Abstract
In this study, we established and characterized human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) from four different donors. However, the hUCB-MSCs showed remarkable variations in their therapeutic efficacy for repairing rat infarcted myocardium (including the process of angiogenesis) 8 weeks after transplantation. In addition, we observed that the level of vascular endothelial growth factor (VEGF) is correlated with the therapeutic efficacy of the four hUCB-MSCs. Next, to investigate the practical application of hUCB-MSCs, we searched for surface signature molecules that could serve as indicators of therapeutic efficacy. The gene for N-cadherin was the only cell surface gene that was highly expressed in the most effective hUCB-MSCs, both at the transcriptional and translational levels. We observed downregulation and upregulation of VEGF in response to N-cadherin blocking and N-cadherin overexpression, respectively. Activation of extracellular signal-regulated kinase (ERK), but not protein kinase B, was increased when N-cadherin expression was increased, whereas disruption of N-cadherin-mediated cell–cell contact induced suppression of ERK activation and led to VEGF downregulation. Moreover, by investigating hUCB-MSCs overexpressing N-cadherin or N-cadherin knockdown hUCB-MSCs, we confirmed the in vivo function of N-cadherin. In addition, we observed that DiI-labeled hUCB-MSCs express N-cadherin in the peri-infarct area and interact with cardiomyocytes.
Introduction
Several preclinical studies have demonstrated that stem cells can improve cardiac function and promote angiogenesis after myocardial infarction (MI).1,2 However, recent human trials have shown conflicting results.3,4,5 There are many potential reasons for such discrepancies, including differences among species, biology, disease models, and cell preparations before delivery. Variations in stem cells from individual patients may be an additional important factor contributing to these unpredictable results. Moreover, cells used in autologous stem cell therapy are acquired from patients with multiple cardiovascular risk factors that are known to suppress the function of stem cells. Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) have recently emerged as a promising solution for allogeneic cell therapy.6 Several studies have reported that hUCB-MSCs can be successfully isolated, expanded, and differentiated into multi-lineages7,8,9,10,11 such as human bone marrow-derived mesenchymal stem cells. Moreover, hUCB-MSCs are extracted from relatively young and healthy donors who have low cardiovascular risk factors. These cells are available from a diverse range of donors. In the future, hUCB-MSCs from many donors could be stored and subsequently used as therapeutic cells. However, donor diversity could be a source of variable therapeutic effects.
There is a paucity of information regarding whether hUCB-MSCs from different donors have different biological characteristics and efficacies in improving myocardial repair after MI, even though they present similar MSC surface markers after isolation and expansion under standard operating procedures.
In this study, we established four hUCB-MSC lines (from different donors) and investigated their biological variations, their therapeutic efficacy in an MI model, and the principal mechanisms underlying these variations.
Results
hUCB-MSCs from four different donors had similar phenotypic characteristics
We established and characterized four hUCB-MSCs (M01, M02, M03, and M04) from four donors (Supplementary Figure S1A) according to standard procedures.10,12 To determine the phenotype of the UCB-derived cells, we examined their surface antigens by using flow cytometric analysis. All cells were observed to express hMSC-specific immunophenotypes, which were positive for CD29, CD44, CD73, CD105, CD166, and human leukocyte antigen (HLA)-ABC and negative for CD34, CD45, and HLA-DR (Figure 1). In addition, all cells exhibited immunosuppressive ability in a mixed lymphocyte reaction test (Supplementary Figure S1B) and showed similar proliferation potency (Supplementary Figure S1C). All the cells had the potential to differentiate into mesoderm lineages, including the osteogenic and chondrogenic lineages (Supplementary Figure S1D).
Figure 1.
Characterization of hUCB-MSCs from four different donors. Cell surface marker analysis. The purple histograms show the fluorescence intensity of hUCB-MSCs reacting with the indicated antibody during flow cytometry. The green histogram represents the isotype control. The phenotypes of hUCB-MSCs from four different donors are positive for CD29, CD44, CD73, CD105, CD166, and HLA-ABC and negative for CD34, CD45, and HLA-DR. HLA, human leukocyte antigen; hUCB-MSCs, human umbilical cord blood-derived mesenchymal stem cells.
Variable therapeutic efficacies of hUCB-MSCs from different donors in improving left ventricle function after MI
We compared the therapeutic efficacy of the four different hUCB-MSCs on postinfarction left ventricle (LV) remodeling in a rat model. In the phase I study (dose–response analysis), the minimum effective cell dose for infarcted myocardium repair was found to be 1 × 105 (data not shown). In the phase II study, we compared the therapeutic efficacy of four different hUCB-MSCs (1 × 105 cells) on postinfarction LV remodeling (Figure 2a). Three days post-MI, there were no significant differences among the five groups with respect to left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular ejection fraction (LVEF), or left ventricular fractional shortening (LVFS) (P > 0.05). Eight weeks after cell transplantation (means post-MI 9 weeks), the akinetic segments of the LV anterior walls were smaller in the M02 group than those in a control group that was not subjected to cell transplantation (Figure 2b). LV systolic function with M02 was improved significantly compared to that of the control group (ΔLVFS = 8.7 ± 1.6% versus −5.6 ± 2.0%, P < 0.05; Figure 2c). The M02 group also showed superior performance in improving LV systolic function compared to the other hUCB-MSCs (ΔLVFS = −5.3 ± 2.1% in groups M01, 8.7 ± 1.6% in groups M02, 2.4 ± 1.0% in groups M03, −2.3 ± 1.9% in groups M04; P < 0.05 between M02 versus M01 or M04; Figure 2c). Only the M02 group showed significant reduction in systolic and diastolic LV dimensions, compared to the control (ΔLVESD = −1.0 ± 0.3 mm versus 1.6 ± 0.4 mm; ΔLVEDD = −0.4 ± 0.2 mm versus 1.4 ± 0.3 mm, P < 0.05), whereas the other hUCB-MSCs showed a tendency to prevent LV dilatation after the infarction (Figure 2d). M02 also showed a significant reduction in the LV dimension compared to the other hUCB-MSCs (ΔLVESD, 1.2 ± 0.5 mm in M01, −1.0 ± 0.3 mm in M02, 0.9 ± 0.2 mm in M03, and 0.7 ± 0.2 mm in M04, P < 0.05 in M02 versus all other groups; ΔLVEDD, 0.9 ± 0.5 mm in M01, −0.4 ± 0.2 mm in M02, 1.4 ± 0.2 mm in M03, 0.7 ± 0.2 mm in M04, P < 0.05 between M02 versus M03; Figure 2d).
Figure 2.
hUCB-MSCs from different donors exhibit variations in their effects on postinfarction left ventricular (LV) remodeling in rats. (a) Scheme for transplantation of hUCB-MSCs from four donors into the rat MI model to assess donor-specific therapeutic variability. (b) Improvement in LV anterior wall motion after transplantation of M02 hUCB-MSC cells (2D-echocardiography; arrows indicate improved LV anterior wall contraction). (c) Systolic function improvement (2D-echocardiography). (d) Dimension reduction (2D-echocardiography). The four groups of hUCB-MSCs exerted variable therapeutic effects on systolic function improvement (ΔLVEF and ΔLVFS) and on LV dimension reduction (ΔLVEDD and ΔLVESD) after MI. The efficacy was the highest in the M02 group. (e) Masson's trichrome staining of infarcted rat heart 8 weeks after hUCB-MSC transplantation (bar is 1 mm). (f) Efficacy of the four hUCB-MSCs in reducing infarct size. (g) Variable efficacy of the four hUCB-MSCs in preserving infarct wall thickness. M02 hUCB-MSCs showed the highest efficacy in reducing infarct size and in preserving infarct wall thickness (n = 7 per group). *P < 0.05 versus control group. hUCB-MSCs, human umbilical cord blood-derived mesenchymal stem cells; MI, myocardial infarction; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; LVESD, left ventricular end-systolic diameter.
To quantify the pathological changes that occur after implantation of hUCB-MSCs, we measured the fibrosis area and the wall thickness of infarcted hearts harvested 8 weeks after cell transplantation. Masson's trichrome staining showed that the M02 group was the only group to show a significantly smaller infarct size and a thicker infarct wall relative to those of the control (infarct size, 12.5 ± 1.5% versus 28.2 ± 2.2%; infarct thickness, 8.3 ± 2.5 mm versus 3.5 ± 0.6 mm; P < 0.05; Figure 2e). The remaining hUCB-MSCs showed a tendency to have a reduced infarct size while maintaining the thickness of the infarct wall relative to the control (Figure 2f,g).
Variable angiogenic efficacies of hUCB-MSCs from different donors in MI heart
MI is caused by insufficient coronary blood supply to the myocardium. This induces the heart cells to die.1 For the progress of MI, angiogenesis including capillary proliferation along the peri-infarct area is essential.13 We assessed the capillary density of the peri-infarct region of the hUCB-MSC-injected MI rat heart using endothelial cell-specific isolectin B4 staining (Figure 3a). To measure the peri-infarct area, we used cardiac troponin-T staining. M02 was the only group that showed significantly increased capillary density compared to that of the control (P < 0.05 versus control; Figure 3b). We observed variations in capillary density among the four hUCB-MSCs, and this variation was found to be correlated with therapeutic efficacy in improving LV function after MI (Figure 3b).
Figure 3.
Variable angiogenic efficacies of hUCB-MSCs from different donors in MI heart. (a) Capillary staining of peri-infarcted tissue (8 weeks after transplantation; n = 7). Rat myocardium was stained with cardiac troponin-T (cTnT) (cardiac muscle cells; red) and isolectin B4 (ILB4) (endothelial cells; green). (b) Capillary staining showing neovascularization in the peri-infarct tissue. M02 hUCB-MSCs achieved the highest capillary density. *P < 0.05 versus control group (bar: 100 µm). hUCB-MSCs, human umbilical cord blood-derived mesenchymal stem cells; MI, myocardial infarction.
VEGF from hUCB-MSCs promotes angiogenesis
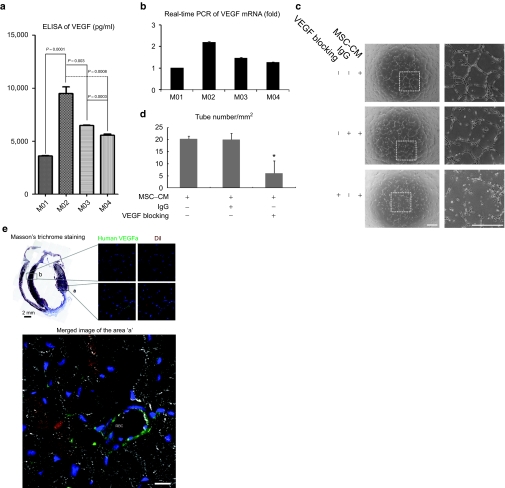
Vascular endothelial growth factor (VEGF) is an endothelial cell-specific mitogenic activator and has been demonstrated to be a major contributor to angiogenesis according to the increased number of capillaries observed in vivo.14 We expected that the VEGF level would be correlated with the therapeutic efficacy, and found that M02 is indeed the most effective with respect to VEGF production (Figure 4a,b). Next, to examine the angiogenic properties of VEGF from the hUCB-MSCs, we treated human umbilical vein endothelial cells with medium isolated from M02 hUCB-MSCs, to assess tube formation on thin Matrigel plates. The cultured medium of hUCB-MSC (conditioned medium) was observed to enhance the process of tube formation. The process was inhibited by the addition of VEGF-blocking antibody (Figure 4c,d). We next examined the MI tissue to assess the role of VEGF produced from hUCB-MSCs in the repair of MI. We used DiI staining to detect the injected hUCB-MSCs and performed immunostaining of the tissue with a human VEGF-specific antibody. In the peri-infarct area, it was observed that the transplanted hUCB-MSCs integrated with the cardiac structure. Interestingly, the transplanted DiI-positive hUCB-MSCs showed expression of VEGF in the vicinity of the vessels (Figure 4e). Furthermore, human VEGF, which was likely released from hUCB-MSCs, was observed to be bound to the endothelial cells of rat vessels containing red blood cells (Figure 4e).
Figure 4.
VEGF from hUCB-MSC contributes to angiogenesis. (a) VEGF secretion from four hUCB-MSCs evaluated by ELISA. M02 has the highest value. (b) Transcriptional level of VEGF mRNA from four hUCB-MSCs evaluated by real-time PCR. (c) Angiogenic effect of VEGF secreted from hUCB-MSCs, assessed by the Matrigel tube formation assay in HUVECs (bar: 500 µm). Tube formation by the culture supernatant from the M02 hUCB-MSCs was inhibited by VEGF-blocking antibody. (d) Quantification of tube formation assay. *P < 0.05 versus control group. (e) VEGF released from hUCB-MSCs was detected in the peri-infarct area injected with hUCB-MSCs (marked “a”) 4 weeks after transplantation; hUCB-MSCs were not transplanted in a noninfarct area (control, “b”). Transplanted DiI-stained hUCB-MSCs expressing VEGF were detected in the vicinity of a vessel. Human VEGF is probably released from hUCB-MSCs bound to rat endothelial cells on the lining of a vessel. Nuclei were stained with Sytox blue (bar: 50 µm) *RBC: red blood cell. CM, conditioned medium; ELISA, enzyme-linked immunosorbent assay; hUCB-MSCs, human umbilical cord blood-derived mesenchymal stem cells; HUVEC, human umbilical vein endothelial cells; mRNA, messenger RNA; VEGF, vascular endothelial growth factor.
Mechanism of donor-specific variations in the therapeutic efficacy of hUCB-MSCs: identification of N-cadherin as a signature molecule
Next, from the viewpoint of practical application of hUCB-MSCs, we searched for a surface signature molecule that might act as a regulator of VEGF. To determine the variations in the signature molecule of individual hUCB-MSCs, we performed complementary DNA microarray analysis of M02 and M01 (cells that showed the highest and lowest efficacy, respectively, in repairing infarcted rat myocardium), and for the M02 cells, we selected the cell-surface genes observed to have high expression levels.
To identify a signature molecule for use as a therapeutic indicator, we carefully validated these differentially expressed genes in another system, both for their potential role in stem cell function and for their usefulness as signature molecules. Therefore, we selected the cluster of differentiation (CD) genes that are commonly used as cell markers. This allows cells to be defined on the basis of the molecules exposed on their surfaces. According to standard selection protocols, we screened five genes: CD9, CD61, CD90, CD146, and CD325 (N-cadherin). These genes have higher expression levels in M02 cells compared to M01 cells (Figure 5a). The RNA and protein levels of these five genes were assessed by real-time PCR and fluorescence-activated cell sorting, respectively (Figure 5b,c). Of these, CD146 and N-cadherin exhibited very high levels of expression of both protein and RNA in M02 cells (Figure 5b,c). We compared the expression of these two markers in the four hUCB-MSCs with the degree of LV remodeling from in vivo data. CD146 expression was observed to be poorly correlated with the efficacy of hUCB-MSCs for repairing infarcted myocardium (Figure 5d).
Figure 5.
Screening of signature molecule for therapeutic efficacy. (a) Scatter plots of whole gene expression profiles between M01 and M02 cells, and histogram of five cell-surface genes. Five cell-surface genes (CD9, CD61, CD90, CD146, and N-cadherin) preferentially expressed in M02 cells are indicated by colored dots in the scatter plot. In the histogram, five surface genes are shown in red and represent the upregulated gene cluster in M02 compared with M01 cells. (b) Real-Time RT-PCR of five cell-surface genes. Consistent with the DNA microarray data, five genes were observed to be preferentially expressed in M02 cells, *P < 0.05. (c) FACS analysis of five cell-surface proteins. Differential protein expression in M02 compared with M01 cells was noted for CD146 and N-cadherin. The protein expression of the other three genes was not significantly different between M02 and M01 cells. (d) Western blotting of CD146 among the hUCB-MSCs; M01, M02, M03, and M04. CD146 protein expression is not correlated with efficacy of the four groups of hUCB-MSCs in repairing the infarcted myocardium. The best therapeutic efficacy was observed for M02, whereas the highest expression of CD146 was observed for M04 cells. Western blots were quantitated using the TINA 2.0 software (Raytest, Straubenhardt, Germany). FACS, fluorescence-activated cell sorting; hUCB-MSCs, human umbilical cord blood-derived mesenchymal stem cells; RT-PCR, reverse transcriptase PCR; STDEV, standard deviation.
N-cadherin-mediated cell–cell contact promotes the expression of VEGF via ERK activation in hUCB-MSCs
Because N-cadherin expression is well correlated with the efficacy of hUCB-MSCs in repairing the infarcted myocardium (Figure 6a,b), we examined whether N-cadherin could regulate VEGF in hUCB-MSCs by performing an N-cadherin-blocking experiment using hUCB-MSCs that exhibit a high level of N-cadherin expression (M02 cells) and with an N-cadherin overexpression experiment using hUCB-MSCs that have a low level of N-cadherin expression (M01 cells).
Figure 6.
N-cadherin, the signature molecule for therapeutic efficacy, regulates VEGF expression. (a) Evaluation of N-cadherin protein was performed by western blotting. (b) Evaluation of N-cadherin mRNA was performed by real-time PCR. N-cadherin expression correlates with the efficacy of repair of infarcted myocardium for the four groups of hUCB-MSCs and was highest in M02 cells. (c) Enhancement of N-cadherin expression using N-cadherin lentiviral vector. Expression increased according to the transduction ratio. (d) The amount of VEGF secreted from hUCB-MSCs was evaluated by ELISA and is represented as a fold change with respect to the naïve group. (e) N-cadherin neutralization. Cells were pretreated with GC-4-blocking antibody (40 µg/ml; Sigma-Aldrich) or IgG (control), 10 minutes before the calcium switch. Treatment with N-cadherin-specific blocking antibody was observed to cause a decrease in VEGF mRNA expression. Western blots and RT-PCR were quantified using TINA 2.0 software (Raytest). ELISA, enzyme-linked immunosorbent assay; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; hUCB-MSCs, human umbilical cord blood-derived mesenchymal stem cells; IgG, immunoglobulin G; mRNA, messenger RNA; RT-PCR, reverse transcriptase PCR; VEGF, vascular endothelial growth factor.
To assess VEGF regulation by N-cadherin-mediated cell–cell contact, we used a lentiviral vector (a kind gift from Nora Heisterkamp, Children's Hospital, Los Angeles). The vector consists of a cytomegalovirus promoter and full-length human N-cadherin fused to enhanced green fluorescent protein. This vector was transfected into M01 cells. Transduction of N-cadherin was observed to significantly increase VEGF expression in a dose-dependent manner (Figure 6c,d).
To confirm that N-cadherin-mediated VEGF expression is regulated by an intercellular interaction, we introduced an N-cadherin blocking antibody into M02 cells. The antibody was observed to suppress VEGF messenger RNA (mRNA) expression, whereas a control treatment (immunoglobulin G) did not (Figure 6e).
We further examined the mechanism underlying VEGF regulation by N-cadherin. Activation of v-akt murine thymoma viral oncogene homolog 1 (AKT) has been reported to be involved in N-cadherin-mediated cell survival and migration in melanomas.15 However, we interestingly observed that although extracellular signal-regulated kinase (ERK) activation is significantly increased with overexpression of N-cadherin (in a dose-dependent manner), AKT was not affected (Figure 7a). To confirm ERK-mediated VEGF regulation, we used an N-cadherin blocking antibody and detected suppressed ERK activity (Figure 7b) that led to the downregulation of VEGF (Figure 7c). Next, to manifest the ERK-VEGF axis as a downstream of N-cadherin, we blocked ERK activation under N-cadherin overexpression (Figure 7d). Although N-cadherin was overexpressed, the amount of VEGF secretion was reduced via inhibition of ERK activation.
Figure 7.
N-cadherin regulates ERK activation. (a) N-cadherin overexpression (by lentiviral vector transfection) results in increased ERK activation in hUCB-MSCs. (b) N-cadherin neutralization: blocking of N-cadherin significantly suppresses ERK activation, but not AKT. (c) N-cadherin blocking decreases ERK activation and leads to the downregulation of VEGF expression. *P < 0.05 versus the IgG group. Western blot data were quantified using TINA 2.0 software (Raytest). (d) Specific inhibition of ERK on N-cadherin overexpression. U0126 was used to inhibit ERK activation. (E) The secreted amount of VEGF was evaluated by ELISA. N-cadherin-induced VEGF production was decreased by ERK inhibition. *P < 0.005 versus the other group. ELISA, enzyme-linked immunosorbent assay; ERK, extracellular signal-regulated kinase; hUCB-MSCs, human umbilical cord blood-derived mesenchymal stem cells; IgG, immunoglobulin; VEGF, vascular endothelial growth factor.
Taken together, these results suggest that N-cadherin promotes ERK activation, which in turn increase the expression of VEGF in hUCB-MSCs.
N-cadherin is a useful marker for the regenerative capacity of hUCB-MSCs in MI
To reveal the in vivo functionality of N-cadherin, we used hUCB-MSCs overexpressing N-cadherin, M01 cells (expressing low levels of N-cadherin), and knockdown M02 cells (expressing high levels of N-cadherin) in the MI model. Overexpression of N-cadherin enhanced repair efficacy via improvement of systolic function (ΔLVEF and ΔLVFS) and reduction of LV dimension (ΔLVEDD and ΔLVESD) after MI (Figure 8a). To observe the N-cadherin function in vivo, we constructed the shN-cadherin lentiviral vector pshNCAD to obtain knockdown of N-cadherin expression. Validation of pshNCAD was performed by real-time PCR for mRNA and a western blot for protein. On pshNCAD transfection, we observed an effective decrease in the mRNA and protein expression levels of N-cadherin (Figures 8b,c). Decreased levels of VEGF mRNA (Figure 8d) and the amount of VEGF secreted (Figure 8e) were indicated by N-cadherin downregulation. In addition, we detected a decrease in the rate of repair efficacy with N-cadherin knockdown in M02 cells via reduction of the systolic function (ΔLVEF and ΔLVFS) and an increase in the LV dimension (ΔLVEDD and ΔLVESD) after MI (Figure 8f).
Figure 8.
N-cadherin is a marker for the high regenerative capacity of hUCB-MSCs in MI. (a) hUCB-MSCs overexpressing N-cadherin enhance the repair efficacy in MI. In N-cadherin-overexpressing M01 cells by pNCAD lentiviral vector, we observed improvement in the systolic function (ΔLVEF and ΔLVFS) and LV dimension reduction (ΔLVEDD and ΔLVESD) after MI. *P < 0.05 versus control and pMock group. (b) N-cadherin mRNA real-time PCR of N-cadherin knockdown M02 cells. M02 cells were transfected with the shN-cadherin lentiviral vector pshNCAD to obtain a knockdown of N-cadherin. (c) Validation of N-cadherin protein in N-cadherin knockdown M02 cells. (d) Real-time PCR of VEGF mRNA in N-cadherin knockdown M02 cells. (e) ELISA of VEGF in N-cadherin knockdown M02 cells. Both transcriptional and translational levels of VEGF were decreased by N-cadherin knockdown. *P < 0.005 versus control and pU6Mock group, n.d; no significant difference. (f) N-cadherin knockdown hUCB-MSCs decrease the repair efficacy in MI. Using N-cadherin knockdown M02 cells (50% pshNCAD), we detected a decrease in systolic function (ΔLVEF and ΔLVFS) and LV dimension (ΔLVEDD and ΔLVESD) after MI. *P < 0.05 versus control and pU6Mock group. (g) To observe the transplanted cells, we performed DiI (red) staining. Four weeks after transplantation of hUCB-MSCs, infarcted rat myocardium was stained for N-cadherin (green) and observed by laser scanning confocal fluorescence microscopy. N-cadherin is normally identified as a line in the intercalated disc between cardiomyocytes (white asterisk). N-cadherin expressing DiI-hUCB-MSCs (white arrow) were detected in peri-infarct rat heart. (h) To detect the cells interacting with transplanted cells, the cardiomyocyte marker a-SA was used. We found that the DiI-labeled transplanted cells express N-cadherin and interact with cardiomyocytes (indicated by asterisk). Nuclei were stained with Sytox blue (bar: 50 µm). ELISA, enzyme-linked immunosorbent assay; hUCB-MSCs, human umbilical cord blood-derived mesenchymal stem cells; LV, left ventricular; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; LVESD, left ventricular end-systolic diameter; MI, myocardial infarction; mRNA, messenger RNA; VEGF, vascular endothelial growth factor.
To detect the maintenance of N-cadherin expression after transplantation, hUCB-MSCs were labeled with a membrane dye, DiI. We observed DiI-labeled hUCB-MSCs expressing N-cadherin 4 weeks after transplantation at the peri-infarct area (Figure 8, white arrow). N-cadherin is normally indicated as a line in the intercalated disc between cardiomyocytes (Figure 8g, white asterisk). The contractile force of the cardiomyocyte is transmitted through the intercalated disc.16 Loss of N-cadherin leads to disassembly of the intercalated disc structure.16 Interestingly, we also detected surviving hUCB-MSCs expressing orienting N-cadherin (Figure 8, white arrow). N-cadherin is a transmembrane cell–cell adhesion receptor, which requires the hemophilic trans-interaction for functional roles in cell survival.17 To reveal the cells interacting with DiI-labeled hUCB-MSCs, we used cardiomyocyte marker a-SA. We found that DiI-labeled hUCB-MSCs interact with cardiomyocytes via N-cadherin as well as a-SA (Figure 8h, white asterisk).
Discussion
Autologous adult progenitor cells are most commonly used as the main source for cell therapy. Although these cells are effective in improving heart function,18 their efficacy is limited because of function and an insufficient supply of progenitor cells from the patient.19 In this context, hUCB-MSCs are potentially good alternatives because they can be obtained in large quantities from healthy young donors. Furthermore, they can be prepared and stored, and are readily available when clinically needed. In our previous study using a rat MI model,6 we confirmed that hUCB-MSCs are good sources of cells for clinical applications. However, hUCB-MSCs are obtained from a range of donors. Therefore, individual variations in therapeutic efficacy raise reasonable concerns.
Individual variations in therapeutic efficacy in hUCB-MSCs
We assumed that hUCB-MSCs from different donors would have different therapeutic efficacy in postinfarction LV remodeling. In this study, we found that hUCB-MSCs derived from different donors have significant variations in therapeutic efficacy in postinfarct LV remodeling, although they were prepared using the same standard protocols. However, no specific parameters for allogeneic cell transplantation can predict the results of transplantation. In clinical practice, the most important issue is the selection of the best among thousands of prepared hUCB-MSCs. Therefore, it is necessary to find signature molecules that can identify hUCB-MSCs with optimal in vivo potency.
N-cadherin as a marker for suitable hUCB-MSCs
To identify a signature molecule for optimal hUCB-MSCs, we performed a DNA microarray analysis of cells that exhibit the best efficacy and cells that exhibit the lowest efficacy in repairing infarcted myocardium in rats. N-cadherin was found to be a signature molecule for the optimal hUCB-MSCs. N-cadherin is a member of a family of Ca2+-dependent cell adhesion molecules that has been identified as an indicator of epithelial-mesenchymal transit. The expression of this molecule leads to a mesenchymal phenotype in carcinomas.20 In this study, we found that N-cadherin is a useful marker for hUCB-MSCs that have good therapeutic efficacy. However, no studies have yet been conducted to evaluate the role of N-cadherin in hUCB-MSCs.
N-cadherin-mediated release of VEGF from hUCB-MSCs contributes to angiogenesis
To determine the function of N-cadherin in hUCB-MSCs, we conducted both knockdown and overexpression of N-cadherin in hUCB-MSCs and concluded that N-cadherin regulates VEGF expression in hUCB-MSCs by activation of ERK. The paracrine effect is one of the best-known mechanisms responsible for the beneficial effects of cell therapy.21,22,23 One study demonstrated that the beneficial effects of stem cell therapy occur as early as 3 days after implantation. These effects, therefore, cannot be attributed to de novo regeneration by cardiomyogenic differentiation of the engrafted cells.24 Another study reported that injection of the conditioned medium from MSCs overexpressing only AKT (which releases significant amounts of beneficial cytokines) markedly improves LV remodeling.25 Furthermore, several studies have reported that MSCs are a rich source of broad-spectrum cytokines.26,27,28,29 In another preport, human bone marrow-derived mesenchymal stem cells were shown to be a practical resource for the production of VEGF and were suggested to be effective in neoangiogenesis.30
In this study, we revealed that transplanted hUCB-MSCs can be engrafted and can survive in infarcted myocardium. Furthermore, these hUCB-MSCs are capable of producing human VEGF, which plays a role in angiogenesis based on the results of our in vitro and in vivo experiments. In addition, we confirmed that VEGF release from hUCB-MSCs is regulated by N-cadherin-mediated cell–cell interactions and activation of ERK. Therefore, N-cadherin could be a useful and practical marker for selecting optimal hUCB-MSCs, not only because it is a surface molecule but also because it plays an important role in angiogenesis.
Possible role of N-cadherin in cell transplantation for treatment of heart failure
N-cadherin is a critical factor in early development of the heart31 and is important for maintaining the structural integrity of the heart, both at the intercalated disc and the end-to-end connections between cardiomyocytes.16 Deletion of N-cadherin causes disassembly of the intercalated disc and leads to heart failure in an animal model.16 In cell transplantation, the formation of new intercalated disc-like cell contacts between host cardiomyocytes and transplanted cells might contribute to cardiac repair after infarction.32,33 We observed oriented N-cadherin-expressing hUCB-MSCs in the peri-infarct area. Moreover, we revealed the in vivo functionality of N-cadherin by overexpressing N-cadherin in hUCB-MSCs and by knockdown of N-cadherin in hUCB-MSCs. N-cadherin hemophilic adhesion can enhance cell survival pathway.17 Therefore, cells with high expression of N-cadherin may be more suitable for transplantation into a damaged heart and could more effectively contribute to interactions with host cardiomyocytes. Transplantation of these cells would lead to improved rates of survival relative to treatment involving transplantation of cells exhibiting low levels of N-cadherin.
Although human MSCs have a unique immunological capacity that allows them to persist even in a xenogenic environment, our xenotransplantation model might have had problems related to the interactions between donor and recipient cells. This complicates the interpretation of our data. Liechty and coworkers have reported that hMSCs could be engrafted and differentiated following in utero transplantation in sheep without the need for immunosuppression. This is consistent with our results.34 Furthermore, we found that hUCB-MSCs showed little immunological response to the presence of rat peripheral blood mononuclear cells (PBMCs; data not shown). Another reason for choosing the xenotransplantation model is that we wanted to avoid variations in the degree of HLA mismatch, which would be encountered in allotransplant models in the real world.
In conclusion, we demonstrated a variation in the therapeutic efficacy of hUCB-MSCs obtained from different donors, and propose the use of N-cadherin as a signature molecule for determining therapeutic efficacy of hUCB-MSCs. Therefore, our findings form an important basis for future improvements in the therapeutic efficacy of hUCB-MSCs and for overcoming the limitations arising from individual variations. Future investigations with the objective of augmenting N-cadherin expression in hUCB-MSCs without genetic manipulation are required to improve the efficacy of hUCB-MSCs for clinical applications.
Materials and Methods
MSC isolation and culture. hUCB-MSCs provided by Medipost were isolated from human umbilical cord vein with informed consents from donors as previously described.10,12 In brief, mononuclear cells were isolated by centrifugation (400g, 50 minutes) in a Ficoll hypaque gradient (density 1.077 g/cm3; Sigma-Aldrich, St Louis, MO). These cells were cultured in α-minimum essential medium (α-minimum essential medium; Gibco, Bethesda, MD), supplemented with 10% fetal bovine serum (Gibco), 10,000 units/ml penicillin G sodium, 10,000 µg/ml streptomycin sulfate, and 25 µg/ml amphotericin B (Invitrogen, San Diego, CA) at a concentration 5.0 × 106 cells/cm2. Cultures were maintained at 37 °C in a humidified cell incubator maintained at an atmosphere of 5% CO2 with a change of culture medium twice each week. Three weeks later, a monolayer of fibroblast-like adherent cells were trypsinized (0.25% trypsin; HyClone, Logan, UT), washed, resuspended in culture medium, and subcultured at a concentration of 1.0 × 105 cells/cm2. A growth curve was established by multiplying the initial number of cells by the amplification fold for each passage.
Quality control of hUCB and hUCB-MSCs. All hUCB donors were characterized by a normal karyotype, and two of them were men. hUCB-MSCs of the 5th–8th passage from four different donors (M01, M02, M03, and M04) were used for this experiment and were produced in a facility conforming to good manufacturing practice. Quality control of cells and quality assurance were performed according to the standards of the Korea Food and Drug Administration. Three hUCB-MSC (M01~M03) and one hUCB-MSC (M04) were collected in March 2005 and January 2006, respectively, and stocked in liquid nitrogen (−196 °C) The hUCB-MSC suspensions were tested and confirmed to be culture negative for bacteria and mycoplasma before infusion.
Flow cytometric analysis. For flow cytometric analysis, the cells were first dissociated by incubation at 37 °C for 1 minute in 0.25% trypsin/ethylenediaminetetraacetic acid (Invitrogen), washed with phosphate-buffered saline (PBS) containing 2.5% fetal bovine serum, and incubated for 30 minutes with various combinations of saturating amounts of antibodies conjugated to fluorescein isothiocyanate (FITC) or phycoerythrin (PE): CD45-FITC, CD34-FITC, HLA-DR-FITC, CD29-PE, CD44-PE, CD73-PE, CD166-PE, and HLA-ABC-PE (BD Biosciences, Sparks, MD), isotype-matched control (Pharmingen, BD Biosciences, Sparks, MD), and CD105-PE (AbD Serotec, Kidlington, UK).
After identification of these cells as hUCB-MSCs, M01 and M02 were stained for CD9-PE (BD Biosciences), CD61 (Abcam, Cambridge, UK), CD90-FITC (BD Biosciences), CD146-PE (BD Biosciences), and N-cadherin (Abcam) to screen for markers of therapeutic efficacy. A flow cytometric analysis was performed. At least 104 events were analyzed using the FACSCalibur system (BD Biosciences) with Cellquest software.
Mixed lymphocyte response test. To assess T-cell reactivity against allogeneic cell populations, human responder PBMCs (1 × 105/well) were cocultured with inactivated allogeneic PBMCs (1 × 105/well) or hUCB-MSCs (1 × 102, 1 × 103 or 1 × 104/well) in 96-well tissue culture plates. The PBMCs were purchased from AllCells (Emeryville, CA) The stimulator PBMCs and hUCB-MSCs were inactivated by treatment with 10 µg/ml mitomycin-C (Sigma-Aldrich) for 1 hour at 37 °C. In some experiments, responder PBMCs were cocultured with allogenic stimulator PBMCs in 96-well tissue culture plates at 1 × 105/well in the presence or absence of 1 × 102, 1 × 103 or 1 × 104/well preplated hUCB-MSCs. Due to the large size of hUCB-MSCs, these cells were not cultured at the same ratio with the PBMC responder cell (1 × 104 hUCB-MSCs in a 96-well plate are 80% confluent). For experiments using transwell chambers (BD Biosciences), PBMCs from two HLA-type-mismatched individuals were seeded at 1 × 105/well in the presence or absence of 1 × 103 or 1 × 104 hUCB-MSCs. The hUCB-MSCs were separated from the PBMCs by a high-density pore membrane (transwell chamber; BD Biosciences) by addition to the upper compartment. T-cell proliferation in response to alloantigens was determined by adding 20 µl of bromodeoxyuridine after 6 days of mixed lymphocyte reaction culture. The tissue culture plates were then incubated at 37 °C in 5% CO2 for an additional 18 hours. Thereafter, the tissue culture plates were centrifuged at 300g for 10 minutes. The substrate was added according to the manufacturer's instructions and colorimetric immunoassays were immediately used to measure bromodeoxyuridine incorporated during DNA synthesis. This provides an estimation of the rate of cellular proliferation (Roche/Boehringer Mannheim, Basel, Swiss). The absorbance of the samples was measured in a UV max kinetic microplate reader (Molecular Devices, Hampshire, UK) at 370 nm (reference wavelength, 492 nm). The data of three replicates are shown as mean and SD.
Rat MI models. All animal protocols were approved by the Institutional Animal Care and Use Committee of Seoul National University (IACUC No. 08-0163), and the investigators adhered to the National Research Council's “Guide for the Care and Use of Laboratory Animals”(revised 1996). Male Sprague-Dawley rats (weighting 240–290 g) aged 8–10 weeks were used. The rats were anesthetized with ketamine hydrochloride (100 mg/kg, Yuhan, Seoul, Korea) and xylazine (10 mg/kg, Bayer, Leverkusen, Germany) by intraperitoneal injection. After intubation, the rats were positive pressure ventilated with room air supplemented with oxygen using a small animal volume controlled ventilator (model 683; Harvard Apparatus, Holliston, MA). A left thoracotomy was performed in the fourth intercostal space and the pericardium was opened. Induction of MI was performed by ligation of the left coronary arteries with 6–0 silk suture as described previously.6,35 After ligation of the proximal left anterior descending, an irreversible pale area was demarcated on the surface of the middle apical portion of LV, and the chest was then closed in layers.
hUCB-MSCs transplantation. hUCB-MSCs were transplanted 7 days after MI. To determine the minimal effective dose for the second phase study, we conducted a phase I study (dose–response experiment). Rats were randomly assigned into six groups (1 × 103, 1 × 104, 1 × 105, 1 × 106, and 1 × 107 hUCB-MSCs (M02) or PBS, n = 5). A total volume of 100 µl (hUCB-MSCs or PBS) was injected with a 24-gauge needle at three different sites (in the peri-infarct area). To detect transplanted cells, the hUCB-MSCs were labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Molecular Probes, Eugene, OR) (2.0 µg/ml). In phase II (donor-specificity experiment), the rats were randomly assigned into five groups 1 week after surgery, and received M01, M02, M03, M04 (each 1 × 105, 100 µl, n = 7), or PBS (100 µl). In addition, for the in vivo functional study of N-cadherin, we used M01 cells overexpressing N-cadherin or N-cadherin knockdown M02 cells.
In vivo echocardiographic evaluation. An echocardiographic study (Vivid I; GE Healthcare, Piscataway, NJ) using a 11.5 MHz transducer was performed 2 days before and 8 weeks after hUCB-MSC transplantation. Two-dimensional M-mode traces were obtained at the level of the papillary muscles in at least three consecutive cardiac cycles. LV fractional shortening (FS) was calculated as FS = (LVEDD−LVESD)/LVEDD.6
Histological preparations and analysis. Eight weeks after hUCB-MSC implantation, rats were euthanized and the hearts were removed. The excised heart was retrograde perfused with PBS for coronary vasculature and LV washing, and fixed with 4% paraformaldehyde overnight at 4 °C and then with 15% sucrose overnight at 4 °C. Each tissue sample was embedded in paraffin or frozen in optimal cutting temperature compound (Tissue-Teck, Sakura, Torrance, CA). Section (7 µm) stained with hematoxylin and eosin and Masson's trichrome were used to calculate infarct size and wall thickness using an Image analysis system (Image Pro version 4.5; Media Cybernetics, Bethesda, MD). Infarct size was defined as the sum of the epicardial and endocardial circumferences of the fibrous scar tissues divided by the sum of the LV epicardial and endocardial circumferences. Three different levels of separate sections (more than 100 µm apart) from each heart were used to calculate average values.36 Wall thickness was measured at five areas the center of infarct, border of the infarct (right and left lateral), and in between (right and left mid-lateral). An average value was then calculated.37 The number of capillary vessels was counted in the peri-infarct area of all groups, using isolectin B4 (Vector Laboratories, Burlingame, CA) and anti-troponin-T (Santa Cruz Biotechnology, Santa Cruz, CA) staining. Five high-power fields in each area were randomly selected, and the number of capillaries in nonconsecutive sections were averaged and expressed as the number of capillary vessels per square millimeter.
To detect hUCB-MSCs injected into the peri-infarct area of the rat heart, hUCB-MSCs were prestained with DiI before transplantation. At a time-point 4 weeks after transplantation of DiI-hUCB-MSCs, the infarcted rat heart was stained for hVEGFa (which does not react with rat VEGFa), (Abcam), N-cadherin (Abcam) and α-sarcomeric actin (a-SA; Sigma-Aldrich) for the cardiomyocyte to reveal the fate of the transplanted cells. All of the examinations were performed using confocal laser scanning microscopy (LSM 510; Carl Zeiss, Oberkochen, Germany).
Tube formation assay. Human umbilical vein endothelial cells were trypsinized and seeded at 3 × 104 cells per well in a 24-well plate on growth factor depleted Matrigel (BD Biosciences) previously polymerized for 30 minutes at 37 °C. Human umbilical vein endothelial cells were incubated in hUCB-MSC cultured supernatant endothelial growth medium (with 2% fetal bovine serum) with or without VEGF blocking. Twelve hours later, the number of tubes was quantitated (a connecting branch between two discrete endothelial cells was counted as a single tube).
Calcium switch and N-cadherin blocking assay. For disruption of N-cadherin-mediated cell–cell interactions, confluent cultured cells were deprived of serum for 12 hours and treated with 4 mmol/l ethylenediaminetetraacetic acid in α-minimum essential medium medium for 30 minutes at 37 °C. For re-establishment of N-cadherin-mediated cell–cell interactions, 2 mmol/l calcium was added with or without a 10 minutes preincubation period with N-cadherin-blocking antibody GC-4 (40 µg/ml; Sigma-Aldrich) or immunoglobulin G. Cells were harvested at different times and used for immunoblot analysis.
Transduction of human N-cadherin gene or shN-cadherin using lentiviral vector. The lentiviral vector expressing human N-cadherin or enhanced green fluorescent protein alone (generous gifts from Dr Nora Heisterkamp of Children's' Hospital, Los Angeles) were cotransfected with packaging plasmid PAX2 and envelope plasmid pMD2G (also generously provided by Dr Nora Heisterkamp) into 293T cells (Invitrogen) for 72 hours.
For knockdown of human N-cadherin, we constructed a shN-cadherin RNA lentiviral vector, pNCAD. The primers used were: sense-TGACTGGATTTCCTGAAGATTT CAAGAGAATCTTCAGGA AATCCAGTCTTTTTTC and antisense-TCGAGAAAAAAG ACTG GATTTC CTGAAGATTCTCTTGAAATCTTCAGGAAAT CCA GTCA. The confluent hUCB-MSCs were introduced into the lentiviral supernatant and cultured for 48 hours. Evaluation of RNA and protein expression was performed by real-time PCR and western blotting, respectively.
Real-time reverse transcriptase PCR analysis. Total RNA from the cultured hUCB-MSCs was extracted using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The complementary DNA was synthesized from ~1 µg of total RNA using the Reverse Transcription System (Promega, Madison, WI) and subjected to PCR amplification with specific primers (Supplementary Table S1). The reactions were performed using the ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) with SYBR Green I dye (Sigma-Aldrich). Glyceraldehyde 3-phosphate dehydrogenase was simultaneously run as a control and used for normalization. Nontemplate control wells without complementary DNA were included as negative controls. Each test sample was run in triplicate. The results are reported as the relative expression after normalization of the transcript amount to the endogenous control using the 2−ΔΔCT method.38 Threshold cycle (CT) indicates the fractional cycle number at which the amount of amplified target reaches a fixed threshold.
Western blot analysis. Cell lysates were obtained by incubation in lysis buffer containing protease inhibitors (Roche) for 20 minutes. Total protein (10–30 µg) was immunoblotted with specific primary antibodies overnight at 4 °C. α-Tubulin was used as an internal control.
Enzyme-linked immunosorbent assay. A sample of conditioned culture media was collected from each plate of hUCB-MSCs after 3 days of culture. Cell-free supernatants were collected from naive hUCB-MSCs and used to detect the level of hVEGF quantitatively determined by Bio-Plex 200 system (Bio-Rad, Hercules, CA) according to the manufacturers' protocol.
Statistical analysis. Continuous variables are expressed as mean ± SE. Differences between continuous variables were analyzed by the unpaired Student's t-test. Group differences of continuous variables were tested by analysis of variance, followed by a Scheffe's post hoc test. A probability value of <0.05 was considered significant. Statistical analysis was performed using SPSS 13.0 statistical package (SPSS, Chicago, IL).
SUPPLEMENTARY MATERIAL Figure S1. Characterization of four hUCB-MSCs. Table S1. Primers used for RNA PCR.
Acknowledgments
We thank Nora Heisterkamp for the lentiviral vectors expressing human N-cadherin as well as the PAX2 plasmid and the envelope plasmid pMD2G. E.J.L., E.-K.C., and H.-S.K. designed the research project; J.Y.P., S.K.K., G.-H.K., K.-H.K., J.S.K., K.H.L., H.-J.L., and H.-J.C. performed the research; S.J.C. and W.I.O. contributed vital new reagents; E.J.L., E.-K.C., H.-J.K., S.-W.L., and Y.A. analyzed the data; E.J.L., Y.-B.P., and H.-S.K. critically revised drafts of the article for intellectual content; and E.J.L., E.-K.C., and H.-S.K. prepared the manuscript. This study was supported by a grant from the “Innovative Research Institute for Cell Therapy” of Seoul National University Hospital (A062260) that was sponsored by the Ministry of Health, Welfare & Family, Republic of Korea. This work was also supported by a National Research Foundation grant funded by the Korea government (MEST) (2010-0020257). The funding agencies themselves did not contribute to the study design, data collection and analysis, decision to publish, or the preparation of the manuscript. The authors declared no conflict of interest.
Supplementary Material
Characterization of four hUCB-MSCs.
Primers used for RNA PCR.
REFERENCES
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B.et al. (2001Bone marrow cells regenerate infarcted myocardium Nature 410701–705. [DOI] [PubMed] [Google Scholar]
- Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T.et al. (1999Autologous transplantation of bone marrow cells improves damaged heart function Circulation 100suppl. 19II247–II256. [DOI] [PubMed] [Google Scholar]
- Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C.et al. (2004Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial Lancet 364141–148. [DOI] [PubMed] [Google Scholar]
- Cleland JG, Freemantle N, Coletta AP., and, Clark AL. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROACTIVE. Eur J Heart Fail. 2006;8:105–110. doi: 10.1016/j.ejheart.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W.et al. (2006Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial Lancet 367113–121. [DOI] [PubMed] [Google Scholar]
- Chang SA, Lee EJ, Kang HJ, Zhang SY, Kim JH, Li L.et al. (2008Impact of myocardial infarct proteins and oscillating pressure on the differentiation of mesenchymal stem cells: effect of acute myocardial infarction on stem cell differentiation Stem Cells 261901–1912. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD.et al. (1999Multilineage potential of adult human mesenchymal stem cells Science 284143–147. [DOI] [PubMed] [Google Scholar]
- Ye ZQ, Burkholder JK, Qiu P, Schultz JC, Shahidi NT., and, Yang NS. Establishment of an adherent cell feeder layer from human umbilical cord blood for support of long-term hematopoietic progenitor cell growth. Proc Natl Acad Sci USA. 1994;91:12140–12144. doi: 10.1073/pnas.91.25.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erices A, Conget P., and, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- Yang SE, Ha CW, Jung M, Jin HJ, Lee M, Song H.et al. (2004Mesenchymal stem/progenitor cells developed in cultures from UC blood Cytotherapy 6476–486. [DOI] [PubMed] [Google Scholar]
- Kögler G, Sensken S, Airey JA, Trapp T, Müschen M, Feldhahn N.et al. (2004A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential J Exp Med 200123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Lee HN, Kang HJ, Kim KH, Hur J, Cho HJ.et al. (2010Novel embryoid body-based method to derive mesenchymal stem cells from human embryonic stem cells Tissue Eng Part A 16705–715. [DOI] [PubMed] [Google Scholar]
- De Boer RA, Pinto YM., and, Van Veldhuisen DJ. The imbalance between oxygen demand and supply as a potential mechanism in the pathophysiology of heart failure: the role of microvascular growth and abnormalities. Microcirculation. 2003;10:113–126. doi: 10.1038/sj.mn.7800188. [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV., and, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Li G, Satyamoorthy K., and, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001;61:3819–3825. [PubMed] [Google Scholar]
- Kostetskii I, Li J, Xiong Y, Zhou R, Ferrari VA, Patel VV.et al. (2005Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure Circ Res 96346–354. [DOI] [PubMed] [Google Scholar]
- Tran NL, Adams DG, Vaillancourt RR., and, Heimark RL. Signal transduction from N-cadherin increases Bcl-2. Regulation of the phosphatidylinositol 3-kinase/Akt pathway by homophilic adhesion and actin cytoskeletal organization. J Biol Chem. 2002;277:32905–32914. doi: 10.1074/jbc.M200300200. [DOI] [PubMed] [Google Scholar]
- Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J.et al. (2007Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials J Am Coll Cardiol 501761–1767. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK.et al. (2004Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial Lancet 363751–756. [DOI] [PubMed] [Google Scholar]
- Rieger-Christ KM, Lee P, Zagha R, Kosakowski M, Moinzadeh A, Stoffel J.et al. (2004Novel expression of N-cadherin elicits in vitro bladder cell invasion via the Akt signaling pathway Oncogene 234745–4753. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, Zhang Z, Ni A., and, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuse T, Peter C, Fedak PW, Doyle T, Reichenspurner H, Zimmermann WH.et al. (2009Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction Circulation 120suppl. 11S247–S254. [DOI] [PubMed] [Google Scholar]
- Katsha AM, Ohkouchi S, Xin H, Kanehira M, Sun R, Nukiwa T.et al. (2011Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model Mol Ther 19196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS.et al. (2003Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts Nat Med 91195–1201. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F.et al. (2006Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement FASEB J 20661–669. [DOI] [PubMed] [Google Scholar]
- Weimar IS, Miranda N, Muller EJ, Hekman A, Kerst JM, de Gast GC.et al. (1998Hepatocyte growth factor/scatter factor (HGF/SF) is produced by human bone marrow stromal cells and promotes proliferation, adhesion and survival of human hematopoietic progenitor cells (CD34+) Exp Hematol 26885–894. [PubMed] [Google Scholar]
- Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H.et al. (2005Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells Nat Med 11367–368. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S.et al. (2004Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms Circulation 1091543–1549. [DOI] [PubMed] [Google Scholar]
- Cheng SL, Zhang SF, Mohan S, Lecanda F, Fausto A, Hunt AH.et al. (1998Regulation of insulin-like growth factors I and II and their binding proteins in human bone marrow stromal cells by dexamethasone J Cell Biochem 71449–458. [DOI] [PubMed] [Google Scholar]
- Kagiwada H, Yashiki T, Ohshima A, Tadokoro M, Nagaya N., and, Ohgushi H. Human mesenchymal stem cells as a stable source of VEGF-producing cells. J Tissue Eng Regen Med. 2008;2:184–189. doi: 10.1002/term.79. [DOI] [PubMed] [Google Scholar]
- Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M., and, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Zuppinger C, Eppenberger-Eberhardt M., and, Eppenberger HM. N-Cadherin: structure, function and importance in the formation of new intercalated disc-like cell contacts in cardiomyocytes. Heart Fail Rev. 2000;5:251–257. doi: 10.1023/A:1009809520194. [DOI] [PubMed] [Google Scholar]
- Pedrotty DM, Klinger RY, Badie N, Hinds S, Kardashian A., and, Bursac N. Structural coupling of cardiomyocytes and noncardiomyocytes: quantitative comparisons using a novel micropatterned cell pair assay. Am J Physiol Heart Circ Physiol. 2008;295:H390–H400. doi: 10.1152/ajpheart.91531.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R.et al. (2000Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep Nat Med 61282–1286. [DOI] [PubMed] [Google Scholar]
- Hahn JY, Cho HJ, Bae JW, Yuk HS, Kim KI, Park KW.et al. (2006Beta-catenin overexpression reduces myocardial infarct size through differential effects on cardiomyocytes and cardiac fibroblasts J Biol Chem 28130979–30989. [DOI] [PubMed] [Google Scholar]
- Uemura R, Xu M, Ahmad N., and, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- Müller-Ehmsen J, Peterson KL, Kedes L, Whittaker P, Dow JS, Long TI.et al. (2002Rebuilding a damaged heart: long-term survival of transplanted neonatal rat cardiomyocytes after myocardial infarction and effect on cardiac function Circulation 1051720–1726. [DOI] [PubMed] [Google Scholar]
- Livak KJ., and, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization of four hUCB-MSCs.
Primers used for RNA PCR.