Abstract
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), a member of the TNF superfamily, induces tumor cell death via death receptors on target cells, without adverse effects on most normal cells. Its receptors are therefore an attractive target for antibody-mediated tumor therapy. Here, we report the creation of a lentivirus vector constructed by linking the heavy chain and the light chain of the antibody with a 2A/furin self-processing peptide in a single open reading frame that expresses a novel chimeric antibody (named as zaptuximab) with tumoricidal activity, which is consisted of the variable region of a mouse anti-human DR5 monoclonal antibody, AD5-10, and the constant region of human immunoglobulin G1. Lentivirus-expressed zaptuximab bound specifically to its antigen, DR5, and exhibited significant apoptosis-inducing activity in various tumor cell lines. The packaged recombinant virus lenti-HF2AL showed strong apoptosis-inducing activity in vitro. Meanwhile, inoculated subcutaneous human colon HCT116 tumor formation in nude mice were inhibited significantly. Moreover, there was a synergistic effect of mitomycin C (MMC) on the observed tumoricidal efficacy, prolonging the life span of nude mice with orthotopic human lung tumor cancers. These data suggest that lentivirus-mediated, 2A peptide-based anti-DR5 chimeric antibody expression may have clinical utility as an anticancer treatment and may represent a rational adjuvant therapy in combination with chemotherapy.
Introduction
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) has been of particular interest with respect to the development of cancer therapies, as it preferentially induces apoptosis of tumor cells, but has little or no effect on normal cells.1–3 TRAIL receptor 2 also known as death receptor 5 (DR5), has emerged as a favorable target for designing anticancer agents in recent years. DR5 can be specifically activated by TRAIL, which also activates TRAIL receptor 1 also known as death receptor 4, to transduce an apoptotic signal. Use of an agonistic monoclonal antibody against DR5 instead of TRAIL could be advantageous because TRAIL targets multiple receptors, including both the death receptors and three decoy receptors (DcR1, DcR2, and osteoprotegerin). Several DR5-specific antibodies are currently investigated in phase I/II clinical trials.4,5 AD5-10 is a mouse monoclonal antibody against the human DR5 extracellular region that was previously reported to induce apoptosis in vitro in various tumor cells. It shows strong tumor inhibition activity in vivo but is not toxic to mouse liver, spleen or kidney, suggesting that AD5-10 is a promising agonistic antibody for cancer therapy.6,7
Antibodies have emerged as an important new class of drugs for therapeutic uses. However, the high cost of antibody therapy had limited its application. Many factors contribute to the high cost of antibody therapeutics, including the great expense of drug development, the high cost of manufacturing these drugs, and the large total dosages that are often required.8 Moreover, chronic diseases, such as cancer, frequently require high dosages of a therapeutic antibody over a long period of time. An alternative approach is to produce such antibodies in vivo, which can provide high concentrations of pure antibodies over a long period of time.
To this end, we previously performed a study addressing antibody gene therapy using adeno-associated virus (AAV)-mediated expression of the single-chain Fv fragment (scFv) of AD5-10; we showed that viral transduction using the AAV-scFv construct triggered significant amounts of apoptosis in various tumor cells and prevented tumor growth in the investigated nude mouse xenograft cancer model.9 However, the apoptotic effect of AAV-mediated scFv expression on tumor cells in vivo and in vitro could only be described as moderate. A possible explanation for this might be the relatively low affinity and short half-life of the smaller scFv fragment compared with the parental antibody. To improve the efficiency of AD5-10-based gene therapy, in the present study we developed a lentivirus vector that expresses a full-length mouse–human chimeric antibody against DR5 (named as zaptuximab) by linking the heavy chain and the light chain with 2A/furin self-processing peptide in a single open reading frame. Our data suggest that lentivirus-mediated, 2A peptide-based zaptuximab expression may have clinical utility as an anticancer treatment and may represent a rational adjuvant therapy in combination with chemotherapy.
Results
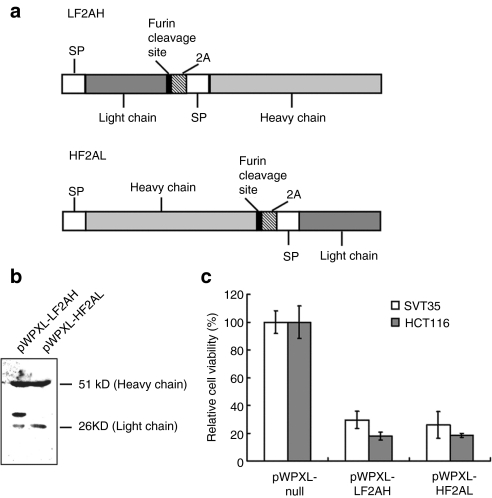
pWPXL-HF2AL-expressed zaptuximab exhibited a better balance of the light and heavy chains compared with pWPXL-LF2AH
A novel mouse–human AD5-10 chimeric antibody gene formed by linkage of the variable region of a mouse monoclonal antibody, AD5-10, and the constant region of human immunoglobulin G1 was cloned (Figure 1a). The heavy chain and light chain of the chimeric antibody were linked together using the foot and mouth disease viral 2A self-cleavage sequence (APVKQTLNFDLLKLAGDVESNPG)10 in a single open reading frame. To eliminate 2A residues, the expression cassettes were engineered such that a furin cleavage site sequence (Arg-Lys/Arg-Arg, RK/RR) was included between the 2A sequence and the chimeric antibody heavy chain or light chain, which were designated HF2AL and LF2AH, respectively. Then HF2AL and LF2AH were cloned into the lentiviral vector pWPXL; the resulting expression vectors were designated pWPXL-HF2AL and pWPXL-LF2AH, respectively. To compare the 2A self-cleavage activity and the removal of the residual 2A amino acids by furin, conditioned media in which HEK 293T cells transfected with pWPXL-HF2AL or pWPXL-LF2AH were collected and subjected to western blot analysis using an anti-human immunoglobulin G antibody. As shown in Figure 1b, the zaptuximab heavy chain and light chain expressed using the pWPXL-HF2AL plasmid exhibited similar molecular weights to the native antibody, suggesting that successful cleavage at the furin cleavage site and the 2A self-cleavage site was accomplished by pWPXL-HF2AL. However, there was an extra band with a slightly higher molecular weight than the light chain in the medium of the HEK 293T cells transfected with pWPXL-LF2AH, suggesting that there was incomplete cleavage of the 2A self-cleavage site, the furin cleavage site or even the signal peptide cleavage site by pWPXL-LF2AH.
Figure 1.
pWPXL-HF2AL-expressed zaptuximab exhibited a better light chain/heavy chain balance than did zaptuximab expressed from pWPXL-LF2AH. (a) Schematic illustration of the full-length chimeric antibody expression cassette using the furin/2A sequence. (b) Expression of zaptuximab in supernatants from pWPXL-HF2AL or pWPXL-LF2AH-transfected HEK 293 cells. The samples were separated by SDS-PAGE under reducing conditions and probed with a goat anti-human IgG (H+L) polyclonal antibody. The data presented are representative of three independent experiments. (c) Cytotoxicity of zaptuximab expressed by pWPXL-HF2AL or pWPXL-LF2AH in tumor cells. HCT116 and SVT35 Jurkat T cell lines were treated with medium from HEK 293 cells transiently transfected with pWPXL-HF2AL or pWPXL-LF2AH for 24 hours. Cell viability was determined by MTS assays (mean ± SD). Replicates were averaged, and the error bars represent the SEM of three independent experiments. H, heavy chain; L, light chain; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; SP, signal peptide.
To detect the tumoricidal activity of 2A peptide-based, lentivirus-expressed zaptuximab, equal volumes of conditional medium from HEK 293T cells transiently transfected with pWPXL-HF2AL or pWPXL-LF2AH were added to cultures of human colon cancer HCT116 or T lymphoma SVT35 cells, and the cytotoxicity was analyzed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). As shown in Figure 1c, both pWPXL-HF2AL- and pWPXL-LF2AH-expressed zaptuximab showed strong cytotoxicity in the cancer cells tested. Because pWPXL-HF2AL-expressed zaptuximab exhibited a better balance of the light chain and heavy chain than did pWPXL-LF2AH, we chose the pWPXL-HF2AL expression vector for the remaining experiments in this study.
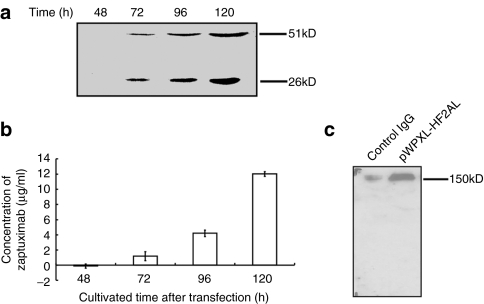
Expression of zaptuximab-mediated by the 2A peptide-based lentivirus vector
To determine the level of zaptuximab expression in lenti-HF2AL particle-infected HEK 293T cells, conditional media from the cells were harvested at 48, 72, 96, and 120 hours postviral infection. The heavy chain and light chain were readily detectable in the medium of cells infected for 72 hours or longer, and the expression level increased in a time-dependent manner (Figure 2a). The concentration of secreted zaptuximab reached levels of 1.18 ± 0.58 µg/ml, 4.19 ± 0.41 µg/ml, and 12.02 ± 0.30 µg/ml at 72, 96, and 120 hours, respectively (Figure 2b). Furthermore, under nonreducing conditions, only a single band, with an apparent molecular weight corresponding to that of the dimerized full-length antibody possessing two heavy and two light chains, were detected in the culture supernatant from the lenti-HF2AL-infected HEK 293T cells (Figure 2c). No additional band was detected under nonreducing conditions, again suggesting that the amounts of the heavy and light chains of zaptuximab produced in the cells were in balance, and neither the heavy nor the light chain was in excess.
Figure 2.
Expression of zaptuximab-mediated by the 2A peptide-based lentivirus vector in vitro. HEK 293 cells were transiently infected with lenti-HF2AL, and the supernatants were harvested for protein analyses. (a) Western blot analysis of zaptuximab expression in the supernatants at the indicated time point after lenti-HF2AL infection. The data presented are representative of three independent experiments. (b) Enzyme-linked immunosorbent assay analysis of secreted zaptuximab at indicated time points after lenti-HF2AL-infection, (mean ± SD). Replicates were averaged, and the error bars represent the SEM of three independent experiments. (c) Western blot analysis of zaptuximab-containing supernatants under nonreducing conditions. Proteins in the supernatants from the HEK 293 cells transfected with the pWPXL-HF2AL were separated by SDS-PAGE under nonreducing conditions and probed with a goat anti-human IgG (H+L) polyclonal antibody. Human immunoglobulin G was used as the positive control. The data presented are representative of three independent experiments. H, heavy chain; L, light chain; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
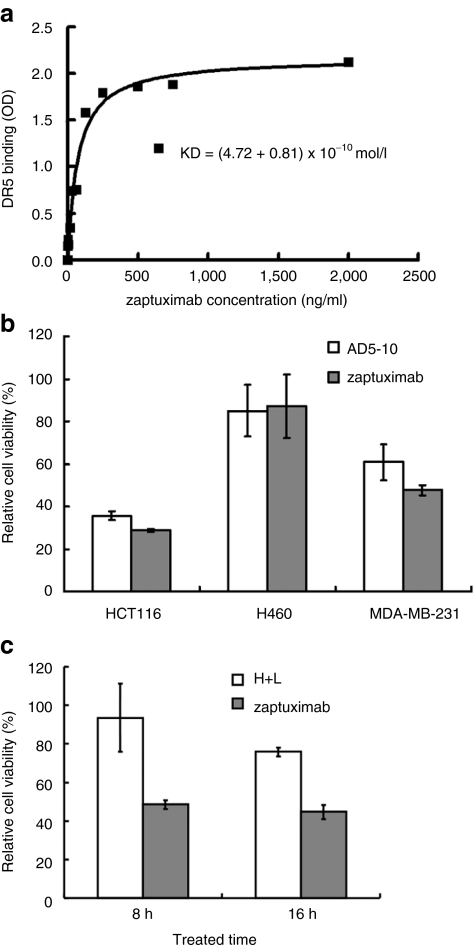
2A peptide-based lentivirus-expressed zaptuximab showed a similar binding affinity and cytotoxicity to the parental antibody
Next, we tested whether virally expressed zaptuximab in the medium of infected cells maintained its affinity to its antigen, DR5. DR5 protein immobilized in a 96-well plate was incubated with medium from lenti-HF2AL-infected HEK 293T cells. The binding affinity between zaptuximab and DR5 was measured by enzyme-linked immunosorbent assay (ELISA). It was shown that zaptuximab efficiently bound to its antigen, DR5 (kD = 4.72 ± 0.81 × 10-10 mol/l) (Figure 3a). The binding affinity of zaptuximab was slightly lower than that of its parental antibody, AD5-10, but it was much higher than that of the AAV-expressed scFv fragment (kD = 4 × 10-9 mol/l)9 or the chimeric antibody (termed H+L) expressed from two separate vectors (kD = 7–9 × 10-10 mol/l). We further compared the cytotoxicity of virally expressed zaptuximab with that of AD5-10 and the chimeric antibody (H+L) expressed from two separate vectors. As shown in Figure 3b,c, zaptuximab showed similar cytotoxicity to AD5-10, but higher cytotoxicity than H+L.
Figure 3.
2A peptide-based, lentivirus vector-expressed zaptuximab showed a binding affinity and cytotoxicity similar to that of its parental antibody. (a) Binding affinity of zaptuximab to DR5. Recombinant human DR5 protein was immobilized in a 96-well plate. The plate was then incubated with conditional medium from pWPXL-HF2AL-transfected HEK 293 cells. The binding affinity was measured with enzyme-linked immunosorbent assay using an anti-human immunoglobulin G Fc-HRP antibody. The presented data are representative of three independent experiments. (b) Comparison of cytotoxicity between zaptuximab and AD5-10. The tumor cell lines H460, HCT116, and MDA-MB-231 were plated in a 96-well plate at a density of 104/well and treated with 1 µg/ml of zaptuximab in conditional medium from pWPXL-HF2AL-transfected HEK 293 cells and AD5-10 for 24 hours. Then cell viability was determined using an MTS assay. Replicates were averaged, and the error bars represent the SEM of three independent experiments. (c) Differences in the cytotoxic activity of zaptuximab and the H+L chimeric antibody were detected in HCT116 cells at the indicated treatment time point. Replicates were averaged, and the error bars represent the SEM of three independent experiments. HRP, horse radish peroxidase; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium.
These data demonstrated that 2A peptide-based, lentivirus-expressed zaptuximab from a single open reading frame showed similar binding affinity and cytotoxicity to its parental antibody, AD5-10.
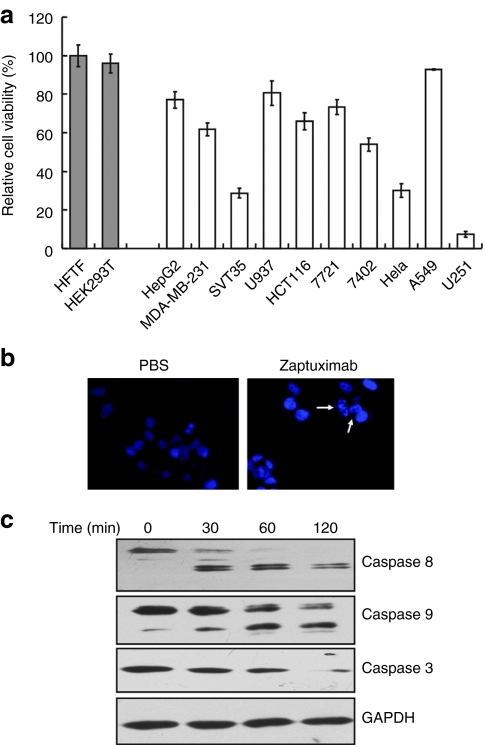
Lenti-HF2AL infection results in tumor cell death by apoptosis in vitro
The cytotoxic activity of lenti-HF2AL was tested in several tumor cell lines. Some cells would be infected and secrete antibodies to mediate cytotoxicity in cells that were not infected. The cytotoxic activity of the supernatant from infected HEK 293 cells was tested in several tumor cell lines including SMMC7721, HCT116, A549, and U251 in our former study.11
The direct cytotoxic activity of lenti-HF2AL was tested in several tumor cell lines in this study. Each cell line was infected with equal numbers of viral particles multiplicity of infection ((MOI) = 25) for 72 hours. As shown in Figure 4a, the relative viabilities of SMMC7721, BEL7402, HepG2, HCT116, MDA-MB-231, U937, SVT35, A549, and U251 cells after 72 hours of transduction were 73.32 ± 3.82%, 54.02 ± 3.45%, 77.03 ± 4.27%, 65.85 ± 4.51%, 61.72 ± 3.3%, 80.56 ± 6.39%, 28.8 ± 2.45%, 92.73 ± 0.22%, and 7.36 ± 1.55%, respectively; there was no cytotoxicity observed in normal human Tenon's fibroblast HFTF and embryonic kidney HEK 293T cells. Additional examination using Hoechst 33258 staining and microscopic analysis revealed that an increased number of condensed nuclei, which are characteristic of apoptosis, were present in the HCT116 cells treated with conditional media from lenti-HF2AL infected HEK 293 cells (Figure 4b). To further evaluate apoptosis resulting from the lenti-HF2AL treatment, HCT116 cells treated with conditional media from lenti-HF2AL infected HEK 293 cells were subjected to western blot analysis to detect the activation of caspases, which are hallmarks of apoptotic cell death. As shown in Figure 4c, procaspase-8, -9, and -3 were cleaved in a time-dependent manner, indicating that zaptuximab treatment could efficiently lead to tumor cell death by apoptosis.
Figure 4.
Lenti-HF2AL infection results in tumor cell death by apoptosis in vitro. (a) Cytotoxicity of lenti-HF2AL in various tumor cell lines, including SMMC7721, BEL7402, HepG2, HCT116, MDA-MB-231, U937, SVT35, A549, and U251 and two normal cell lines, HFTF and HEK 293. Tumor cells or normal cells were infected with lenti-HF2AL at a MOI of 25 for 24 hours, and the media were then replaced with fresh media, and cell viability was determined by an MTS assay 48 hours later. Replicates were averaged, and the error bars represent the SEM of three independent experiments. (b) HCT116 cells infected with the indicated recombinant vectors (MOI = 25) were stained with Hoechst and examined under fluorescent microscopy. Arrows indicate typical apoptotic cells exhibiting nuclear condensation. (c) Caspase-8, -9, and -3 activation in HCT116 cells treated with conditional medium from lenti-HF2AL-infected HEK 293 cells at the indicated time point was detected by western blot analysis using corresponding antibodies. GAPDH was used as the loading control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MOI, multiplicity of infection; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; PBS, phosphate-buffered saline.
The cytotoxic activity of lenti-HF2AL had been measured against infected cells, however the principle of the approach was that some cells would be infected and secrete antibodies to mediate cytotoxicity in cells that were not infected. The cytotoxic activity of the supernatant from infected HEK 293 cells was tested in several tumor cell lines including SMMC7721, HCT116, A549, and U251.11
Tumor-suppressing activity of lenti-HF2AL in vivo
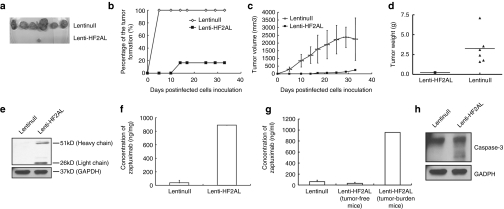
We further investigated the effects of lenti-HF2AL infection on tumor formation and growth in nude mice. HCT116 cells incubated with lenti-HF2AL/null (MOI = 25) for 4 hours were inoculated subcutaneously into the right dorsal flanks of nude mice, and tumor formation was monitored. Tumors became palpable in all six mice in the control group at 5 days, but only one mouse in the lenti-HF2AL infected group presented a visible tumor by day 14 (Figure 5a). This significant difference in the tumor incidence rate remained to the end of the experiment (32 days) (Figure 5b). Moreover, tumor growth was inhibited by lenti-HF2AL infection (Figure 5c). Thirty-two days after the mice were inoculated with the cells, the tumor volume and tumor weight in the control group were 2255.02 ± 1379.64 mm3 and 3.26 ± 2.03 g, respectively, compared with 249.40 mm3 and 0.24 g in the lenti-H2AL treated group (Figure 5c,d), which indicated that lenti-HF2AL infection notably suppressed tumor formation.
Figure 5.
Tumor-suppressing activity of lenti-HF2AL in vivo. HCT116 cells (5 × 105) infected with either lenti-HF2AL or lenti-null (MOI = 25) were injected into subcutaneous tissue of the right dorsal flank of 6-week-old female nude mice (n = 6). On day 32, the mice were killed, and serum and tumor tissues were obtained and analyzed. (a) Tumor formation in the mice at day 32 (b) or throughout the whole time course of the experiment is shown. (c) Tumor size and (d) tumor weight were decreased by lenti-HF2AL infection. (e) Expression of zaptuximab in the tumor tissues was detected by western blot analysis and (f) enzyme-linked immunosorbent assay (ELISA). (g) Quantitative expression of zaptuximab in the sera samples were detected by ELISA. (h) Activation of caspase-3 was detected in the tumor tissue. A total of 100 µg of protein from the lysates prepared from tumor tissues of the above mice was separated by SDS-PAGE. Activation of caspase-3 was examined using immunoblotting with the corresponding antibodies. GAPDH was used as the loading control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MOI, multiplicity of infection; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
ELISA and western blot assays showed that the expression level of the chimeric antibody in tumor tissue isolated from the only mouse with a tumor in the lenti-H2AL-infected group had reached 890 ng/mg, 32 days after virus infection (Figure 5e,f). Moreover, the concentration of zaptuximab reached 1 µg/ml in the serum of tumor-bearing mouse in the lenti-HF2AL-infected group, and there was almost no expression of the chimeric antibody detected in the blood of tumor-free animals in this group (Figure 5g). In addition, we observed activation of the apoptosis-related protease caspase-3 in the tumor cells using immunoblotting, which suggests that lenti-HF2AL infection induced apoptosis through activation of the caspase signaling pathway cascade (Figure 5h). These data demonstrated that lenti-HF2AL infection resulted in significant tumor inhibition in vivo.
Synergistic cytotoxicity of zaptuximab and mitomycin in zaptuximab-resistant tumor cells
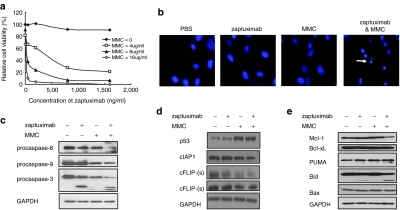
As shown in Figure 4a, several cancer cell lines were somewhat resistant to zaptuximab, including A549 and U937 etc. Chemotherapy agents can act synergistically with TRAIL or anti-TRAIL receptor antibodies. When A549 cells were treated with a combination of zaptuximab and a subtoxic dose of mitomycin C (MMC), significant synergistic cytotoxicity was observed (Figure 6a). Hoechst 33258 staining provided additional evidence of the synergistic apoptosis-inducing activity of zaptuximab and MMC (Figure 6b). To determine whether the apoptosis induced by the combination of MMC and zaptuximab was mediated by caspase activation, we performed western blot analysis for specific caspases. Activation of caspase-3, -8, and -9 was easily identified in A549 cells exposed to MMC and zaptuximab in combination, as demonstrated by decreased expression of the preforms of these caspases (Figure 6c). We further analyzed the levels of intracellular proteins that may regulate the zaptuximab death pathway. As shown in Figure 6d, c-FLIP, which inhibits caspase-8 activity, and c-IAP1, which is an inhibitor of caspase-3, caspase-7, and caspase-9, was downregulated in MMC-treated A549 cells. Meanwhile, basal p53 expression was enhanced after MMC treatment. Moreover, expression of Bcl-2 family members, which are key players in the mitochondrial apoptosis pathway, was detected. As shown in Figure 6e, the proapoptotic protein PUMA was upregulated after MMC treatment, and the antiapoptotic protein Mcl-1 was downregulated in the combination group. Importantly, Bid, a Bcl-2 family member that links the extrinsic and intrinsic apoptosis pathways via the death receptor-activated caspase-8 cleavage of Bid to truncated Bid, was cleaved in the zaptuximab alone treatment group and further cleaved after combined treatment with MMC. These findings suggest that the observed MMC-mediated sensitization to zaptuximab-mediated apoptotic signaling may be due in part to enhanced expression of p53 and decreased expression of the antiapoptotic proteins, c-FLIP(L), c-FLIP(s), and c-IAP1. Furthermore, the mitochondrial apoptosis pathway was activated during the MMC plus zaptuximab-induced apoptosis of A549 cells.
Figure 6.
Synergistic cytotoxicity of zaptuximab plus mitomycin in zaptuximab-resistant A549 cells (a) The relative viability of A549 cells treated with zaptuximab from conditional medium of lenti-HF2AL-infected HEK 293 cells in combination with MMC at the indicated doses for 24 hours was determined using MTT assay. (b) A549 cells were treated with buffer (control), zaptuximab (50 ng/ml) from conditional medium of lenti-HF2AL-infected HEK 293 cells, MMC (4 µg/ml), or the combination of zaptuximab and MMC for 8 hours and subjected to Hoechst staining. The arrows indicate typical apoptotic cells exhibiting nuclear condensation. (c) Western blotting analysis to determine the effects of zaptuximab (50 ng/ml), MMC (4 µg/ml), or the combination of both on caspase-8, -9, and -3 activation, (d) p53, c-IAP1, c-FLIP and (e) Bcl-2 family members. MMC, mitomycin C; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
MMC augments lenti-HF2AL tumoricidal activity in an orthotopic lung cancer mouse model
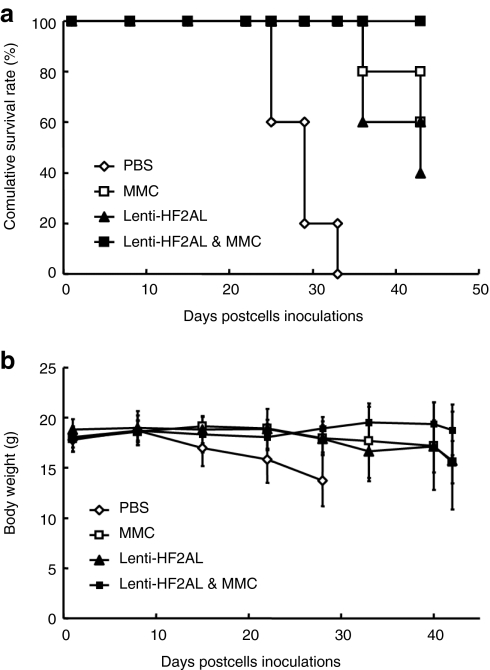
An orthotopic lung tumor model12 was established to evaluate the synergistic cytotoxicity of MMC and lenti-HF2AL infection in vivo. The orthotopic lung cancer mouse model showed initial mortality on day 28 in the phosphate-buffered saline (PBS) group and on day 35 in the MMC-treated or lenti-HF2AL alone-treated group, whereas no death had occurred in the combined treatment group at day 42 (Figure 7a, P = 0.0019 for the combination group versus the control groups). Moreover, the body weights of the mice in the PBS control group decreased by over 30%, from ~18 g at the beginning of the experiment to 13.73 ± 2.57 g at day 28. Treatment with MMC or lenti-HF2AL alone decreased the body weights of the mice to 15.57 ± 2.12 g and 15.75 ± 4.88 g at day 42, respectively. However, the body weight of the mice in the combined treatment group remained at 18.7 ± 0.55 g (Figure 7b) to the end of the experiment (P = 0.0002 versus PBS group, P = 0.00249 versus lenti-HF2AL alone group, P = 0.0219 versus MMC alone group). These data indicate that MMC significantly augments lenti-HF2AL tumoricidal activity in the orthotopic lung cancer model.
Figure 7.
MMC enhances lenti-HF2AL activity in the orthotopic lung tumor model. A549 cells (1 × 106) were injected into the lung parenchyma. Three days later, mice were randomized into four groups (n = 5) and treated as described in the materials and methods. (a) The introduction of lenti-HF2AL in combination with MMC prolonged the survival of mice with orthotopic A549 lung tumors. (b) Body weight decreased in the lenti-HF2AL and MMC combination group. MMC, mitomycin C.
Discussion
In the present study, we developed a novel therapeutic strategy involving the expression of an anti-DR5 full-length chimeric antibody, zaptuximab, through lentivirus-based, vector-mediated gene transfer. We showed that lentivirus-mediated zaptuximab expression significantly suppressed tumor cell growth both in vitro and in vivo.
AD5-10 is a monoclonal antibody against human DR5 that is promising for cancer therapy. However, in a clinical setting, mouse monoclonal antibodies can cause a human anti-mouse antibody response to occur. One approach for reducing the potential for human anti-mouse antibody induction is to generate a remodeled antibody that contains the antigen-binding variable region from an immunized animal grafted onto a human constant region. Zaptuximab is a mouse–human chimeric antibody engineered based on AD5-10. The affinity of virally expressed zaptuximab for DR5 was 0.472 ± 0.081 nmol/l, which is very close to the affinity of the parental antibody AD5-10 (0.3 nmol/l) but is significantly higher than the affinity of the scFv of AD5-10 (4 nmol/l) that we reported previously.9 Moreover, the cytotoxicity of virally expressed zaptuximab was similar to that of AD5-10, suggesting that the lentivirus-expressed chimeric antibody zaptuximab retains the properties of the parental antibody AD5-10.
Expressing antibodies in vivo is an approach that can provide a high concentration and pure antibodies over a long period of time and decrease the cost of engineered antibody development.13–15 For vector based antibody expression in vivo, a one-vector system expressing the heavy chain and light chain in one open reading frame has advantages over a dual-vector system. A one-vector system represents the best way of avoiding imbalances of heavy chain and light chain expression and ensures that the complete regulation system is delivered to transduced cells. In addition, it simplifies the manufacturing process. An internal ribosome entry site is one of the elements employed to construct bicistronic vectors for the coexpression of antibody heavy and light chains in a single vector. However, the use of an internal ribosome entry site is reported to lead to lower expression of the second gene than the first gene,16 which may cause concern about possible toxic effects due to imbalances of heavy chain and light chain expression, particularly with respect to toxicity from extra heavy chain molecules.17 Fang et al. developed a novel furin/2A sequence allowing monocistronic expression of a full-length antibody by AAV.10 We adapted the furin/2A technology in the present study and demonstrated that well-balanced coexpression of heavy and light chains could be achieved. We also constructed a dual-vector system expressing the heavy chain and light chain of the AD5-10 chimeric antibody separately (data not shown). As shown in the results, the antigen affinity and cytotoxicity of zaptuximab expressed in a one-vector system were higher than that observed for zaptuximab expressed in a dual-vector system. One possible explanation for this might be an imbalance of heavy and light chain expression in the dual-vector system, such that an excess of amount of the heavy or light chain caused inaccurate quantitation of the chimeric antibody. Moreover, equal volumes of conditional medium from HEK 293T cells transiently cotransfected with different ratios of heavy chain vector and light chain vector were added to cultures of HCT116 cells. As shown in Supplementary Figure S1, the larger the ratios of heavy chain vector and light chain vector cotransfected, the lower the cytotoxicity to HCT116 cells of the conditional medium from cotransfected cells. This result further demonstrated that balanced production is better than imbalanced expression of the heavy and light chain.
More interestingly, the sequences of the heavy and light chains in the construct used in the one-vector system based on the furin/2A technique influenced the resulting cleavage activity. There was an extra band with a slightly higher molecular weight than the light chain in the medium of the cells transfected with pWPXL-LF2AH, suggesting that there was incomplete cleavage of the 2A self-cleavage site, the furin cleavage site or even the signal peptide cleavage site by pWPXL-LF2AH. The molecular weight of extra band was about 31 kD, and predicted molecular weight of the signal peptide plus 2A peptide is 5.6 kD. So the incomplete cleavage of the signal peptide was able to account for the molecular weight change. However, we still supposed there were some other possible mechanism responsible for the extra band. The mechanism responsible for this phenomenon remains to be elucidated. The underlying mechanism may involve the amino acid sequence or particular amino acids of the light chain and requires further analysis in detail. Further study is now underway in our laboratory to clarify the mechanism thus to establish a universal vector can be used in various chimeric antibody construction.
Fang et al. reported that if the furin cleavage site RAKR used in the construction, the final antibody product would contain two additional amino acid residues derived from the furin cleavage sequence at the C-terminus of the antibody heavy chain that could affect the activity of the antibody. However, the mass spectrometry analysis showed that native antibodies, without additional amino acid residues remaining at the C-terminus of the heavy chain, were generated in >99% of mAb protein from expression cassettes that contained three different furin cleavage sites, RRRR, RKRR, and RRKR.18 Therefore, the furin cleavage site RKRR was used in our one-vector system to ensure the complete cleavage.
Lentiviral vectors (LVs) are derived from the human immunodeficiency virus (HIV-1) and have been extensively studied for use as a vehicle for gene transfer, especially in studies related to short hairpin RNA. There are distinct advantages of using LVs for gene therapy. LVs display wide host range tropism, and they offer integrative capabilities, resulting in long-term stable transgene expression.19–22 To our knowledge, most of the reported gene therapy strategies based on lentiviruses involve transduction of a target cell in vitro followed by delivery of the infected cells in vivo.23–26 The difficulty of virus production and the low stability27 of LVs might account for the rarity of reports of direct delivery of LVs in vivo, which requires a higher MOI. Moreover, we found that the yield of LVs decreased markedly as the inserted fragment length increased. Therefore, several steps are still needed to make lentiviral gene therapy useful at the clinical level, including improving the production of the lentiviral packaging and enhancing its stability.
As we previously reported, sensitivity toward anti-DR5 antibody induced cell death is not proportional to the level of DR5 expression on the cell surface. Cells may incorporate additional regulatory signaling pathways to control DR5 receptor functions in a cell type–specific manner. A549 cells are resistant to zaptuximab stimulation, despite exhibiting a high level of cell surface DR5 (data not shown). Some intracellular molecules might inhibit zaptuximab-induced cell death. It has been reported that the antiapoptotic molecule c-FLIP can interfere with the binding of caspase-8 to FADD28 and thus suppress apoptosis induced by the death ligand. The c-IAP1 protein is a member of the inhibitor of apoptosis protein family and is an endogenous caspase inhibitor.29 It was apparent from our findings that zaptuximab resistance was overcome by adjuvant treatment with MMC, which downregulated the expression of c-FLIP and c-IAP1 and upregulated the expression of p53. And the mitochondrial apoptosis pathway was activated during the MMC plus zaptuximab-induced apoptosis of A549 cells. Synergy between anticancer drugs and lenti-HF2AL may help to overcome most mechanisms related to zaptuximab resistance or decrease the effective dose of chemotherapy drugs required, thereby reducing the side-effects of chemotherapy.
In summary, the present study provided a lentivirus-mediated delivery strategy incorporating furin/2A technology and expression of zaptuximab, a novel full-length chimeric anti-DR5 antibody. We demonstrated that lenti-HF2AL infection of HCT116 cells resulted in significant tumor inhibition in vivo, and treatment with MMC plus lenti-HF2AL prolonged the survival of mice with orthotopic A549 lung tumors, suggesting that lentivirus-mediated zaptuximab expression gene therapy may provide a feasible and effective form of treatment for cancer and may represent a rational adjuvant therapy in combination with chemotherapy.
Materials and Methods
Cells and reagents. The human lung cancer cell lines A549 and H460, liver cancer cell line HepG2, colon cancer cell line HCT116, breast cancer cell line MDA-MB-231, cervical cancer cell line HeLa, promyelocytic leukemia cell line HL-60, leukemic monocyte lymphoma cell line U937, and HEK293T embryonic kidney cells were purchased from the American Type Culture Collection (Manassas, VA). The human liver cancer cell lines SMMC-7721 and BEL7402 were supplied by the Institute of Cell and Biochemistry, Chinese Academy of Sciences (Shanghai, China). The human Tenon's fibroblast (HFTF) cells were obtained from the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences (Beijing, China). The KB cells were a gift from Wuhan University (Wuhan, China). These cells were cultured at 37 °C in RPMI 1640, F12, or Dulbecco's modified Eagle medium (Life Technologies, Grand Island, NY). Anti-human immunoglobulin G (H+L) was purchased from KPL (Gaithersburg, MD), and the anti-human immunoglobulin Gκ chain antibody used in this study was from Sigma Aldrich (Taufkirchen, Germany).
Vector construction. The heavy chain and the light chain of the chimeric antibody were synthesized by PCR from the plasmids hmAD5-10H/PCI-neo and hmAD5-10L/PCI-neo, respectively, and linked together by the FMDV 2A self-cleavage sequence.10 To further eliminate remaining 2A residues, this expression cassette was engineered to include a furin cleavage site sequence located between the 2A sequence and the chimeric antibody heavy chain or light chain; these constructs were referred to as HF2AL and LF2AH, respectively. Next, HF2AL and LF2AH were cloned into the LV pWPXL and designated pWPXL-HF2AL and pWPXL-LF2AH, respectively.
Production of recombinant lentivirus particles. Eighty to ninety percent confluent HEK 293T cells were cotransfected with the expression plasmid pWPXL-HF2AL or pWPXL-LF2AH and the helper plasmids pSPAX2 and pMD2.G. Sixty hours later, the conditioned medium was harvested, concentrated at 2,000 rpm for 20 minutes and filtered through a 0.45 µm filter. Next, the concentrated supernatant was ultracentrifuged at 70,000g at 4 °C for 2 hours. The virus titer was quantified using the HIV-1 P24 ELISA kit (Zeptometrix, Buffalo, NY).
Quantification detection of chimeric antibody expression. The expression of zaptuximab in cells transfected with pWPXL-HF2AL plasmid or cells infected with the recombinant lentivirus lenti-HF2AL and in the sera of mice and tumor tissues injected with lenti-HF2AL was detected using a commercial ELISA assay kit for human immunoglobulin G (Bethyl Laboratories, Montgomery, TX) according to the manufacturer's instructions.
Analysis of DR5 binding by zaptuximab. To evaluate the binding activity of zaptuximab with respect to human DR5, 96-well plates were coated with 300 ng/well of recombinant His-tagged DR5 protein, blocked with 5% nonfat dry milk, and incubated with various concentrations of zaptuximab at room temperature. After incubation with an horse radish peroxidase-conjugated goat anti-human Fc antibody, binding was determined by adding peroxidase substrate. The absorbance at 490 nm was measured on a microplate reader. The data obtained were analyzed using GraphPad Prism software (GraphPad Software, San Diego, CA).
Infection of various cell lines with lenti-HF2AL/null. Various cell lines were cultured in complete medium before addition of the lentivirus. The cells were washed once with 1 × PBS, and the vectors were added at the indicated MOI to the culture medium. For assessment of the cytotoxicity of lenti-HF2AL in various tumor cell lines, cells were infected with lenti-HF2AL at a MOI of 25 for 24 hours. The media were then replaced with complete medium, and cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assays 48 hours later.
Subcutaneous tumor xenograft and assessment of tumor growth. Female BALB/C-nude mice aged 4–6 weeks were provided by Vital River Laboratories Animal Technology (Beijing, China) or the Institute of Laboratory Animal Sciences, Chinese Academy of Medical Sciences (Beijing, China) and used in accordance with the Institutional guidelines for animal care approved by the Committee on the use and care of animals, Chinese Academy of Medical Sciences. HCT116 cells infected with lenti-HF2AL or lenti-null for 4 hours (MOI = 25) were inoculated into subcutaneous tissue of the right dorsal flank of the female BALB/C nude mice (5 × 106 cells/animal). Tumor volume was measured three times/week with calipers and was calculated using the formula V = length × (width)2/2. The experiments were terminated when the tumors in the control group became ulcerated. After the animals were killed, their tumors were removed for subsequent analysis.
Orthotopic lung cancer model. The orthotopic lung cancer model was established as described previously.11 Briefly, following anesthetization, 20 µl aliquots of an A549 cell suspension (5 × 107 cells/ml) containing 1 mg/ml of matrigel (BD Biosciences, Bedford, MA) were incised into the left side of the chest (lung parenchyma) of 6-week-old female BALB/C nude mice. Three days later, the animals were randomized into four groups receiving intramuscular injection of 5 × 107 TU of either lenti-H2AL or lenti-null in addition to intravenous injection of MMC, 20 mg/kg or PBS 3 days later. Administration of MMC or PBS was carried out every week for 3 weeks.
Statistical analysis. Data were expressed as mean values ± SD. Student's t test was used to evaluate statistical significance. Body weight data were analyzed using two-way analysis of variance. Log rank tests were performed for survival data. P < 0.05 was considered significant.
SUPPLEMENTARY MATERIAL Figure S1. Comparison of cytotoxicity of conditional medium from HEK 293T cells transiently cotransfected with different ratios of heavy chain vector and light chain vector.
Acknowledgments
This work was partially supported by the Natural Science Foundation (nos. 30772495, 30972684, and 30972699) and the State Key Basic Research Program (no. 2007CB507404). The authors have declared that no conflict of interest exists.
Supplementary Material
. Comparison of cytotoxicity of conditional medium from HEK 293T cells transiently cotransfected with different ratios of heavy chain vector and light chain vector.
REFERENCES
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK.et al. (1995Identification and characterization of a new member of the TNF family that induces apoptosis Immunity 3673–682. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA.et al. (1999Safety and antitumor activity of recombinant soluble Apo2 ligand J Clin Invest 104155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M.et al. (1999Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo Nat Med 5157–163. [DOI] [PubMed] [Google Scholar]
- Bellail AC, Qi L, Mulligan P, Chhabra V., and, Hao C. TRAIL agonists on clinical trials for cancer therapy: the promises and the challenges. Rev Recent Clin Trials. 2009;4:34–41. doi: 10.2174/157488709787047530. [DOI] [PubMed] [Google Scholar]
- Forero-Torres A, Shah J, Wood T, Posey J, Carlisle R, Copigneaux C.et al. (2010Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5) Cancer Biother Radiopharm 2513–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Chen C, Zheng Y, Zhang J, Tao X, Liu S.et al. (2005A novel anti-human DR5 monoclonal antibody with tumoricidal activity induces caspase-dependent and caspase-independent cell death J Biol Chem 28041940–41952. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zheng Y, Shi J, Zhang Y, Liu S, Liu Y.et al. (2010Targeting a novel N-terminal epitope of death receptor 5 triggers tumor cell death J Biol Chem 2858953–8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- Shi J, Liu Y, Zheng Y, Guo Y, Zhang J, Cheung PT.et al. (2006Therapeutic expression of an anti-death receptor 5 single-chain fixed-variable region prevents tumor growth in mice Cancer Res 6611946–11953. [DOI] [PubMed] [Google Scholar]
- Fang J, Qian JJ, Yi S, Harding TC, Tu GH, VanRoey M.et al. (2005Stable antibody expression at therapeutic levels using the 2A peptide Nat Biotechnol 23584–590. [DOI] [PubMed] [Google Scholar]
- Li M, Lu FJ, Shi J, Liu YX., and, Zheng DX.2011Expression and tumoricidal activity of a human-mouse chimeric anti-DR5 antibody through a Furin/2A peptide Zonghua Yi Xue Za Zhi 91707–710.Chinese]. [PubMed] [Google Scholar]
- Shi J, Zheng D, Liu Y, Sham MH, Tam P, Farzaneh F.et al. (2005Overexpression of soluble TRAIL induces apoptosis in human lung adenocarcinoma and inhibits growth of tumor xenografts in nude mice Cancer Res 651687–1692. [DOI] [PubMed] [Google Scholar]
- Tjelle TE, Corthay A, Lunde E, Sandlie I, Michaelsen TE, Mathiesen I.et al. (2004Monoclonal antibodies produced by muscle after plasmid injection and electroporation Mol Ther 9328–336. [DOI] [PubMed] [Google Scholar]
- Ho DT, Wykoff-Clary S, Gross CS, Schneider D, Jin F, Kretschmer PJ.et al. (2009Growth inhibition of an established A431 xenograft tumor by a full-length anti-EGFR antibody following gene delivery by AAV Cancer Gene Ther 16184–194. [DOI] [PubMed] [Google Scholar]
- Wang G, Qiu J, Wang R, Krause A, Boyer JL, Hackett NR.et al. (2010Persistent expression of biologically active anti-HER2 antibody by AAVrh.10-mediated gene transfer Cancer Gene Ther 17559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi H, Xu Z, Ishii-Watabe A, Uchida E., and, Hayakawa T. IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol Ther. 2000;1:376–382. doi: 10.1006/mthe.2000.0050. [DOI] [PubMed] [Google Scholar]
- Hale G, Berrie E., and, Bird P. Design and manufacture of monoclonal antibodies for radioimmunotherapy. Q J Nucl Med Mol Imaging. 2004;48:258–266. [PubMed] [Google Scholar]
- Fang J, Yi S, Simmons A, Tu GH, Nguyen M, Harding TC.et al. (2007An antibody delivery system for regulated expression of therapeutic levels of monoclonal antibodies in vivo Mol Ther 151153–1159. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blömer U, Gage FH, Trono D., and, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafri T, Blömer U, Peterson DA, Gage FH., and, Verma IM. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH.et al. (1996In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector Science 272263–267. [DOI] [PubMed] [Google Scholar]
- Reiser J, Zhang XY, Hemenway CS, Mondal D, Pradhan L., and, La Russa VF. Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases. Expert Opin Biol Ther. 2005;5:1571–1584. doi: 10.1517/14712598.5.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock N, Kasmieh R, Weissleder R., and, Shah K. Tumor therapy mediated by lentiviral expression of shBcl-2 and S-TRAIL. Neoplasia. 2007;9:435–442. doi: 10.1593/neo.07223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhao P, Kennedy C, Chen K, Wiegand J, Washington G.et al. (2008Treatment of pulmonary metastatic tumors in mice using lentiviral vector-engineered stem cells Cancer Gene Ther 1573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardella C, Dettori D, Olivero M, Coltella N, Mazzone M., and, Di Renzo MF. The therapeutic potential of hepatocyte growth factor to sensitize ovarian cancer cells to cisplatin and paclitaxel in vivo. Clin Cancer Res. 2007;13:2191–2198. doi: 10.1158/1078-0432.CCR-06-1915. [DOI] [PubMed] [Google Scholar]
- Visigalli I, Delai S, Politi LS, Di Domenico C, Cerri F, Mrak E.et al. (2010Gene therapy augments the efficacy of hematopoietic cell transplantation and fully corrects mucopolysaccharidosis type I phenotype in the mouse model Blood 1165130–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo M, Alves A, Rodrigues AF, Coroadinha AS, Carrondo MJ, Alves PM.et al. (2009Stabilization of gammaretroviral and lentiviral vectors: from production to gene transfer J Gene Med 11670–678. [DOI] [PubMed] [Google Scholar]
- Krueger A, Baumann S, Krammer PH., and, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001;21:8247–8254. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Tschopp J., and, Lin SC. Smac mimetics and TNFalpha: a dangerous liaison. Cell. 2007;131:655–658. doi: 10.1016/j.cell.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
. Comparison of cytotoxicity of conditional medium from HEK 293T cells transiently cotransfected with different ratios of heavy chain vector and light chain vector.