Abstract
Mesenchymal stem cells (MSCs) are emerging as a promising immunotherapeutic, based largely on their overt suppression of T lymphocytes under inflammatory and autoimmune conditions. While paracrine cross-talk between MSCs and T cells has been well-studied, an intrinsic transcriptional switch that programs MSCs for immunomodulation has remained undefined. Here we show that bone marrow-derived MSCs require the transcriptional regulator Aire to suppress T cell-mediated pathogenesis in a mouse model of chronic colitis. Surprisingly, Aire did not control MSC suppression of T cell proliferation in vitro. Instead, Aire reduced T cell mitochondrial reductase by negatively regulating a proinflammatory cytokine, early T cell activation factor (Eta)-1. Neutralization of Eta-1 enabled Aire−/− MSCs to ameliorate colitis, reducing the number of infiltrating effector T cells in the colon, and normalizing T cell reductase levels. We propose that Aire represents an early molecular switch imposing a suppressive MSC phenotype via regulation of Eta-1. Monitoring Aire expression in MSCs may thus be a critical parameter for clinical use.
Introduction
Transplantation of mesenchymal stem cells (MSCs; also called mesenchymal stromal cells) is considered as a new cellular therapeutic for suppressing unwanted inflammation. MSCs self-renew in vitro and can be differentiated to bone, cartilage, adipose, and connective tissue;1,2 yet it is their capacity for immunosuppression that has generated such strong clinical interest. MSC transplantation has been reported to improve clinical and preclinical outcomes across an array of inflammatory and autoimmune conditions, including graft versus host disease, organ transplantation, and following tissue injury;3,4,5,6,7 as well as in autoimmune models for multiple sclerosis, type-1 diabetes, systemic lupus erythematosus, rheumatoid arthritis, and gastroenteritis.8,9,10,11,12 However, recent pivotal trials using MSCs as a treatment for graft versus host disease did not meet primary efficacy endpoints13 causing a more critical assessment of the formulations of clinical-grade MSCs and their mechanism of action with the prospect of optimizing this promising therapy.
MSCs have been shown to impose many immunomodulatory effects on leukocytes,14,15,16,17 but paracrine MSC-T cell interactions have been studied most closely. Early studies revealed that T cell proliferation was profoundly reduced following coculture with MSCs. In vivo, MSC transfer broadly impacts T cells, particularly at stages of activation and differentiation occurring within lymph nodes. These include reduced T cell activation and proliferation, skewed differentiation to particular T cell subtypes, and increased regulatory T cell (Treg) generation in acute settings.11,18,19,20,21 Persistent engraftment or transdifferentiation at inflammatory sites has been observed only rarely12,22,23,24 suggesting this is unlikely to be a conserved mechanism of action. Instead, evidence suggests that MSCs act early after transplantation.25,26
Mechanistically, a bidirectional cross-talk exists between MSCs and T cells: activated T cells produce interferon γ (IFNγ) and other cytokines that together induce MSCs to secrete T cell suppressive factors like nitric oxide (mouse) or kynurenine by the enzymatic activity of indoleamine 2,3-dioxygenase (human).27,28 A similar response of MSCs to other inflammatory mediators can induce the release of prostanoids and soluble tumor necrosis factor (TNF) receptors that act on innate immune cells;4,29 however, intrinsic mechanisms underlying the ability of MSCs to suppress T cells are largely undefined.
Aire is an important transcriptional regulator expressed by stromal cells in primary30 and secondary31 immune organs; however, Aire expression in bone marrow (BM) stroma or other MSC populations has not been assessed. In the thymus, spleen and lymph nodes, Aire induces the destruction or suppression of autoreactive T cells by permitting transcriptional machinery to access genomic regions rich in rare peripheral tissue-restricted antigens (PTAs).30,32 PTAs are then presented via MHC, and responding T cells are functionally silenced, although signals governing this response are unknown. Thymic stromal cells strongly express Aire, preventing fulminant, spontaneous autoimmunity.30 Aire is also functional in lymph node and splenic stroma, inducing PTA expression and deletion of autoreactive CD8+ T cells.31
Here, we demonstrate that the transcriptional regulator Aire is essential to bone marrow MSC-mediated immunosuppression in the gut, using a clinically relevant model of Crohn's disease directly induced by T cells. Aire−/− MSCs could not suppress severe T cell-mediated colitis in vivo, but still retained the capacity to reduce T cell proliferation in vitro. Our findings link disease-relevant immunosuppression to altered T cell reductase, through Aire-controlled regulation of proinflammatory cytokine Eta-1.
Results
MSCs require expression of Aire in order to suppress inflammation in a T-cell mediated model of Crohn's disease
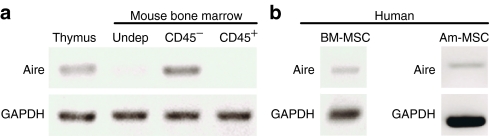
This study aimed to address the hypothesis that, as in suppressive stroma from thymus and lymph node, Aire controls the suppressive phenotype of bone marrow MSCs. We first analyzed messenger RNA (mRNA) expression of Aire in MSCs after isolation and long-term ex vivo culture. After 7 days of culture, adherent CD4− MSCs derived from mouse (Figure 1a) or human (Figure 1b) BM expressed Aire mRNA. Human MSCs derived from amniotic fluid also expressed PTAs (Figure 1b), which suggests that cells exhibiting MSC phenotypes from other tissue sources also express Aire.
Figure 1.
Mouse and human MSCs express Aire. (a) RT-PCR was performed comparing whole undepleted bone marrow (Undep) or sorted bone marrow stroma. Unsorted thymus was a positive control. (b) RT-PCR of human bone marrow (BM) and amniotic fluid-derived (Am) MSCs. Gene expression data representative of n = 3–5 subjects. MSC, mesenchymal stem cells; RT-PCR, reverse transcriptase PCR.
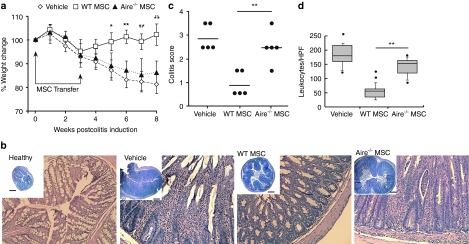
As Aire is primarily linked to T cell suppression, we chose to test its function in mouse MSCs using a model of Crohn's disease solely mediated by pathogenic T cells, induced by CD4+ CD45RBhi T cell transfer to Rag1−/− mice. Wild type (WT) or Aire−/− mouse MSCs were intravenously injected between 0 and 21 days after T cell transfer. Within 8 weeks, saline-treated (control) mice developed hallmarks of colitis including significant weight loss (Figure 2a) and severe intestinal pathology (Figure 2b–d). Mice treated with WT mouse MSCs showed a significant improvement in body mass and colon pathology.
Figure 2.
MSC-mediated immunosuppression of chronic colitis is Aire-dependent. (a) Chronic colitis was induced by transfer of CD4+ CD45RBhi T cells to Rag−/− mice. Mouse MSCs or saline were infused post-T cell transfer (arrows) and body mass measured. (b) Representative transverse colon sections (H&E) of healthy and colitic mice 8 weeks after colitis induction. A higher original magnification at ×20 of the corresponding group is provided along with an inset is at ×4 original magnification (Inset bar = 500 µm). (c) Colitis pathology scores of colon sections at 8 weeks. (d) Quantification of infiltrating mononuclear cells in intestine. Results represent five sections with five random fields per group. Data depict median, 10th, 25th, 75th, and 90th percentiles with standard deviation and outliers. For mouse data, n = 5–15 per group, across at least three independent trials. *P < 0.05; **P < 0.01, by Tukey's Method a or Student's t-test b,d. H&E, hematoxylin and eosin; HPF, high power field; MSC, mesenchymal stem cells.
Strikingly, mice treated with Aire−/− mouse MSCs developed severe colitis similar to vehicle controls. Unlike mice given WT MSCs, mice receiving Aire−/− MSCs exhibited significant weight loss (Figure 2a) with extensive colon pathology, including mucosal hyperplasia, goblet cell loss, and abnormal villi morphology (Figure 2b). These pathological findings were quantified by an independent pathologist and found to denote higher colitis scores (Figure 2c). The number of leukocytes in the colon was also significantly increased compared with mice receiving WT MSCs (Figure 2d). These data establish that Aire is required for MSC-mediated immunotherapy in this model of Crohn's disease.
We performed a short-term analysis of mouse MSC distribution to determine whether Aire−/− MSCs were distributed differently compared to WT MSCs, which could lead to altered potency in this model. carboxyfluorescein succinimidyl ester (CFSE)-labeled cells were intravenously administered to Rag1−/− mice and after 3 hours, the spleen, liver, lungs, and large intestine were harvested and digested for analysis of transferred cells. In concordance with other MSC biodistribution studies,25,26 we observed that the majority of both Aire−/− and WT MSCs were found in the lung with infrequent events observed in the colon, liver, and spleen at this time point (Supplementary Figure S1). Since both cell populations distributed similarly after injection, we began to seek other explanations for the role of Aire in MSC immunomodulation.
MSCs reduce activated T cells and intestinal inflammation in an Aire-dependent fashion
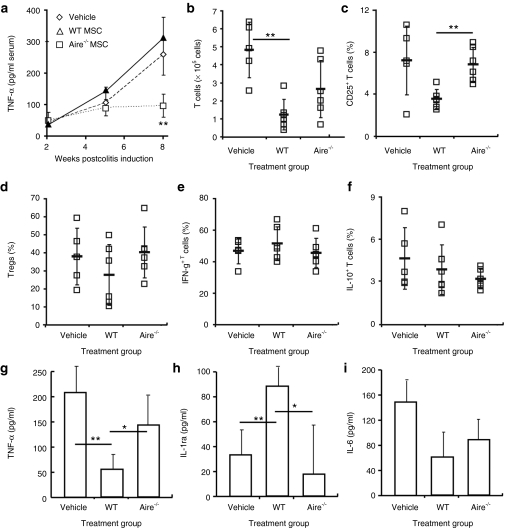
We analyzed a number of systemic and local parameters that have been reported to be affected by MSCs in search of durable disease-modifying targets in this model. We found a strong correlation between TNF-α levels and WT MSC treatment. Serum TNF-α levels were decreased in mice treated with WT MSCs and elevated in mice given Aire−/− MSCs, again reflecting inflammation levels across all groups (Figure 3a). Based on the stratification in the systemic TNF-α response, we examined local indexes of inflammation in the intestinal tract. In line with decreased pathology and inflammation, mice receiving WT MSCs had significantly smaller colon-draining mesenteric lymph nodes compared to vehicle-treated controls and contained fewer T cells overall (Figure 3b). The activation state of lymph node T cells was also altered. A reduced proportion of T cells expressed the activation marker CD25 (Figure 3c), indicating lower interleukin-2 (IL-2) receptor expression. While activated cells and Tregs both express CD25, there was no change in the proportion of CD25+ cells expressing FoxP3 (and therefore no proportional changes in Tregs) (Figure 3d), showing that the decrease in CD25+ cells was equally due to a reduction in activated T cells. Activated lymph node T cells were still present in mice receiving Aire−/− MSCs compared to WT MSCs (Figure 3b). The difference in the numbers of activated T cells as a function of Aire expression in MSCs was the resounding conclusion from these experiments.
Figure 3.
Aire mediates suppression through T cell activation rather than effector T cell skewing or Treg induction. Colitis was induced and mice were treated as depicted in Figure 2a. Tissues were harvested 6 weeks postdisease induction. Mesenteric lymph nodes were isolated and cells activated in culture before analysis of intracellular cytokines. (a) Serum TNF-a levels were tested by ELISA following colitis induction. Flow cytometry of (b) mesenteric lymph node T cell numbers and (c) CD25+ T cells, shown as % total CD4+ T cells. (d) FoxP3+ Tregs, shown as % total CD4+CD25+ T cells. Intracellular cytokine flow cytometry of (e) IFN-g and (f) IL-10, shown as % total CD4+ T cells. Intestinal lesions were isolated during active colitis at 6 weeks after induction and cultured overnight in basal media. ELISA of (g) TNF-α, (h) IL-1ra, and (i) IL-6 release. Data depict mean ± SD and are representative of n = 3–5 per group from two experiments. *P < 0.05; **P < 0.01, Student's t-test. ELISA. Enzyme-linked immunosorbent assay; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; WT, wild type.
In Crohn's disease patients, and in the mouse model used for this study, the T cell repertoire is known to be skewed toward T helper (Th)1 (i.e., IFNγ+) and deficient in Tregs. Indeed, we observed a predominant IFNγ+ T cell response in mesenteric (gut-draining) lymph nodes (Figure 3e) in all treated mice, with very few T cells producing the Th2 cytokine IL-10 (Figure 3f). Although WT MSCs did not alter the dominant Th1 T cell response, we can speculate that colitis severity was reduced because a decreased number of activated T cells, even with similar proportions of IFNγ producing cells, still amounts to a decreased overall number of pathogenic Th1 effectors. Since Aire−/− MSCs had a significantly increased number of activated T cells we can also conclude that the total numbers of pathogenic Th1 effectors were also elevated. We also looked to other tissues and inflammatory mediators for clues to Aire's suppressive mechanism.
We next tested production of inflammatory and suppressive cytokines directly from intestinal lesions, focusing on those linked to MSC-mediated protection in chemical-induced colitis.18 Similar to serum, intestinal TNF-α was profoundly decreased in mice given WT MSCs and increased in mice given Aire−/− MSCs (Figure 3g). Treatment with WT MSCs induced higher levels of anti-inflammatory IL-1ra in intestinal epithelia (Figure 3h), while MSCs did not appreciably alter inflammatory IL-6 production (Figure 3i).
Aire imposes immunosuppression through transcriptional repression of proinflammatory cytokine Eta-1
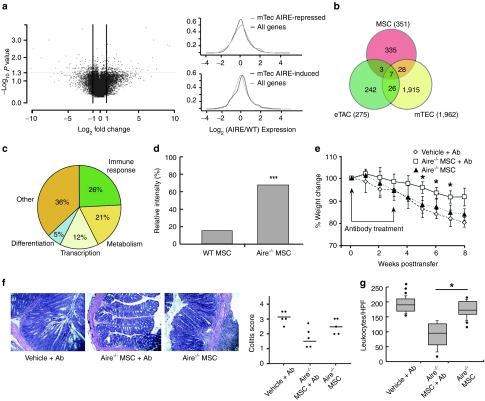
To uncover Aire's mechanisms, we performed a comprehensive analysis of Aire-controlled gene expression in MSCs, using genome-wide mRNA analysis. WT MSCs differentially expressed 351 genes compared to Aire−/− MSCs (Figure 4a). In keeping with Aire's molecular mechanism, which dictates expression of vastly different genes for each cell type in which it is expressed, we saw a significant shift towards Aire-induced genes with little overlap in Aire-regulated gene expression between MSCs and stroma from the thymus and lymph nodes (Figure 4b). Among the genes regulated by Aire in MSCs, only 9% of genes were classified as PTAs, while 26% corresponded to immune response genes that included soluble factors with known pro- or anti-inflammatory functions (Figure 4c). Given prior studies demonstrating that MSCs suppress T cell activation independently of cell contact, we posited that one or more of these Aire-regulated soluble factors may be involved in suppression. One such candidate soluble factor, significantly upregulated in Aire−/− MSCs in our gene expression profiling (i.e., downregulated by Aire in WT cells), was a well-recognized proinflammatory cytokine called early T cell activation factor (Eta)-1, also known as osteopontin. To first validate the gene array, we confirmed increased expression of the secreted form of Eta-1 protein in Aire−/− MSCs by immunoblots (Figure 4d). Next, we tested whether the T cell suppressive function of Aire−/− MSCs could be restored when Eta-1 was neutralized by antibodies. Indeed, we found that colitis symptoms and colon pathology (Figure 4e–g) were significantly improved when Eta-1 was neutralized upon administration of Aire−/− MSCs. These results suggest that Aire mediates immune suppression in MSCs, at least partially, by maintaining transcriptional control of Eta-1, although it was not yet clear how this suppression was imposed.
Figure 4.
Aire regulates Eta-1 secretion, which mediates immunosuppression in vivo. (a) Volcano plot of Aire-regulated genes in CD45− MSCs; and nonparametric equality testing of microarray results for (top) Aire-repressed and (bottom) Aire-induced transcripts showing global shifts in Aire-induced genes compared to other lymphoid tissues from prior studies. (b) Venn-diagram of Aire-regulated compared to other lymphoid tissues from prior studies. (c) Pie chart grouping function of Aire-regulated MSC transcripts based on gene ontology, relative to Aire-regulated genes. (d) Densitometry of intracellular Eta-1 protein stores by antibody array, n = 3 independent cell cultures. Relative intensity is shown normalized to internal controls. (e) Mouse weight over time after colitis induction. Mice were treated with Aire−/− MSCs and Eta-1 neutralizing antibody at 0 and 3 weeks after colitis induction, indicated by arrows. (f) Histology of colon sections after antibody neutralization and colitis pathology scores. (g) Quantification of leukocytes infiltrating colon sections following antibody therapy trials. *P < 0.05; ***P < 0.001, Student's t-test.MSC, mesenchymal stem cell; WT, wild type.
MSCs induce an oxidative state in activated T cells which is reversed by coculture with Aire−/− MSCs through the effects of Eta-1
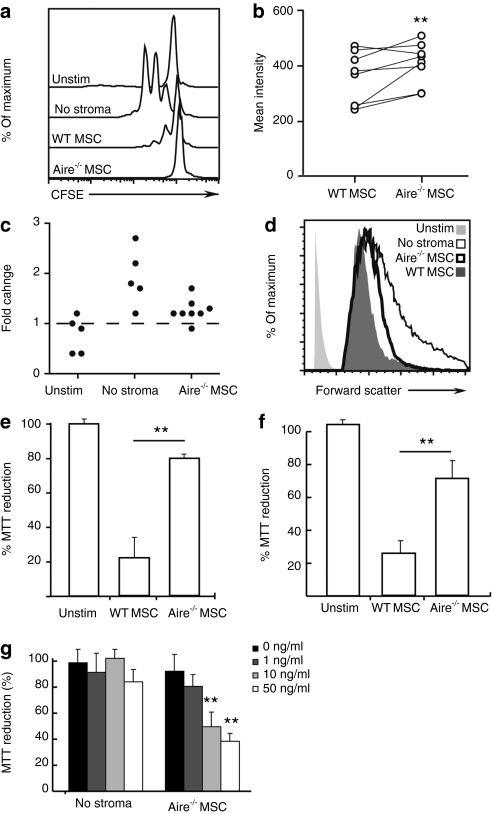
Next we sought to directly assess the effect of Aire and Eta-1 on newly activated T cells. To this end, we used a classic in vitro proliferation assay, where T cells are stimulated to divide using plate-bound anti-CD3ε in the presence or absence of MSCs. T cells were labeled with the fluorescent dye CFSE to quantitatively monitor cell division by flow cytometry. Surprisingly, despite their profound inability to suppress T cell-mediated inflammation in vivo, Aire−/− MSCs were still able to suppress T cell proliferation in vitro, equivalent to WT MSCs (Figure 5a). This held true regardless of the T cell activation method used, as we evaluated both anti-CD3ε/anti-CD28 and soluble anti-CD3ε at various concentrations, with or without other antigen presenting cells and leukocytes, and across multiple ratios of MSCs to T cells (data not shown). Instead, we found evidence for a profound alteration in T cell metabolic activity. T cells cocultured with WT MSCs were small, as befits nondividing, quiescent lymphocytes (Figure 5b–d). However, T cells cocultured with Aire−/− MSCs were significantly larger by forward scatter, despite similar lack of cell division. We further confirmed that this size difference was not related to cell division itself, or related to the capacity of the T cells to be stimulated, by assessing the size of T cells that had gone through at least one cell division (Figure 5d). The differences persisted, and therefore occurred independent of their capacity to be stimulated, as suggested by the proliferation data (Figure 5a).
Figure 5.
Aire-regulated Eta-1 alters T cell size and mitochondrial reductase. T cells stimulated with anti-CD3ε were cocultured with WT or Aire−/− MSCs for 3 days. Unstimulated (Unstim) and cells stimulated but not cultured with stroma (“No stroma”) were used as negative and positive controls, respectively. (a) T cell proliferation was detected as CFSE dilution. (b) Mean forward scatter intensity for live T cells. (c) Fold change in live T cell mean forward scatter intensity, compared to T cells cocultured with WT MSCs (normalized to one). (d) Histograms showing forward scatter profiles gated on live CD4+ T cells that have undergone at least one cell division. (e) MTT reduction of CD3ε-activated T cells cocultured with WT or Aire−/− MSCs. (f) MTT reduction of CD3ε-activated T cells separated from MSCs by a porous membrane. (g) MTT reduction of CD3ε activated T cells, indirectly cocultured with Aire−/− MSCs, with increasing doses of Eta-1 neutralizing antibody. Data represent at least two independent experiments and three mice. (**P = 0.01, two-tailed Student's t-test; Aire−/− MSCs vs. WT (b,e,f) or 0 ng/ml antibody (g)).CFSE, carboxyfluorescein succinimidyl ester; MSC, mesenchymal stem cell; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide; WT, wild type.
Altered cell size independent of proliferation suggested altered mitochondrial function. Eta-1 inhibits apoptosis-inducing factor, a pro-apoptotic mitochondrial flavoprotein that also promotes nicotinamide adenine dinucleotide oxidase33 a predominant inducible nitric oxide synthase-independent oxidant source in activated T cells.34 Accordingly, analysis of mitochondrial function using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay showed a global reductive state in T cells cocultured with either no stroma or Aire−/− stroma, but not WT stroma (Figure 5b). Validating this, we measured global oxidation levels and found decreased total reactive oxygen species in T cells cultured with Aire−/− MSCs (Supplementary Figure S2). This was not due to lower levels of inducible nitric oxide synthase (data not shown). We next tested whether secreted Eta-1 controlled this antioxidative effect of Aire−/− MSCs on activated T cells. We first asked whether Aire altered intracellular T cell mitochondrial function through control of a secreted factor, by separating MSCs from stimulated T cells with a semipermeable membrane, and indeed, Aire−/− MSCs still induced a profoundly reductive mitochondrial response in T cells (Figure 5f). Accordingly, we found that antibody neutralization of secreted Eta-1 restored the oxidative microenvironment in T cells cocultured with Aire−/− MSCs in a dose-dependent fashion (Figure 5g), confirming that the alteration in intracellular T cell oxidation was mediated by Eta-1. These experiments revealed that Aire negatively regulates Eta-1 in MSCs that induces important biochemical changes to T cells by a paracrine mechanism.
Discussion
MSCs are considered to be a progenitor to many adult mesenchymal-derived cells. Here, we have found that MSCs express Aire and specialized PTAs similar to other lymphoid organ stromal cells. Similar to other peripheral stroma expressing low levels of Aire,35 transcribed protein was below detection limits (data not shown). In investigating the biological significance for Aire expression, we found that PTAs expressed by thymic stroma were also expressed by mouse and human MSCs (Supplementary Table S1 and Supplementary Figure S3a,b) and clonal expression analysis involving subculture of undifferentiated colony-forming units revealed a mosaic PTA expression pattern (Supplementary Figure S3c), also reminiscent of thymic stromal cells, which preserve tolerance by deleting T cells reactive to these rare antigens. Ontologically, MSCs may be related to stromal cells found in lymph nodes and the thymus, although we have not provided such evidence in this study. Certainly, promiscuous gene expression has been observed in other stem cell populations as a preceding step to lineage commitment36 and previous reports showed that MSCs express neuronal-specific proteins reminiscent of PTAs in their undifferentiated state.37 We believe that MSCs may express PTAs not because of stem cell plasticity per se, but as a heretofore-unrecognized antigen expression pattern similar to other nonhematopoietic cells in lymphoid organs.
While Aire is best known for its role in mediating thymic PTA expression, Aire's influences in the thymus and periphery do demonstrably extend beyond PTA expression. Aire alters thymic chemokine expression, antigen presentation, stromal cell development, stromal cell lifespan,38 and thymocyte migration.39 In peripheral dendritic cells, Aire changes IFNγ signaling, increasing B cell activation.40 Aire protein is also strongly expressed in spermatogonia41, early embryos, human embryonic stem cells and induced pluripotent stem cells42 with unknown purpose. Since MSCs suppress T cells in xenogenic and allogeneic settings, independent of major histocompatibility complex and antigen specificity, we were interested in the existence of antigen-independent roles for Aire in driving T cell suppression. Here we show that Aire is required for MSC-mediated amelioration of colitis in vivo through its regulation of an important proinflammatory cytokine, Eta-1. Aire's expression in cultured MSCs, before T cells are added or activated, suggests it is an early transcriptional switch encoding intrinsic suppressive capability.
Eta-1, also known as secreted phosphoprotein-1 and osteopontin, has pleiotropic functions in health and disease. The secreted form of this protein can drive the differentiation of Th1 effector cells and plays causal roles in autoimmunity43 and cancer progression.44 An intracellular isoform of Eta-1, expressed by plasmacytoid dendritic cells, has been recently discovered to regulate the expression of proinflammatory IFN-α.45 Eta-1 has also been associated with osteoblastic differentiation; however, our gene expression data did not report any corresponding upregulation of osteoblast-related transcription factors such as Runx2 or osterix in Aire−/− MSCs. The independent rise of Eta-1 without Runx2 or osterix led us to conclude that increased Eta-1 was independent of differentiation and may therefore impinge on immune function. Indeed, we showed that Eta-1 neutralization restores the ability of Aire−/− MSCs to induce a reductive intracellular microenvironment in responding T cells.
These data also mechanistically separate suppression of T cell proliferation in vitro from suppression of a purely T cell-mediated disease in vivo, since Aire−/− MSCs failed to suppress disease while retaining the ability to strongly suppress T cell proliferation in vitro. This finding has important clinical relevance, given the number of ongoing trials using MSCs, and the field's necessary but heavy reliance on in vitro studies to further dissect preclinical and clinical trial results. Precedent for this finding exists46,47 where in vitro suppressive WT MSCs were unable to suppress an analogous disease in vivo. Comparison of Aire−/− MSCs with their WT controls permitted us to further examine potential molecular and cellular contributors to this phenomenon. In the T cell transfer model of colitis, the global oxidation/reduction state of responding T cells best predicted immunotherapeutic potential and would form a valuable adjunctive in vitro test.
Human colitis is often chronic, progressive, and the source of significant morbidity, creating an urgent clinical need for new therapeutic avenues. Phase II clinical trials for MSCs in drug-resistant Crohn's disease showed a rapid, statistically significant improvement in symptoms48 with more trials ongoing (see http://www.clinicaltrials.gov). MSC transfer reliably reduces symptoms in markedly different preclinical models of gastrointestinal inflammation, varying in disease induction, pathogenesis, and disease duration.10,18,21 Colitis in the model described in our study is purely immune-mediated, chronic, and degenerative without requiring administration of cytotoxic chemicals. This is the first demonstration that MSCs suppress disease in a T cell transfer model of colitis. MSC therapy is known to have measurable short-term effects after transplantation. In our chronic model of colitis, we observed that two injections of MSCs had a durable response that lasted several weeks after treatment. The need to optimize dosing given the short half-life of MSCs in order to realize clinical endpoints is critical to the success of this therapy in larger cohorts.
Here we show that Aire plays an important role in the molecular regulation of MSC-mediated immune suppression in the gut in vivo. Expression of Aire could be maintained in culture after multiple passages, with potential therapeutic importance, as MSCs are expanded ex vivo before transfer into patients. Aire represents a molecular switch of MSC phenotype from a suppressive to a stimulatory state and may be a critical parameter to monitor while processing MSCs for therapeutic use.
Materials and Methods
Mice. C57BL/6 mice (6–10 weeks) were purchased from Charles River Laboratory (Wilmington, MA). Aire−/−, and Rag-1−/− mice were bred at the Dana Farber Cancer Institute. Animals were maintained in accordance with Institutional and National Institutes of Health guidelines, and experiments were approved by the subcommittee on research animal care and laboratory animal resources of Dana Farber Cancer Institute and the Massachusetts General Hospital.
MSC isolation and culture. Bone marrow was flushed from tibias and femurs with MSC medium (α-MEM (Gibco, Carlsbad, CA), 10% lot selected fetal bovine serum (Atlanta Biologicals, Norcross, GA), 100 U/ml penicillin (Sigma, St Louis, MO), and 100 µg/ml streptomycin (Sigma)), then dissociated and filtered. The overall cellularity of Aire−/− and WT BM did not differ. Cells were plated at a density of 2.6 × 106 cells/cm2 in MSC medium and cultured for 3 days, then washed, and the adherent population cultured for another 4–10 days. Cells were passaged using 0.1% trypsin/0.1% ethylenediaminetetraaceticacid, and subcultured at 5 × 103 cells/cm2. All cultures were used between passages 2–8. Before use, stromal cells were depleted of CD45+ cells using magnetic-activated cell sorting (Miltenyi Biotec, Auburn, CA) per vendor's instructions. The phenotype of Aire−/− MSCs was identical to C57BL/6 MSCs49,50 (Supplementary Figure S4). Human BM (Lonza Biologics, Walkersville, MD) was separated using a Ficoll gradient, centrifuged at 1500 rpm for 30 minutes. Mononuclear cells were harvested, washed in MSC medium and plated at 1 × 104 cells/cm2. After 7 days of culture, nonhematopoietic cells were washed away and purified human MSCs were cultured and maintained as per mouse MSCs. Human amniotic fluid-derived MSCs were a kind gift from Dr. Myriam Armant, Center for Human Cell Therapy, Immune Disease Institute, Boston. These cells were prepared in a similar fashion to BM MSCs.
Colony-forming units assays. Limiting dilutions of freshly isolated BM cells (9.6 × 104 cells/cm2) were allowed to adhere and expand in MSC medium for 10 days. Adherent colonies were enumerated using a Giemsa stain. When expanded in culture, Aire−/− MSCs were not growth deficient, showing similar colony-forming efficiency compared to WT when placed in limiting dilution cultures.
Preparation of thymus. Thymi were mechanically disrupted into a saline solution, red cell lysed (ACK lysis buffer, 1–2 minutes) before being washed, filtered, and counted. Cells were used for RNA extraction and PCR.
RNA isolation and PCR. RNA was extracted from 0.1–1.0 × 106 cells using the Nucleospin RNA purification kit (BD Biosciences, San Diego, CA) per vendor's instructions. 0.5–1 µg mRNA was reverse transcribed to complementary DNA (cDNA) using the Two-Step RT-PCR Kit (Qiagen, Valencia, CA) and amplified in a Perkin Etus Thermal Cycler 480 or Stratagene Light Cycler. Cycling conditions for PCR were: (i) 50 °C for 30 minutes; (ii) 95 °C for 15 minutes; (iii) 40 cycles at 94 °C for 30 seconds, 55 °C for 30 seconds, 72 °C for 1 minute; and (iv) 72 °C for 10 minutes. Primers are listed in Supplementary Table S1. For endpoint analysis, amplified cDNA mixed with SybrGreen (Molecular Probes, Carlsbad, CA) was run on a 1.2% agarose gel and visualized using a gel imager. For quantitative analysis of colony forming units, we used a ΔΔCt method with stated controls with results stated as relative differences between experimental groups.
Microarrays. RNA was extracted from 0.1–1.0 × 106 MSCs using Trizol (Invitrogen, Carlsbad, CA) then chloroform. Purity was determined using spectrophotometry and capillary electrophoresis (Bioanalyzer 2100; Agilent Technologies, Santa Clara, CA). First strand cDNA was prepared using a DNA/RNA chimeric primer and reverse transcriptase. Synthesis of a second strand resulted in double stranded cDNA with a unique DNA/RNA heteroduplex at the 5′end of the antisense strand. cDNA was amplified (signaling pathway impact analysis) and purified (DNA Clean & Concentrator; Zymo Research, Orange, CA), then fragmented through a chemical and enzymatic process and labeled via enzymatic attachment of a biotin-labeled nucleotide to the 3′-hydroxyl end. Biotinylated cDNA was added to a hybridization solution containing several biotinylated control oligonucleotides (for quality control), and hybridized to a microarray chip overnight at 45 °C. Chips were washed, and bound cDNA was fluorescently labeled using phycoerythrin-conjugated streptavidin; additional fluorophores were then added using biotinylated anti-streptavidin antibody and additional streptavidin–phycoerythrin. Each bound cDNA was excited using a confocal laser scanner, and the positions and intensities of the fluorescent emissions were captured.
Antibodies and conjugates. Antibodies to CD3 (clone 145-2C110, CD90 (Thy1) (clone 30-H12), CD106 (VCAM-1) (clone 429 MVCAM.A), CD117 (c-kit) (clone 2B8), CD279 (PD-1) (clone J43), Flk-1 (clone Avas 12α1), Gr-1 (clone clone RB6-8C5), I-A/I-E (clone 2G9), TCRb (clone H97-597), and Sca-1 (clone D7) were purchased from BD Biosciences. Antibodies to CD45 (clone 30-F11), CD31 (clone MEC13.3), CD44 (clone IM7), IFNg (clone XMG1.2), IL-4 (clone 11B11), and IL-10 (clone JES5-16E3) were purchased from Biolegend (San Diego, CA), and antibodies to CD140a (clone APA5) and FoxP3 (FJK-165) were from eBioscience (San Diego, CA). The antibody MTS-15, which detects the Forssman antigen on some mesenchmal cells, was a kind gift from Dr. Richard Boyd. UEA-1 lectin was obtained from Vector laboratories (Burlingame, CA). Secondary reagents used were anti-rat IgG2c (Southern Biotech, Birmingham, AL) and streptavidin-allophycocyanin (BD Biosciences). Cells were fixed and permeabilized using the BD Cytofix/Cytoperm kit, or the eBioscience FoxP3 staining kit, according to manufacturer's instructions.
T cell stimulation assays. 96-well culture plates were coated overnight with 0.5 mg/ml anti-CD3ε (BD Biosciences, clone 145-2C11) and washed in phosphate-buffered saline. Splenocytes were depleted of B cells and dendritic cells using magnetic-activated cell sorting (Miltenyi Biotec), then incubated for 10 minutes with 1 µmol/l CFDA-SE (Molecular Probes) at 37 °C. 2.5 × 105 T cells (>90% pure) were cultured in the presence of anti-CD3ε (BD Biosciences, clone 145-2C11) and cocultured with WT or Aire−/− CD45− MSCs (2.5 × 104 cells; day 10 postisolation). Cell proliferation was measured using carboxyfluorescein succinimidyl ester dilution. The MTT assay was performed using the CellTiter96 kit (Promega, Madison, WI). In certain experiments, MSCs were separated from T cells using a 0.4 µm Transwell membrane (Corning, Big Flats, NY) with or without neutralizing Spp1 antibody (R&D Systems, Minneapolis, MN, clone AF-808). To assess reactive oxygen species, T cells were loaded with cell-permeable H2DCFH-DA (10 µmol/l, 30 minutes, 37 °C). H2DCFH-DA is oxidized by free radicals to dichlorofluorescein. Cells were washed, cultured for 60 minutes, then analyzed by flow cytometry. Positive control T cells were incubated with H2O2 (100 µmol/l) for 2 hours before loading with H2DCFH-DA.
Secretome analysis. MSC lysates were analyzed for Eta-1 protein expression using a customized mouse antibody array for specified proteins following vendor's instructions (RayBiotech, Norcross, GA).
Colitis induction. WT C57BL/6 mice (female, 8–12 weeks old) were killed, and splenic cells were isolated by mechanical disruption of the tissue into a saline solution. Cells were filtered, red cell lysed (ACK buffer) and enriched for T cells using a T cell enrichment column (R&D Systems) per vendor's instructions, then stained with CD4 (BD Biosciences, clone GK1.5) and CD45RB (BD Biosciences, clone 16A). CD4+CD45RBhi cells were sorted using the Massachusetts General Hospital Pathology Core Facility, and 5 × 105 cells intravenously injected into Rag-1−/− mice. At that timepoint and 3 weeks later, MSCs (2.5 × 105/mouse) or vehicle alone were infused intravenously. Animals were killed 8 weeks after cell transfer. Ascending, transverse, and descending colons were isolated for analysis. Tissue was formalin fixed and paraffin embedded, sectioned to 5 µm, and stained with hematoxylin and eosin. Images were taken with a Nikon Eclipse 800 and slides were blindly scored by a pathologist as follows: 0: normal colon; 1: slight epithelial hyperplasia, increased leukocyte infiltrate; 2: pronounced epithelial cell hyperplasia with two to threefold increase in crypt length, significant leukocyte infiltrate in mucosa and submucosa, goblet cell depletion; 3: marked epithelial cell hyperplasia with up to tenfold increase in crypt length, extensive leukocytic infiltrate in mucosa, submucosa, and tunica muscularis, ulceration, significant goblet cell loss; 4: marked epithelial cell hyperplasia and ulceration; extensive transmural leukocytic infiltrate extending from submucosa to serosa (sometimes present in mesentary and sometimes granulomatous), substantial goblet cell depletion (>50%), crypt abscesses.
Digital cell quantification. Cells in stained intestinal sections were quantified from five random ×40 images per animal group using ImageJ (http://rsb.info.nih.gov/ij/). Nuclei of area >700 pixels2 identified parenchymal cells from infiltrating inflammatory cells.
Biodistribution analysis. MSCs were harvested and incubated with carboxyfluorescein succinimidyl ester (10 µmol/l) for 10 minutes. Cells were washed and intravenously (1.5 × 105 cells per mouse) administered to immunocompromised mice by a tail vein injection. Organs were harvested 3 hours after the cell injection and digested in a collagenase/dispase buffer for 1 hour before single cell suspensions were analyzed for flow cytometry.
Statistical analysis. Quantitative PCR and T cell stimulation results were analyzed using an unpaired Student's t-test given an unskewed data set and assuming a normal distribution. Changes in body mass were analyzed using Tukey's method. P < 0.05 was considered significant. Robust Multichip Analysis (RMA) normalization was performed for microarray data. Probe sets in mouse Aire−/− MSC vs. WT MSC were filtered based on >twofold change with false discovery rate cutoff of <0.15. EASE analysis was performed to enrich MSC Aire-regulated genes with a significance threshold of 0.001. eTAC and mTEC Aire induced/repressed probeset lists were determined from previously published studies (Gardner et al. 2008; Anderson et al. 2002). Expression data for this study was RMA normalized (GSE12388, GSE8563). Differentially expressed genes were determined with false discovery rate <0.15 cutoff.
SUPPLEMENTARY MATERIAL Figure S1 In vivo tracking of AIRE−/− and WT MSCs. Figure S2 Reactive oxygen species in T cells after coculture with MSCs. Figure S3 Characterization of mouse and human BMSC PTA expression. Figure S4 Immunophenotype of WT and Aire−/− stroma. Table S1 Oligonucleotide sequences to study peripheral tissue antigen expression
Acknowledgments
We thank the Dana Farber Microarray and Biostatistics Core Facility for hybridizing cDNA chips and performing computational analysis. We thank Myriam Armant for kindly providing amniotic fluid-derived MSCs. We thank Veronika Lukacs-Kornek for thoughtful opinions on the manuscript. This work was supported by the National Institutes of Health (DK074500, AI045757, DK059766-06, and DK087770), NHMRC Australia: (546259), the Broad Medical Research Program of The Broad Foundation (BMRP498382) and Shriners Hospitals for Children.
Supplementary Material
In vivo tracking of AIRE−/− and WT MSCs.
Reactive oxygen species in T cells after coculture with MSCs.
Characterization of mouse and human BMSC PTA expression.
Immunophenotype of WT and Aire−/− stroma.
Oligonucleotide sequences to study peripheral tissue antigen expression
REFERENCES
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD.et al. (1999Multilineage potential of adult human mesenchymal stem cells Science 284143–147. [DOI] [PubMed] [Google Scholar]
- Dennis JE., and, Caplan AI. Analysis of the developmental potential of conditionally immortal marrow-derived mesenchymal progenitor cells isolated from the H-2Kb-tsA58 transgenic mouse. Connect Tissue Res. 1996;35:93–99. doi: 10.3109/03008209609029179. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M.et al. (2004Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells Lancet 3631439–1441. [DOI] [PubMed] [Google Scholar]
- Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K.et al. (2009Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production Nat Med 1542–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N.et al. (2003Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects Proc Natl Acad Sci USA 1008407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H.et al. (2005Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells Nat Med 11367–368. [DOI] [PubMed] [Google Scholar]
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S.et al. (2002Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo Exp Hematol 3042–48. [DOI] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E.et al. (2005Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy Blood 1061755–1761. [DOI] [PubMed] [Google Scholar]
- Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD.et al. (2006Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice Proc Natl Acad Sci USA 10317438–17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekkadan B, Tilles AW., and, Yarmush ML. Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independently of regulatory T cells. Stem Cells. 2008;26:1913–1919. doi: 10.1634/stemcells.2007-0790. [DOI] [PubMed] [Google Scholar]
- Augello A, Tasso R, Negrini SM, Cancedda R., and, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- Gu Z, Akiyama K, Ma X, Zhang H, Feng X, Yao G.et al. (2010Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice Lupus 191502–1514. [DOI] [PubMed] [Google Scholar]
- Parekkadan B., and, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ge W, Li C, You S, Liao L, Han Q.et al. (2004Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells Stem Cells Dev 13263–271. [DOI] [PubMed] [Google Scholar]
- Aggarwal S., and, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H.et al. (2005Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness Blood 1052214–2219. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN., and, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- González MA, Gonzalez-Rey E, Rico L, Büscher D., and, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E.et al. (2003Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide Blood 1013722–3729. [DOI] [PubMed] [Google Scholar]
- Rasmusson I, Ringdén O, Sundberg B., and, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- Parekkadan B, Upadhyay R, Dunham J, Iwamoto Y, Mizoguchi E, Mizoguchi A.et al. (2011Bone marrow stromal cell transplants prevent experimental enterocolitis and require host CD11b+ splenocytes Gastroenterology 140966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li Y, Lu M, Cui Y, Chen J, Noffsinger L.et al. (2006Bone marrow stromal cells reduce axonal loss in experimental autoimmune encephalomyelitis mice J Neurosci Res 84587–595. [DOI] [PubMed] [Google Scholar]
- Kassis I, Grigoriadis N, Gowda-Kurkalli B, Mizrachi-Kol R, Ben-Hur T, Slavin S.et al. (2008Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis Arch Neurol 65753–761. [DOI] [PubMed] [Google Scholar]
- Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H.et al. (2004Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction Blood 1043581–3587. [DOI] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL.et al. (2009Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6 Cell Stem Cell 554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangi L, Margalit R, Reich-Zeliger S, Bachar-Lustig E, Beilhack A, Negrin R.et al. (2009Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells Stem Cells 272865–2874. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI.et al. (2008Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide Cell Stem Cell 2141–150. [DOI] [PubMed] [Google Scholar]
- Meisel R, Zibert A, Laryea M, Göbel U, Däubener W., and, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, Nahmias Y, Goldwasser Y, Kitagawa Y.et al. (2010Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1 Mol Ther 181857–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ.et al. (2002Projection of an immunological self shadow within the thymus by the aire protein Science 2981395–1401. [DOI] [PubMed] [Google Scholar]
- Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X.et al. (2008Deletional tolerance mediated by extrathymic Aire-expressing cells Science 321843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson J, Giraud M, Benoist C., and, Mathis D. Aire's partners in the molecular control of immunological tolerance. Cell. 2010;140:123–135. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Hangen E, Blomgren K, Bénit P, Kroemer G., and, Modjtahedi N. Life with or without AIF. Trends Biochem Sci. 2010;35:278–287. doi: 10.1016/j.tibs.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Jackson SH, Devadas S, Kwon J, Pinto LA., and, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS.et al. (2010Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions J Exp Med 207689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Iwasaki H, Reizis B, Ye M, Graf T, Weissman IL.et al. (2002Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment Dev Cell 3137–147. [DOI] [PubMed] [Google Scholar]
- Tondreau T, Lagneaux L, Dejeneffe M, Massy M, Mortier C, Delforge A.et al. (2004Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation Differentiation 72319–326. [DOI] [PubMed] [Google Scholar]
- Gardner JM, Fletcher AL, Anderson MS., and, Turley SJ. AIRE in the thymus and beyond. Curr Opin Immunol. 2009;21:582–589. doi: 10.1016/j.coi.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan M, Kisand K, Kont V, Möll K, Tserel L, Scott HS.et al. (2009Autoimmune regulator deficiency results in decreased expression of CCR4 and CCR7 ligands and in delayed migration of CD4+ thymocytes J Immunol 1837682–7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh E, Lind SM, Lindmark E, Hässler S, Perheentupa J, Peltonen L.et al. (2008AIRE regulates T-cell-independent B-cell responses through BAFF Proc Natl Acad Sci USA 10518466–18471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller CE, Wang CL, Beck-Engeser G, Goss L, Scott HS, Anderson MS.et al. (2008Expression of Aire and the early wave of apoptosis in spermatogenesis J Immunol 1801338–1343. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Hirota F, Yano M, Kitajima H, Miyazaki J, Kawamoto H.et al. (2010Biphasic Aire expression in early embryos and in medullary thymic epithelial cells before end-stage terminal differentiation J Exp Med 207963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., and, Shinohara ML. Regulation of T-helper-cell lineage development by osteopontin: the inside story. Nat Rev Immunol. 2009;9:137–141. doi: 10.1038/nri2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA.et al. (2008Systemic endocrine instigation of indolent tumor growth requires osteopontin Cell 133994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH.et al. (2006Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells Nat Immunol 7498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudres M, Norol F, Trenado A, Grégoire S, Charlotte F, Levacher B.et al. (2006Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice J Immunol 1767761–7767. [DOI] [PubMed] [Google Scholar]
- Schurgers E, Kelchtermans H, Mitera T, Geboes L., and, Matthys P. Discrepancy between the in vitro and in vivo effects of murine mesenchymal stem cells on T-cell proliferation and collagen-induced arthritis. Arthritis Res Ther. 2010;12:R31. doi: 10.1186/ar2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman RE, Yoo D, LeRoux MA., and, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8:110–123. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC.et al. (2003Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection J Cell Biochem 891235–1249. [DOI] [PubMed] [Google Scholar]
- Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF., and, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vivo tracking of AIRE−/− and WT MSCs.
Reactive oxygen species in T cells after coculture with MSCs.
Characterization of mouse and human BMSC PTA expression.
Immunophenotype of WT and Aire−/− stroma.
Oligonucleotide sequences to study peripheral tissue antigen expression