8% per each unit increase of adiponectin (odds ratio [OR], 0.92; 95% CI, 0.86 to 0.98; P = .007, adjusted for BMI and mammographic percent density). The observed association remained statistically significant after adjustment for other variables shown in Table 3.
Known breast cancer risk factors and new breast events, mostly second breast neoplasms, according to median adiponectin level are summarized in Table 4. We observed that participants with lower adiponectin values presented greater WHR and HOMA index but lower mammographic percent density and a higher frequency of new diagnosed breast events. Adjusting for BMI, mammographic percent density, and disease status, we found a significantly lower risk of breast events for women with adiponectin higher than the median value of 10 μg/mL (OR, 0.87; 95% CI, 0.79 to 0.95; P = .003). The association did not change when we adjusted for treatment arm allocation (OR, 0.86; 95% CI, 0.78 to 0.95; P = .002). A similar trend was observed when adiponectin was categorized into quartiles (7, 10, and 14 μg/mL). Women with baseline adiponectin levels in the higher quartile were at decreased risk for breast neoplastic events compared with women with adiponectin levels in the lowest quartile (OR, 0.20; 95% CI, 0.07 to 0.62; P = .002).
Table 4.
Known Breast Cancer Risk Factors and New Breast Events by Adiponectin Level
| Risk Factor | Patients |
Adiponectin (μg/mL) |
P* | ||||
|---|---|---|---|---|---|---|---|
| > 10 |
≤ 10 |
||||||
| No. | % | No. | % | No. | % | ||
| Total | 235 | 117 | 118 | ||||
| Age, years | .75 | ||||||
| Median | 46 | 46 | |||||
| Q1-Q3 | 42-49 | 42-49 | |||||
| BMI | .05 | ||||||
| Median | 23 | 23 | |||||
| Q1-Q3 | 21-24 | 22-26 | |||||
| WHR | .02 | ||||||
| Median | 0.81 | 0.83 | |||||
| Q1-Q3 | 0.76-0.87 | 0.79-0.90 | |||||
| HOMA index | .02 | ||||||
| Median | 2.74 | 3.10 | |||||
| Q1-Q3 | 2.05-3.65 | 2.37-4.01 | |||||
| MX % density | .01 | ||||||
| Median | 51.1 | 43.8 | |||||
| Q1-Q3 | 38.6-61.2 | 33.9-54.1 | |||||
| Breast cancer family history | 85 | 43 | 37 | 42 | 36 | .9 | |
| Any pregnancy | 197 | 98 | 84 | 99 | 84 | .9 | |
| IEN/T1 stratum | 181 | 85 | 73 | 96 | 81 | .11 | |
| Gail stratum | 54 | 32 | 27 | 22 | 19 | ||
| New breast events | 57 | 20 | 17 | 37 | 31 | .01 | |
| IEN/T1 stratum | 53 of 181 | 29 | 19 of 85 | 22 | 34 of 96 | 35 | .05 |
| New breast event in Gail stratum | 4 of 54 | 7 | 1 of 32 | 3 | 3 of 22 | 14 | .15 |
Abbreviations: BMI, body mass index; HOMA, homeostasis model assessment; IEN, intraepithelial neoplasia; MX, mammographic; Q1-Q3, first to third quartile; WHR, waist–hip ratio.
χ2 or Wilcoxon test.
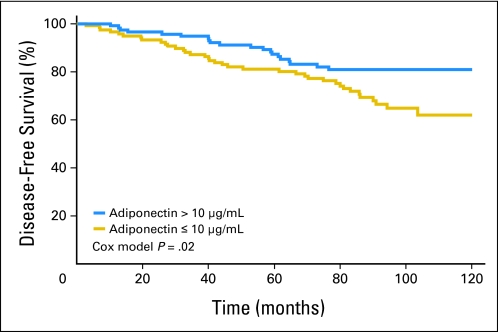
Disease-free survival curves according to median adiponectin levels are shown in Figure 2. There was a reduced breast cancer risk with increasing baseline adiponectin level. Log-rank test for median and quartile values of adiponectin indicated a significant association with disease-free survival (P = .02; P for trend among quartile values = .005). None of the potential confounding factors considered in Table 4 achieved statistical significance when included in the multivariate Cox model. The hazard ratio (HR) from the Cox model, adjusting for disease status and mammographic percent density, was 0.88 (95% CI, 0.81 to 0.96; P = .002) for one unit increase of adiponectin level and 0.40 (95% CI, 0.24 to 0.85) for adiponectin higher than 10 μg/mL versus lower values. When we further adjusted for BMI and treatment arm allocation, the risk remained significant (HR, 0.88; 95% CI, 0.81 to 0.96; P = .003 for one unit of increase). When we included WHR instead of BMI, among the confounders, the HR did not change (HR, 0.86; 95% CI, 0.77 to 0.94). Adiponectin was associated with new breast events independently from all other confounding factors, including BMI, WHR, and HOMA index. The change in adiponectin at different times (2 and 3 years) was not significantly associated with disease-free survival. However, there was a tendency for more women without an event to show an increase in adiponectin level compared with women with an event (60% v 50%). Further analysis was carried out excluding LIN as a breast neoplastic event. Results for disease-free survival excluding LIN as outcome measure did not change (HR, 0.87; 95% CI, 0.80 to 0.95; P = .0011 for one unit increase of adiponectin). When radiotherapy was included in the subgroup analysis of affected women, the HR for adiponectin level remained significant (HR, 0.93; 95% CI, 0.87 to 0.99; P = .02). HOMA index and mammographic percent density were not associated with breast neoplastic events.
Fig 2.
Disease-free survival according to median baseline adiponectin level of 10 μg/mL.
DISCUSSION
In this prospective analysis within our randomized chemoprevention trial in premenopausal women, we found that participants with low plasma adiponectin levels had a significantly higher risk of a breast neoplastic event. The association seemed to be independent of known risk factors such as BMI and mammographic percent density. We observed that at the baseline time point, adiponectin levels were lower in women with a history of breast neoplastic events compared with unaffected women at risk according to the Gail model. More importantly, baseline adiponectin levels predicted new breast events during follow-up; women with adiponectin less than 10 μg/mL were at 2.47-fold increased risk of developing a new breast neoplastic event.
Our results are consistent with some,18–20,22,35 but not all,21,23,32,36 case-control studies showing an inverse association between adiponectin level and breast cancer risk. Moreover, significantly lower adiponectin levels were found only in postmenopausal women with breast cancer compared with healthy controls,18–21,23 whereas other reports have shown a stronger association in premenopausal and obese women.22,36 It has also been suggested that the L-A ratio could be a suitable marker to determine breast cancer risk rather than adiponectin alone.36
We found that adiponectin levels were directly associated with mammographic percent density, whereas leptin and L-A ratio were inversely correlated. This is in line with a recent report by Maskarinec et al,37 although they conversely found no more association after adjustment for BMI. We also found that adiponectin was inversely correlated with BMI, whereas leptin and L-A ratio showed direct correlations, in line with previous reports.18,20,23,32,35,36 A positive correlation between adiponectin level and percent breast density, a strong predictor of subsequent breast cancer risk, may mean that the potential protective effect of adiponectin on breast cancer is not mediated by mammographic density.
In the NHS (Nurses Health Study) and NHS II cohorts,21 the relationship between adiponectin level and breast cancer risk significantly differed by ductal versus lobular breast cancer type among postmenopausal women, with an inverse association noted only in ductal cancers. No differences were observed by in situ versus invasive status.20,21 Our results cannot support any definitive conclusion because of the limited power of this subgroup analysis.
Studies evaluating concentrations and expression of adiponectin and its receptors in breast cancer tissue further support the inverse association between adiponectin and breast cancer risk. Adiponectin expression has been found to be higher in the adjacent adipose tissue compared with breast cancer tissues, in which it is weak or not expressed.22,38 Conversely, adiponectin receptors AdipoR1 and AdipoR2 have been found to be expressed more highly in in vitro malignant cells and in breast cancer tissue compared with normal breast tissue.22,39 The higher AdipoR1 expression in epithelial and stromal cells of invasive breast cancer tissue compared with preinvasive lesion tissues40 suggests that lower adiponectin levels in patients with invasive breast cancer may trigger upregulation of AdipoR1 expression in breast cancer tissue as a feedback loop.
The mechanisms linking adiponectin with insulin resistance, obesity, and human diseases have been studied extensively.6,41–46 Accumulating evidence indicates that adiponectin plays a remarkable role in glucose and lipid metabolism. In the liver, adiponectin enhances insulin sensitivity, reduces hepatic glucose output, increases fatty acid oxidation,43,47 and exerts anti-inflammatory and antiatherogenic properties.42,44 In our study, adiponectin levels were inversely correlated with the HOMA index, supporting the close connection between adiponectin and insulin resistance. A recent report from the HEAL (Health, Eating, Activity, and Lifestyle) study, a prospective cohort study to evaluate whether diet, weight, physical activity, lifestyle, hormones, or other factors affect breast cancer prognosis,48 showed that elevated HOMA score and low adiponectin levels were associated with increased breast cancer mortality. Interestingly, a causal role of adiponectin reduction in the development of insulin resistance, type 2 diabetes, metabolic syndrome, and atherosclerosis has been proposed. Adiponectin levels were reduced in obesity, which is also associated with decreased expression levels of adiponectin receptors, thereby reducing adiponectin signaling and finally leading to insulin resistance through a “vicious cycle.”42(p446)
To date, the mechanisms underlying the protective role of adiponectin in carcinogenesis are not fully understood. Adiponectin may act either directly on cancer cells or indirectly by regulating whole-body insulin sensitivity.44 Another potential mechanism could involve regulating the bioavailability of growth factors and steroid hormones.49 Overall, available data suggest a possible protective role of adiponectin in breast cancer development, and our results are consistent with previous such findings.
In our study, we found that both tamoxifen and fenretinide did not affect adiponectin level. Different compounds have been shown to modulate adiponectin levels in humans. We previously showed that fenretinide decreased the HOMA index, thus reducing insulin resistance, in women with BMI greater than 25 kg/m2 in the same study, whereas tamoxifen had an opposite effect.26 Conversely, metformin alone in young women with polycystic ovary syndrome or in diabetic patients50,51 and metformin plus pioglitazone or pioglitazone alone in diabetic patients have been associated with adiponectin increase.52,53 Also, rosiglitazone and imatinib have been shown to increase adiponectin concentrations in nondiabetic patients with metabolic syndrome54 and in patients with chronic myeloid leukemia,55 respectively, whereas atorvastatin has decreased adiponectin in hypercholesterolemic patients.56 Moreover, diet, weight loss, and physical exercise have been shown to increase adiponectin levels in obese patients,11,57,58 and adiponectin increase has been observed with safflower oil,59 stabilized rice bran,60 and dairy-supplemented diet61 in obese or diabetic patients. Further investigation is necessary to assess the role of adiponectin as a breast cancer risk biomarker and its potential direct effect on breast carcinogenesis pathways.
In conclusion, we found that participants with low plasma adiponectin levels had a significantly higher risk of breast neoplastic events in our randomized chemoprevention trial in premenopausal women. Our findings suggest that adiponectin is a risk biomarker for breast cancer, but additional prospective studies should be conducted before the conclusion can be made that low adiponectin has a causal or promotional role in premenopausal breast cancer.
Supplementary Material
Acknowledgment
Fenretinide was manufactured and donated by the R.W. Johnson Pharmaceutical Research Institute, Spring House, PA; tamoxifen was donated by Laboratori MAG, Garbagnate, and manufactured by Cosmo SpA, Lainate, Italy. We thank all personnel from the Unit of Laboratory Medicine of the European Institute of Oncology. This article is dedicated to the lovely memory of Dr Karen Audrey Johnson, DCP, NCI, for her continuous support to our breast cancer chemoprevention studies.
Footnotes
See accompanying editorial on page 124
Supported by National Cancer Institute Grant No. CA-77188; a contract from the Italian Foundation for Cancer Research; Associazione Italiana per la Ricerca sul Cancro Regional Grant No. 1068/2005 on second tumors; and Progetto Integrato Oncologia, Italian Health Ministry, Contracts No. RFPS-2006-1-339898 and RFPS-2006-1-339856.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Debora Macis, Paolo Magni, Andrea DeCensi
Financial support: Bernardo Bonanni, Andrea DeCensi
Administrative support: Aliana Guerrieri-Gonzaga, Serena Mora
Provision of study materials or patients: Debora Macis, Harriet Johansson, Matteo Lazzeroni, Davide Serrano, Massimiliano Cazzaniga, Irene Feroce, Maria Pizzamiglio, Maria Teresa Sandri, Marcella Gulisano, Bernardo Bonanni, Andrea DeCensi
Collection and assembly of data: Debora Macis, Aliana Guerrieri-Gonzaga, Harriet Johansson, Paolo Magni, Massimiliano Ruscica, Matteo Lazzeroni, Davide Serrano, Massimiliano Cazzaniga, Serena Mora, Irene Feroce, Maria Pizzamiglio, Maria Teresa Sandri, Marcella Gulisano, Bernardo Bonanni
Data analysis and interpretation: Debora Macis, Sara Gandini, Aliana Guerrieri-Gonzaga, Harriet Johansson, Paolo Magni, Davide Serrano, Bernardo Bonanni, Andrea DeCensi
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 2.Huang Z, Hankinson SE, Colditz GA, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–1411. [PubMed] [Google Scholar]
- 3.Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 4.van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 5.Harvie M, Hooper L, Howell AH. Central obesity and breast cancer risk: A systematic review. Obes Rev. 2003;4:157–173. doi: 10.1046/j.1467-789x.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 6.Lafontan M, Viguerie N. Role of adipokines in the control of energy metabolism: Focus on adiponectin. Curr Opin Pharmacol. 2006;6:580–585. doi: 10.1016/j.coph.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 8.Sonnenberg GE, Krakower GR, Hoffmann RG, et al. Plasma leptin concentrations during extended fasting and graded glucose infusions: Relationships with changes in glucose, insulin, and FFA. J Clin Endocrinol Metab. 2001;86:4895–4900. doi: 10.1210/jcem.86.10.7951. [DOI] [PubMed] [Google Scholar]
- 9.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 10.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 11.Christiansen T, Paulsen SK, Bruun JM, et al. Diet-induced weight loss and exercise alone and in combination enhance the expression of adiponectin receptors in adipose tissue and skeletal muscle, but only diet-induced weight loss enhanced circulating adiponectin. J Clin Endocrinol Metab. 2010;95:911–919. doi: 10.1210/jc.2008-2505. [DOI] [PubMed] [Google Scholar]
- 12.Oda N, Imamura S, Fujita T, et al. The ratio of leptin to adiponectin can be used as an index of insulin resistance. Metabolism. 2008;57:268–273. doi: 10.1016/j.metabol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Lawlor DA, Smith GD, Ebrahim S. Hyperinsulinaemia and increased risk of breast cancer: Findings from the British Women's Heart and Health Study. Cancer Causes Control. 2004;15:267–275. doi: 10.1023/B:CACO.0000024225.14618.a8. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 15.Stoll BA. Upper abdominal obesity, insulin resistance and breast cancer risk. Int J Obes Relat Metab Disord. 2002;26:747–753. doi: 10.1038/sj.ijo.0801998. [DOI] [PubMed] [Google Scholar]
- 16.DeCensi A, Gennari A. Insulin breast cancer connection: Confirmatory data set the stage for better care. J Clin Oncol. 2011;29:7–10. doi: 10.1200/JCO.2010.32.3022. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Wang Y. Adiponectin and breast cancer. Med Oncol. doi: 10.1007/s12032-010-9617-x. [epub ahead of print on July 13, 2010] [DOI] [PubMed] [Google Scholar]
- 18.Miyoshi Y, Funahashi T, Kihara S, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–5704. [PubMed] [Google Scholar]
- 19.Mantzoros C, Petridou E, Dessypris N, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 20.Tian YF, Chu CH, Wu MH, et al. Anthropometric measures, plasma adiponectin, and breast cancer risk. Endocr Relat Cancer. 2007;14:669–677. doi: 10.1677/ERC-06-0089. [DOI] [PubMed] [Google Scholar]
- 21.Tworoger SS, Eliassen AH, Kelesidis T, et al. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 2007;92:1510–1516. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]
- 22.Korner A, Pazaitou-Panayiotou K, Kelesidis T, et al. Total and high-molecular-weight adiponectin in breast cancer: In vitro and in vivo studies. J Clin Endocrinol Metab. 2007;92:1041–1048. doi: 10.1210/jc.2006-1858. [DOI] [PubMed] [Google Scholar]
- 23.Cust AE, Stocks T, Lukanova A, et al. The influence of overweight and insulin resistance on breast cancer risk and tumour stage at diagnosis: A prospective study. Breast Cancer Res Treat. 2009;113:567–576. doi: 10.1007/s10549-008-9958-8. [DOI] [PubMed] [Google Scholar]
- 24.Guerrieri-Gonzaga A, Robertson C, Bonanni B, et al. Preliminary results on safety and activity of a randomized, double-blind, 2 × 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in premenopausal women. J Clin Oncol. 2006;24:129–135. doi: 10.1200/JCO.2005.02.9934. [DOI] [PubMed] [Google Scholar]
- 25.DeCensi A, Robertson C, Guerrieri-Gonzaga A, et al. Randomized double-blind 2 × 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol. 2009;27:3749–3756. doi: 10.1200/JCO.2008.19.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson H, Gandini S, Guerrieri-Gonzaga A, et al. Effect of fenretinide and low-dose tamoxifen on insulin sensitivity in premenopausal women at high risk for breast cancer. Cancer Res. 2008;68:9512–9518. doi: 10.1158/0008-5472.CAN-08-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Ma Z, Gingerich RL, Santiago JV, et al. Radioimmunoassay of leptin in human plasma. Clin Chem. 1996;42:942–946. [PubMed] [Google Scholar]
- 29.Shand B, Elder P, Scott R, et al. Biovariability of plasma adiponectin. Clin Chem Lab Med. 2006;44:1264–1268. doi: 10.1515/CCLM.2006.227. [DOI] [PubMed] [Google Scholar]
- 30.Lissner L, Karlsson C, Lindroos AK, et al. Birth weight, adulthood BMI, and subsequent weight gain in relation to leptin levels in Swedish women. Obes Res. 1999;7:150–154. doi: 10.1002/j.1550-8528.1999.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 31.Sattar N, Wannamethee G, Sarwar N, et al. Adiponectin and coronary heart disease: A prospective study and meta-analysis. Circulation. 2006;114:623–629. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 32.Gaudet MM, Falk RT, Gierach GL, et al. Do adipokines underlie the association between known risk factors and breast cancer among a cohort of United States women? Cancer Epidemiol. 2010;34:580–586. doi: 10.1016/j.canep.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyd NF, Byng JW, Jong RA, et al. Quantitative classification of mammographic densities and breast cancer risk: Results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670–675. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 34.DeCensi A, Bonanni B, Baglietto L, et al. A two-by-two factorial trial comparing oral with transdermal estrogen therapy and fenretinide with placebo on breast cancer biomarkers. Clin Cancer Res. 2004;10:4389–4397. doi: 10.1158/1078-0432.CCR-04-0087. [DOI] [PubMed] [Google Scholar]
- 35.Chen DC, Chung YF, Yeh YT, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 36.Hancke K, Grubeck D, Hauser N, et al. Adipocyte fatty acid-binding protein as a novel prognostic factor in obese breast cancer patients. Breast Cancer Res Treat. 2010;119:367. doi: 10.1007/s10549-009-0577-9. [DOI] [PubMed] [Google Scholar]
- 37.Maskarinec G, Woolcott C, Steude JS, et al. The relation of leptin and adiponectin with breast density among premenopausal women. Eur J Cancer Prev. 2010;19:55–60. doi: 10.1097/CEJ.0b013e328333fb0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahata C, Miyoshi Y, Irahara N, et al. Demonstration of adiponectin receptors 1 and 2 mRNA expression in human breast cancer cells. Cancer Lett. 2007;250:229–236. doi: 10.1016/j.canlet.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Jardé T, Caldefie-Chézet F, Goncalves-Mendes N, et al. Involvement of adiponectin and leptin in breast cancer: Clinical and in vitro studies. Endocr Relat Cancer. 2009;16:1197–1210. doi: 10.1677/ERC-09-0043. [DOI] [PubMed] [Google Scholar]
- 40.Pfeiler G, Hudelist G, Wulfing P, et al. Impact of AdipoR1 expression on breast cancer development. Gynecol Oncol. 2010;117:134–138. doi: 10.1016/j.ygyno.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 41.Schaffler A, Scholmerich J, Buechler C. Mechanisms of disease: Adipokines and breast cancer—Endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat Clin Pract Endocrinol Metab. 2007;3:345–354. doi: 10.1038/ncpendmet0456. [DOI] [PubMed] [Google Scholar]
- 42.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 43.Diez JJ, Iglesias P. The role of the novel adipocyte-derived protein adiponectin in human disease: An update. Mini Rev Med Chem. 2010;10:856–869. doi: 10.2174/138955710791608325. [DOI] [PubMed] [Google Scholar]
- 44.Barb D, Williams CJ, Neuwirth AK, et al. Adiponectin in relation to malignancies: A review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858–s866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 45.Grossmann ME, Ray A, Nkhata KJ, et al. Obesity and breast cancer: Status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29:641–653. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 46.Brochu-Gaudreau K, Rehfeldt C, Blouin R, et al. Adiponectin action from head to toe. Endocrine. 2010;37:11–32. doi: 10.1007/s12020-009-9278-8. [DOI] [PubMed] [Google Scholar]
- 47.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 48.Duggan C, Irwin ML, Xiao L, et al. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J Clin Oncol. 2011;29:32–39. doi: 10.1200/JCO.2009.26.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barb D, Pazaitou-Panayiotou K, Mantzoros CS. Adiponectin: A link between obesity and cancer. Expert Opin Investig Drugs. 2006;15:917–931. doi: 10.1517/13543784.15.8.917. [DOI] [PubMed] [Google Scholar]
- 50.Agarwal N, Rice SP, Bolusani H, et al. Metformin reduces arterial stiffness and improves endothelial function in young women with polycystic ovary syndrome: A randomized, placebo-controlled, crossover trial. J Clin Endocrinol Metab. 2010;95:722–730. doi: 10.1210/jc.2009-1985. [DOI] [PubMed] [Google Scholar]
- 51.Mather KJ, Funahashi T, Matsuzawa Y, et al. Adiponectin, change in adiponectin, and progression to diabetes in the Diabetes Prevention Program. Diabetes. 2008;57:980–986. doi: 10.2337/db07-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaku K. Efficacy and safety of therapy with metformin plus pioglitazone in the treatment of patients with type 2 diabetes: A double-blind, placebo-controlled, clinical trial. Curr Med Res Opin. 2009;25:1111–1119. doi: 10.1185/03007990902820816. [DOI] [PubMed] [Google Scholar]
- 53.Park JS, Cho MH, Nam JS, et al. Effect of pioglitazone on serum concentrations of osteoprotegerin in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2011;164:69–74. doi: 10.1530/EJE-10-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aquilante CL, Kosmiski LA, Zineh I, et al. Pharmacodynamic effects of rosiglitazone in nondiabetic patients with metabolic syndrome. Pharmacotherapy. 2010;30:236–247. doi: 10.1592/phco.30.3.236. [DOI] [PubMed] [Google Scholar]
- 55.Fitter S, Vandyke K, Schultz CG, et al. Plasma adiponectin levels are markedly elevated in imatinib-treated chronic myeloid leukemia (CML) patients: A mechanism for improved insulin sensitivity in type 2 diabetic CML patients? J Clin Endocrinol Metab. 2010;95:3763–3767. doi: 10.1210/jc.2010-0086. [DOI] [PubMed] [Google Scholar]
- 56.Koh KK, Quon MJ, Han SH, et al. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol. 2010;55:1209–1216. doi: 10.1016/j.jacc.2009.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shalitin S, Ashkenazi-Hoffnung L, Yackobovitch-Gavan M, et al. Effects of a twelve-week randomized intervention of exercise and/or diet on weight loss and weight maintenance, and other metabolic parameters in obese preadolescent children. Horm Res. 2009;72:287–301. doi: 10.1159/000245931. [DOI] [PubMed] [Google Scholar]
- 58.Christiansen T, Paulsen SK, Bruun JM, et al. Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: A 12-week randomized intervention study. Am J Physiol Endocrinol Metab. 2010;298:E824–E831. doi: 10.1152/ajpendo.00574.2009. [DOI] [PubMed] [Google Scholar]
- 59.Norris LE, Collene AL, Asp ML, et al. Comparison of dietary conjugated linoleic acid with safflower oil on body composition in obese postmenopausal women with type 2 diabetes mellitus. Am J Clin Nutr. 2009;90:468–476. doi: 10.3945/ajcn.2008.27371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng HH, Huang HY, Chen YY, et al. Ameliorative effects of stabilized rice bran on type 2 diabetes patients. Ann Nutr Metab. 2010;56:45–51. doi: 10.1159/000265850. [DOI] [PubMed] [Google Scholar]
- 61.Zemel MB, Sun X, Sobhani T, et al. Effects of dairy compared with soy on oxidative and inflammatory stress in overweight and obese subjects. Am J Clin Nutr. 2010;91:16–22. doi: 10.3945/ajcn.2009.28468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.