Abstract
Purpose
This is the first randomized phase II/III trial comparing two carboplatin-based chemotherapy regimens in patients with urothelial cancer who are ineligible (“unfit”) for cisplatin chemotherapy.
Patients and Methods
The primary objective of the phase III part of this study was to compare the overall survival (OS) of chemotherapy-naive patients with measurable disease and an impaired renal function (glomerular filtration rate < 60 but > 30 mL/min) and/or performance score of 2 who were randomly assigned to receive either gemcitabine/carboplatin (GC) or methotrexate/carboplatin/vinblastine (M-CAVI). To detect an increase of 50% in median survival with GC compared with M-CAVI (13.5 v 9 months) based on a two-sided log-rank test at error rates α = .05 and β = .20, 225 patients were required. Secondary end points were overall response rate (ORR), progression-free survival (PFS), toxicity, and quality of life.
Results
In all, 238 patients were randomly assigned by 29 institutions over a period of 7 years. The median follow-up was 4.5 years. Best ORRs were 41.2% (36.1% confirmed response) for patients receiving GC versus 30.3% (21.0% confirmed response) for patients receiving M-CAVI (P = .08). Median OS was 9.3 months in the GC arm and 8.1 months in the M-CAVI arm (P = .64). There was no difference in PFS (P = .78) between the two arms. Severe acute toxicity (death, grade 4 thrombocytopenia with bleeding, grade 3 or 4 renal toxicity, neutropenic fever, or mucositis) was observed in 9.3% of patients receiving GC and 21.2% of patients receiving M-CAVI.
Conclusion
There were no significant differences in efficacy between the two treatment groups. The incidence of severe acute toxicities was higher for those receiving M-CAVI.
INTRODUCTION
Cisplatin-containing combination chemotherapy has been the standard of care in the treatment of advanced or metastatic urothelial cancer (UC) since the late 1980s. However, more than 50% of patients are ineligible (“unfit”) for cisplatin because of poor performance status (PS), impaired renal function, or comorbidity that forbids high-volume hydration.1–4 So far, no standard chemotherapy has been established for this patient group.5
To the best of our knowledge, the first randomized phase II/III trial in this setting has now been conducted by the European Organisation for Research and Treatment of Cancer-Genitourinary Tract Cancer (EORTC GU) group. Patients with UC were categorized as ineligible (“unfit”) for cisplatin-containing chemotherapy6,7 because they had a PS of 2 and/or impaired renal function (glomerular filtration rate [GFR] < 60 mL/min). Two carboplatin-based chemotherapy regimens—gemcitabine/carboplatin (GC) and methotrexate/carboplatin/vinblastine (M-CAVI)—were compared. Carboplatin is a less nephrotoxic platinum analog than cisplatin. M-CAVI is a well-tolerated and widely used palliative combination chemotherapy regimen.7–12 Several new agents and combinations have been explored to reduce toxicity and improve efficacy in the treatment of UC. Among them is gemcitabine, a pyrimidine antimetabolite.13–17 Gemcitabine is well tolerated and can be safely used in patients with impaired renal function (GFR ≥ 30 mL/min).18 Trial history and background of this study were presented earlier together with the analysis of the phase II results.19
This phase II/III study was initiated to evaluate the efficacy and toxicity of the two treatment arms. The phase II part included 178 patients. Both treatment combinations were shown to be active and safe in this group of unfit patients, and it was decided to proceed to phase III, the results of which are reported here.
PATIENTS AND METHODS
Patients
Detailed inclusion and exclusion criteria were published elsewhere.19 In short, patients with histologically proven UC of the urinary tract (including renal pelvis, ureter, and urinary bladder), unresected lymph nodes (N+), distant metastases (M1, stage IV), or unresectable primary bladder cancer (T3-4) with measurable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST)20 were included. No previous cytotoxic or biologic systemic treatment was allowed. All patients had to be ineligible (unfit) for cisplatin-based chemotherapy, defined by a WHO PS of 2 and/or impaired renal function (GFR > 30 but < 60 mL/min). GFR could be assessed by direct measurement (EDTA or creatinine clearance) if available or by calculation from serum or plasma creatinine.21
The protocol was approved by the ethics review boards of the participating institutions. Before random assignment, written informed consent was obtained from all patients in accordance with the Declaration of Helsinki, applicable guidelines for good clinical practice, or laws and regulations of the countries where the study was conducted, whichever represented the greater protection of the individual.
Treatment Schedule
Patients who were given M-CAVI received methotrexate 30 mg/m2 intravenously (IV) on days 1, 15, and 22. It was omitted in patients presenting with pleural effusions or ascites until complete resolution. Carboplatin was dosed in milligrams (4.5 × [GFR + 25]) and given over 1 hour IV on day 1 in both treatment arms, once every 4 weeks. Vinblastine 3 mg/m2 IV was given on days 1, 15, and 22. Patients allocated to the GC arm received gemcitabine 1,000 mg/m2 over 30 minutes IV on days 1 and 8, followed by carboplatin on day 1, every 3 weeks. Treatment was continued until disease progression or intolerable toxicity. In case of complete response, two more cycles were to be given. Granulocyte colony-stimulating factor was allowed and documented but was reserved for those patients in whom the recommended dose modifications were insufficient. Detailed protocol requirements for dose adjustments and dose delays as well as information about amendments were detailed in a previous article in the Journal of Clinical Oncology.19
Treatment Evaluation
The main objective of this phase III study was to compare overall survival (OS) in the two treatment groups. Adverse effects and quality of life (QoL) were secondary end points. Furthermore, response rates and progression-free survival (PFS) were also assessed. The main end points were also analyzed taking into account the stratification factors (WHO PS, renal function, and institution) and, in a post hoc analysis, the Bajorin risk groups.22 Severe acute toxicity (SAT) was defined by death resulting from toxicity, grade 4 thrombocytopenia with bleeding, grade 3 to 4 renal toxicity, neutropenic fever, or grade 3 to 4 mucositis. All patients were evaluated by the study coordinators who took into account eligibility, response to treatment, and the date of first progression and/or death.
Statistical Considerations
The median duration of survival on the M-CAVI arm was assumed to be 9 months. To detect an increase of 50% in median survival on the GC arm to 13.5 months, based on a two-sided log-rank test at error rates α = .05 and β = .20, a total of 192 deaths were required. Assuming that 85% of the patients would be followed to death, a total of 225 patients were required. With an expected entry rate of 45 patients per year, the required number of patients would be entered in 5 years.
Patients were centrally randomly assigned at the EORTC Headquarters to receive either GC or M-CAVI by using the minimization technique, with stratification for WHO PS, renal function (GFR), and institution. No formal interim efficacy analyses were planned.
OS in the two treatment groups was compared by using all randomly assigned patients on the basis of an intent-to-treat analysis; a sensitivity analysis was also performed in all patients according to WHO PS and GFR. In a post hoc attempt to evaluate outcome measures in this unfit patient population by using the Bajorin risk groups on the basis of PS and visceral metastases, PS 0 and 1 were transformed into Karnofsky performance status ≥ 80% and PS 2 into Karnofsky performance status less than 80%. When adding presence or absence of visceral metastases, patients were regrouped into three prognostic groups depending on their number of adverse prognostic factors (Bajorin risk groups 0, 1, or 2).19,22
RESULTS
A total of 238 patients were recruited by 29 centers (12 countries) between March 2001 and March 2008; 119 patients were randomly assigned to each treatment group (GC or M-CAVI). Two ineligible patients on M-CAVI had no lesions. The median follow-up was 4.5 years, and the maximum follow-up was 7.8 years.
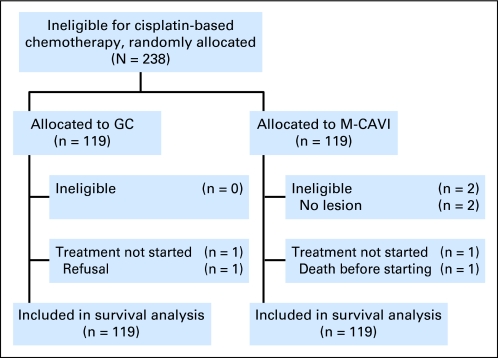
Patient characteristics were generally well balanced between the arms, as were the stratification factors. There was only a slight imbalance in the distribution of liver and visceral metastases (P = .15; Table 1). Of the randomly assigned patients, 236 of 238 started the protocol treatment (one patient refusal, one patient died before the first cycle of treatment; Fig 1). The majority of patients received four cycles of chemotherapy. Fifty-one patients (21.4%) stopped the treatment due to toxicity, 25 (21.0%) in the GC arm and 26 (21.8%) in the M-CAVI arm. Dose reductions were required in 78.8% (72.9% in the GC arm and 84.7% in the M-CAVI arm) and delays were required in 65.7% (71.2% in the GC arm and 60.2% in the M-CAVI arm) of patients. Detailed information about patient characteristics, number of cycles, dose reductions, and dose delays is given in Tables 1 and 2.
Table 1.
Patient and Disease Characteristics
| Characteristic | GC (n = 119) |
M-CAVI (n = 119) |
Total (N = 238) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age, years | ||||||
| Median | 70 | 72 | 71 | |||
| Range | 36-87 | 34-86 | 34-87 | |||
| Sex | ||||||
| Male | 90 | 75.6 | 96 | 80.7 | 186 | 78.2 |
| Female | 29 | 24.4 | 23 | 19.3 | 52 | 21.8 |
| Associated chronic disease | ||||||
| No | 59 | 49.6 | 64 | 53.8 | 123 | 51.7 |
| Yes | 60 | 50.4 | 55 | 46.2 | 115 | 48.3 |
| WHO PS | ||||||
| 0 | 20 | 16.8 | 19 | 16.0 | 39 | 16.4 |
| 1 | 46 | 38.7 | 46 | 38.7 | 92 | 38.7 |
| 2 | 53 | 44.5 | 54 | 45.4 | 107 | 45.0 |
| GFR, mL/min | ||||||
| Median | 50.0 | 48.0 | 49.0 | |||
| Range | 30.8-128.0 | 30.0-126.0 | 30.0-128.0 | |||
| Reason unfit for cisplatin therapy | ||||||
| WHO PS 2 | 21 | 17.6 | 21 | 17.6 | 42 | 17.6 |
| GFR 30-60 mL/min | 66 | 55.5 | 65 | 54.6 | 131 | 55.0 |
| Both | 32 | 26.9 | 33 | 27.7 | 65 | 27.3 |
| Site of primary tumor | ||||||
| Bladder | 90 | 75.6 | 87 | 73.1 | 177 | 74.4 |
| Renal pelvis | 12 | 10.1 | 17 | 14.3 | 29 | 12.2 |
| Ureter | 12 | 10.1 | 11 | 9.2 | 23 | 9.7 |
| Urethra | 3 | 2.5 | 2 | 1.7 | 5 | 2.1 |
| Other | 2 | 1.7 | 2 | 1.7 | 4 | 1.7 |
| Liver metastases | ||||||
| No | 99 | 83.2 | 90 | 75.6 | 189 | 79.4 |
| Yes | 20 | 16.8 | 29 | 24.4 | 49 | 20.6 |
| Visceral metastases | ||||||
| No | 64 | 53.8 | 53 | 44.5 | 117 | 49.2 |
| Yes | 55 | 46.2 | 66 | 55.5 | 121 | 50.8 |
| Bajorin risk group | ||||||
| 0 | 45 | 37.8 | 36 | 30.3 | 81 | 34.0 |
| 1 | 40 | 33.6 | 46 | 38.7 | 86 | 36.1 |
| 2 | 34 | 28.6 | 37 | 31.1 | 71 | 29.8 |
Abbreviations: GC, gemcitabine/carboplatin; GFR, glomerular filtration rate; M-CAVI, methotrexate/carboplatin/vinblastine; PS, performance status.
Fig 1.
CONSORT diagram. GC, gemcitabine/carboplatin; M-CAVI, methotrexate/carboplatin/vinblastine.
Table 2.
Amount of Treatment Received and Toxicity
| Amount of Treatment Received | GC (n = 118) |
M-CAVI (n = 118) |
Total (N = 236) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| No. of cycles of therapy | ||||||
| 1 | 12 | 10.2 | 23 | 19.5 | 35 | 14.7 |
| 2 | 17 | 14.4 | 23 | 19.5 | 40 | 16.8 |
| 3 | 10 | 8.5 | 11 | 9.3 | 21 | 8.8 |
| 4 | 18 | 15.3 | 18 | 15.3 | 36 | 15.1 |
| 5 | 10 | 8.5 | 11 | 9.3 | 21 | 8.8 |
| 6 | 38 | 32.2 | 22 | 18.6 | 60 | 25.4 |
| > 6 | 13 | 11.0 | 10 | 8.5 | 23 | 9.7 |
| Median | 4.0 | 5.0 | 4.0 | |||
| Range | 1.0-23.0 | 1.0-10.0 | 1.0-23.0 | |||
| Duration of treatment, weeks | ||||||
| Median | 13.9 | 15.0 | 14.3 | |||
| Range | 1.0-36.1 | 0.1-98.0 | 0.1-98.0 | |||
| Dose reduction (any reason) | ||||||
| No | 32 | 27.1 | 18 | 15.3 | 50 | 21.2 |
| Yes | 86 | 72.9 | 100 | 84.7 | 186 | 78.8 |
| Treatment delay (any reason) | ||||||
| No | 34 | 28.8 | 47 | 39.8 | 81 | 34.3 |
| Yes | 84 | 71.2 | 71 | 60.2 | 155 | 65.7 |
| Severe acute toxicity* | ||||||
| No | 107 | 90.7 | 93 | 78.8 | 200 | 84.7 |
| Yes | 11 | 9.3 | 25 | 21.2 | 36 | 15.3 |
| Leucopenia grade† | ||||||
| 0-2 | 65 | 55.1 | 63 | 53.4 | 128 | 54.2 |
| 3 | 40 | 33.9 | 34 | 28.8 | 74 | 31.4 |
| 4 | 13 | 11.0 | 21 | 17.8 | 34 | 14.4 |
| Neutropenia grade† | ||||||
| 0-2 | 54 | 45.8 | 38 | 32.2 | 92 | 39.0 |
| 3 | 38 | 32.2 | 30 | 25.4 | 68 | 28.8 |
| 4 | 24 | 20.3 | 45 | 38.1 | 69 | 29.2 |
| Missing | 2 | 1.7 | 5 | 4.2 | 7 | 3.0 |
| Thrombocytopenia grade† | ||||||
| 0-2 | 61 | 51.7 | 95 | 80.5 | 156 | 66.1 |
| 3 | 47 | 39.8 | 22 | 18.6 | 69 | 29.2 |
| 4 | 10 | 8.5 | 1 | 0.8 | 11 | 4.7 |
| Febrile neutropenia grade† | ||||||
| 0-2 | 112 | 94.9 | 99 | 83.9 | 211 | 89.4 |
| 3 | 2 | 1.7 | 14 | 11.9 | 16 | 6.8 |
| 4 | 3 | 2.5 | 3 | 2.5 | 6 | 2.5 |
| Missing | 1 | 0.8 | 2 | 1.7 | 3 | 1.3 |
| Infection grade† | ||||||
| 0-2 | 103 | 87.3 | 101 | 85.6 | 204 | 86.4 |
| 3 | 13 | 11.0 | 15 | 12.7 | 28 | 11.9 |
| 4 | 1 | 0.8 | 0 | 0.0 | 1 | 0.4 |
| Missing | 1 | 0.8 | 2 | 1.7 | 3 | 1.3 |
Abbreviations: GC, gemcitabine/carboplatin; M-CAVI, methotrexate/carboplatin/vinblastine.
Severe acute toxicity, death as a result of toxicity, renal toxicity (grade 3 to 4), febrile neutropenia (grade 3 to 4), hemorrhage/bleeding with thrombocytopenia (grade 4), or mucositis (grade 3 to 4).
Common Toxicity Criteria v2.0.
Toxicity
SAT was observed in 9.3% of patients in the GC arm (including two deaths resulting from toxicity) and 21.2% in the M-CAVI arm (including four deaths resulting from toxicity). The most common grade 3 to 4 toxicities were leucopenia (44.9%, 46.6%), neutropenia (52.5%, 63.5%), febrile neutropenia (4.2%, 14.4%), thrombocytopenia (48.3%, 19.4%), and infection (11.8%, 12.7%) in the GC and M-CAVI arms, respectively. There were more SATs in patients with impaired renal function, and there were also more SATs in the M-CAVI arm, both overall and also in subgroups, according to the reason for being unfit for cisplatin therapy and Bajorin risk groups. Details can be found in Tables 2 and 3.
Table 3.
Impact of Reason Ineligible (unfit) for Cisplatin and Bajorin Risk Groups by Treatment Group (severe acute toxicity, response rate, survival)
| Variable | GC |
M-CAVI |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO PS ≥ 2 (n = 21) |
GFR (< 60 mL/min) (n = 66) |
Both (n = 32) |
WHO PS ≥ 2 (n = 21) |
GFR (< 60 mL/min) (n = 65) |
Both (n = 33) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Severe acute toxicity | ||||||||||||
| No | 20 | 95.2 | 60 | 90.9 | 28 | 87.5 | 19 | 90.5 | 51 | 78.5 | 24 | 72.7 |
| Yes | 1 | 4.8 | 6 | 9.1 | 4 | 12.5 | 2 | 9.5 | 14 | 21.5 | 9 | 27.3 |
| Best overall response | ||||||||||||
| Complete response | 0 | 0.0 | 4 | 6.1 | 0 | 0.0 | 0 | 0.0 | 4 | 6.2 | 0 | 0.0 |
| Confirmed | 0 | 3 | 0 | 0 | 3 | 0 | ||||||
| Unconfirmed | 0 | 1 | 0 | 0 | 1 | 0 | ||||||
| Partial response | 10 | 47.6 | 27 | 40.9 | 8 | 25.0 | 4 | 19.0 | 19 | 29.2 | 9 | 27.3 |
| Confirmed | 9 | 26 | 5 | 3 | 14 | 5 | ||||||
| Unconfirmed | 1 | 1 | 3 | 1 | 5 | 4 | ||||||
| Stable disease | 6 | 28.6 | 24 | 36.4 | 9 | 28.1 | 7 | 33.3 | 23 | 35.4 | 11 | 33.3 |
| Progression | 3 | 14.3 | 8 | 12.1 | 7 | 21.9 | 7 | 33.3 | 9 | 13.8 | 1 | 3.0 |
| Early death | 1 | 4.8 | 2 | 3.0 | 1 | 3.1 | 0 | 0.0 | 4 | 6.1 | 6 | 18.1 |
| Not assessable | 1 | 4.8 | 1 | 1.5 | 7 | 21.9 | 3 | 14.3 | 6 | 9.2 | 6 | 18.2 |
| Survival status | ||||||||||||
| Alive | 0 | 0.0 | 6 | 9.1 | 3 | 9.4 | 3 | 14.3 | 5 | 7.7 | 3 | 9.1 |
| Dead | 21 | 100.0 | 60 | 90.9 | 29 | 90.6 | 18 | 85.7 | 60 | 92.3 | 30 | 90.9 |
| Bajorin Risk Group | 0 (n = 45) | 1 (n = 40) | 2 (n = 34) | 0 (n = 36) | 1 (n = 46) | 2 (n = 37) | ||||||
| Severe acute toxicity* | ||||||||||||
| No | 42 | 93.3 | 35 | 87.5 | 31 | 91.2 | 29 | 80.6 | 37 | 80.4 | 28 | 75.7 |
| Yes | 3 | 6.7 | 5 | 12.5 | 3 | 8.8 | 7 | 19.4 | 9 | 19.6 | 9 | 24.3 |
| Best overall response | ||||||||||||
| Complete response | 3 | 6.7 | 1 | 2.5 | 0 | 0.0 | 4 | 11.1 | 0 | 0.0 | 0 | 0.0 |
| Confirmed | 3 | 0 | 0 | 3 | 0 | 0 | ||||||
| Unconfirmed | 0 | 1 | 0 | 1 | 0 | 0 | ||||||
| Partial response | 17 | 37.8 | 19 | 47.5 | 9 | 26.5 | 14 | 38.9 | 12 | 26.1 | 6 | 16.2 |
| Confirmed | 16 | 18 | 6 | 10 | 9 | 3 | ||||||
| Unconfirmed | 1 | 1 | 3 | 4 | 3 | 3 | ||||||
| Stable disease | 18 | 40.0 | 11 | 27.5 | 10 | 29.4 | 10 | 27.8 | 19 | 41.3 | 12 | 32.4 |
| Progression | 4 | 8.9 | 6 | 15.0 | 8 | 23.5 | 3 | 8.3 | 8 | 17.4 | 6 | 16.2 |
| Early death | 2 | 4.4 | 0 | 0.0 | 2 | 5.9 | 1 | 2.8 | 4 | 8.7 | 5 | 13.5 |
| Not assessable | 1 | 2.2 | 3 | 7.5 | 5 | 14.7 | 4 | 11.1 | 3 | 6.5 | 8 | 21.6 |
| Survival status | ||||||||||||
| Alive | 5 | 11.1 | 4 | 10.0 | 0 | 0.0 | 4 | 11.1 | 5 | 10.9 | 2 | 5.4 |
| Dead | 40 | 88.9 | 36 | 90.0 | 34 | 100.0 | 32 | 88.9 | 41 | 89.1 | 35 | 94.6 |
Abbreviations: GC, gemcitabine/carboplatin; GFR, glomerular filtration rate ; M-CAVI, methotrexate/carboplatin/vinblastine ; PS, performance status.
Severe acute toxicity, death resulting from toxicity, renal toxicity (grade 3 to 4), febrile neutropenia (grade 3 to 4), hemorrhage/bleeding with thrombocytopenia (grade 4), or mucositis (grade 3 to 4).
Efficacy
The main reason for stopping treatment was treatment failure (recurrence, progression, or death resulting from malignant disease) in 73 patients (25.2% in the GC arm and 36.1% in the M-CAVI arm; Table 4).
Table 4.
End of Treatment, Response Rate, and Disease Status
| Variable | GC (n = 119) |
M-CAVI (n = 119) |
Total (N = 238) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Reason for treatment discontinuation | ||||||
| Progression/relapse/death resulting from PD | 30 | 25.2 | 43 | 36.1 | 73 | 30.7 |
| Toxicity | 25 | 21.0 | 26 | 21.8 | 51 | 21.4 |
| Patient's refusal | 14 | 11.8 | 10 | 8.4 | 24 | 10.1 |
| End of protocol treatment | 5 | 4.2 | 3 | 2.5 | 8 | 3.4 |
| Intercurrent death | 7 | 5.9 | 4 | 3.4 | 11 | 4.6 |
| Major protocol violation | 0 | 0.0 | 3 | 2.5 | 3 | 1.3 |
| Other* | 38 | 31.9 | 29 | 24.4 | 67 | 28.2 |
| Missing | 0 | 0.0 | 1 | 0.8 | 1 | 0.4 |
| Best overall response | ||||||
| Complete response | 4 | 3.4 | 4 | 3.4 | 8 | 3.4 |
| Confirmed | 3 | 3 | 6 | |||
| Unconfirmed | 1 | 1 | 2 | |||
| Partial response | 45 | 37.8 | 32 | 26.9 | 77 | 32.4 |
| Confirmed | 40 | 22 | 62 | |||
| Unconfirmed | 5 | 10 | 15 | |||
| Stable disease | 39 | 32.8 | 41 | 34.5 | 80 | 33.6 |
| Progression | 18 | 15.1 | 17 | 14.3 | 35 | 14.7 |
| Early death | 4 | 3.4 | 10 | 8.4 | 14 | 5.9 |
| Not assessable | 9 | 7.6 | 15 | 12.6 | 24 | 10.1 |
| Progression-free survival status | ||||||
| Alive without progression | 4 | 3.4 | 6 | 5.0 | 10 | 4.2 |
| Progression | 84 | 70.6 | 79 | 66.4 | 163 | 68.5 |
| Death resulting from progression | 11 | 9.2 | 10 | 8.4 | 21 | 8.8 |
| Death resulting from other cause | 20 | 16.8 | 24 | 20.2 | 44 | 18.5 |
| Survival status | ||||||
| Alive | 9 | 7.6 | 11 | 9.2 | 20 | 8.4 |
| Dead | 110 | 92.4 | 108 | 90.8 | 218 | 91.6 |
| Progression | 82 | 75 | 157 | |||
| Toxicity | 3 | 4 | 7 | |||
| Chronic disease | 2 | 3 | 5 | |||
| Other† | 12 | 16 | 28 | |||
| Missing | 11 | 10 | 21 | |||
Abbreviations: GC, gemcitabine/carboplatin; M-CAVI, methotrexate/carboplatin/vinblastine; PD, progressive disease.
Most common reasons: stable disease after more than six chemotherapy cycles, no further clinical benefit at discretion of local investigator, general deterioration.
Most common reasons: cardiac events, pulmonary embolism, clinical deterioration.
Of the patients receiving GC, 41.2% had a complete or partial response (including six unconfirmed responses). Of the patients receiving M-CAVI, 30.3% had a complete or partial response (including 11 unconfirmed responses). The difference between the two treatment arms was not statistically significant (P = .08). However, considering only confirmed responses, this difference became significant (P = .01) favoring GC. Patients in Bajorin risk group 2 had a lower response rate (Table 3).
OS and PFS
Death was reported in 218 patients (110 in the GC arm and 108 in the M-CAVI arm). The main cause of death was progression of malignant disease (72%).
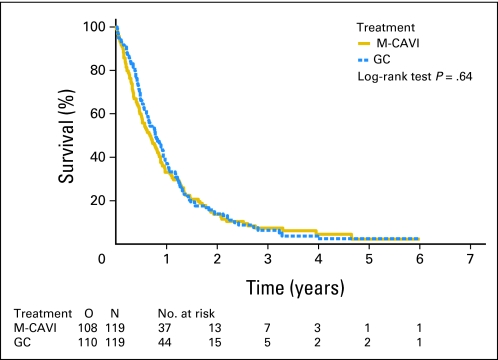
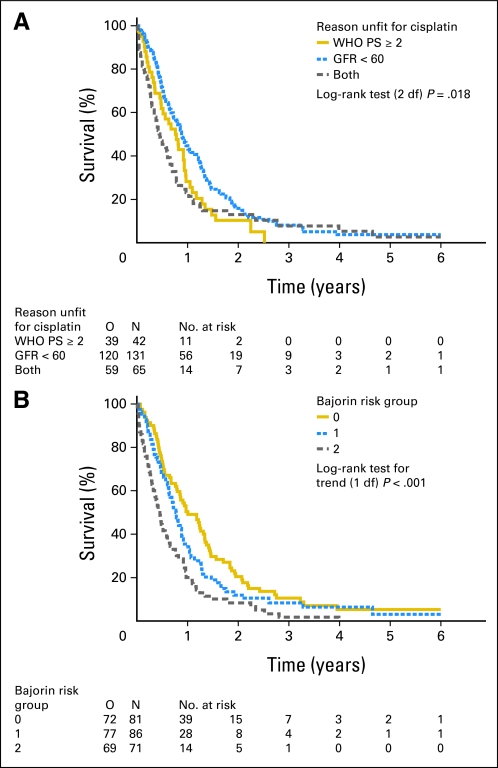
The intent-to-treat analysis of the primary end point showed a median OS of 9.3 months in the GC arm and 8.1 months in the M-CAVI arm, with a hazard ratio of 0.94 (95% CI, 0.72 to 1.22; P = .64; Fig 2). Median PFS was 5.8 months in the GC arm and 4.2 months in the M-CAVI arm in the intent-to-treat analysis, with a hazard ratio of 1.04 (95% CI, 0.80 to 1.35). We also evaluated the differences in OS according to the number of reasons for being unfit (PS 2, GFR < 60 mL/min, or both) and the Bajorin risk groups. Patients with only one reason for being unfit for cisplatin had a better OS than patients with both reasons (GFR < 60 mL/min and WHO PS 2; Fig 3). The post hoc analysis of OS by the Bajorin risk groups showed that, as the number of Bajorin risk factors increased, OS decreased significantly (Fig 3).
Fig 2.
Duration of survival by treatment group. GC, gemcitabine/carboplatin; M-CAVI, methotrexate/carboplatin/vinblastine; O, observed number of deaths.
Fig 3.
(A) Impact of stratification factors and (B) Bajorin risk groups on survival. GFR, glomerular filtration rate (mL/min); O, observed number of deaths; PS, performance status.
QoL Analysis
QoL was assessed at baseline, after every two cycles, and at the time of stopping treatment by using the EORTC Quality of Life Questionnaire C30 (QLQ-C30) Version 3.0 to which four trial-specific questions were added. The available data revealed no differences (P = .47) between the two treatment arms for changes in the primary scale global health status/QoL from baseline to the end of cycle 2. However, because of low compliance (90% at baseline and less than 50% afterward), the results remain inconclusive.
DISCUSSION
We have conducted, to the best of our knowledge, the first randomized phase II/III trial comparing two carboplatin-based combination chemotherapies in patients with advanced UC who were ineligible for cisplatin therapy. This study was designed to establish a treatment standard in patients unfit for therapy with cisplatin. Valuable information in a clear-cut group of cisplatin-ineligible patients was collected and analyzed and, for the first time, well-grounded reference figures for PFS and OS in this patient population have been generated.
The hypothesized increase in OS from 9 months with the older M-CAVI regimen to 13.5 months with GC was not reached. The primary end point of the study, OS, showed no statistically significant difference between the two treatment arms. Median survival was 8.1 months in the M-CAVI arm and 9.3 months in the GC arm. On the basis of the number of patients included in the study, it is not possible to determine whether GC therapy might provide a survival benefit in any of the patient subgroups. PFS was also short, with no statistically significant difference between treatments.
Although the most effective treatment for patients ineligible for cisplatin remains to be defined, the results of this randomized phase II/III study are still a major step forward. This study with GC and M-CAVI, the two most studied regimens in this setting, has shown that M-CAVI is more toxic than GC and, in particular, more toxic in patients with impaired renal function. SAT occurred more often in patients with both factors for being unfit for cisplatin and being in Bajorin risk group 2 and even more often when patients were treated with M-CAVI. Because there were more SATs in the M-CAVI arm, these results make GC the preferred treatment and reference regimen for patients ineligible for cisplatin therapy. This is in line with the experience for patients who are eligible for cisplatin therapy in whom GC was found to be less toxic than methotrexate/vinblastine/doxorubicin/cisplatin (MVAC).23
However, in view of the results of several single-arm phase II studies,15,24 it remains uncertain to what extent carboplatin adds to the effect of gemcitabine monotherapy. Only a randomized phase III study will be able to answer this question.
Platinum-free chemotherapy has, so far, not been particularly promising in the first-line setting of patients with UC. In a recent study by Calabro et al,25 the combination of gemcitabine/paclitaxel in the first-line setting for advanced disease in patients with mostly PS 0 to 1, a median GFR of 62 mL/min, and a 15% rate of liver metastases showed a response rate of 37% and a median survival of 13.5 months. These results are rather disappointing in the context of a single-arm phase II trial. The non-nephrotoxic combination chemotherapy oxaliplatin/gemcitabine,26,27 has been studied in fit as well as in unfit patients. In both settings, this combination was well tolerated but only modestly effective, and it needs to be compared with platinum-based standard chemotherapy in randomized controlled trials.
The definition of being unfit for cisplatin has been a matter of controversy. In our study, the definition for being ineligible for cisplatin included the factors PS 2 and/or impaired renal function (GFR > 30 but < 60 mL/min). Patients with comorbidities such as congestive heart failure, cerebrovascular disease, or severe hearing impairment are usually precluded from treatment with cisplatin. There is consensus that the use of cisplatin is contraindicated in patients with impaired renal function. However, there is still dissent about the absolute figures—whether cisplatin is safe in patients with a GFR as low as 50 mL/min or even less if given in split dose and which method to use for determining the creatinine clearance. According to the manufacturer, drugs like cisplatin that are primarily excreted through the kidney, need to be reduced in dose when the estimated GFR falls below 60 mL/min.28
In the International Society of Geriatric Oncology (SIOG) recommendations for dose adjustment in elderly patients with cancer who have renal insufficiency,29 cisplatin is not recommended if the estimated GFR is less than 60 mL/min. In view of this, including a GFR of less than 60 mL/min in the definition for patients being unfit for cisplatin seems to be appropriate. Recent publications indicate that in patients older than age 70 years, calculated creatinine clearance tends to underestimate the GFR. Creatinine clearance measurement by 24-hour urine collection seems to be more appropriate.30
The true reason for the short duration of OS and PFS in our study compared with that in patients treated with cisplatin-based chemotherapy remains a matter of speculation. It might be due to patient selection (unfit) or the use of carboplatin instead of cisplatin. The question of whether carboplatin is as effective as cisplatin combination chemotherapy in patients eligible for cisplatin has, so far, not been answered sufficiently,8,31–33 but there is the general belief, supported by limited data, that it probably is not. Patients treated with cisplatin-based chemotherapy in randomized trials had a nearly 50% longer median survival than those in our trial. Moreover, patients receiving cisplatin have a small but realistic chance of long-term survival.34,35 At a median follow-up of 4.5 years, nine patients receiving GC and 11 patients receiving M-CAVI were still alive. These few long-term survivors (8.4%) were observed among patients with only one reason for being unfit for cisplatin and in those with 0 or 1 Bajorin risk factors.
The post hoc analysis of OS by Bajorin risk groups showed that as the number of Bajorin risk factors increased, OS significantly decreased. Our data thus suggest that the Bajorin risk groups are also valid in this population of patients ineligible for cisplatin therapy. Fit patients with no Bajorin risk factors have been found to have a median OS of 33.0 months when treated with MVAC.22 In this subgroup in our trial, the median survival was only 12.0 months for both carboplatin-based regimens. The small number of patients in each risk group ruled out a definitive treatment comparison within these subgroups.
Concerning the reason for being unfit for cisplatin, the difference between the three OS curves was statistically significant, with patients who had only one reason for being unfit appearing to have a better OS than patients who had both reasons (GFR < 60 and WHO PS 2).
The questions of whether renal dysfunction is an adverse prognostic factor by itself and whether the inability to administer cisplatin has an adverse impact on the outcome have not been explored systematically thus far and are, indeed, matters of debate.36 The subgroup of patients with no Bajorin risk factors had the longest OS, suggesting that renal insufficiency probably has the least adverse impact on outcome compared with a lowered PS and/or the presence of visceral metastases. Conversely, patients with two Bajorin risk factors had the lowest response rate.
Because these are post hoc findings, they are only hypothesis generating, and further investigation in prospective study cohorts is still needed and should be addressed in future trials. A formal prognostic factor analysis of these current data will be the subject of a future report.
In the phase III part of this trial, several of the phase II findings were confirmed. Patients with two reasons for being ineligible for cisplatin therapy and patients in Bajorin risk group 2 derived little, if any, benefit from combination chemotherapy with a low response, a high rate of SATs, and low OS (Table 4). This new knowledge about ineligible patients and the respective subgroups should guide future trial design. Ineligible patients should no longer be studied as a uniform group.
The median age in this study was 10 years older compared with that in cisplatin-based chemotherapy trials. As previously discussed,19 comprehensive geriatric assessment tools have been recommended by several societies and might be integrated into study designs to better select elderly patients with bladder cancer (those older than age 70 years) for trials and different schedules of treatment.37–39
In conclusion, this is the first randomized phase II/III trial in patients ineligible for cisplatin therapy. There were no significant differences between the GC and M-CAVI arms in OS or for the secondary end points of response and PFS. Both regimens were active. However, SAT was higher in patients treated with M-CAVI, which makes GC the preferred and reference treatment in patients ineligible for cisplatin. Further studies should be designed to find more effective treatment options in this patient population.
Supplementary Material
Acknowledgment
We thank G. Kaiser for temporary coordination of the study and J.J. Croles, J.B. Vermorken, P. Carpentier, I. Billiet, M. Nogue-Aliguer, J.H. Schornagel, W. Kirkels, Th.M. De Reijke, B. Mellado, A. Horwich, P. Harper, J. Graham, V. Serretta, A. Bono, I. Bodrogi, A. Sella, O. Koriakine, and the study coordinators and data managers for their contributions.
Footnotes
Author affiliations appear at the end of this article.
Supported by Grants No. 2U10 CA11488-28 through 5U10 CA011488-40 from the National Cancer Institute (Bethesda, MD), by the European Organisation for Research and Treatment of Cancer Charitable Trust and by Eli Lilly (study code B9E-MC-S018).
Presented in part at the 46th Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, June 4-8, 2010 (preliminary results of phase III); presented at the ASCO Genitourinary Cancers Symposium, San Francisco, CA, February 14-16, 2008, and at the 44th Annual Meeting of ASCO, Chicago, IL, May 30-June 3, 2008 (results of phase II).
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00111787.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Joaquim Bellmunt, Eli Lilly (C); Ronald de Wit, Eli Lilly (C) Stock Ownership: None Honoraria: Maria De Santis, Eli Lilly; Joaquim Bellmunt, Eli Lilly; Iwona Skoneczna, Eli Lilly; Ronald de Wit, Eli Lilly Research Funding: Iwona Skoneczna, Eli Lilly Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Maria De Santis, Joaquim Bellmunt, Ronald de Wit, Richard Sylvester
Administrative support: Richard Sylvester
Provision of study materials or patients: Maria De Santis, Joaquim Bellmunt, Graham Mead, J. Martijn Kerst, Michael Leahy, Pablo Maroto, Thierry Gil, Gedske Daugaard, Iwona Skoneczna, Ronald de Wit
Collection and assembly of data: Maria De Santis, Sandrine Marreaud, Sandra Collette, Richard Sylvester
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–513. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
- 2.Balducci L. Evidence-based management of cancer in the elderly. Cancer Control. 2000;7:368–376. doi: 10.1177/107327480000700412. [DOI] [PubMed] [Google Scholar]
- 3.Balducci L, Extermann M. Management of cancer in the older person: A practical approach. Oncologist. 2000;5:224–237. doi: 10.1634/theoncologist.5-3-224. [DOI] [PubMed] [Google Scholar]
- 4.Balducci L, Yates J. General guidelines for the management of older patients with cancer. Oncology (Williston Park) 2000;14:221–227. [PubMed] [Google Scholar]
- 5.De Santis M, Bachner M. New developments in first- and second-line chemotherapy for transitional cell, squamous cell and adenocarcinoma of the bladder. Curr Opin Urol. 2007;17:363–368. doi: 10.1097/MOU.0b013e3282c4b0cb. [DOI] [PubMed] [Google Scholar]
- 6.de Wit R European Organization for Research and Treatment. Overview of bladder cancer trials in the European Organization for Research and Treatment. Cancer. 2003;97:2120–2126. doi: 10.1002/cncr.11288. [DOI] [PubMed] [Google Scholar]
- 7.Bellmunt J, Albanell J, Gallego OS, et al. Carboplatin, methotrexate, and vinblastine in patients with bladder cancer who were ineligible for cisplatin-based chemotherapy. Cancer. 1992;70:1974–1979. doi: 10.1002/1097-0142(19921001)70:7<1974::aid-cncr2820700727>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Bellmunt J, Ribas A, Eres N, et al. Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer. 1997;80:1966–1972. doi: 10.1002/(sici)1097-0142(19971115)80:10<1966::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 9.de Wit R, Tesselaar M, Kok TC, et al. Randomised phase II trial of carboplatin and iproplatin in advanced urothelial cancer. Eur J Cancer. 1991;27:1383–1385. doi: 10.1016/0277-5379(91)90015-6. [DOI] [PubMed] [Google Scholar]
- 10.Mottet-Auselo N, Bons-Rosset F, Costa P, et al. Carboplatin and urothelial tumors. Oncology. 1993;50(suppl 2):28–36. doi: 10.1159/000227258. [DOI] [PubMed] [Google Scholar]
- 11.Klocker J, Pont J, Schumer J, et al. Carboplatin, methotrexate and vinblastin (Carbo-MV) for advanced urothelial cancer: A phase II trial. Am J Clin Oncol. 1991;14:328–330. doi: 10.1097/00000421-199108000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Small EJ, Fippin LJ, Ernest ML, et al. A carboplatin-based regimen for the treatment of patients with advanced transitional cell carcinoma of the urothelium. Cancer. 1996;78:1775–1780. doi: 10.1002/(sici)1097-0142(19961015)78:8<1775::aid-cncr18>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Lorusso V, Pollera CF, Antimi M, et al. A phase II study of gemcitabine in patients with transitional cell carcinoma of the urinary tract previously treated with platinum: Italian Co-operative Group on Bladder Cancer. Eur J Cancer. 1998;34:1208–1212. doi: 10.1016/s0959-8049(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 14.Pollera CF, Ceribelli A, Crecco M, et al. Weekly gemcitabine in advanced bladder cancer: A preliminary report from a phase I study. Ann Oncol. 1994;5:182–184. doi: 10.1093/oxfordjournals.annonc.a058775. [DOI] [PubMed] [Google Scholar]
- 15.Stadler WM, Kuzel T, Roth B, et al. Phase II study of single-agent gemcitabine in previously untreated patients with metastatic urothelial cancer. J Clin Oncol. 1997;15:3394–3398. doi: 10.1200/JCO.1997.15.11.3394. [DOI] [PubMed] [Google Scholar]
- 16.Moore MJ, Tannock IF, Ernst DS, et al. Gemcitabine: A promising new agent in the treatment of advanced urothelial cancer. J Clin Oncol. 1997;15:3441–3445. doi: 10.1200/JCO.1997.15.12.3441. [DOI] [PubMed] [Google Scholar]
- 17.Albers P, Siener R, Härtlein M, et al. Gemcitabine monotherapy as second-line treatment in cisplatin-refractory transitional cell carcinoma: Prognostic factors for response and improvement of quality of life. Onkologie. 2002;25:47–52. doi: 10.1159/000055202. [DOI] [PubMed] [Google Scholar]
- 18.von der Maase H. Gemcitabine in transitional cell carcinoma of the urothelium. Expert Rev Anticancer Ther. 2003;3:11–19. doi: 10.1586/14737140.3.1.11. [DOI] [PubMed] [Google Scholar]
- 19.De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer “unfit” for cisplatin-based chemotherapy: Phase II—Results of EORTC study 30986. J Clin Oncol. 2009;27:5634–5639. doi: 10.1200/JCO.2008.21.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–3181. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 23.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 24.Castagneto B, Zai S, Marenco D, et al. Single-agent gemcitabine in previously untreated elderly patients with advanced bladder carcinoma: Response to treatment and correlation with the comprehensive geriatric assessment. Oncology. 2004;67:27–32. doi: 10.1159/000080282. [DOI] [PubMed] [Google Scholar]
- 25.Calabrò F, Lorusso V, Rosati G, et al. Gemcitabine and paclitaxel every 2 weeks in patients with previously untreated urothelial carcinoma. Cancer. 2009;115:2652–2659. doi: 10.1002/cncr.24313. [DOI] [PubMed] [Google Scholar]
- 26.Mir O, Alexandre J, Ropert S, et al. Combination of gemcitabine and oxaliplatin in urothelial cancer patients with severe renal or cardiac comorbidities. Anticancer Drugs. 2005;16:1017–1021. doi: 10.1097/01.cad.0000176503.48433.74. [DOI] [PubMed] [Google Scholar]
- 27.Theodore C, Bidault F, Bouvet-Forteau N, et al. A phase II monocentric study of oxaliplatin in combination with gemcitabine (GEMOX) in patients with advanced/metastatic transitional cell carcinoma (TCC) of the urothelial tract. Ann Oncol. 2006;17:990–994. doi: 10.1093/annonc/mdl057. [DOI] [PubMed] [Google Scholar]
- 28.Cancer Care Ontario (CCO) Cisplatin User File, 2010.
- 29.Lichtman SM, Wildiers H, Launay-Vacher V, et al. International Society of Geriatric Oncology (SIOG) recommendations for the adjustment of dosing in elderly cancer patients with renal insufficiency. Eur J Cancer. 2007;43:14–34. doi: 10.1016/j.ejca.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Raj GV, Iasonos A, Herr H, et al. Formulas calculating creatinine clearance are inadequate for determining eligibility for Cisplatin-based chemotherapy in bladder cancer. J Clin Oncol. 2006;24:3095–3100. doi: 10.1200/JCO.2005.04.3091. [DOI] [PubMed] [Google Scholar]
- 31.Dreicer R, Manola J, Roth BJ, et al. Phase III trial of methotrexate, vinblastine, doxorubicin, and cisplatin versus carboplatin and paclitaxel in patients with advanced carcinoma of the urothelium. Cancer. 2004;100:1639–1645. doi: 10.1002/cncr.20123. [DOI] [PubMed] [Google Scholar]
- 32.Dogliotti L, Cartenì G, Siena S, et al. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: Results of a randomized phase 2 trial. Eur Urol. 2007;52:134–141. doi: 10.1016/j.eururo.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 33.Petrioli R, Frediani B, Manganelli A, et al. Comparison between a cisplatin-containing regimen and a carboplatin-containing regimen for recurrent or metastatic bladder cancer patients: A randomized phase II study. Cancer. 1996;77:344–351. doi: 10.1002/(SICI)1097-0142(19960115)77:2<344::AID-CNCR18>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 34.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 35.Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006;42:50–54. doi: 10.1016/j.ejca.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 36.De Santis M, Sylvester R, Bellmunt J. Reply to G. Sonpavde et al. J Clin Oncol. 2010;28:e443–e444. [Google Scholar]
- 37.Wedding U, Ködding D, Pientka L, et al. Physicians' judgement and comprehensive geriatric assessment (CGA) select different patients as fit for chemotherapy. Crit Rev Oncol Hematol. 2007;64:1–9. doi: 10.1016/j.critrevonc.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Roehrig B, Hoeffken K, Pientka L, et al. How many and which items of activities of daily living (ADL) and instrumental activities of daily living (IADL) are necessary for screening. Crit Rev Oncol Hematol. 2007;62:164–171. doi: 10.1016/j.critrevonc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–252. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.