Abstract
Bacteriocins are an abundant and diverse group of ribosomally synthesized antimicrobial peptides produced by bacteria and archaea. Traditionally, bacteriocin production has been considered an important trait in the selection of probiotic strains, but until recently, few studies have definitively demonstrated the impact of bacteriocin production on the ability of a strain to compete within complex microbial communities and/or positively influence the health of the host. Although research in this area is still in its infancy, there is intriguing evidence to suggest that bacteriocins may function in a number of ways within the gastrointestinal tract. Bacteriocins may facilitate the introduction of a producer into an established niche, directly inhibit the invasion of competing strains or pathogens, or modulate the composition of the microbiota and influence the host immune system. Here we review the role of bacteriocin production in complex microbial communities and their potential to enhance human health.
INTRODUCTION
Probiotics are defined as “live microorganisms, which when consumed in adequate amounts, confer a health benefit on the host” (45). They are believed to enhance or maintain the ratio of beneficial to undesirable components in the human gastrointestinal (GI) microbiota (44). The majority of probiotics in use today include species of lactic acid bacteria (LAB), including lactobacilli, as well as bifidobacteria, nonpathogenic Escherichia coli, bacilli, and yeasts such as Saccharomyces boulardii. The scientific and clinical evidence in support of the therapeutic potential of probiotic bacteria in human health, and most notably with respect to GI health, has been increasing steadily (7). It is not surprising, then, that there is an ever greater interest in these potential biotherapeutic agents and the mechanisms by which they elicit their beneficial effects.
Several mechanisms of probiotic action have been described, the most common relating to their abilities to strengthen the intestinal barrier, to modulate the host immune system, and to produce antimicrobial substances (8). Indeed, the production of antimicrobials is often regarded a priori as an important trait in the context of bacterial fitness but also in terms of probiotic efficacy. Several probiotic bacteria produce a variety of antimicrobial compounds (e.g., short-chain fatty acids, hydrogen peroxide, nitric oxide, bacteriocins) that may enhance their ability to compete against other GI microbes and which could potentially inhibit pathogenic (disease-causing) bacteria (1, 6). Traditionally, bacteriocin production has been an important criterion in the selection of a probiotic strain, albeit that few studies have definitively demonstrated the impact of bacteriocin production on the ability of a strain to compete within the GI tract and/or positively influence the health of the host (9).
Bacteriocins are bacterially produced peptides that are active against other bacteria and against which the producer has a specific immunity mechanism (10, 30). They are produced by all major lineages of bacteria and archaea and constitute a heterogeneous group of peptides with respect to size, structure, mode of action, antimicrobial potency, immunity mechanisms and target cell receptors (21). Here we review the literature with respect to the role of bacteriocin production within complex microbial niches, and in particular in the GI tract, in terms of their impact on the prevalence of the producing strain, as well as on microbial diversity and the survival of pathogens. We conclude with suggestions for future work and the possible ways in which bacteriocins could potentially be applied to enhance health.
BACTERIOCIN FUNCTION: AN ECOLOGICAL PERSPECTIVE
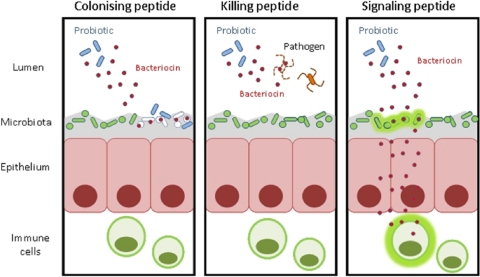
It has been estimated that the vast majority of all bacteria and archaea produce at least one bacteriocin (29). The apparent ubiquity of this trait implies that bacteriocins play an important role, despite the associated energy costs imposed by their production (23). However, their exact ecological function has been the subject of much debate. It is possible that bacteriocins could contribute to probiotic functionality in a number of ways (Fig. 1). Bacteriocins may function as colonizing peptides, facilitating the introduction and/or dominance of a producer into an already occupied niche (48). Alternatively, bacteriocins may act as antimicrobial or killing peptides, directly inhibiting competing strains or pathogens (38). Lastly, bacteriocins may function as signaling peptides, either signaling other bacteria through quorum sensing and bacterial cross talk within microbial communities or signaling cells of the host immune system (12, 17, 24, 38, 40).
Fig 1.
Mechanisms via which bacteriocin production could contribute to probiotic functionality. Bacteriocins may act as colonizing peptides, facilitating the competition of a probiotic with the resident microbiota (23); they may function as killing peptides, directly eliminating pathogens (9); or they may serve as signaling peptides, signaling other bacteria or the immune system (32, 40, 56).
Bacteriocins as colonizing peptides.
The high cell density typically associated with the GI tract may result in close cell-cell contact between members of the same or different species, promoting both cooperative and antagonistic microbial interactions (33). The production of antimicrobials may provide a mechanism by which producers can gain a competitive advantage over neighboring sensitive strains within this environment. In support of this hypothesis, Gillor et al. (23) demonstrated that E. coli producing the bacteriocin colicin was able to persist in the large intestine of streptomycin-treated mice for an extended period of time relative to their non-colicin-producing counterparts. Over time, the density of the noncolicinogenic strains decreased from 106 to 102 CFU/g feces, while that of colicin-producing strains remained significantly higher (23). In a similar study, Hillman et al. (25) noted a strong correlation between the ability of a Streptococcus mutans strain to colonize the oral cavity and the production of the bacteriocin mutacin 1140. One mutacin-producing strain was shown to be stably maintained in human subjects, persisting for 14 years following a single administration (25–27). Although direct competition studies using isogenic non-bacteriocin-producing mutants were not performed in this case, the fact that no other strains of mutans streptococci were observed in saliva and plaque samples is indicative of the competitive dominance of this strain. It has also been established that the production of BlpMN bacteriocins by the S. pneumoniae type 6A strain contributes to the ability of this strain to colonize and compete in the mouse nasopharynx (14). In this study, a non-BlpMN-producing mutant failed to compete with its bacteriocin-producing parent strain when the strains were administered in equivalent numbers. Cocolonization with a nonisogenic, non-BlpMN-producing strain, S. pneumoniae TIGR4, yielded similar results, thereby confirming that the production of the BlpMN bacteriocins contributes to the competitiveness of the associated strain within the complex microbial environment of the nasopharynx (14).
As one might expect, a number of GI-related studies have also been performed. A five-strain probiotic mixture composed of Lactobacillus murinus DPC6002 and DPC6003, Lactobacullus pentosus DPC6004, Lactobacillus salivarius DPC6005, and Pediococcus pentosaceus DPC6006 has been shown to improve the clinical and microbiological outcome of Salmonella infection in pigs (5). It was subsequently established that the only bacteriocin producer, L. salivarius DPC6005, dominated over strains coadministered with it in both the ileum digesta and mucosa of weaned pigs (57). Although an isogenic nonproducing mutant of L. salivarius DPC6005 was not employed, the authors suggested that the superior ileal survival of this strain could be attributed to bacteriocin production, indicating that this antimicrobial confers a competitive advantage over the other coadministered probiotics (57).
Bacteriocin producers may also modulate insensitive species within the GI tract. A recent study demonstrated that the bacteriocin-producing strain Enterococcus faecium KH24 significantly affected the fecal microbiota of mice (3). In this study, mice received 108 CFU/day of bacteriocinogenic E. faecium KH24 (Bac+) and a nonbacteriocinogenic variant (Bac−) for a period of 12 days. It was established that Lactobacillus populations were significantly greater in mice fed bacteriocinogenic E. faecium than those in the nonproducer. The authors concluded that E. faecium KH24 could be exploited as a probiotic and hypothesized that bacteriocinogenic enterococci may help to control the indigenous microbiota in a beneficial manner.
Evidence that bacteriocin production can contribute to microbial survival in the human GI tract has also been reported. One such study stemmed from functional genomic analyses of the intestinal isolate Bifidobacterium longum subsp. longum DJO10A which indicated that bacteriocin production might be an important adaptation for GI survival. Competitive growth rate experiments in a model fecal environment revealed that B. longum DJO10A had a significantly greater ability to compete against the intestinal isolates Clostridium difficile DJOcd1 and E. coli DJOec1 than did the nonproducing isogenic variant B. longum DJO10-JH1 (36). Although additional in vivo studies are necessary, these results provide a further indication that bacteriocin production is an important trait with regard to microbial competition in the human intestine.
Bacteriocins as killing peptides.
The ability of bacteriocin-producing microorganisms to inhibit pathogens in vitro has been well documented (23, 35, 52). However, studies involving direct correlations between in vitro efficacy and in vivo protection are somewhat scarce. For instance, it has recently been shown that lactococci producing the broad-spectrum antimicrobial peptide lacticin 3147 fail to confer protection against Listeria monocytogenes infection in a mouse model, despite the efficacy of the bacteriocin against this pathogen in vitro (18, 50). However, it should be noted that, in this case, the producing strain is not a GI-associated microorganism. Likewise, it has also been revealed that although pediocin PA-1 production by Pediococcus acidilactici UL5 reduces L. monocytogenes viability by approximately 3 logs in vitro, a corresponding effect was not observed in vivo (13). In this instance, the intragastric administration of 1010 CFU/animal of P. acidilactici UL5 failed to provide protection against L. monocytogenes infection in mice, despite the fact that the administration of purified pediocin PA-1 to mice resulted in a ca. 2-log reduction of fecal listerial counts. Indeed, an increase in L. monocytogenes invasion was observed in the intestines, livers, and spleens of P. acidilactici-treated mice compared to those of control mice. These findings are in agreement with an earlier study by Bernbom et al. (2) that investigated the ability of the pediocin AcH producer Lactobacillus plantarum DDEN 11007 to prevent L. monocytogenes EP2 infection in germfree rats. In this case, the introduction of L. plantarum DDEN 1007 prior to L. monocytogenes inoculation resulted in a subsequent relative increase in L. monocytogenes numbers in the livers and spleens of gnotobiotic animals over those of control animals. The authors of both studies attributed the increase in pathogen numbers to a lowering of the intestinal pH, suggesting that the production of lactic acid by Pediococcus spp. and Lactobacillus spp. induced virulence gene production in the pathogen. This theory remains quite speculative, although it has been noted that acid-treated L. monocytogenes has been shown to be more invasive than its non-acid-adapted counterparts (43). There are some studies which demonstrated the ability of bacteriocin producers to inhibit pathogens in the GI tract. Most notably, Corr et al. (9) found that L. salivarius UCC118 provides protection against L. monocytogenes infection in mice (Fig. 2). The inhibition of the pathogen was shown to be the direct result of the production of the two-peptide bacteriocin Abp118, as it was demonstrated that a non-bacteriocin-producing isogenic derivative failed to protect mice from infection. Similarly, the bacteriocin-producing human isolates P. acidilactici MM33 and L. lactis MM19 were shown to reduce vancomycin-resistant enterococci (VRE) populations in vivo (41). P. acidilactici MM33 produces the bacteriocin pediocin PA-1/AcH, while L. lactis MM19 produces the bacteriocin nisin Z. In these studies, mice received daily intragastric doses of L. lactis MM19, P. acidilactici MM33, or P. acidilactici MM33A (a non-pediocin-producing mutant) for a total of 16 days. Within the first 6 days of administration, levels of VRE in the groups of animals administered the bacteriocin-producing strains were below the detection threshold whereas VRE levels in mice fed the non-pediocin-producing strain were similar to those of control mice.
Fig 2.
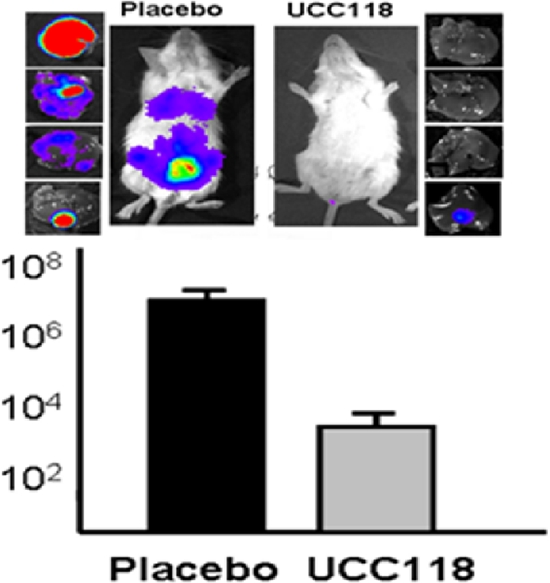
Bacteriocin-mediated anti-infective activity of L. salivarius UCC118. Survival of luminescent L. monocytogenes EGDe in the livers of mice administered a placebo (no bacterium; black bar) or 109 CFU L. salivarius UCC118 (gray bar) for 6 days prior to Listeria infection.
As bacteriocins produced by Gram-positive bacteria usually possess little or no activity against Gram-negative pathogens, Gram-negative bacteriocin producers seem to have greater potential with respect to controlling such pathogens (28). One strain of particular note is E. coli H22. H22 produces several bacteriocins, including microcin C7 and colicins 1b and E1, and inhibits a number of pathogenic enterobacteria in vitro, including Klebsiella pneumoniae and Salmonella spp. (11). Studies involving a germfree mouse model demonstrated that E. coli H22 reduced fecal populations of Shigella flexneri 4 to undetectable levels within 6 days of administration. Additionally, in vitro inhibition assays confirmed that E. coli H22 lacked activity against members of the normal human microbiota such as members of the phylum “Bacteroidetes” and Bifidobacterium species. As a result, the authors concluded that E. coli H22 is a promising probiotic with respect to preventing intestinal infections in humans and livestock (11). Similarly, a mixture of eight colicin E7-producing E. coli strains was recently found to exhibit anti-E. coli O157:H7 activity in cattle (51). A daily dose of a mixture of colicinogenic strains to calves (108 CFU/g of feed) resulted in a 2-log reduction in fecal shedding of E. coli O157:H7 compared to that of a control group. Furthermore, tissue analysis revealed that the colicin E7-producing E. coli strains significantly reduced the extent of pathogenic colonization. Although colicin E7 production was not directly implicated, the authors suggested that the inclusion of colicin E7-producing bacteria in feed may be an effective means of controlling E. coli O157:H7 (51). Likewise, Stern and colleagues (53) investigated the ability of bacteriocin-producing L. salivarius or Paenibacillus polymyxa to perturb Campylobacter jejuni colonization in broiler chicks. Despite successfully inhibiting the pathogen in vitro, treatments with the bacteriocin-producing strains did not effectively reduce C. jejuni levels in chickens. However, complete elimination of the pathogen was achieved when chickens were administered 250 mg of the encapsulated bacteriocins. Therefore, the authors hypothesized that the bacteriocins in question may not be produced in sufficient quantities in vivo to elicit a positive effect in the intestinal environment (53).
Bacteriocins as signaling peptides.
Bacterial communication via extracellular diffusible signaling molecules (quorum sensing) allows populations of bacteria to synchronize group behavior and can facilitate coordinated multicellular functionality (22). In Gram-negative bacteria, (N-acyl) homoserine lactone typically serves as a signal molecule, while in Gram-positive bacteria, peptides, including some bacteriocins, frequently serve as signaling agents (54). Thus, it has been suggested that at least some bacteriocins have a dual role, acting as inhibitors at high concentrations and as signaling compounds at lower concentrations (19). Therefore, bacteriocins produced by probiotic strains may also act as quorum-sensing molecules or autoinducing peptides in the intestinal environment.
In general, peptide-based quorum sensing in Gram-positive bacteria involves a two-component regulatory signal transduction system composed of a histidine protein kinase (HPK) located on the cell membrane and an intracellularly located response regulator (RR) (32). These are responsible for sensing of the signaling peptide and inducing an appropriate cellular response. In the case of autoinducing systems, it is thought that the signaling peptide is produced at a low level during normal growth but when present above a certain concentration threshold, the autoinducing peptide binds to the N-terminal domain of the HPK, resulting in autophosphorylation and activation. The HPK then transfers a phosphoryl group to the RR, which ultimately provokes a response, often at the level of transcription. The autoinducing peptide normally has no function other than its signaling role, but it is known that some autoinducing peptides also function as antimicrobials. A classic example of this dual functionality relates to the bacteriocin nisin. Nisin acts both as a killing molecule and as a signal molecule, inducing its own biosynthesis in a cell density-dependent manner (32). This phenomenon has also been associated with other bacteriocins, including subtilin, produced by some Bacillus subtilis strains, and salivaricin Abp118 and plantaricin A, produced by L. salivarius UCC118 and L. plantarum C11, respectively (20, 24, 31).
Recent evidence has suggested that, in addition to regulating their own synthesis, bacteriocins may also engage in interspecies communication or bacterial cross talk. Cocultivation of the plantaricin A producer L. plantarum DC400 with several species of sourdough LAB revealed that bacteriocin production was induced in DC400 to various extents, depending on the microbial partner (15). Production of plantaricin A was induced most strongly by L. sanfranciscensis DPPMA174, a plantaricin A-sensitive strain. The presence of the plantaricin A peptide, in turn, induced a response in L. sanfranciscensis leading to the overexpression of proteins involved in the stress response, as well as amino acid and energy metabolism. It thus seems that plantaricin A production serves as a means via which the bacteria communicate, shaping the phenotypic traits of the starter LAB population and their subsequent contribution to fermentation (16). This phenomenon has also been associated with other bacteriocin systems, including plantaricin NC8 and lactacin B, produced by L. plantarum NC9 and L. acidophilus La-5, respectively (39, 55). Since sourdough fermentation represents a complex ecosystem in which different species of LAB interact, it is conceivable that similar interactions also occur between closely related bacteria within the GI tract.
Evidence has recently emerged regarding the impact of bacteriocins on the immune system. More specifically, studies by Meijerink et al. (40) and van Hemert et al. (56) identified a number of L. plantarum genes that may influence the immune response of dendritic and peripheral blood mononuclear cells, respectively. Deletion of these genes from the L. plantarum WCFSI genome resulted in substantial changes in cytokine profiles. Notably, the majority of the candidate genes identified were involved in bacteriocin production and/or secretion. The authors of both studies speculated that the bacteriocin produced by L. plantarum may modulate immune responses in a manner similar to that of human antimicrobial peptides secreted in the GI tract. It is important to note that previous results demonstrate that L. plantarum WCFS1 plantaricin genes were indeed induced in the GI tracts of mice, and thus, plantaricin production does indeed occur in the intestine (4, 56). Future investigations with different bacteriocinogenic strains are necessary to determine if the impact that this plantaricin has on immune functionality is simply an isolated case or a more common feature of bacteriocin peptides.
APPLICATIONS OF BACTERIOCINS IN HUMAN HEALTH AND FUTURE DIRECTIONS
Bacteriocins in human health.
In addition to bacteriocinogenic probiotics, purified or partially purified bacteriocins also hold great promise with respect to the treatment of target pathogenic bacteria and may ultimately be employed as pharmabiotics and/or novel alternatives to existing antibiotics (22). Recent studies using a mouse model of Salmonella Newport infection have shown that treatment with microcin J25 resulted in a 2- to 3-log reduction in viable numbers of the pathogen in both the spleen and the liver compared to those of control mice (37). Mersacidin, produced by Bacillus sp. strain HIL Y85, was also active against methicillin-resistant Staphylococcus aureus (MRSA) in a hydrocortisone-treated mouse rhinitis model (34). This bacteriocin was able to completely eradicate MRSA from the nasal epithelium of the mouse, independent of the colonization time and number of inoculations. It has also been shown that a single dose of mutacin B-Ny266, produced by Streptococcus mutans, was 100% protective when administered intraperitoneally to mice previously infected with methicillin-susceptible S. aureus Smith (42). Finally, it is noteworthy that both lacticin 3147 and thuricin CD, produced by Lactococcus lactis DPC3147 and Bacillus thuringiensis DPC6431, respectively, exhibited inhibitory activity against C. difficile in an ex vivo model of the colon (46, 47). Thuricin CD is of particular interest, as this two-peptide bacteriocin was shown to be as effective as conventional antibiotics (e.g., metronidazole and vancomycin) in an ex vivo model of C. difficile infection. However, in contrast to conventional antibiotics, thuricin CD did not result in major alterations of GI populations, a contributing factor in recurrent C. difficile infection (49). Although further studies are required to fully determine the value of bacteriocins in clinical practice, these results highlight their potential in human health.
Future directions.
Although extensive progress has been made with respect to our understanding of bacteriocin structure/function, regulation, and immunity, additional research is required to gain a full understanding of the factors which control bacteriocin production in the GI tract. For instance, a standardized method of assessing bacteriocin activity in vivo would be useful since variations in animal models, effective dosage, and quantification methods have made the direct comparison of data from different laboratories problematic. Future investigations in this regard may help to resolve the variability and inconsistencies historically associated with bacteriocin production in the mammalian host. Additionally, as functionality is likely to change, depending on the individual probiotic, the further use of in vivo models directly comparing bacteriocin-producing and -nonproducing isogenic strains will be important. This information may ultimately lead to human trials where the health implications of bacteriocin producers may be accurately assessed.
CONCLUSIONS
Collectively, the results presented in this review highlight the complexity associated with bacteriocin production in the mammalian GI tract. It is likely that mitigating factors including strain survival, the specific activity of the bacteriocin, the dosing regimen, the animal model, and the target organism all influence the ability of a bacteriocin to function in vivo. Additionally, bacteriocins actively produced in vitro may not necessarily be produced in sufficiently high quantities, or at all, within the GI tract (28). A thorough investigation of the factors influencing probiotic survival, bacteriocin production, and bacteriocin activity is required to bring about a better correlation between in vitro inhibition and in vivo results. Ultimately, a number of unanswered questions still remain regarding the efficacy of bacteriocin production in vivo. Further investigations will further unravel the role of bacteriocin-producing strains in the GI tract, possibly leading to the development of superior probiotics with enhanced bacteriocin functionality. This information will ultimately lead to a greater understanding of bacteriocinogenic probiotics and their potential applications in human and veterinary health.
ACKNOWLEDGMENT
This work was supported by the Science Foundation of Ireland-funded Centre for Science, Engineering and Technology, the Alimentary Pharmabiotic Centre.
Footnotes
Published ahead of print 28 October 2011
REFERENCES
- 1. Atassi F, Servin AL. 2010. Individual and co-operative roles of lactic acid and hydrogen peroxide in the killing activity of enteric strain Lactobacillus johnsonii NCC933 and vaginal strain Lactobacillus gasseri KS120.1 against enteric, uropathogenic and vaginosis-associated pathogens. FEMS Microbiol. Lett. 304:29–38 [DOI] [PubMed] [Google Scholar]
- 2. Bernbom N, et al. 2006. Effects of Lactococcus lactis on composition of intestinal microbiota: role of nisin. Appl. Environ. Microbiol. 72:239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhardwaj A, et al. 2010. Safety assessment and evaluation of probiotic potential of bacteriocinogenic Enterococcus faecium KH 24 strain under in vitro and in vivo conditions. Int. J. Food Microbiol. 141:156–164 [DOI] [PubMed] [Google Scholar]
- 4. Bron PA, Grangette C, Mercenier A, de Vos WM, Kleerebezem M. 2004. Identification of Lactobacillus plantarum genes that are induced in the gastrointestinal tract of mice. J. Bacteriol. 186:5721–5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casey PG, et al. 2007. A five-strain probiotic combination reduces pathogen shedding and alleviates disease signs in pigs challenged with Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 73:1858–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chenoll E, et al. 2011. A novel probiotic Bifidobacterium bifidum CECT 7366 strain active against the pathogenic bacteria Helicobacter pylori. Appl. Environ. Microbiol. 77:1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collado MC, Isolauri E, Salminen S, Sanz Y. 2009. The impact of probiotic on gut health. Curr. Drug Metab. 10:68–78 [DOI] [PubMed] [Google Scholar]
- 8. Corr SC, Hill C, Gahan CGM. 2009. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv. Food Nutr. Res. 56:1–15 [DOI] [PubMed] [Google Scholar]
- 9. Corr SC, et al. 2007. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. U. S. A. 104:7617–7621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777–788 [DOI] [PubMed] [Google Scholar]
- 11. Cursino L, et al. 2006. Exoproducts of the Escherichia coli strain H22 inhibiting some enteric pathogens both in vitro and in vivo. J. Appl. Microbiol. 100:821–829 [DOI] [PubMed] [Google Scholar]
- 12. Czárán TL, Hoekstra RF, Pagie L. 2002. Chemical warfare between microbes promotes biodiversity. Proc. Natl. Acad. Sci. U. S. A. 99:786–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dabour N, Zihler A, Kheadr E, Lacroix C, Fliss I. 2009. In vivo study on the effectiveness of pediocin PA-1 and Pediococcus acidilactici UL5 at inhibiting Listeria monocytogenes. Int. J. Food Microbiol. 133:225–233 [DOI] [PubMed] [Google Scholar]
- 14. Dawid S, Roche AM, Weiser JN. 2007. The blp bacteriocins of Streptococcus pneumoniae mediate intraspecies competition both in vitro and in vivo. Infect. Immun. 75:443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Cagno R, De Angelis M, Calasso M, Gobbetti M. 2011. Proteomics of the bacterial cross-talk by quorum sensing. J. Proteomics 74:19–34 [DOI] [PubMed] [Google Scholar]
- 16. Di Cagno R, et al. 2010. Quorum sensing in sourdough Lactobacillus plantarum DC400: induction of plantaricin A (PlnA) under co-cultivation with other lactic acid bacteria and effect of PlnA on bacterial and Caco-2 cells. Proteomics 10:2175–2190 [DOI] [PubMed] [Google Scholar]
- 17. Di Cagno R, et al. 2007. Cell-cell communication in sourdough lactic acid bacteria: a proteomic study in Lactobacillus sanfranciscensis CB1. Proteomics 7:2430–2446 [DOI] [PubMed] [Google Scholar]
- 18. Dobson A, et al. 2011. Fate and efficacy of lacticin 3147-producing Lactococcus lactis in the mammalian gastrointestinal tract. FEMS Microbiol. Ecol. 76:602–614 [DOI] [PubMed] [Google Scholar]
- 19. Fajardo A, Martinez JL. 2008. Antibiotics as signals that trigger specific bacterial responses. Curr. Opin. Microbiol. 11:161–167 [DOI] [PubMed] [Google Scholar]
- 20. Flynn S, et al. 2002. Characterization of the genetic locus responsible for the production of ABP-118, a novel bacteriocin produced by the probiotic bacterium Lactobacillus salivarius subsp. salivarius UCC118. Microbiology 148:973–984 [DOI] [PubMed] [Google Scholar]
- 21. Gillor O, Etzion A, Riley MA. 2008. The dual role of bacteriocins as anti- and probiotics. Appl. Microbiol. Biotechnol. 81:591–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gillor O, Ghazaryan L. 2007. Recent advances in bacteriocin application as antimicrobials. Recent Pat. Antiinfect. Drug Discov. 2:115–122 [DOI] [PubMed] [Google Scholar]
- 23. Gillor O, Giladi I, Riley MA. 2009. Persistence of colicinogenic Escherichia coli in the mouse gastrointestinal tract. BMC Microbiol. 9:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gobbetti M, De Angelis M, Di Cagno R, Minervini F, Limitone A. 2007. Cell-cell communication in food related bacteria. Int. J. Food Microbiol. 120:34–45 [DOI] [PubMed] [Google Scholar]
- 25. Hillman J, Dzuback A, Andrews S. 1987. Colonization of the human oral cavity by a Streptococcus mutans mutant producing increased bacteriocin. J. Dent. Res. 66:1092–1094 [DOI] [PubMed] [Google Scholar]
- 26. Hillman J, et al. 1998. Genetic and biochemical analysis of mutacin 1140, a lantibiotic from Streptococcus mutans. Infect. Immun. 66:2743–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hillman JD. 2002. Genetically modified Streptococcus mutans for the prevention of dental caries. Antonie Van Leeuwenhoek 82:361–366 [PubMed] [Google Scholar]
- 28. Kirkup BC., Jr 2006. Bacteriocins as oral and gastrointestinal antibiotics: theoretical considerations, applied research, and practical applications. Curr. Med. Chem. 13:3335–3350 [DOI] [PubMed] [Google Scholar]
- 29. Klaenhammer TR. 1988. Bacteriocins of lactic acid bacteria. Biochimie 70:337–349 [DOI] [PubMed] [Google Scholar]
- 30. Klaenhammer TR. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39–85 [DOI] [PubMed] [Google Scholar]
- 31. Kleerebezem M. 2004. Quorum sensing control of lantibiotic production; nisin and subtilin autoregulate their own biosynthesis. Peptides 25:1405–1414 [DOI] [PubMed] [Google Scholar]
- 32. Kleerebezem M, Quadri LE, Kuipers OP, de Vos WM. 1997. Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Mol. Microbiol. 24:895–904 [DOI] [PubMed] [Google Scholar]
- 33. Kreth J, Merritt J, Shi W, Qi F. 2005. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 187:7193–7203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kruszewska D, et al. 2004. Mersacidin eradicates methicillin-resistant Staphylococcus aureus (MRSA) in a mouse rhinitis model. J. Antimicrob. Chemother. 54:648–653 [DOI] [PubMed] [Google Scholar]
- 35. Le Blay G, Lacroix C, Zihler A, Fliss I. 2007. In vitro inhibition activity of nisin A, nisin Z, pediocin PA-1 and antibiotics against common intestinal bacteria. Lett. Appl. Microbiol. 45:252–257 [DOI] [PubMed] [Google Scholar]
- 36. Lee JH, et al. 2008. Comparative genomic analysis of the gut bacterium Bifidobacterium longum reveals loci susceptible to deletion during pure culture growth. BMC Genomics 9:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lopez FE, Vincent PA, Zenoff AM, Salomon RA, Farias RN. 2007. Efficacy of microcin J25 in biomatrices and in a mouse model of Salmonella infection. J. Antimicrob. Chemother. 59:676–680 [DOI] [PubMed] [Google Scholar]
- 38. Majeed H, Gillor O, Kerr B, Riley MA. 2011. Competitive interactions in Escherichia coli populations: the role of bacteriocins. ISME J. 5:71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maldonado A, Ruiz-Barba JL, Jimenez-Diaz R. 2004. Production of plantaricin NC8 by Lactobacillus plantarum NC8 is induced in the presence of different types of gram-positive bacteria. Arch. Microbiol. 181:8–16 [DOI] [PubMed] [Google Scholar]
- 40. Meijerink M, et al. 2010. Identification of genetic loci in Lactobacillus plantarum that modulate the immune response of dendritic cells using comparative genome hybridization. PLoS One 5:e10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Millette M, et al. 2008. Capacity of human nisin- and pediocin-producing lactic acid bacteria to reduce intestinal colonization by vancomycin-resistant enterococci. Appl. Environ. Microbiol. 74:1997–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mota-Meira M, Morency H, Lavoie MC. 2005. In vivo activity of mutacin B-Ny266. J. Antimicrob. Chemother. 56:869–871 [DOI] [PubMed] [Google Scholar]
- 43. O'Driscoll B, Gahan CG, Hill C. 1996. Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl. Environ. Microbiol. 62:1693–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O'Hara AM, Shanahan F. 2007. Mechanisms of action of probiotics in intestinal diseases. ScientificWorldJournal 7:31–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pineiro M, Stanton C. 2007. Probiotic bacteria: legislative framework—requirements to evidence basis. J. Nutr. 137:850S–853S [DOI] [PubMed] [Google Scholar]
- 46. Rea MC, et al. 2011. Effect of broad and narrow spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc. Natl. Acad. Sci. U. S. A. 108:4639–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rea MC, et al. 2010. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc. Natl. Acad. Sci. U. S. A. 107:9352–9357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Riley MA, Wertz JE. 2002. Bacteriocin diversity: ecological and evolutionary perspectives. Biochimie 84:357–364 [DOI] [PubMed] [Google Scholar]
- 49. Rupnik M, Wilcox MH, Gerding DN. 2009. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7:526–536 [DOI] [PubMed] [Google Scholar]
- 50. Ryan MP, Rea MC, Hill C, Ross RP. 1996. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl. Environ. Microbiol. 62:612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schamberger GP, Diez-Gonzalez F. 2004. Characterization of colicinogenic Escherichia coli strains inhibitory to enterohemorrhagic E. coli. J. Food Prot. 67:486–492 [DOI] [PubMed] [Google Scholar]
- 52. Servin AL. 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 28:405–440 [DOI] [PubMed] [Google Scholar]
- 53. Stern NJ, et al. 2008. Bacteriocins reduce Campylobacter jejuni colonization while bacteria producing bacteriocins are ineffective. Microb. Ecol. Health Dis. 20:74–79 [Google Scholar]
- 54. Sturme MH, et al. 2002. Cell to cell communication by autoinducing peptides in gram-positive bacteria. Antonie Van Leeuwenhoek 81:233–243 [DOI] [PubMed] [Google Scholar]
- 55. Tabasco R, Garcia-Cayuela T, Pelaez C, Requena T. 2009. Lactobacillus acidophilus La-5 increases lactacin B production when it senses live target bacteria. Int. J. Food Microbiol. 132:109–116 [DOI] [PubMed] [Google Scholar]
- 56. van Hemert S, et al. 2010. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol. 10:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Walsh MC, et al. 2008. Predominance of a bacteriocin-producing Lactobacillus salivarius component of a five-strain probiotic in the porcine ileum and effects on host immune phenotype. FEMS Microbiol. Ecol. 64:317–327 [DOI] [PubMed] [Google Scholar]