Abstract
Adeno-associated virus (AAV)-based vectors are promising gene delivery vehicles for human gene transfer. One significant obstacle to AAV-based gene therapy is the high prevalence of neutralizing antibodies in humans. Until now, it was thought that, except for nonhuman primates, pre-existing neutralizing antibodies are not a problem in small or large animal models for gene therapy. Here, we demonstrate that sera of several animal models of cardiovascular diseases harbor pre-existing antibodies against the cardiotropic AAV serotypes AAV1, AAV6, and AAV9 and against AAV2. The neutralizing antibody titers vary widely both between species and between serotypes. Of all species tested, rats displayed the lowest levels of neutralizing antibodies. Surprisingly, naive mice obtained directly from commercial vendors harbored neutralizing antibodies. Of the large animal models tested, the neutralization of AAV6 transduction by dog sera was especially pronounced. Sera of sheep and rabbits showed modest neutralization of AAV transduction whereas porcine sera strongly inhibited transduction by all AAV serotypes and displayed the largest variation between individual animals. Importantly, neutralizing antibody titers as low as 1/4 completely prevented in vivo transduction by AAV9 in rats. Our results suggest that prescreening of animals for neutralizing antibodies will be important for future gene transfer experiments in these animal models.
Introduction
Over the past decade, adeno-associated virus (AAV)-based vectors have gained increasing attention in both basic, preclinical and clinical research and AAV vectors are now among the most promising vector systems for gene therapy applications. This is partially due to the fact that AAV is not associated with any known human disease, that AAV vectors can, at least in nondividing cells, generate long-term transgene expression—even in the absence of genome integration—and that AAVs display relatively low immunogenicity. The relatively low immunogenicity notwithstanding, it has been recognized that the high prevalence of neutralizing antibodies against AAV in the human population presents a considerable obstacle to the broad use of AAV vectors in clinical gene therapy. Whereas the prevalence depends somewhat on the serotype, most studies report that between 20% and 40% of the population has neutralizing antibody titers of ≥1/20 against any given serotype.1–3 It is important to point out, however, that it has been demonstrated in both animal studies4–7 and clinical trials8,9 that neutralizing titers as low as 1/2–1/4 can prevent successful transduction. Hence, the percentage of patients that need to be excluded from clinical trials is likely substantially higher than the 20–40% of individuals reported to harbor neutralizing antibodies against AAV.1–3
Until now, it was assumed that in animal models neutralizing antibodies against AAVs only play a role in transduction in nonhuman primate models.4,10 The presence of neutralizing antibodies against AAVs in nonhuman primates is not unexpected because a large number of AAV variants have been isolated from nonhuman primates.11,12 Here, we show that, somewhat surprisingly, neutralizing antibodies against AAVs are present in the sera of several small and large animal models, including mice, rats, rabbits, dogs, sheep, and pigs. The prevalence of neutralizing antibodies depends both on the AAV serotype and the species. Importantly, we show that not only do the pre-existing neutralizing antibodies neutralize transduction in vitro but also in vivo in a rat model as well as in pigs.
Taken together, our results suggest that the existence of neutralizing antibodies against AAV, and potentially other serum factors that can neutralize AAV transduction, has to be taken into account when planning and executing in vivo experiments that use AAV vectors. Based on our data we propose that, especially for large animal models, which use outbred animals and do not come from a germ-free environment, sera of individual animals destined to be included into preclinical studies should be prescreened for the presence of neutralizing antibodies. Furthermore, we propose that animals with neutralizing antibody titers >1/2 should be excluded, at least if the neutralizing antibody titers are determined similarly to the assay described in this manuscript. (For the purpose of this article, we define the neutralizing titers as the dilution at which transduction is inhibited by >50% when compared to transduction controls in the absence of serum).
Results
Pooled mouse serum inhibits transduction by AAV serotypes
During our study of the prevalence of antibodies against AAV1 in a human population we made the surprising discovery that pooled mouse serum inhibits AAV1 transduction (Figure 1a). Strikingly, even mouse serum that was diluted 1/16 inhibited transduction by >50%. Because fetal bovine serum (FBS) also inhibited AAV1 transduction (Supplementary Figure S1), we omitted FBS from the medium for all our neutralizing antibody assays of sera from other species (Supplementary Materials and Methods). To test whether the surprising inhibition of AAV1 transduction by mouse serum was unique to AAV1 we decided to analyze the effect of pooled mouse serum on transduction by the prototypical AAV, AAV2, and by AAV6 and AAV9, both of which are cardiotropic (as is AAV1). Interestingly, of these additional serotypes, only transduction by AAV6 was inhibited by pooled mouse serum, whereas transduction by AAV2 and AAV9 were unaffected and strongly stimulated, respectively. Because all the recombinant AAVs used carried the same genome, a luciferase expression cassette driven by a hybrid cytomegalovirus/chicken β-actin promoter flanked by AAV2 inverted terminal repeats, whatever the factor was that inhibited transduction had to act on the viral capsid.
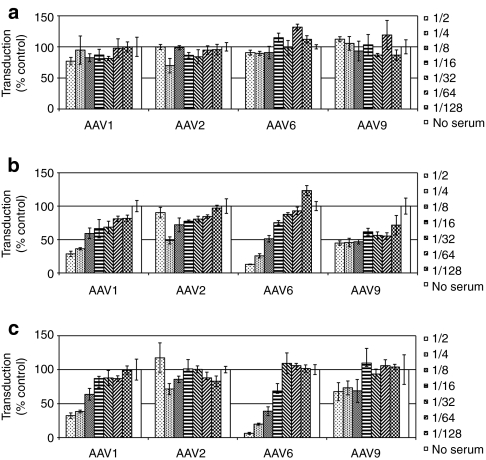
Figure 1.
Pooled mouse serum inhibits transduction by adeno-associated virus (AAV) serotypes 1 and 6, but not by serotypes 2 and 9. (a) Pooled mouse serum inhibits AAV transduction. Twofold dilutions of pooled mouse serum were incubated with constant amounts of AAV serotypes 1, 2, 6, and 9 and tested for neutralization of transduction using the in vitro assay described in Materials and Methods. Transduction is expressed as mean percent transduction of no-serum control samples ± SD. (b) Depletion of immunoglobulins drastically reduces the inhibition of AAV transduction by mouse serum. Pooled mouse serum was depleted of immunoglobulins by incubation with protein G resin and a rabbit anti-mouse immunoglobulin M (IgM) antibody. The effect of twofold dilutions of the precleared serum on AAV transduction was assayed as described in (a).
Although most AAVs have been isolated either from humans or nonhuman primates,11,12 several AAVs from other animal species have also been described, for instance bovine AAV,13 which is related to AAV4 and AAV5, and caprine AAV (AAV-Go.1),14,15 which is highly similar to AAV5. Because of the similarity of the bovine AAV capsid to the AAV4 capsid (76% identical) and of the high similarity of AAV-Go.1 capsid to the AAV5 capsid (94% identical), it is possible that animals that have been exposed to bovine AAV or AAV-Go.1 would not only harbor antibodies against these viruses but also against AAV4 or AAV5, respectively. Mice could also harbor antibodies against AAV serotypes isolated from humans or nonhuman primates because a murine AAV has also been described;16 although, the amino acid identity of this murine AAV to AAV1–9 is only ~50%.16 Furthermore, considering that mice are easily transduced in vivo by primate AAVs,17 it is also possible that they are infected by these AAV serotypes and, as a result, harbor antibodies against them. Based on this rational, we decided to test whether the inhibition of transduction by AAV1 and AAV6 by pooled mouse serum is due, at least in part, to the presence of neutralizing antibodies against these serotypes. In a first step, we depleted immunoglobulin G (IgGs) and IgMs by preincubating mouse serum with protein G beads in combination with a rabbit anti-mouse IgM antibody because protein G does not bind mouse IgMs.18 As can be seen from Figure 1b, this preclearing step completely eliminated the inhibition of transduction by AAV1 and AAV6 by mouse serum, which indicates that the inhibition of transduction was mostly, if not exclusively, due to neutralizing antibodies in pooled mouse serum.
Pooled sera of several animal species inhibit transduction by AAV serotypes
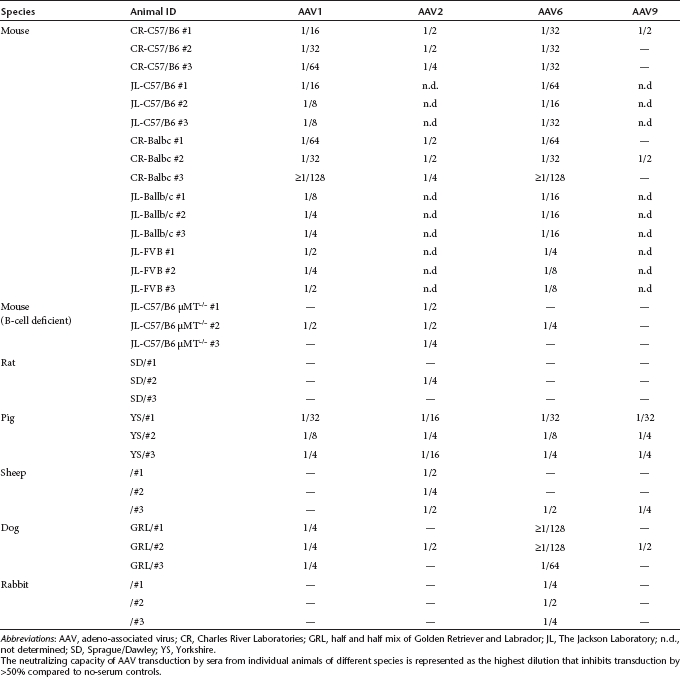
Because the presence of neutralizing antibodies against AAVs can have important implications for preclinical experiments, we decided to test sera of other animal models for gene transfer, particularly animal models commonly used to evaluate gene transfer for cardiac diseases. As can be seen from Table 1, the inhibition of transduction by the cardiotropic AAVs (AAV1, AAV6, and AAV9) and AAV2 varies widely among both animal species and AAV serotypes.
Table 1. Neutralization of AAV1, 2, 6, and 9 transduction by pooled sera from animal species used as preclinical animal models for AAV gene transfer.

Of the six animal species tested (mouse, rats, pigs, sheep, dogs, and rabbits), rat and sheep had the lowest neutralizing capacity (Table 1). For rats, only 1/2 and 1/8 dilutions of sera inhibited transduction by AAV2 and AAV6 and no inhibition was observed for AAV1 and AAV9. For sheep, the neutralizing titers for AAV2 and AAV6 were 1/8 and 1/2, respectively, whereas transduction by AAV1 and AAV9 was not inhibited. In sharp contrast, the neutralizing titers of pooled pig serum were equal to or higher than 1/16 for all serotypes tested. Neutralizing titers for pooled dog serum were 1/16 for AAV1, 1/2 for AAV2, and 1/1,024 for AAV6 whereas transduction by AAV9 was unaffected.
To confirm that the observed inhibition in transduction by these animal sera was due to the presence of anti-AAV immunoglobulins, we followed the same strategy that we used for mouse serum: we depleted immunoglobulins in the pooled sera and then tested for neutralization of transduction by AAV serotypes 1, 2, 6, and 9. To deplete the serum immunoglobulins, we incubated the animal sera with either protein A or G resin. Because neither protein A nor protein G exhibit a high affinity for rat IgGs,18 we used a rabbit anti-rat IgG antibody in addition to protein G to enhance binding of rat IgGs to the protein G resin. To deplete immunoglobulins from the other animal sera the choice of resin was based on the strong affinity of protein G for sheep IgG and of protein A for pig, dog, and rabbit IgG. As shown in Table 1, the modest inhibition of AAV6 transduction by rat serum and the inhibition of AAV2 transduction by sheep serum was not completely relieved by preclearing the pooled sera. One potential explanation for this could be the incomplete depletion of rat IgGs by the combination of protein G and rabbit anti-rat IgG antibodies. Alternatively, the inhibition of transduction could be a result of other immunoglobulin isotypes or nonimmunoglobulin serum factors. For the other pooled sera, with the exception of rabbit serum, preclearing resulted in greatly reduced neutralization (Table 1). Interestingly, using the standard immunoglobulin depletion protocol, preincubation of dog serum with protein A beads to remove immunoglobulins completely eliminated the inhibitory effect of the serum on AAV1 and AAV2 transduction but did not affect inhibition of AAV6 transduction. To evaluate whether this was due to insufficient depletion of anti-AAV6 immunoglobulins by protein G, we repeated the preclearing with diluted serum (1/32), thereby reducing the total amount of immunoglobulins that needed to be depleted. As shown in Supplementary Figure S2 and Table 1, this treatment resulted in a shift of the neutralizing titers from 1/1,024 for untreated serum to 1/128 for precleared serum. This eightfold reduction in neutralization suggests that, at a minimum, >85% of the inhibition of transduction by AAV6 by dog serum is due to neutralizing antibodies. At the same time, the presence of additional non-immunoglobulin inhibitory factors cannot be excluded.
Preclearing of rabbit serum with protein A beads only partially eliminated the inhibition of transduction by AAV1 and AAV6 (Table 1). Similarly, preclearing of sheep serum did not completely alleviate the inhibition of transduction by AAV2. One possible explanation for these results is that rabbits, and other species for this matter, have anti-AAV1/6 IgMs, which do not bind to protein A (or protein G).18 Alternatively, additional factors in certain animal sera might inhibit transduction by these serotypes.
Purified immunoglobulins from several animal species inhibit transduction by AAV serotypes 1, 2, 6, and 9
To confirm further that the inhibition of transduction by the AAV serotypes was the result of pre-existing neutralizing antibodies, we performed the in vitro neutralization assay using commercially available purified immunoglobulin preparations. The purified immunoglobulins were first diluted to a concentration corresponding to the approximate concentration found in the serum of the animal species tested. As was done for the pooled animal sera, the reconstituted immunoglobulins were then diluted in twofold increments. As shown in Figure 2 (mouse immunoglobulins) and Table 2 (aggregate data), purified immunoglobulins inhibit transduction by AAVs to varying degrees. The extent of inhibition qualitatively correlated with the reduction in transduction with pooled animal sera, although there was a general trend towards decreased inhibition by purified immunoglobulins when compared to animal sera. This difference was especially pronounced for rabbit IgGs versus rabbit serum. In contrast to rabbit serum, rabbit IgGs did not inhibit AAV1 and AAV6 transduction. This might be either due to the fact that, similarly to rodents, the inhibitory immunoglobulins in rabbit serum are IgMs, not IgGs, or that the reduction is a result of some unknown serum factor. Interestingly, in the case of rodent immunoglobulins, the inhibition is largely exerted by IgMs (and/or other immunoglobulin isotypes) rather than IgGs, which could be indicative of a recent infection. Unexpectedly, rat IgMs inhibit transduction by AAV1, AAV6, and AAV9 whereas rat serum did not. The easiest explanation for this discrepancy is that the purified rat IgM were not purified from the same batch of pooled serum. It is also possible, however, that serum factors that stimulate transduction are present in rat serum and compensate for the inhibition of transduction by rat IgMs. We did indeed observe stimulation of AAV transduction by some sera of individual rats (data not shown). Finally, it is striking that of all the immunoglobulins tested with AAV serotypes 1, 2, 6, and 9, the inhibition of AAV6 transduction by dog IgGs was most pronounced, mirroring the results obtained with pooled animal sera.
Figure 2.
Mouse immunoglobulins M (IgMs) inhibit transduction by adeno-associated virus (AAV) serotypes. (a) Mouse IgMs but no IgGs inhibit AAV transduction. Purified mouse IgG was diluted in Dulbecco's modified Eagle's medium (DMEM) to a concentration found in mouse serum. Twofold dilutions were incubated with constant amounts of AAV serotypes 1, 2, 6, and tested for neutralization of transduction using the in vitro assay described in Materials and Methods. Transduction is expressed as mean percent transduction of no IgG control ± SD. (b) As in (a) but with mouse IgM. (c) As in (b) but with mouse IgG and IgM combined.
Table 2. Inhibition of AAV transduction by purified immunoglobulins from different animal models for AAV gene transfer.

Sera from individual mice neutralize transduction by AAV serotypes 1, 2, 6 and, to a lesser extent, serotype 9
The neutralization experiments described above were performed using pooled sera that were commercially available. Because the presence of neutralizing antibodies in individual animals—especially if they vary between animals—could have significant effects on gene transfer experiments with these animals, we next sought to investigate the neutralization of AAV transduction by sera of individual animals. For our initial experiments, we used the two most commonly used inbred strains for mice, C57/B6 and Balb/c, which we obtained from Charles River Laboratories (Wilmington, MA). These naive mice were sacrificed and their blood harvested within less than 48 hours of their arrival at our facility. We considered using these two commonly used strains of mice relevant because variations in the immune responses between strains have been reported previously.19 As shown in Table 3, C57/B6 and Balb/c mice from Charles River Laboratories display similar patterns of neutralization against each AAV serotype. In particular, AAV9 is the least inhibited by individual mouse sera followed by AAV2, which is mildly inhibited. The closely related AAV serotypes 1 and 6 show a similar pattern: they are both inhibited strongly, with Balb/c sera demonstrating a slightly stronger inhibition. Strikingly, the titers varied between animals and, for AAV1, the neutralizing titers ranged from 1/16 to ≥1/128. Especially at lower vector doses, it is difficult to imagine that this eightfold difference in neutralizing titers observed would not affect AAV1 transduction levels between these two particular mice.
Table 3. Aggregate data of neutralization assays by serum from individual animals of different species against AAV serotypes 1, 2, 6, and 9.
We next wanted to test whether the strong inhibition of AAV1 and AAV6 transduction by sera of individual mice was specific for mice obtained from Charles River Laboratories. Hence, we obtained mice of three different strains (C57/B6J, Balb/c, and FVB/NJ) from the Jackson Laboratory (Bar Harbor, ME). As can be seen from Table 3, similarly to mice obtained from Charles River Laboratories, sera from C57/B6 and Balb/c mice strongly inhibited AAV1 and AAV6 transduction, although the inhibition of AAV1 transduction was less pronounced with sera from Balb/c mice from Jackson Laboratories. Finally, sera from FVB/NJ mice inhibited AAV1 and AAV6 transduction as well, albeit to a somewhat lesser extent. Strikingly, the sera from all 15 naive mice of three different strains that were obtained directly from two major vendors inhibited—to varying degrees—AAV1 and AAV6 transduction.
The experiments with pooled mouse serum that was depleted of IgGs and IgMs and the results of the neutralization assays with purified immunoglobulins strongly suggested that the observed inhibition was, to a significant part, due to neutralizing antibodies. To confirm further that the inhibition of transduction by pooled and individual mouse sera is due to neutralizing antibodies we took advantage of µMT–/– mice.20 Because of a disruption of the transmembrane exon of the immunoglobulin µ chain, these mice largely lack mature B-cells and fail to produce IgMs,20,21 although they are, to a variable extent, able to produce IgAs.21
Interestingly, all three B-cell deficient mice modestly inhibited AAV2 transduction. This suggests that the modest inhibition of AAV2 transduction that we observed in wild-type mice was not a result of pre-existing neutralizing antibodies but rather due to an unknown serum factor. Only one of the three µMT–/– mice modestly inhibited AAV1 and AAV6 transduction (Table 3). It has to be pointed out, however, that this particular mouse had a large tumor in its chest cavity, making the results difficult to interpret. Most importantly, of all the sera from the 18 individual mice test only the two tumor-free µMT–/– mice did not inhibit AAV1 or AAV6 transduction. In contrast, the sera of these mice stimulated transduction by AAV1 and AAV6 by up to fourfold. These data lend further support to the notion that the inhibition of AAV1 and AAV6 transduction is, to a significant extent, due to neutralizing antibodies in the serum of naive mice from commercial vendors. At the same time, the data also suggests that the modest inhibition of AAV2 transduction by individual mouse sera is the result of an unidentified serum factor or factors and not due to pre-existing neutralizing antibodies.
Sera from individual animals of nonmurine species neutralize transduction by AAV serotypes 1, 2, 6, and 9
We also examined another rodent species, the rat, as it serves as an experimental model in a wide variety of disease studies, including cardiovascular diseases. We observed that the serum from Sprague/Dawley strain rats did not inhibit transduction by AAV serotypes 1, 6, and 9, whereas AAV2 was neutralized moderately by the serum from only one animal out of the three animals tested. Interestingly, both pooled and individual rat sera were the least neutralizing compared to all other species tested in this study (Tables 1 and 3). We also analyzed serum from pigs, which—because of the similarities of the porcine and human cardiovascular system—are a widely used large animal model for cardiovascular diseases.22–24 In contrast to mice and rats, individual pig sera inhibited transduction by all serotypes and without drastic differences between the serotypes studied. Individual sera from another large animal model, sheep, consistently inhibited AAV2 but only one out of three sheep sera tested also inhibited transduction by the other serotypes studied. Because dogs are an important animal model for several diseases, including hemophilia,25 cardiovascular disease,26 and muscular dystrophy,27,28 we also tested the neutralizing capacity of individual dog sera. Sera from individual dogs showed an interesting neutralization pattern that was similar to the results obtained with pooled dog serum. In particular, AAV6 transduction was inhibited very strongly by the serum from all dogs. The inhibition of AAV6 transduction was by far the highest when compared to any other serotype or animal. Interestingly, transduction by AAV1, whose capsid sequence differs by only six amino acids from AAV6,29 was only mildly inhibited by dog serum and only one individual dog serum inhibited AAV2 or AAV9 transduction. Finally, we tested individual rabbit sera and, similar to individual rat sera, the neutralization was low. Only AAV6 transduction was mildly inhibited by rabbit sera. Overall, rat, rabbit, and sheep displayed the least inhibition of AAV transduction among the animal species tested. Mouse serum strongly inhibited AAV1 and AAV6, whereas dog serum very strongly inhibited AAV6. Finally, pig sera displayed the highest levels of variability between animals, which has important implications when comparing results between individual animals in an experimental group.
Very low in vitro neutralizing antibody titers neutralize AAV transduction in vivo
We next decided to test the effects of a range of neutralizing antibody titers on in vivo transduction. Of all species tested, rats were the species with the lowest neutralizing titers against AAVs and neither pooled serum nor sera from individual rats showed any neutralizing antibodies against AAV9 (Tables 1 and 3). Consequently, rats allowed us to control predictably neutralizing antibody titers upon immunization with AAV9 viral-like particles (VLPs, i.e., empty capsids). In order to generate a wide range of neutralizing titers against the capsid, we used different amounts of AAV9-VLPs and two time-points (5 and 7 days postimmunization) at which we analyzed the neutralizing antibody titers and injected AAV9scEGFP. With this approach we obtained neutralizing titers ranging from <1/2 to 1/128. Rats were sacrificed 1 month postinjection, a time-point known to be sufficient for high-level transgene expression with self-complementary AAV vectors.30 The heart was harvested and green fluorescent protein (GFP) expression levels in the cardiac tissue were determined by western blot (protein levels) (Figure 3a,b). AAV genome copies in the cardiac tissue were quantified by quantitative PCR (qPCR) (Figure 3c).
Figure 3.
Even rats with low in vitro neutralizing titers show strongly reduced in vivo transduction. Rats were immunized with different amounts of empty adeno-associated virus serotype 9 (AAV9) capsids or were left untreated (rats 7, 8, and 15). Five or seven days after immunization the rats were injected with AAV9scEGFP and sacrificed 1 month after vector injection. Protein levels and AAV viral genomes in the heart were quantified as described in Materials and Methods. (a) Western blot against enhanced green fluorescent protein (EGFP) and GAPDH. (b) Quantification of EGFP/GAPDH expression in (a). (c) Viral vector genomes in the heart measured by quantitative PCR (qPCR) ; β actin was used as an internal control. Values in (c) are expressed relative to the levels found in the nonimmunized rat 7 (mean ± SD). The asterisk indicates a nonspecific band recognized by the anti-GFP antibody.
One month after systemic injection of AAV9scEGFP, rats that were not immunized (rats 7, 8, and 15) expressed high levels of enhanced GFP (EGFP) (Figure 3a,b). Strikingly, even very low neutralizing titers—i.e., <1/2—could result in an inhibition of gene expression, and none of the animals with a neutralizing titer of >1/2 showed any EGFP expression in the heart. To confirm that the inhibition of transduction is a result of the virions not being taken up by cardiac myocytes we isolated viral DNA from the heart tissue and determined the number of AAV genomes. As shown in Figure 3c, rats that were not immunized (rats 7, 8, and 15) exhibited high levels of viral DNA in the heart, whereas rats with low neutralization titers (rats 6, 11, 13) had significantly fewer viral genomes in the heart, matching the EGFP expression levels in these animals. These results suggest strongly that the neutralization of AAVs in vivo mostly occurs before virus endocytosis. Rat 10 demonstrated low levels of viral DNA, although no EGFP gene expression was detected. Whereas the precise reason for this is unknown, a similar observation has been reported by others.31 One possible explanation is that while the virus was successfully taken up by cardiomyocytes, a step downstream of endocytosis is inhibited by antibodies in the serum of this rat.
The inhibition of in vivo transduction by low levels of neutralizing antibodies also appears to translate into large animal models. When Göttingen minipigs were injected with an AAV1 vector encoding for the sarcoplasmatic reticulum ATPase, SERCA2a, the vector genome copy numbers in the heart, 2 days after vector injection, were lower in animals with neutralizing antibodies (Supplementary Figure S3). Importantly, barely any vector genomes could be detected in the heart of an animal with a neutralizing titer of 1/4 again demonstrating that even low neutralizing titers can significantly affect AAV transduction. Because the animals were sacrificed 2 days postvector injection, SERCA2a expression levels could not be measured.
Discussion
It has recently been recognized that pre-existing neutralizing antibodies against AAV present a significant hurdle to the broad application of AAV gene therapy. For instance, in the first human gene therapy trial to treat heart failure, the Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) trial, almost 50% of patients had to be excluded because they had neutralizing antibody titers of >1/2.8,32 Similarly, in the high-dose vector group in a clinical trial aimed at the treatment of factor IX deficiency (hemophilia B) with a liver-targeted AAV vector only the subject with a neutralizing titer of 1/2, but not the patient with a titer of 1/17, showed significant, albeit transient, factor IX expression.9 Furthermore, several studies demonstrated that neutralizing antibodies are highly prevalent in the human population.1,2,33,34
Until now, however, it has been assumed that, with the exception of nonhuman primates,4,10 animals used in preclinical research do not have pre-existing neutralizing antibodies against AAV. In rats, this notion was supported by Palomeque et al.,35 who detected no antecedent neutralizing antibodies against AAV1–8. However, in the present manuscript, we show that rats are the exception rather than the rule as far as pre-existing neutralizing antibodies directed against AAVs are concerned.
Maybe the most surprising finding of our study is the presence of neutralizing antibodies in the sera of individual, naive mice that were obtained directly from commercial vendors and that were sacrificed within less than 48 hours after arrival at our facility. While there is a murine AAV described in the literature,16 the sequence identity of the capsid proteins is only ~50% when compared to the primate AAVs. The reason for the presence of neutralizing antibodies against AAVs in mice is unclear. However, the simplest explanation is that there are as of yet unidentified murine AAVs with higher sequence similarity to the AAV serotypes and variants isolated from humans and nonhuman primates. Because AAVs are nonpathogenic, infections by such viruses could easily be missed and only screening by PCR could detect the presence of such viruses. Going forward, it will be important to identify such candidates—if indeed present—and raise mice under conditions that prevent infection by these viruses thus eliminating a potentially confounding variable in AAV gene transfer experiments in mice. It is also possible that the human or nonhuman primate AAVs, which under experimental conditions transduce mice very efficiently,17 have infected the mice, through their handling by the animal facility personnel.
AAVs have also been isolated from sheep36 and pigs37 providing a potential explanation for the presence of (crossreacting) neutralizing antibodies in the sera of these species. In dogs the situation is unclear. Whereas a canine AAV is listed by the International Committee on Taxonomy of Viruses (http://www.ictvdb.rothamsted.ac.uk/Ictv/fslowbar;parvo.htm) and a similarity between bovine AAV and canine AAV has been reported,38 we were unable to find the genome sequence for this virus. Nonetheless, given the wide-spread presence of AAVs in the animal kingdom the easiest explanation for the presence of neutralizing antibodies in the animal species discussed in this report is the infection of these animals with either species-specific AAVs or AAVs with broad species tropism.
The results in this article demonstrate clearly that neutralizing antibodies against several AAV serotypes are present in the sera of animals that are commonly used in preclinical gene therapy research. However, our results also provide evidence for the presence of additional neutralizing factors in animal sera. For instance, preclearing of rabbit and dog sera with protein G and protein A beads, respectively did not completely eliminate the neutralizing capacity of these sera. Similarly, the inhibition of transduction by purified immunoglobulins was generally lower when compared to the corresponding animal sera, and the sera of B-cell-deficient µMT–/– inhibit AAV2 modestly but as efficiently as sera from wild-type mice. Clearly, serum factors, in addition to neutralizing antibodies, exist that can either inhibit transduction or, as in the case of AAV9 and mouse serum, strongly stimulate transduction. The precise nature of these factors is currently unknown and future studies will have to identify and characterize those factors. However, it has recently been reported that the serum factor galectin-3-binding protein in human and dog sera and C-reactive protein in mouse sera can interact with AAV6 and potentially inhibit transduction.39 Therefore, it is possible, if not likely, that in dog and mouse sera AAV6 transduction is inhibited by galectin-3-binding protein and C-reactive protein in addition to neutralizing antibodies; although the majority of inhibitory capacity in the pooled dog serum used in our study seems to be a result of pre-existing neutralizing antibodies. Furthermore, both AAV1 and AAV6 have been shown to bind to N-linked sialic acids and removal of sialic acid from the cell surface or competition with lectins inhibits AAV1 and AAV6 transduction.40 In this context, it is noteworthy that the majority of secreted proteins are glycosylated and that the N-linked glycosyl units terminate in sialic acid. It is not unreasonable to assume then that at least certain glycosylated serum proteins might compete with receptor binding. It has indeed been shown that AAV1 can bind to apotransferrin, a serum glycoprotein, although it has not been tested whether apotransferrin can inhibit AAV1 transduction.40 Furthermore, the EGF receptor has recently been reported to function as a coreceptor for AAV6.41 At present, it is not known whether binding of EGF competes with AAV6 transduction. It has, however, been described that soluble laminin and platelet-derived growth factor compete with the binding of AAV9 and AAV5 to their putative (co-)receptors, the 37/67 kDa laminin receptor and the platelet-derived growth factor receptor.42,43 Similarly, it cannot be excluded completely that EGF binding could compete with AAV6 transduction. In addition, AAV2 capsids have been shown to interact directly with the complement factors C3, C3b, iC3b, and factor H in pooled human serum.44 However, at present, it is not known if binding of these complement factors to the viral capsid interferes with AAV2 transduction and it remains to be shown if the same factors in animal sera share this property. Future research aimed at elucidating the nature of serum factors that influence AAV transduction will be crucial because the knowledge of the identity of inhibitory serum factors will be essential in the development of AAV variants that are resistant to these factors.
In summary, our data reveal the surprisingly high levels of neutralizing antibodies against several AAV serotypes in multiple animal species used in preclinical gene therapy research. The unexpectedly high prevalence of neutralizing antibodies notwithstanding, we do not want to imply that our results put into question the results of the enormous amount of AAV gene transfer experiments reported in the literature because the presence of neutralizing antibodies in many—though not all cases—might not matter because of either the route of administration or the large vector doses per kg body weight used. Instead, we hope that our results will bring to the attention of the gene therapy field information that will prove beneficial in the design and analysis of future experiments.
We also believe that, if the presence of neutralizing antibodies is the result of an infection by naturally occurring AAVs, then it should be possible , at least for mice, to eliminate this confounding variable by preventing infection by these AAVs. For large animal models, such as dogs and pigs, this is much more challenging, if at all possible. In many ways, these animal models mimic the situation observed in the human population; individual animals can present with largely varying neutralizing antibody titers. Clearly, in the future, it will be essential to screen such animals to be included into preclinical research for pre-existing neutralizing antibodies against the AAV serotype to be used. Essentially, especially with large animals, the same kind of exclusion criteria that are currently being used in clinical trials using AAV vectors have to be put into practice in preclinical gene therapy research. While somewhat cumbersome, it seems likely that this will result in smaller variations in experimental outcomes within any given experimental group of animals as well as in an increase in the reproducibility of experiments between different laboratories. There can be little doubt that the implementation of these protocols will facilitate the translation of preclinical results into the clinic.
Materials and Methods
All procedures were performed according to local Institutional Animal Care and Use Committees in accordance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical research and the “Guide for the Care and Use of Laboratory Animals” released by the Institute of Laboratory Animal Resources, the Commission on Life Sciences and the National Research Council.
Animal sera. Pooled mouse and canine serum was obtained from Sigma-Aldrich (cat. nos.: S3509, S1757, respectively; St Louis, MO), rat, rabbit, and sheep serum were from Equitech-Bio, Kerrville, TX (cat. nos.: SRT-0050, SR-0100, SS-0100, respectively) and pig serum was from Pel Freez, Rogers, AR (cat. no.: 39526-1).
Individual mouse and rat serum samples were collected from animals using serum separator tubes. For mice and rats, the serum was prepared with BD Microtainer Serum Separator tubes according to manufacturer's instructions (365956; BD Biosciences, Franklin Lakes, NJ). For pigs, serum was collected with BD Vacutainer tubes (366430; BD Biosciences), let coagulate for 20 minutes at room temperature and then centrifuged at 700g for 10 minutes. Serum samples from rabbits, dogs, and sheep were kindly provided by Juan J. Badimon (Mount Sinai School of Medicine, New York, NY), Dongsheng Duan (University of Missouri, Columbia, MO) and Robert A. Levine (Massachusetts General Hospital, Boston, MA). Samples were stored at -20 °C and thawed on the day of the experiment.
Purified immunoglobulins. Lyophilized immunoglobulins were reconstituted in Dulbecco's modified Eagle's medium (DMEM) according to the manufacturers instructions: mouse IgGs (cat. no.: SLM66-0100), rat IgGs (cat. no.: SLRT66-0100), rabbit IgGs (cat. no.: SLR66-0001), sheep IgGs (cat. no.: SLS66-0100), dog IgGs (cat. no.: SLCA66-0100), and pig IgGs (cat. no.: SLP66) were from Equitech-Bio, whereas mouse IgMs (cat. no.: 010-0107) and rat IgMs (cat. no.: 012-0107) were from Rockland Immunochemicals (Gilbertsville, PA).
Serum preclearing. Sera were depleted of immunoglobulins by incubation with protein A resin (cat. no.: L00210) or protein G resin (cat. no.: L00209) from Genscript (Piscataway, NJ). Briefly, protein A or protein G beads were washed three times in phosphate-buffered saline and equal volumes of resin and serum were mixed and incubated overnight at 4 °C with end over end rotation. The protein A or G resin was pelleted by centrifugation and the serum collected with an insulin syringe. This depletion was repeated once. The mouse serum was incubated with protein G resin and 100 µg rabbit anti-mouse IgM (cat. no.: 315-005-020; Jackson Immunoresearch, West Grove, PA) per 500 µl mouse serum.. The rat serum was incubated with protein G resin and 100 µg/500 µl rat serum of rabbit anti-rat IgG (cat. no.: 312-005-003; Jackson Immunoresearch). The sheep serum was incubated with protein G resin, whereas the dog, pig, and rabbit pooled sera were incubated with protein A resin.
AAV production, purification, and characterization. 293T cells were grown in DMEM, 10% FBS, and penicillin/streptomycin at 37 °C and 5% CO2. Viruses were prepared with the so-called two-plasmid system as previously described with some modifications.45 Transfection was achieved by transfection of 293T cells grown in triple flasks (cat. no.: 12-565-541; Thermo Fisher Scientific, Rochester, NY) using Polyethylenimine (linear, MW ~25,000; cat. no.: 23966; Polysciences, Warrington, PA) as a transfection reagent. 150 µg of the helper plasmid and 50 µg of the transgene plasmid were mixed with 20 ml of DMEM and 700 µl of polyethylenimine (1 mg/ml) and vortexed for 30 seconds. The mixture was incubated at room temperature for 15 minutes, added to DMEM, 2% FBS, penicillin/streptomycin, and added to the triple flasks with 50–80% confluent 293T cells. The cells were collected 3 days later at 300g for 10 minutes (Sorval RC-4, LH-4000 rotor) and resuspended in 10 ml of lysis buffer (150 mmol/l sodium chloride, 50 mmol/l Tris–HCl pH 8.5), subjected to three freeze-thaw cycles and treated with 1,500 u of Benzonase Nuclease (cat. no. E1014; Sigma-Aldrich) in the presence of 1 mmol/l magnesium chloride for 1 hour at 37 °C. Cellular debris was removed by centrifugation for 10 minutes at 5,000g (Sorval RC-4, LH-4000 rotor). The AAV particles in the supernatant of the cell culture medium were precipitated by ammonium sulfate precipitation (31.3 g/100 ml of medium, centrifugation at 8,300g (Sorval RC-4, LH-4000 rotor) for 30 minutes at 4 °C) and the viral pellet from 200 ml of medium was resuspended in 10 ml of lysis buffer. For AAV1 and AAV6, the virus in the cell lysate was concentrated before purification by precipitation with 8% (wt/vol) polyethylene glycol (PEG, PEG-8000). The final PEG concentration was achieved by the addition of one quarter volume of a PEG stock solution (40% PEG-8000, 2.5 mol/l sodium chloride). After a 2-hour incubation on ice the AAVs were collected by centrifugation at 2,500g (Sorval RC-4, LH-4000 rotor) for 10 minutes. The pellet of three triple flasks was resuspended in 10 ml of lysis buffer and the virus was isolated as described below. The virus was purified by a four-step iodixanol gradient centrifugation (7.3 ml of 15%, 4.9 ml of 25%, 4 ml of 40%, and 4 ml of 60% iodixanol (Optiprep; Sigma-Aldrich, cat. no. D1556) were overlayed with 10 ml of cell lysate in lysis buffer) in a 70Ti rotor (Beckman Coulter, Brea, CA) at 68,000 r.p.m. for 1 hour using OptiSeal Polyallomer Tubes (cat. no.: 361625; Beckman Coulter). The 40–60% interphase of the gradient was collected and the buffer was exchanged by dialysis (Spectrum × Spectra/Por × 2 RC Dialysis Membrane Tubing 12,000 to 14,000 Da MWCO, 132676; Thermo Fisher Scientific) against lactated Ringer's solution (two-times 500 volumes of the virus solution). The titers were determined by qPCR using the SYBR Advantage qPCR Premix (cat. no.: 639676; Clontech, Mountain View, CA) with an Applied Biosystems (Carlsbad, CA) 7500 real-time PCR system with primers against the Simian Virus 40 (SV40) polyA sequence (Forward: AACCTCCCACACCTCCC, Reverse: TTGGACAAACCACAACTAGAA) and the rAAV2 reference standard stock (RSS) (VR-1616; ATCC, Manassas, VA). The purity of the viral preparation was assessed by Coomassie staining.
Helper plasmids. AAV1: pXYZ 1,46 AAV2: pDG plasmid (kindly provided by Jürgen Kleinschmidt, Deutsches Krebsforschungszentrum, Heidelberg, Germany),47 AAV6: pDP6rs (Plasmid Factory, Bielefeld, Germany) and AAV9: pDG9 plasmid (kindly provided by James M. Wilson, University of Pennsylvania School of Medicine, Philadelphia, PA). Transgene plasmids: CBA-Luc encodes for firefly luciferase under the control of a hybrid cytomegalovirus/chicken β-actin promoter (kindly provided by M. Nonnenmacher, Mount Sinai School of Medicine). dsAAV-GFP (kindly provided by X. Xiao, University of North Carolina, Chapel Hill, NC): encodes for the EGFP under the control of a cytomegalovirus promoter. Deletion of the left-hand D-region in this construct results in the generation of self-complementary, i.e., double-stranded AAV vectors.30
VLP production, purification, and characterization. Sf9 cells were grown in Sf900 II SFM cell culture medium (Carlsbad, CA) to a density of 2 × 106 cells/ml and were infected with BacCapAAV948 with a multiplicity of infection of 5. Three days after infection, the AAV9-VLPs were harvested from cells and media. The cells were pelleted at 2,000g for 10 minutes (Sorval RC-4, LH-4000 rotor) and the pellet was resuspended in lysis buffer 2 (20 mmol/l Tris–HCl (pH 7.5), 150 mmol/l sodium chloride, 1% (vol/vol) Triton X-100, 10 mmol/l magnesium chloride). After a 10 minutes incubation at room temperature, cell debris was pelleted at 2,100g (Sorval RC-4, LH-4000 rotor) for 10 minutes. VLPs were isolated by iodixanol gradient centrifugation as described below. The VLPs from the cell culture media were harvested by PEG precipitation. To the cell culture supernatant one quarter volume of a PEG stock solution (40% PEG-8000, 2.5 mol/l sodium chloride) was added for a final PEG concentration of 8%. After 2-hour incubation on ice, the VLPs were collected by centrifugation at 2,500 g (Sorval RC-4, LH-4000 rotor) for 30 minutes. The pellet containing the VLPs was resuspended in lysis buffer and then further purified by centrifugation on an iodixanol gradient. The solution containing the resuspended VLPs, in Optiseal tubes (cat. no.: 361625, Beckman Coulter), was underlaid sequentially with 5 ml 15%, 10 ml 25%, and 5 ml 40% iodixanol. The gradient was centrifuged at 69,000 r.p.m. for 1 hour in a 70Ti rotor at 18 °C. Fractions were collected from the bottom of the tube. The peak fractions containing the VLPs, near the 25–40% interface, were pooled and diluted to 10 ml in 150 mmol/l sodium chloride, 50 mmol/l Tris–hydrochloride pH 8.5 and further purified on a second, identical iodixanol gradient. The pure VLP peak fractions were concentrated and the buffer was exchanged to lactated Ringer's solution by three rounds of dilution/concentration in Vivaspin-20 100 KDa MWCO centrifugal concentrators (cat. no.: VS2041; Sartorius, Bohemia, NY).
In vitro AAV neutralization assay. 293T cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C and 5% CO2. 2 × 104 cells/well in DMEM + penicillin/streptomycin were seeded into 96 well-plates (cat. no.: 08-772-2C; Thermo Fisher Scientific) the morning of the infection. Five hours later, in a separate 96 well-plate, twofold serial dilutions of the animal sera in DMEM were prepared. The concentrations ranged from 10 µl undiluted serum to 1/128 diluted serum. Wells with the same volume of DMEM served as no-serum controls. 2 × 108/well viral genomes of AAV-Luc in an equal volume of DMEM were added to the sera samples or the DMEM control wells (in triplicate), incubated at 37 °C for 30 minutes and then added to the 293T cells. Twenty four hours later, the transduction efficiency was analyzed by quantifying luciferase activity. The percentage of inhibition was calculated relative to positive, no-serum control samples.
Luciferase assay. The cells were lysed in the wells of the 96-well plate by adding an equal volume of 2× luciferase lysis buffer [10 mmol/l Tris–hydrochloride pH 8.0, 150 mmol/l NaCl, 1% (wt/vol) NP40, 10 mmol/l DTT] and incubated at room temperature on a shaker for 10 minutes before the assay. The luciferase assay was performed as previously described49 with minor modifications. Briefly, 20 µl of the cell lysate were added to a black 96-well FluoroNuc plate (cat. no.: 12-565-275; Thermo Fisher Scientific). The luciferase reaction was initiated by the addition of 100 µl of the reaction buffer (25 mmol/l Tricine–hydrochloride pH 7.8, 5 mmol/l magnesium sulfate, 0.5 mmol/l EDTA (pH 8.0), 3.3 mmol/l DTT, 0.5 mmol/l ATP pH ~7–8 (A26209; Sigma-Aldrich), 1 mg/ml bovine serum albumin, 0.05 mg/ml 𝒟-luciferin (cat. no.: LUCK-100; Gold Biotechnology, St Louis, MO), 0.05 mmol/l CoA (cat. no.: 13787, United States Biological, Swampscott, MA) and was read in a luminescence counter (Microbeta Trilux 1450 LSC and Luminescence Counter; Perkin Elmer, Carlsbad CA).
Immunization of rats and analysis of in vivo AAV transduction. 200–250g male rats (Sprague–Dawley strain; Charles River Laboratories) were injected with AAV9-VLPs at the following concentrations: rats 1 and 2: 100 ng protein, rats 3 and 4: 10 ng, rats 5 and 6: 1 ng, rats 9 and 10: 10 ng, rats 11 and 12: 1 ng, rats 13 and 14: 1.25 ng. The injections were performed using a 24G catheter and, before injection, blood was collected through the catheter for the in vitro neutralization assays. Five to seven days later the animals were injected with 1011 viral genomes of AAV9scEGFP through the tail vein using a 24G catheter. Before vector injection, blood was collected to determine the neutralization titer with the in vitro neutralization assay described above. Vector was injected at day 5 after immunization for rats 9 and 10 and at day 7 after immunization for rats 1 to 6 and rats 11 to 14. One month postinjection the rats were sacrificed and the heart collected.
Western blot. Heart tissues were washed in phosphate-buffered saline and snap-frozen in liquid nitrogen. The tissue was ground in liquid nitrogen and stored at -80 °C. The pulverized tissue was homogenized in RIPA buffer [50 mmol/l Tris–hydrochloride 50 mmol/l, 150 mmol/l sodium chloride, 0.1% (wt/vol) SDS, 0.5% (wt/vol) sodium deoxycholate, 1% (vol/vol) NP40], with protease inhibitors (cat. no.: 118361700001; Invitrogen, Carlsbad, CA), using Lysing Matrix D tubes (cat. no.: 6913-500; MP Biochemicals, Solon, OH) and a FastPrep homogenizer (MP Biochemicals). Protein concentration was quantified by using the BCA Protein Assay Reagent (cat. no.: 23227; Pierce, Thermo Fisher Scientific). Forty microgram of total protein extracts were mixed with sample buffer (62.5 mmol/l Tris–hydrochloride, pH 6.8, 10% (vol/vol) glycerol, 2% (wt/vol) SDS, 5% (vol/vol) β-mercaptoethanol and 0.005% (wt/vol) bromophenol blue), heated at 95 °C for 5 minutes and then loaded onto 12% SDS-PAGE gels. They were then transferred onto Immobilon-FL Transfer Membrane (cat. no.: IPFL00010; Millipore, Billerica, MA). Membranes were blocked with 5% fat-free milk in Tris-buffered saline (TBS) for 2 hours at room temperature and incubated with the primary antibodies diluted in blocking buffer overnight at 4 °C (mouse anti-GFP 1:2,000, rabbit anti-GAPDH 1:10,000; cat. no.: 632375, Invitrogen and cat. no.: G9545; Sigma-Aldrich, respectively). The membranes were washed three times in TBS-Tween [0.05% (vol/vol)] and then incubated for 1 hour at room temperature with the horseradish peroxidase-conjugated secondary antibodies (1:10,000 in 2.5% fat-free milk in TBS-Tween). The membranes were then washed three times with TBS-Tween and visualized using the Immobilon Western Chemiluminescent horseradish peroxidase Substrate (cat. no.: WBKLS0050; Millipore). Contrast and brightness of the western blot in Figure 3a was adjusted uniformly across the entire picture in Adobe Photoshop CS2 (Adobe Systems Incorporated, San Jose, CA). To quantify the intensity of the bands, we calculated the integrated intensity parameter using in Adobe Photoshop CS2 before adjusting for brightness and contrast. The integrated intensity of each band was calculated as the mean intensity multiplied by the number of pixels.
Viral DNA extraction and qPCR. Total DNA was extracted from the pulverized tissue using the Hirt extraction method as previously described.50 Briefly, the pulverized tissue was homogenized in 500 µl of Hirt extraction buffer [10 mmol/l Tris-hydrochloride, 1% (wt/vol) SDS, 10 mmol/l EDTA, pH 8.0] using Eppendorf tube pestles. The lysates were incubated with RNAse A (10 µg/ml, cat. no.: 10109169001; Roche, Indianapolis, IN) and after 30 minutes. Proteinase K (100 µg/ml. cat. no. EO0491; Fermentas, Glen Burnie, MA) for 4 hours at 37 °C. The homogenates were then extracted with an equal volume of phenol: chloroform: isoamyl alcohol (25:24:1) (cat. no.: P3803; Sigma-Aldrich) and the aqueous phase was collected. The DNA was precipitated by the addition of sodium chloride to a final concentration of 0.2 mol/l, glycogen to 50 µg/ml and three volumes of prechilled (-20 °C) ethanol. Samples were centrifuged in an Eppendorf centrifuge at 14,000g for 15 minutes at 4 °C and the pellet was washed with 70% ethanol and air-dried. The DNA was resuspended in 50 µl of water and used for real-time PCR. For the real-time PCR the following primers were used: EGFP (Forward: 5′ TGACCCTGAAGTTCATCTGCACCA 3′, Reverse: 5′ TCTTGTAGTTGCCGTCGTCCTTGA 3′), and gbActin (Forward 5′ GGAGGGGCCGGACTCATCGT 3′, Reverse 5′ ACACAGTGCTGTCTGGTGGCA 3′). The PCR targets (2 µl, equal to 100 ng DNA) were amplified using the SYBR Advantage qPCR Premix (cat. no.: 639676; Clontech) in the 7500 Real-Time PCR system (Applied Biosystems). No-template, negative control samples were also included.
Acknowledgments
We thank Mathieu Nonnenmacher (Mount Sinai School of Medicine, New York, NY), Jürgen Kleinschmidt (Deutsches Krebsforschungszentrum, Heidelberg, Germany), James M. Wilson, (University of Pennsylvania School of Medicine, Philadelphia, PA) and Xiao Xiao (University of North Carolina, Chapel Hill, NC) for providing us with the plasmids CBA-Luc, pDG, pDG9, and dsAAV-GFP, respectively. We gratefully acknowledge Juan J. Badimon and Chiara Giannarelli (Mount Sinai School of Medicine, New York, NY), Dongsheng Duan and Jinhong Shin (University of Missouri, Columbia, MO), Robert A. Levine, Suzanne Sullivan, and Nicholas Palmeri (Massachusetts General Hospital, Boston, MA) and James Lough (Mount Sinai School of Medicine, New York, NY) for providing us with serum samples from rabbits, dogs, sheep, and pigs, respectively. This work was supported by US National Institutes of Health Grants HL100396 (to R.J.H.), HL088434 (to R.J.H.), HL007824 (to V.L.-J.), HL097088 (to S.Z.), and GM082946 (to S.Z.).
Supplementary Material
FBS inhibits transduction by AAV serotypes 1 and 6 but not by serotypes 2 and 9.
Immunoglobulin depletion of 1/32 diluted dog serum alleviates inhibitory activity on AAV6 transduction.
Even pigs with low neutralizing antibody titers show reduced virus uptake into cardiomyocytes.
REFERENCES
- Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF.et al. (2010Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors Hum Gene Ther 21704–712. [DOI] [PubMed] [Google Scholar]
- Calcedo R, Vandenberghe LH, Gao G, Lin J., and, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Marel S, Comijn EM, Verspaget HW, van Deventer S, van den Brink GR, Petry H.et al. (2011Neutralizing antibodies against adeno-associated viruses in inflammatory bowel disease patients: Implications for gene therapy Inflamm Bowel Disepub ahead of print). [DOI] [PubMed]
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA.et al. (2006Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy Blood 1083321–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Calcedo R, Vandenberghe LH, Figueredo JM., and, Wilson JM. Impact of preexisting vector immunity on the efficacy of adeno-associated virus-based HIV-1 Gag vaccines. Hum Gene Ther. 2008;19:663–669. doi: 10.1089/hum.2008.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry H, Brooks A, Orme A, Wang P, Liu P, Xie J.et al. (2008Effect of viral dose on neutralizing antibody response and transgene expression after AAV1 vector re-administration in mice Gene Ther 1554–60. [DOI] [PubMed] [Google Scholar]
- Scallan CD, Jiang H, Liu T, Patarroyo-White S, Sommer JM, Zhou S.et al. (2006Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice Blood 1071810–1817. [DOI] [PubMed] [Google Scholar]
- Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease (CUPID) Trial Investigators et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase ½ clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ.et al. (2006Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response Nat Med 12342–347. [DOI] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Wang H, Bell P, Grant R, Vandenberghe LH.et al. (2010The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques Mol Ther 18126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Alvira MR, Somanathan S, Lu Y, Vandenberghe LH, Rux JJ.et al. (2003Adeno-associated viruses undergo substantial evolution in primates during natural infections Proc Natl Acad Sci USA 1006081–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J., and, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Katano H, Bossis I., and, Chiorini JA. Cloning and characterization of a bovine adeno-associated virus. J Virol. 2004;78:6509–6516. doi: 10.1128/JVI.78.12.6509-6516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbetman AE, Lochrie M, Zhou S, Wellman J, Scallan C, Doroudchi MM.et al. (2005Novel caprine adeno-associated virus (AAV) capsid (AAV-Go.1) is closely related to the primate AAV-5 and has unique tropism and neutralization properties J Virol 7915238–15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Cheng F., and, Pintel D. Molecular characterization of caprine adeno-associated virus (AAV-Go.1) reveals striking similarity to human AAV5. Virology. 2006;356:208–216. doi: 10.1016/j.virol.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Lochrie MA, Tatsuno GP, Arbetman AE, Jones K, Pater C, Smith PH.et al. (2006Adeno-associated virus (AAV) capsid genes isolated from rat and mouse liver genomic DNA define two new AAV species distantly related to AAV-5 Virology 35368–82. [DOI] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, Rengo G, Koch WJ., and, Rabinowitz JE. Comparative cardiac gene delivery of adeno-associated virus serotypes 1-9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin Transl Sci. 2010;3:81–89. doi: 10.1111/j.1752-8062.2010.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M, Metzger D.1994Antibody-binding bacterial proteins as immunoreagentsIn: Malik, V and Lillehoj, E (eds.). Antibody Techniques Academic Press: San Diego, CA; 177–209. [Google Scholar]
- Breous E, Somanathan S., and, Wilson JM. BALB/c mice show impaired hepatic tolerogenic response following AAV gene transfer to the liver. Mol Ther. 2010;18:766–774. doi: 10.1038/mt.2009.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura D, Roes J, Kühn R., and, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Lamarre A, McCoy K, Harriman GR, Odermatt B, Dougan G.et al. (2001IgA production without mu or delta chain expression in developing B cells Nat Immunol 2625–631. [DOI] [PubMed] [Google Scholar]
- Hadri L, Bobe R, Kawase Y, Ladage D, Ishikawa K, Atassi F.et al. (2010SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells Mol Ther 181284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Ladage D, Tilemann L, Fish K, Kawase Y., and, Hajjar RJ. Gene transfer for ischemic heart failure in a preclinical model. J Vis Exp. 2011. [DOI] [PMC free article] [PubMed]
- Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H.et al. (2008Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure J Am Coll Cardiol 511112–1119. [DOI] [PubMed] [Google Scholar]
- Nichols TC, Dillow AM, Franck HW, Merricks EP, Raymer RA, Bellinger DA.et al. (2009Protein replacement therapy and gene transfer in canine models of hemophilia A, hemophilia B, von willebrand disease, and factor VII deficiency ILAR J 50144–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeper MM, Bish LT., and, Sweeney HL. Gene therapy in large animal models of human cardiovascular genetic disease. ILAR J. 2009;50:199–205. doi: 10.1093/ilar.50.2.199. [DOI] [PubMed] [Google Scholar]
- Lu QL, Yokota T, Takeda S, Garcia L, Muntoni F., and, Partridge T. The status of exon skipping as a therapeutic approach to duchenne muscular dystrophy. Mol Ther. 2011;19:9–15. doi: 10.1038/mt.2010.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Shin JH., and, Duan D. Whole body skeletal muscle transduction in neonatal dogs with AAV-9. Methods Mol Biol. 2011;709:313–329. doi: 10.1007/978-1-61737-982-6_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Grieger JC, Govindasamy L, Agbandje-McKenna M., and, Samulski RJ. Single amino acid changes can influence titer, heparin binding, and tissue tropism in different adeno-associated virus serotypes. J Virol. 2006;80:11393–11397. doi: 10.1128/JVI.01288-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ma HI, Li J, Sun L, Zhang J., and, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- Peden CS, Burger C, Muzyczka N., and, Mandel RJ. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J Virol. 2004;78:6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar RJ, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A.et al. (2008Design of a phase ½ trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure J Card Fail 14355–367. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Miller AD, McNamara S, Emerson J, Gibson RL, Ramsey B.et al. (2006Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors Hum Gene Ther 17440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SL, Li H, Mingozzi F, Sabatino DE, Hui DJ, Edmonson SA.et al. (2009Diverse IgG subclass responses to adeno-associated virus infection and vector administration J Med Virol 8165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomeque J, Chemaly ER, Colosi P, Wellman JA, Zhou S, Del Monte F.et al. (2007Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo Gene Ther 14989–997. [DOI] [PubMed] [Google Scholar]
- Clarke JK, McFerran JB, McKillop ER., and, Curran WL. Isolation of an adeno associated virus from sheep. Brief report. Arch Virol. 1979;60:171–176. doi: 10.1007/BF01348034. [DOI] [PubMed] [Google Scholar]
- Bello A, Tran K, Chand A, Doria M, Allocca M, Hildinger M.et al. (2009Isolation and evaluation of novel adeno-associated virus sequences from porcine tissues Gene Ther 161320–1328. [DOI] [PubMed] [Google Scholar]
- Myrup AC, Mohanty SB., and, Hetrick FM. Isolation and characterization of adeno-associated viruses from bovine adenovirus types 1 and 2. Am J Vet Res. 1976;37:907–910. [PubMed] [Google Scholar]
- Denard J, Beley C, Kotin RM, Moullier P, Voit T, Garcia L.et al. (2011New proteins providing host defense function against rAAVs Mol Ther 19S254Abstract 661. [Google Scholar]
- Wu Z, Miller E, Agbandje-McKenna M., and, Samulski RJ. Alpha2,3 and alpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol. 2006;80:9093–9103. doi: 10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller ML, Amornphimoltham P, Schmidt M, Wilson PA, Gutkind JS., and, Chiorini JA. Epidermal growth factor receptor is a co-receptor for adeno-associated virus serotype 6. Nat Med. 2010;16:662–664. doi: 10.1038/nm.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akache B, Grimm D, Pandey K, Yant SR, Xu H., and, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale G, Davidson BL, Stein CS, Martins I, Scudiero D, Monks A.et al. (2003Identification of PDGFR as a receptor for AAV-5 transduction Nat Med 91306–1312. [DOI] [PubMed] [Google Scholar]
- Zaiss AK, Cotter MJ, White LR, Clark SA, Wong NC, Holers VM.et al. (2008Complement is an essential component of the immune response to adeno-associated virus vectors J Virol 822727–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger JC, Choi VW., and, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ., Jret al. (2002Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors Methods 28158–167. [DOI] [PubMed] [Google Scholar]
- Grimm D, Kern A, Rittner K., and, Kleinschmidt JA. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- Mitchell M, Nam HJ, Carter A, McCall A, Rence C, Bennett A.et al. (2009Production, purification and preliminary X-ray crystallographic studies of adeno-associated virus serotype 9 Acta Crystallogr Sect F Struct Biol Cryst Commun 65Pt 7715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford SR., and, Leach FR. Improvements in the application of firefly luciferase assays. Methods Mol Biol. 1998;102:3–20. doi: 10.1385/0-89603-520-4:3. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FBS inhibits transduction by AAV serotypes 1 and 6 but not by serotypes 2 and 9.
Immunoglobulin depletion of 1/32 diluted dog serum alleviates inhibitory activity on AAV6 transduction.
Even pigs with low neutralizing antibody titers show reduced virus uptake into cardiomyocytes.