Abstract
The safety and efficacy of hematopoietic stem cell (HSC) mobilization was investigated in adult splenectomized (SPL) and non-SPL patients with thalassemia major, in two clinical trials, using different mobilization modes: granulocyte-colony-stimulating factor (G-CSF)-alone, G-CSF following pretreatment with hydroxyurea (HU), plerixafor-alone. G-CSF-mobilization was both safe and effective in non-SPL patients. However, in SPL patients the procedure resulted in excessive response to G-CSF, expressed as early hyperleukocytosis necessitating significant dose reduction, and suboptimal CD34+ cells yields. One-month HU-pretreatment prevented hyperleukocytosis and allowed successful CD34+ cell collections when an optimal washout period was maintained, but it significantly prolonged the mobilization procedure. Plerixafor resulted in rapid and effective mobilization in both SPL and non-SPL patients and was well-tolerated. For gene therapy of thalassemia, G-CSF or Plerixafor could be used as mobilization agents in non-SPL patients whereas Plerixafor appears to be the mobilization agent of choice in SPL adult thalassemics in terms of safety and efficacy.
Introduction
Gene therapy for thalassemia will require optimal numbers of transduced hematopoietic stem cells (HSCs) to be infused to the patient. Mobilized peripheral blood (PB) represents a desired source of HSCs, due to the higher yields of CD34+ cells1,2,3 and the atraumatic collection procedure, as compared to conventional bone marrow (BM) harvest. In nonthalassemic individuals, serious adverse events are rare following granulocyte-colony-stimulating factor (G-CSF) mobilization,4 but there is a scarcity of information on the safety and efficacy of mobilization in adult patients with β-thalassemia major. Adult thalassemic patients often present with advanced organ damage due to accumulated iron and may possibly have a decreased BM stem cell reservoir, due to the BM suppression in response to multiple transfusions. In addition, a great proportion of adult patients have undergone splenectomy and there is a lack of information on the safety and efficacy of mobilization in asplenic individuals.
Until recently, G-CSF was the only agent available for stem cell mobilization in humans. Although G-CSF is generally well tolerated, the rare events of splenic rupture5,6,7,8,9 or thrombosis10,11,12 during G-CSF-mobilization in normal donors or patients with hematologic malignancies, raise concerns for its use in thalassemia where chronic splenomegaly and hypercoagulability exist. The recently available mobilizing agent, plerixafor (Mozobil; Genzyme, Cambridge, MA and Cambridge, UK formerly known as AMD3100) which reversibly inhibits the CXCR4–SDF1 interaction within the BM microenvironment resulting in rapid mobilization, could represent an attractive alternative to G-CSF due to its different mode of action and its emerging safety profile.13,14
The goals of our studies were first to investigate approaches for safe collection of high numbers of CD34+ cells from adult splenectomized (SPL) or non-SPL patients with severe thalassemia. Second, to cryopreserve these cells for use in a planned globin gene therapy trial for thalassemia. We first investigated the safety and efficacy of G-CSF mobilization with or without pretreatment with hydroxyurea (HU) and subsequently we explored the safety and efficacy of mobilization with plerixafor.
Results
Patients
From the 26 patients, enrolled from February 2007 to August 2010, in the G-CSF study, 23 were evaluable: 10 non-SPL (6 without HU-pretreatment and 4 with HU-pretreatment) and 13 SPL (4 without HU-pretreatment and 9 with HU-pretreatment). Three patients dropped out during the study; one after HU-pretreatment, because of thalassemia-associated hypersplenism with subsequent increase in spleen volume exceeding the eligibility threshold; the second because of a greater than 80% increase in spleen volume during G-CSF administration; and the third due to noncompliance.
Ten patients enrolled in the Plerixafor study, since September 2010. Nine patients, 5 SPL and 4 non-SPL, were treated with Plerixafor alone. One SPL patient who had previously mobilized with G-CSF-alone was remobilized with Plerixafor+G-CSF.
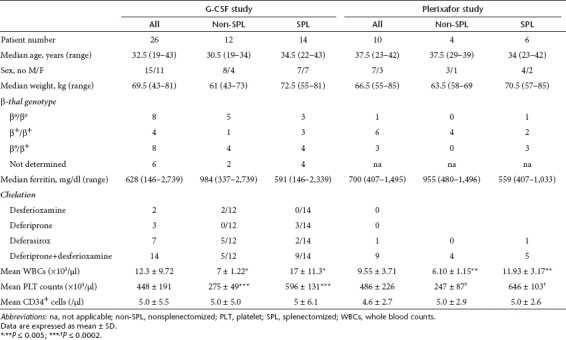
Patient characteristics at baseline are shown in Table 1.
Table 1. Patient characteristics at baseline.
Safety
No serious adverse events occurred. Toxicity was graded according to the Common Terminology Criteria for Adverse Events v3.0. The most common adverse events following G-CSF administration were bone pain, low-grade fever, and grade 1 thrombocytopenia during G-CSF-leukapheresis. HU was generally well tolerated, resulting in uncomplicated neutropenia and thrombocytopenia (grade 1–3). Plerixafor has been very well tolerated and only mild toxicities, most commonly nausea, diarrhea and injection site erythema were encountered.
CD34+ cell yields in G-CSF treated, non-SPL subjects with thalassemia
Six non-SPL patients were mobilized with G-CSF alone and all but one yielded adequate CD34+ cell numbers. These yields were comparable to those of 21 normal adult sibling donors in consecutive allogeneic blood HSC transplantations performed in our department during September 2009–September 2010 (CD34+ cells × 106/kg of donor/2 aphereses: thalassemia patients 6.67 ± 2.87 versus normal donors 5.00 ± 1.75, P = 0.1). The maximum mean white blood cells (WBC) (×103/µl) and PB CD34+ cells (/µl) during the procedure were 39.34 ± 13.74 and 37.1 ± 0.8, respectively (Figure 1a, left panel, and Tables 2 and 3).
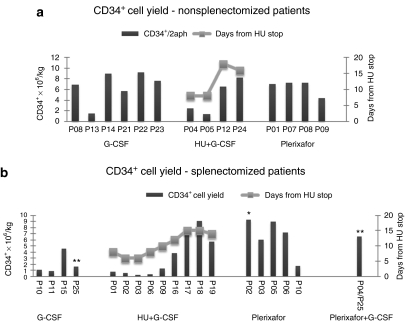
Figure 1.
CD34+ cell yield. (a) Non-SPL patients. The yields from non-SPL subjects, in total 2 aphereses, are shown individually in bars. Left panel: G-CSF-treated subjects; middle panel: HU+G-CSF-treated subjects; right panel: plerixafor-treated subjects. The mobilization outcome in the HU+G-CSF-treated group is directly correlated with the length of the washout period after HU (gray line). (b) SPL patients. The yields from SPL subjects, in total 2 aphereses, are shown individually in bars. Left panel: G-CSF-treated subjects; left-middle panel: HU+G-CSF-treated subjects; right-middle panel: Plerixafor-treated subjects; right panel: Plerixafor+G-CSF-treated subject. The mobilization outcome in the HU+G-CSF-treated group is directly correlated with the length of the washout period after HU (gray line). The primary y-axis represents the CD34+ cell yield ×106/kg and the secondary y-axis the days of washout period after HU-pretreatment. P25 and P04/P25 (**) is the same patient mobilized initially with G-CSF and later with Plerixafor+G-CSF. Patients P02 (*) and P04/P25 (**) reached the target cell dose in 1 apheresis. G-CSF, granulocyte-colony stimulating factor; HU, hydroxyurea; non-SPL, nonsplenectomized; SPL, splenectomized.
Table 2. Cumulative data on mobilization parameters.
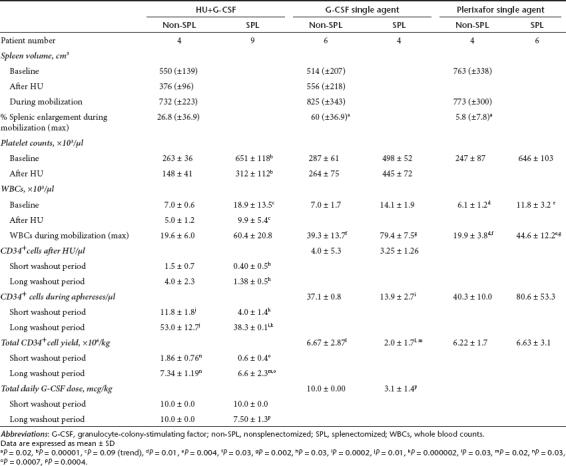
Table 3. Individual characteristics and mobilization parameters in nonsplenectomized patients.
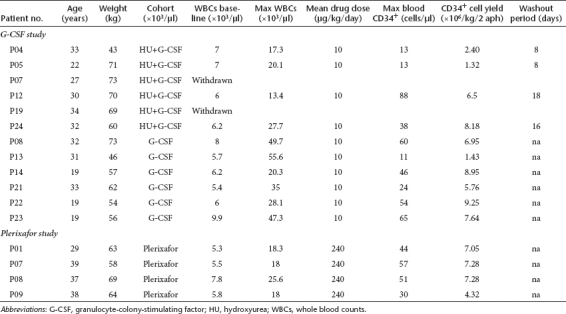
Six non-SPL patients were pretreated with HU. The rationale was that HU would result in an increase of CD34+ cells after its cessation15 and would reduce the degree of splenomegaly (by reducing extramedullary hematopoiesis),16,17 potentially minimizing the risk of splenic rupture and of cell sequestration during G-CSF-mobilization. Patients who started G-CSF after a washout period of 8 days from HU cessation yielded low CD34+ cells numbers, whereas those who received G-CSF after a longer interval period (16 and 18 days) mobilized successfully (CD34+ cells × 106/kg: 1.86 ± 0.76 versus 7.34 ± 1.19, respectively, P = 0.03) (Figure 1a, middle panel; Tables 2 and 3). This finding suggests that there is a critical washout period to allow BM recovery after the myelosuppressive effect of HU, which greatly affects the mobilization efficiency. The mean blood CD34+ cells count after HU and before G-CSF was 1.5 ± 0.71/µl in patients who received G-CSF after the short washout period and 4.0 ± 2.35/µl in those who were mobilized after the long washout period (Table 2).
HU resulted in a reduction of spleen volume before G-CSF administration (376 ± 96 cm3 versus 556 ± 218 cm3, P = 0.1) and in less splenic enlargement (26.8% versus 60%, P = 0.1) during mobilization as compared to non-HU-pretreated subjects, although these differences did not reach statistical significance (Table 2).
The CD34+ yields from patients who did not receive HU-pretreatment and patients who were optimally pretreated with HU, were similar, indicating that HU has no additional beneficial role in the mobilization efficiency or that there is a need for a longer washout period between HU treatment and G-CSF administration (Table 2).
G-CSF-mobilization in SPL thalassemic patients results in excessive leukocytosis, indirectly affecting the CD34+ cell yield
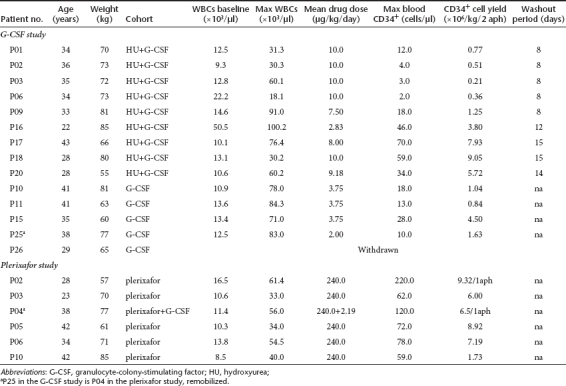
SPL patients presented with reactive leukocytosis and thrombocytosis at baseline (Table 1). The first treated patient was started at the standard G-CSF dose of 10 µg/kg. After just two doses, G-CSF triggered early hyperleukocytosis (WBC 71 × 103/µl) which necessitated significant subsequent dose reductions and adjustments based on the daily WBC levels, in an effort to avoid a thrombotic complication. In the subsequent SPL patients, the dose of G-CSF was initiated at 2.5 µg/kg/day, and adjusted according to the degree of leukocytosis. Although the daily G-CSF dose in the SPL patients was reduced ~70% (Tables 2 and 4), the mean maximum WBC level still reached 79 ± 7.5 × 103/µl whereas it was not accompanied by a proportional increase in PB CD34+ cells (Figure 2a, left panel and Table 2). As a consequence of the reduced G-CSF doses, mobilization was affected in all but one case, resulting in poor yields (mean CD34+ cells × 106/kg: 2.0 ± 1.7, range 0.84–4.5) (Figure 1b, left panel and Tables 2 and 4).
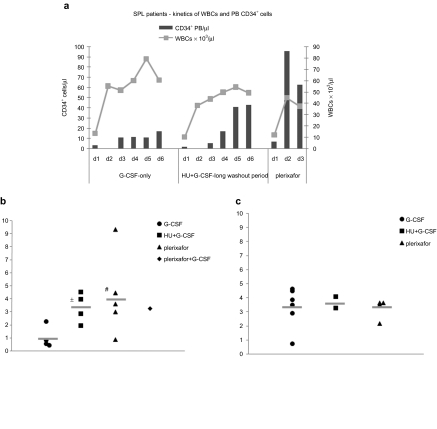
Figure 2.
Kinetics of white blood cells (WBCs) and peripheral blood CD34+ cells in splenectomized (SPL) patients and the mean yield of CD34+ cells per patient per apheresis. (a) Kinetics of WBCs and peripheral blood CD34+ cells in the SPL patients treated with G-CSF or HU+G-CSF (with the long washout period) or plerixafor. (b) The mean yield per patient per apheresis in SPL subjects treated with granulocyte-colony stimulating factor (G-CSF), HU+G-CSF (with the long washout period), plerixafor and plerixafor + G-CSF. (c) The mean yield per patient per apheresis in non-SPL subjects treated with G-CSF, HU+G-CSF (with the long washout period), and plerixafor. *P = 0.02 versus G-CSF, #P = 0.09 versus G-CSF. HU, hydroxyurea.
Table 4. Individual characteristics and mobilization parameters in splenectomized patients.
HU-pretreatment can prevent G-CSF-induced hyperleukocytosis in SPL thalassemic patients but successful mobilization depends on the length of the washout period
The length of the washout period between HU discontinuation and initiation of G-CSF, proved to be critical for successful mobilization; SPL patients who started G-CSF 8 days after HU cessation, mobilized extremely poorly, whereas those for whom the washout period was extended to 12–15 days mobilized successfully (CD34+ cells × 10/kg6: 0.62 ± 0.41 versus 6.63 ± 2.34, respectively, P = 0.0007) (Figure 1b, middle-left panel and Tables 2 and 4). The mean blood CD34+ cells count after HU and before G-CSF was 0.40 ± 0.54/µl in patients who received G-CSF after the short washout period and 1.38 ± 0.48/µl in those who received G-CSF after the long washout period (Table 2, P = 0.03).
HU-pretreatment reversed splenectomy-associated thrombocytosis and leukocytosis before G-CSF (platelets (×103/µl): 312 ± 112.1 versus 651 ± 118.21, P = 0.00001/WBCs (×103/µl): 9.94 ± 5.4 versus 18.88 ± 13.0, P = 0.09, Table 2). In addition, SPL patients treated with G-CSF after the optimal interval period from HU, developed “smooth ” increases in WBCs and less exuberant leukocytosis during the mobilization procedure as compared to non-HU-pretreated patients (Figure 2a, left and middle panel). PB CD34+ cells peaked, as expected, on days 5 and 6 in accordance to the increase in WBCs (Figure 2a, middle panel and Table 4). In addition, these subjects were able to tolerate close to standard daily G-CSF doses compared to non-HU-pretreated SPL patients (total mean daily dose 7.50 ± 1.28 µg/kg versus 3.10 ± 1.39 µg/kg, respectively, P = 0.0004) (Tables 2 and 4).
Plerixafor results in rapid and efficient CD34+ cell mobilization in subjects with thalassemia without inducing excessive leukocytosis in SPL patients
Nine patients were mobilized with plerixafor as single agent and one (SPL) patient received low-dose G-CSF+plerixafor (P04/P25). Of these 10 patients, eight reached the target cell dose of 6 × 106 CD34+ cells/kg in either one or two aphereses. One non-SPL patient (P09) failed to reach the cell target whereas one SPL patient (P10) experienced a mobilization failure yielding a total of 1.73 × 106/kg CD34+ cells (Figure 1a, right panel, Figure 1b, right-middle panel, and Tables 3 and 4). One SPL patient (P25), who was unsuccesfuly mobilized with G-CSF-alone in the G-CSF study (CD34+ cell yield: 1.63 × 106/kg2 aphereses) due to receiving substandard drug doses to control hyperleukocytosis, and who was remobilized in the plerixafor study (P04/P25) with low-dose G-CSF+plerixafor, yielded 6.5 × 106 CD34+ cells/kg in one apheresis without excessive increases of WBCs (max WBCs 56 × 103/µl) (Figure 1b, right-middle panel and Table 4).
The mean value of CD34+ cells with plerixafor was 6.22 ± 1.65 × 106/kg (in a mean of 2 aphereses) in non-SPL patients and 6.63 ± 3,05 × 106/kg in SPL patients (in a mean of 1.8 aphereses) (Table 2). In SPL subjects, the mean yield per apheresis with plerixafor was higher, with a trend to significance, over its G-CSF counterpart and similar to the mean yield obtained by the optimally HU+G-CSF-treated patients in the G-CSF study (CD34+ cells/kg × 106/apheresis: 4.25 ± 3.13 versus 1.01 ± 0.85 versus 3.32 ± 1.16, P = 0.09 and P = 0.6, respectively) (Figure 2b). No difference in terms of yield per apheresis was observed in non-SPL patients mobilized with G-CSF or plerixafor (Figure 2c).
The maximum WBC (×103/µl) reached during mobilization with plerixafor was 44.58 ± 12.20 and 19.98 ± 3.75 in SPL and non-SPL patients, respectively (Table 2). Although these values represent a significant increase over baseline, they are significantly lower than the corresponding values of SPL and non-SPL patients treated with adjusted or standard doses of G-CSF-alone, respectively (Figure 2a and Table 2), confirming the observation that plerixafor does not induce leukocytosis to the same extent as G-CSF. The mean PB CD34+ cells during aphereses were 40.25 ± 10.00/µl and 80.56 ± 53.26/µl in non-SPL and SPL subjects, respectively (Table 2).
Importantly for the non-SPL patients treated with plerixafor, a mean increase of splenic volume of only 5.8 ± 7.8% (range 0–19%) was encountered which was significantly lower than the 60% mean spleen volume increase during G-CSF mobilization (Table 2).
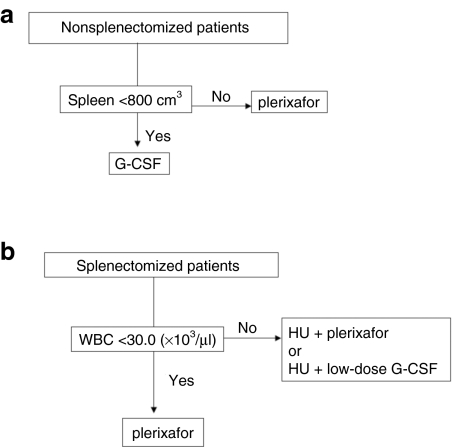
A suggested algorithm for mobilizing SPL and non-SPL patients with thalassemia is provided as Figure 3.
Figure 3.
Suggested HSC mobilization algorithm for patients with thalassemia. (a) Nonsplenectomized patients. Non-SPL patients with spleen volume <800 cm3 (arbitrary cutoff) could be mobilized with G-CSF whereas patients with excessive splenomegaly (>800 cm3) would better receive Plerixafor which results in significantly less splenic enlargement. (b) Splenectomized patients. SPL patients with baseline WBC <30.0 × 103/µl (arbitrary cutoff) could be mobilized with Plerixafor which triggers less leukocytosis compared to G-CSF, whereas SPL patients with >30.0 × 103/µl baseline WBCs would better receive pretreatment with HU (with the optimal washout interval) to reduce WBCs before mobilization, either with Plerixafor or low-dose G-CSF. HSC, hematopoietic stem cell; HU, hydroxyurea; non-SPL, nonsplenectomized; SPL, splenectomized.
CD34+ cell recovery and purity, expression of primitive hematopoietic cell surface markers and clonogenic capacity of thalassemic CD34+ cells
In both the G-CSF and the Plerixafor trials, postselection CD34+ cell recovery ranged from 46 to 100% (mean 71%) and purity from 72 to 100% (mean 92%) in all patients. No significant differences in recovery and purity of CD34+ cells were detected between patient subsets.
Comparison of primitive hematopoietic cell surface marker expression (CD34+CD38-/CD34+CD38-HLADR-/CD34+CD38-CD45RA+) in purified thawed cells mobilized with G-CSF or HU+G-CSF or Plerixafor, demonstrated a trend for higher frequencies of cells expressing primitive phenotypic markers in the Plerixafor-treated patients (Table 5).
Table 5. Clonogenic capacity and expression of primitive hematopoietic cell surface markers of CD34+ cells.
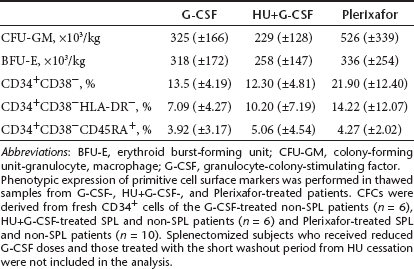
Absolute numbers of colony-forming cells derived from G-CSF-, HU+G-CSF-, or Plerixafor-mobilized fresh CD34+ cells were comparable (Table 5), without differences in clonogenic potential between SPL and non-SPL patients mobilized with the three different modes (data not shown). Patients who mobilized poorly due to receiving substandard G-CSF doses (SPL) or because of the short washout period from HU were not included in the analysis.
Discussion
The current information on autologous HSC collection from G-CSF-mobilized blood18 or steady-state BM19 of thalassemic patients is almost exclusively based on a pediatric patient population. Adult thalassemics constitute a distinct population however, in which the age-dependent disease sequelae due to accumulated iron, the suppressive effect of long-term transfusions and chelation on the stem cell compartment, and the “aged ” stem cells, could compromise the safety and success of HSC collection and subsequently of gene therapy.
In these trials, we used different mobilization strategies in order to determine an optimal approach for collecting an autologous graft from adult subjects with thalassemia before proceeding with β-globin gene transfer into the collected HSCs in a future clinical collaborative trial.
G-CSF-mobilized PB represents the most widely used source of HSC for autologous or allogeneic transplantation.20 Compared to BM harvest, G-CSF-mobilized PB yields higher numbers of CD34+ cells and demonstrates a shorter time to engraftment, whereas, importantly for the patient, the mobilization-leukapheresis procedure is less invasive.1,2,3 Thus, in thalassemia GT, where high numbers of transduced HSCs will need to be introduced into the patients, G-CSF-mobilized PB could be the preferred source of HSCs.
However, the specific features of the disease such as splenomegaly and procoagulability could lead to unpredictable responses in terms of adverse events and CD34+ cell yield. Furthermore, G-CSF-related enlargements of the spleen have been reported21,22,23 which have very rarely resulted in splenic rupture.5,6,7,8,9 Conceivably, a pre-existing splenic enlargement as occurs in thalassemia, could increase the risk of splenic rupture because of further organ enlargement during mobilization. For these reasons, we explored the possibility, that HU could reduce spleen volume before G-CSF administration, thus preventing excessive splenomegaly during mobilization. In this study, G-CSF-mobilization resulted in a maximum 60% spleen volume increase; pretreatment with HU decreased splenic enlargement but this decrease was not statistically significant.
Thalassemia has been associated with a chronic procoagulant state which is more prevalent in SPL patients with the intermediate form of the disease.24,25 Moreover, G-CSF has been reported to precipitate thrombotic events in high-risk individuals,10,11,12 severe sickle cell crisis in patients with sickle cell disease (SCD)26,27,28 and splenic infarcts in thalassemic mice.29 SPL thalassemic patients could theoretically be at increased risk for thromboembolic events during G-CSF-mobilization because of the secondary thrombocytosis and leukocytosis, the latter further exacerbated by G-CSF, and the high numbers of circulating damaged thalassemic red cells (that ordinarily would have been removed by the spleen).30,31 In our study, HU played a safety role in SPL subjects, by normalizing the high numbers of circulating WBCs and platelets before G-CSF administration, thus preventing the steep increases of WBCs shown in SPL subjects during G-GSF mobilization.
SPL patients would have been—a priori—considered as effective mobilizers because of the absence of a site which could sequester HSCs from the periphery. Surprisingly, mobilization in SPL, non-HU-pretreated subjects was poor in all but one patient. This effect however, was not a mobilization deficit inherent to splenectomy, but probably the result of the mandatory G-CSF dose reductions required to avoid hyperleukocytosis. Indeed, SPL subjects mobilized following the optimal washout period from HU yielded high numbers of CD34+ cells and tolerated almost regular daily G-CSF doses without developing abrupt increases in WBCs. In both SPL and non-SPL patients, the interval period between HU discontinuation and G-CSF initiation was critical to allow for BM recovery after HU myelosuppresion and therefore successful mobilization. Although optimal HU-pretreatment overcame the limitation of hyperleukocytosis during G-CSF mobilization in SPL subjects and resulted in safe and successful mobilization, it significantly prolonged the mobilized procedure by 6 weeks.
Plerixafor, due to its different mode of action and kinetics as compared to G-CSF, resulted in rapid and successful mobilization in the majority of thalassemic patients without inducing excessive leukocytosis in the SPL patients. Importantly also, in non-SPL subjects, Plerixafor resulted in a 10-fold lower splenic enlargement as compared to the G-CSF-mobilized patients.
Mobilization with single-agent Plerixafor has been reported to provide modest and, in several cases, inferior CD34+ cells yields over single-agent G-CSF in healthy donors or in patients with lymphomas or multiple myeloma.14,32,33 Interestingly, in our study, almost all non-SPL and SPL thalassemic patients treated with Plerixafor-alone yielded sufficient numbers of CD34+ cells. The CD34+ cell yields were similar to those obtained in the G-CSF-treated non-SPL patients and in the optimally HU+G-CSF-treated SPL patients.
Importantly also, Plerixafor-mobilized HSCs have been reported to provide durable engraftment both in preclinical models and in clinical settings and to achieve long-term repopulating capacity with higher gene marking levels as compared to G-CSF-mobilized CD34+ cells.34,35,36
Overall, in our studies, Plerixafor was well-tolerated and associated with successful HSC collections in both non-SPL patients and the SPL thalassemic subjects. Taking into consideration, the emerging safety profile of Plerixafor,14 the rapidity of mobilization and the successful CD34+ collections procured with single-agent Plerixafor, as well as the long-term repopulating capacity of Plerixafor-mobilized HSCs.34,35,36 mobilization with this agent potentially represents the optimal mobilization treatment to enable gene therapy of SPL patients with thalassemia. In addition, its use could be also considered for gene therapy of SCD in which documented complications preclude G-CSF mobilization.
Materials and Methods
Study approval. The studies were registered with the European clinical trials database (EudraCT number 2005-000315-10, 2009-014136-37) and at www.clinicaltrials.gov (NCT00336362, NCT01206075) and were reviewed and approved by the Greek IEC at George Papanicolaou Hospital, the US institutional review board at the University of Washington, and the National Organization for Medicines in Greece. The study was conducted at the George Papanicolaou Hospital in Thessaloniki, Greece in compliance with the Declaration of Helsinki. All patients provided written informed consent.
Studies design and patients. The G-CSF study was designed to assess the safety and efficacy of mobilization with G-CSF-alone or after HU-pretreatment, in SPL or non-SPL adults with β-thalassemia major. SPL patients with platelet counts in excess of 550 × 103/µl, were enrolled in the HU arm due to a putative increased risk of thrombosis. HU was administered at 10 mg/kg/day and escalated during the second week of treatment up to 20 mg/kg/day in the non-SPL patients and up to 25 mg/kg/day in the SPL patients, adjusted weekly to the degree of hematologic toxicity. Initially, HU was stopped 1 week prior to treatment with G-CSF but, following evaluation of the mobilization data, this interval period was increased up to 15 days or until the CD34+ cells became ³1/µl accompanied by initial recovery from the hematologic toxicity. G-CSF was administered at 10 µg/kg/day by two subcutaneous injections for 4–7 days. Because of the development of early, excessive leukocytosis by the first SPL patient who was mobilized with G-CSF, SPL subjects were started on low doses of G-CSF (2.5 µg/kg/day) which were subsequently adjusted according to the degree of leukocytosis. Two consecutive leukapheresis were conducted, with a target cell yield of at least 2 × 106 CD34+ cells/kg (Supplementary Figure S1).
Plerixafor (provided by Genzyme, gratis), was administered subcutaneously at 0.24 mg/kg/day on two consecutive evenings followed by leukapheresis 10 hours later, unless ≥6 × 106 CD34+ cells/kg were collected in 1 apheresis. Subjects who failed to achieve 6 × 106/kg CD34+ cells per two aphereses or those who had failed to mobilize effectively with G-CSF-alone, were offered the opportunity to be remobilized with the combination of G-CSF+Plerixafor (Supplementary Figure S2).
Patient eligibility in either study was based on common criteria shown in Supplementary Table S1.
In patients who mobilized successfully, 2 × 106/kg CD34+ cells from the combined leukapheresis product were cryopreserved as unmanipulated “back-up ” cells. The rest of the product was enriched for CD34+ cells by a CliniMacs device (Miltenyi Biotech, Auburn, CA and Bergisch Gladbach, Germany) and the bulk of the CD34+ selected cells was stored separately for a future β-globin gene transfer trial, for which these participants would be eligible and willing to participate. Small aliquots of the final product was sampled for sterility, CD34+ cell content by flow cytometry, clonogenic capacity (colony-forming unit-granulocyte, macrophage, erythroid burst-forming unit) and later transduction with an optimized β-globin lentiviral vector for in vitro and xenotransplant studies (Supplementary Figures S1 and S2).
Patients were followed by weekly physical and laboratory evaluations for up to one month after completion of mobilization.
Safety. Safety was monitored by the incidence of adverse events and serious adverse events in terms of changes from baseline, clinical laboratory measurements, and physical examination findings. In non-SPL patients receiving G-CSF, spleen size was determined by physical examination daily, and by ultrasound every other day (V = 0.523 × length × thickness × width37). If the spleen volume increased by >80% as compared to baseline, G-CSF was discontinued and the patient was withdrawn. In SPL patients, the development of excessive leukocytosis (≥100 × 103/µl) would trigger drug discontinuation and leukapheresis for the purpose of leukoreduction, regardless of CD34+ counts.
CFU assays and flow cytometry analysis. Fresh, purified CD34+ cells were plated at 1 × 103 cell/ml in complete methylcellulose medium (GF H4434; StemCell Technologies, Tukwila, WA and Vancouver, British Columbia, Canada) and colony-forming unit-granulocyte, macrophage and erythroid burst-forming unit colonies were scored after 14 days.
Flow cytometry analysis was performed on thawed CD34+ cell samples from G-CSF-, HU+G-CSF- and Plerixafor-treated individuals, labeled with PE-Cy7-conjugated anti-CD34 antibody and analyzed for the expression of primitive markers using the following antibodies: APC-conjugated anti-CD38 and PE-conjugated HLA-DR and CD45RA (BD Biosciences Pharmingen, San Jose, CA and Heidelberg, Germany). Results were obtained on a FACSCanto flow cytometer (BD, Franklin Lakes, NJ and Oxford, UK) and analyzed with the FACSDiva 6 software.
Statistics. Descriptive statistics were used to define characteristics of patients and patient subsets. Data are expressed as means ± SD. Means of continuous variables were compared using paired t-test.
SUPPLEMENTARY MATERIAL Figure S1. G-CSF study scheme. Figure S2. Plerixafor study scheme. Table S1. Eligibility criteria.
Acknowledgments
We thank the patients for their participation and the physicians who referred patients to this program. We thank Zoe Moustakoudaki and Anastasia Papadopoulou (G. Papanicolaou Hospital) for technical assistance and Miranda Athanasiou-Metaxa (Hippokration Hospital) for performing the medical monitoring locally. We thank Genzyme for providing Plerixafor gratis. This work was supported by NIH grant #U01 HL66947. The authors declared no conflict of interest.
Supplementary Materials
G-CSF study scheme.
Plerixafor study scheme.
Eligibility criteria.
REFERENCES
- To LB, Haylock DN, Simmons PJ., and, Juttner CA. The biology and clinical uses of blood stem cells. Blood. 1997;89:2233–2258. [PubMed] [Google Scholar]
- Champlin RE, Schmitz N, Horowitz MM, Chapuis B, Chopra R, Cornelissen JJ.et al. (2000Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT) Blood 953702–3709. [PubMed] [Google Scholar]
- Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R.et al. (2001Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers N Engl J Med 344175–181. [DOI] [PubMed] [Google Scholar]
- Miller JP, Perry EH, Price TH, Bolan CD, Jr, Karanes C, Boyd TM.et al. (2008Recovery and safety profiles of marrow and PBSC donors: experience of the National Marrow Donor Program Biol Blood Marrow Transplant 149 Suppl29–36. [DOI] [PubMed] [Google Scholar]
- Falzetti F, Aversa F, Minelli O., and, Tabilio A. Spontaneous rupture of spleen during peripheral blood stem-cell mobilisation in a healthy donor. Lancet. 1999;353:555. doi: 10.1016/S0140-6736(99)00268-8. [DOI] [PubMed] [Google Scholar]
- Becker PS, Wagle M, Matous S, Swanson RS, Pihan G, Lowry PA.et al. (1997Spontaneous splenic rupture following administration of granulocyte colony-stimulating factor (G-CSF): occurrence in an allogeneic donor of peripheral blood stem cells Biol Blood Marrow Transplant 345–49. [PubMed] [Google Scholar]
- Brown SL., and, Dale DC. Spontaneous splenic rupture following administration of granulocyte colony-stimulating factor (G-CSF): occurrence in an allogeneic donor of peripheral blood stem cells. Biol Blood Marrow Transplant. 1997;3:341–343. [PubMed] [Google Scholar]
- Balaguer H, Galmes A, Ventayol G, Bargay J., and, Besalduch J. Splenic rupture after granulocyte-colony-stimulating factor mobilization in a peripheral blood progenitor cell donor. Transfusion. 2004;44:1260–1261. doi: 10.1111/j.1537-2995.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- Dincer AP, Gottschall J., and, Margolis DA. Splenic rupture in a parental donor undergoing peripheral blood progenitor cell mobilization. J Pediatr Hematol Oncol. 2004;26:761–763. doi: 10.1097/00043426-200411000-00015. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y, Miyamoto T, Okamura T, Gondo H, Iwasaki H, Horiuchi T.et al. (1997Angina pectoris occurring during granulocyte colony-stimulating factor-combined preparatory regimen for autologous peripheral blood stem cell transplantation in a patient with acute myelogenous leukaemia Br J Haematol 97666–668. [DOI] [PubMed] [Google Scholar]
- Lindemann A., and, Rumberger B. Vascular complications in patients treated with granulocyte colony-stimulating factor (G-CSF) Eur J Cancer. 1993;29A:2338–2339. doi: 10.1016/0959-8049(93)90236-9. [DOI] [PubMed] [Google Scholar]
- Sinha S, Poh KK, Sodano D, Flanagan J, Ouilette C, Kearney M.et al. (2006Safety and efficacy of peripheral blood progenitor cell mobilization and collection in patients with advanced coronary heart disease J Clin Apher 21116–120. [DOI] [PubMed] [Google Scholar]
- Liles WC, Broxmeyer HE, Rodger E, Wood B, Hübel K, Cooper S.et al. (2003Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist Blood 1022728–2730. [DOI] [PubMed] [Google Scholar]
- Devine SM, Vij R, Rettig M, Todt L, McGlauchlen K, Fisher N.et al. (2008Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction Blood 112990–998. [DOI] [PubMed] [Google Scholar]
- Richard RE, Siritanaratkul N, Jonlin E, Skarpidi E, Heimfeld S., and, Blau CA. Collection of blood stem cells from patients with sickle cell anemia. Blood Cells Mol Dis. 2005;35:384–388. doi: 10.1016/j.bcmd.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Lima CS, Saad ST., and, Costa FF. Successful use of hydroxyurea in beta-thalassemia major. N Engl J Med. 1997;336:964. doi: 10.1056/NEJM199703273361318. [DOI] [PubMed] [Google Scholar]
- Saxon BR, Rees D., and, Olivieri NF. Regression of extramedullary haemopoiesis and augmentation of fetal haemoglobin concentration during hydroxyurea therapy in beta thalassaemia. Br J Haematol. 1998;101:416–419. doi: 10.1046/j.1365-2141.1998.00719.x. [DOI] [PubMed] [Google Scholar]
- Li K, Wong A, Li CK, Shing MM, Chik KW, Tsang KS.et al. (1999Granulocyte colony-stimulating factor-mobilized peripheral blood stem cells in beta-thalassemia patients: kinetics of mobilization and composition of apheresis product Exp Hematol 27526–532. [DOI] [PubMed] [Google Scholar]
- Frittoli MC, Biral E, Cappelli B, Zambelli M, Roncarolo MG, Ferrari G.et al. (2011Bone marrow as a source of hematopoietic stem cells for human gene therapy of ß-thalassemia Hum Gene Ther 22507–513. [DOI] [PubMed] [Google Scholar]
- Gratwohl A, Baldomero H, Frauendorfer K., and, Urbano-Ispizua A, Joint Accreditation Committee, International Society for Cellular Therapy; European Group for Blood and Marrow Transplantation 2006). EBMT activity survey 2004 and changes in disease indication over the past 15 years. Bone Marrow Transplant37: 1069–1085. [DOI] [PubMed]
- Picardi M, De Rosa G, Selleri C, Scarpato N, Soscia E, Martinelli V.et al. (2003Spleen enlargement following recombinant human granulocyte colony-stimulating factor administration for peripheral blood stem cell mobilization Haematologica 88794–800. [PubMed] [Google Scholar]
- Platzbecker U, Prange-Krex G, Bornhäuser M, Koch R, Soucek S, Aikele P.et al. (2001Spleen enlargement in healthy donors during G-CSF mobilization of PBPCs Transfusion 41184–189. [DOI] [PubMed] [Google Scholar]
- Stiff PJ, Bensinger W, Abidi MH, Gingrich R, Artz AS, Nademanee A.et al. (2009Clinical and ultrasonic evaluation of spleen size during peripheral blood progenitor cell mobilization by filgrastim: results of an open-label trial in normal donors Biol Blood Marrow Transplant 15827–834. [DOI] [PubMed] [Google Scholar]
- Taher AT, Otrock ZK, Uthman I., and, Cappellini MD. Thalassemia and hypercoagulability. Blood Rev. 2008;22:283–292. doi: 10.1016/j.blre.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Eldor A., and, Rachmilewitz EA. The hypercoagulable state in thalassemia. Blood. 2002;99:36–43. doi: 10.1182/blood.v99.1.36. [DOI] [PubMed] [Google Scholar]
- Adler BK, Salzman DE, Carabasi MH, Vaughan WP, Reddy VV., and, Prchal JT. Fatal sickle cell crisis after granulocyte colony-stimulating factor administration. Blood. 2001;97:3313–3314. doi: 10.1182/blood.v97.10.3313. [DOI] [PubMed] [Google Scholar]
- Abboud M, Laver J., and, Blau CA. Granulocytosis causing sickle-cell crisis. Lancet. 1998;351:959. doi: 10.1016/S0140-6736(05)60614-9. [DOI] [PubMed] [Google Scholar]
- Rosenbaum C, Peace D, Rich E., and, Van Besien K. Granulocyte colony-stimulating factor-based stem cell mobilization in patients with sickle cell disease. Biol Blood Marrow Transplant. 2008;14:719–723. doi: 10.1016/j.bbmt.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Yannaki E, Psatha N, Athanasiou E, Karponi G, Constantinou V, Papadopoulou A.et al. (2010Mobilization of hematopoietic stem cells in a thalassemic mouse model: implications for human gene therapy of thalassemia Hum Gene Ther 21299–310. [DOI] [PubMed] [Google Scholar]
- Cappellini MD, Robbiolo L, Bottasso BM, Coppola R, Fiorelli G., and, Mannucci AP. Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br J Haematol. 2000;111:467–473. doi: 10.1046/j.1365-2141.2000.02376.x. [DOI] [PubMed] [Google Scholar]
- Cappellini MD, Motta I, Musallam KM., and, Taher AT. Redefining thalassemia as a hypercoagulable state. Ann N Y Acad Sci. 2010;1202:231–236. doi: 10.1111/j.1749-6632.2010.05548.x. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA.et al. (2005Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist J Exp Med 2011307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak GW, Knopinska-Posluszny W, Matuszak M, Kisiel E, Hawrylecka D, Szmigielska-Kaplon A.et al. (2011Hematopoietic stem cell mobilization with the reversible CXCR4 receptor inhibitor plerixafor (AMD3100)-Polish compassionate use experience Ann Hematol 90557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle A, Krouse A, Metzger M, Orlic D, Donahue RE, Fricker S.et al. (2006AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates Blood 1073772–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs L, Mielcarek M, Little MT, Bridger G, Macfarland R, Fricker S.et al. (2005Durable engraftment of AMD3100-mobilized autologous and allogeneic peripheral-blood mononuclear cells in a canine transplantation model Blood 1064002–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DA, Bonde J, Craft TP, Craft TC, Wirthlin L, Hohm S.et al. (2007Human progenitor cells rapidly mobilized by AMD3100 repopulate NOD/SCID mice with increased frequency in comparison to cells from the same donor mobilized by granulocyte colony stimulating factor Biol Blood Marrow Transplant 13398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Odorico I, Spaulding KA, Pretorius DH, Lev-Toaff AS, Bailey TB., and, Nelson TR. Normal splenic volumes estimated using three-dimensional ultrasonography. J Ultrasound Med. 1999;18:231–236. doi: 10.7863/jum.1999.18.3.231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
G-CSF study scheme.
Plerixafor study scheme.
Eligibility criteria.