Abstract
Toll interleukin-1 receptor (IL-1R) 8 (TIR8), also known as single Ig IL-1 receptor (IL-R)-related molecule, or SIGIRR, is a member of the IL-1R-like family, primarily expressed by epithelial cells. Current evidence suggests that TIR8 plays a nonredundant role as a negative regulator in vivo under different inflammatory conditions that are dependent on IL-R and Toll-like receptor (TLR) activation. In the present study, we examined the role of TIR8 in innate resistance to acute lung infections caused by Pseudomonas aeruginosa, a Gram-negative pathogen responsible for life-threatening infections in immunocompromised individuals and cystic fibrosis patients. We show that Tir8 deficiency in mice was associated with increased susceptibility to acute P. aeruginosa infection, in terms of mortality and bacterial load, and to exacerbated local and systemic production of proinflammatory cytokines (gamma interferon [IFN-γ], tumor necrosis factor alpha [TNF-α], IL-1β, and IL-6) and chemokines (CXCL1, CXCL2, and CCL2). It has been reported that host defense against P. aeruginosa acute lung infection can be improved by blocking IL-1 since exaggerated IL-1β production may be harmful for the host in this infection. In agreement with these data, IL-1RI deficiency rescues the phenotype observed in Tir8-deficient mice: in Tir8−/− IL-1RI−/− double knockout mice we observed higher survival rates, enhanced bacterial clearance, and reduced levels of local and systemic cytokine and chemokine levels than in Tir8-deficient mice. These results suggest that TIR8 has a nonredundant effect in modulating the inflammation caused by P. aeruginosa, in particular, by negatively regulating IL-1RI signaling, which plays a major role in the pathogenesis of this infectious disease.
INTRODUCTION
Pseudomonas aeruginosa is a common pathogen in different clinical settings. In particular, it is a frequent cause of nosocomial infections in hospitalized and immunocompromised patients and the major pathogen associated with respiratory tract infections in cystic fibrosis (CF) patients, playing a critical role in the development and progression of pulmonary disease (12, 18).
The pathogenesis of P. aeruginosa pneumonia is complex, and the outcome of an infection depends on the virulence factors displayed by the bacteria as well as on the host response (46). The Toll-like receptor (TLR)-MyD88 pathway plays nonredundant roles in resistance to P. aeruginosa infection. In particular, TLR2, TLR4, and TLR5 and the common adaptor molecule MyD88 expressed by alveolar macrophages, neutrophils, and epithelial cells are involved in the production of inflammatory mediators following P. aeruginosa infection, in leukocyte recruitment, and in bacterial clearance (36, 40, 52). On the other hand, the role of interleukin-1 receptor type I (IL-1RI), which belongs to the IL-1R-like (ILR) superfamily and also signals through MyD88, is controversial in P. aeruginosa lung infection. Schultz et al. showed that IL-1RI deficiency had a protective effect in P. aeruginosa pulmonary infection and was associated with a decreased influx of neutrophils and lower levels of cytokines and chemokines in the lungs (49). In contrast, Sutterwala et al. demonstrated the protective role of the interleukin-converting enzyme protease-activating factor (IPAF) inflammasome/caspase-1 axis in inducing macrophage cell death and IL-1β secretion, an innate immune response which is circumvented by virulent ExoU-expressing P. aeruginosa strains (55).
The activation of the signaling cascade leading to the production of proteins related to inflammation and immunity by ILRs or TLRs potentially causes devastating inflammatory reactions and is tightly regulated. For the IL-1 system, the control is exerted at different levels, both extracellularly and intracellularly (11, 14, 30, 51). Toll IL-1R 8 (TIR8), also known as single Ig IL-1-related receptor (SIGIRR), is a molecule acting intracellularly to inhibit ILR and TLR signaling (14, 57). Inhibition requires the intracellular portion of TIR8, possibly obtained by trapping IRAK-1 and TRAF-6, while the extracellular domain may interfere with heterodimerization of IL-1R1 and IL-1 accessory protein (IL-1AcP) (37).
Gene-targeted mice demonstrated that Tir8 acts as a nonredundant negative regulator in vivo under different inflammatory conditions which are dependent on ILR and TLR activation. These include intestinal inflammation and colon cancer (16, 17), autoimmune diseases (29), allergic reactions (8), kidney ischemia/reperfusion injury, and allotransplantation (28, 33). In infections (tuberculosis, fungal infections, P. aeruginosa-induced keratitis, and endotoxemia), Tir8 emerged as a key molecule involved in dampening ILR- and TLR-induced inflammation and tissue damage (6, 15, 25, 59).
In the present work, we examined the effect of Tir8 deficiency in resistance to acute P. aeruginosa lung infection. Our results suggest that the lack of a negative regulator of TLRs and ILR results in exacerbated local and systemic production of proinflammatory cytokines and chemokines, leading to increased mortality and lung bacterial load. Moreover, results obtained in mice deficient in both Tir8 and IL-1RI showed that the abrogation of the IL-1-dependent inflammatory cascade reverted the phenotype of Tir8-deficient mice.
These results suggest that the modulation of IL-1RI signaling exerted by TIR8 has a major role in the pathogenesis of the inflammation associated with P. aeruginosa lung infection.
MATERIALS AND METHODS
Ethics statement.
Procedures involving animals and their care conformed with institutional guidelines in compliance with national (25a) and international law and policies (12a, 31)). The protocol was approved by the Italian Ministry of Health (Protocol 192/2008-B). All efforts were made to minimize the number of animals used and their suffering.
Animals.
Tir8-deficient (Tir8−/−) mice were generated as described previously (16). Mice (20 to 22 g) used in this study were 8 to 12 weeks old and were bred on the C57BL/6J background (backcrossed for 11 generations). Wild-type controls (Tir8+/+) were C57BL/6J mice obtained from Charles River Laboratories (Lyon, France). IL-1RI-deficient (IL-1R−/−) mice were kindly provided by J. E. Sims (Amgen, Seattle, WA). Mice deficient in both Tir8 and IL-1R (Tir8−/− IL-1R−/−) were obtained by crossing Tir8−/− and IL-1R−/− mice. Mice were housed in a specific-pathogen-free (SPF) animal facility of the Istituto Clinico Humanitas in individually ventilated cages.
Acute lung infection.
The reference laboratory P. aeruginosa strain PAO1 (54) was used. Bacteria were cultured in Trypticase soy broth (TSB) and plated on Pseudomonas isolation agar (PIA) or Trypticase soy agar (TSA) plates at 37°C.
Mice were anesthetized with 375 mg/kg of body weight with Avertin (97% 2,2,2-treidromoethanol; Aldrich) and injected intratracheally (i.t.) with a 20-μl inoculum. The challenge inoculum (106 CFU/mouse of P. aeruginosa) was established by pilot experiments. Mice were sacrificed after 8, 20, or 36 h of infection. Mortality was recorded every 6 to 12 h.
BALF and lung analysis.
The bronchoalveolar lavage was performed three times with 1 ml of RPMI 1640 medium with protease inhibitors (complete tablets [Roche Diagnostic] and phenylmethylsulfonyl fluoride [PMSF; Sigma]) with a 22-gauge venous catheter. Total cells present in the bronchoalveolar lavage fluid (BALF) were counted, and a differential cell count was performed on cytospins stained with Diff Quick (Dade, Biomap, Italy). Lungs were removed and homogenized in 1 ml of phosphate-buffered saline (PBS) with Ca2+/Mg2+-containing protease inhibitors. BALF and lung samples were serially diluted 1:10 in PBS and plated for CFU counts. Samples were then centrifuged, and supernatants were stored at −20°C for quantification of total protein content with Coomassie Plus protein assay reagent (Thermo Scientific, Rockford, IL) or for cytokine analysis. Murine cytokines and chemokines (gamma interferon [IFN-γ], tumor necrosis factor alpha [TNF-α], IL-1β, IL-6, IL-2, IL-12p70, IL-23, IL-17, IL-4, IL-5, transforming growth factor β [TGF-β], IL-10, keratinocyte-derived chemokine [KC]/CXCL1, MIP-2/CXCL2, IP10/CXCL10, JE/CCL2, MIP1α/CCL3, RANTES/CCL5, and eotaxin/CCL11) were measured in whole lung tissue homogenates and serum by enzyme-linked immunosorbent assay ([ELISA] R&D DuoSet ELISA Development Systems), according to the manufacturer's instructions.
To analyze the myeloperoxidase (MPO) activity, pellets from lung homogenates were resuspended and homogenized in 0.5% cetyltrimethylammonium chloride (CTAC) (4 μl/mg of tissue) and centrifuged. The clear extract (50 μl) was mixed with an equal volume of 3 mM TMB (3,3′,5,5′-tetramethyl-benzidine dihydrochloride; Sigma-Aldrich) for 2 min. The reaction was stopped by the addition of 100 μl of 2 M H2SO4. The optical density (OD) was measured at 450 nm.
Histological and immunohistochemical examination.
Lungs were removed, fixed in 4% paraformaldehyde (PFA) for at least 24 h, and embedded in paraffin. Consecutive sections from the middle of the five lung lobes were stained by hematoxylin and eosin (H&E) and examined blindly by a pathologist (M. Nebuloni). The presence of acute alveolitis and bronchitis was used as a parameter for the comparative analysis, with a score ranging (degree of inflammation) from 0 to 3 (0, no lesions; 1, low; 2, moderate; and 3, severe).
For Tir8 immunohistochemistry, sections were deparaffinized and treated with 2% H2O2 for 20 min to block endogenous peroxidase. For antigen retrieval, sections were pretreated in a microwave oven (two cycles for 3 min each at 800 W, in 0.25 mM EDTA buffer, pH 8.0). Nonspecific binding sites were blocked with a solution of 1% bovine serum albumin, 0.02% NP-40, and PBS, pH 7.0. Then, sections were incubated with goat anti-murine SIGIRR ([mSIGIRR] 100 μg/ml; R&D Systems) for 2 h at room temperature. The reaction was revealed by the application of an anti-goat polymer kit (Biocare Medical) and 3,3′-diaminobenzidine (DAB) free base as the chromogen.
Quantitative PCR of whole-lung and neutrophil mRNAs.
Lungs were removed immediately after euthanasia, preserved in RNAlater (Ambion, Inc./Applied Biosystems, Foster City, CA), and stored at −20°C until homogenization and total RNA extraction by TRIzol (Invitrogen, San Diego, CA).
Bone marrow cells were separated by centrifugation at 1,000 to 1,200 × g for 30 min at room temperature over discontinuous Percoll gradients consisting of 55% (vol/vol) and 80% (vol/vol) Percoll in PBS. Eighty percent pure neutrophils were recovered from the interface fractions. Neutrophils were then isolated by using an anti-Ly6G Micro Bead kit (Miltenyi Biotec, Bergisch Gladbach, Germany).
A reverse transcription reaction from 1 μg of lung or neutrophil RNA template was done using a High Capacity cDNA Archive kit (Applied Biosystems) according to the manufacturer's instructions. The sequences of primer pairs specific for the Tir8 gene sequence (Invitrogen) were designed with Beacon Designer (Premier Biosoft International). Real-time PCR was performed using the SYBR green PCR Master Mix (Applied Biosystems) with a 7900HT Fast Real-Time PCR system (Applied Biosystems). Data were normalized for β-actin expression. Analysis of all samples was performed in triplicate. The following primer sequences were used for Tir8: forward, 5′-TGCTTTGGAAGCCTGGCTCCGT-3′; reverse, 5′-GGTTTCCTGCAGTGGAGTTGGT-3′.
Analysis of apoptosis.
Apoptosis of BALF neutrophils was determined by flow cytometry using rat IgG2a anti-mouse Ly6G-phycoerythrin ([PE] BD Pharmingen, San Diego, CA), annexin V-allophycocyanin ([APC] BD Pharmingen, San Diego, CA), and propidium iodide (PI) (Apoptosis Detection Kit; Immunostep Research, Salamanca, Spain) according to the manufacturer's instructions. Multicolor flow cytometric analyses were performed using a FACS (fluorescence-activated cell sorter) Canto (BD Biosciences) instrument equipped with a solid diode laser (488 nm, 20 mW) and an Elio-Neon laser (633 nm, 17 mW).
The evaluation of apoptosis in tissues was performed on paraffin-embedded tissues using a QIA33 FragEL DNA Fragmentation Detection Kit (using colorimetric detection and terminal deoxynucleotidyltransferase [TdT] enzyme for a TdT dUTP-biotin nick end labeling [TUNEL] assay; Calbiochem/Merck Chemicals, Nottingham, United Kingdom). The percentage of apoptotic cells per field (magnification, ×40) was analyzed in each mouse.
Statistical analysis.
Five to 12 animals per experimental group were used throughout the study. Data are expressed as means ± standard errors (SE), and two-tailed or one-tailed Student's t or log rank tests were used as specified the legends. Experiments were repeated two to five times.
RESULTS
Tir8 deficiency impairs the resistance to acute P. aeruginosa lung infection.
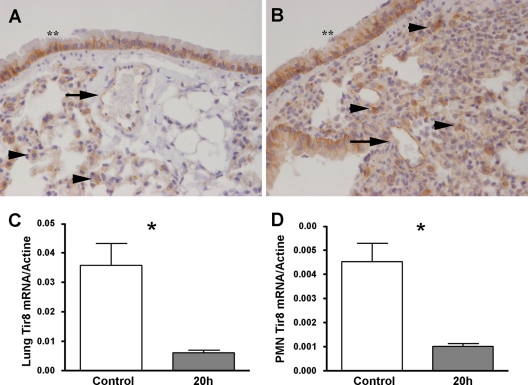
To address the potential involvement of Tir8 in P. aeruginosa lung infection, we first analyzed the expression of Tir8 at the protein and mRNA levels in the lungs of control and infected mice. As shown in Fig. 1A and B, the immunohistochemical analysis indicated that, in the lung, Tir8 was expressed by bronchial epithelium, blood endothelial cells, and leukocytes in both noninfected (Fig. 1A) and infected (Fig. 1B) mice. In the latter, the inflammatory infiltrate expressed Tir8. Under these experimental conditions, the expression of Tir8 protein was not modified 20 h after infection. In contrast, the Tir8 mRNA level in the lung was significantly reduced at 20 h postinjection compared to the level in noninfected lungs (Fig. 1C). Similarly, BALF neutrophils collected at 20 h postinjection had significantly lower Tir8 mRNA levels than noninfected bone marrow neutrophils (Fig. 1D), in agreement with previous observations (35).
Fig 1.
Tir8 expression in infected lungs and neutrophils. (A and B) Immunostaining analysis for Tir8 in lung sections of noninfected mice (A) and mice infected by P. aeruginosa (106 CFU/mice; 20 h postinjection) (B) (original magnification, ×40). Lungs were fixed in 4% PFA overnight at 4°C and paraffin embedded. Bronchial epithelium (**), blood endothelial cells (arrows), and leukocytes (arrowheads) are indicated. (C) Tir8 mRNA expression by reverse transcription-PCR in total lung of noninfected mice (Control) and at 20 h postinjection of P. aeruginosa (106 CFU/mice). (D) Tir8 mRNA expression by reverse transcription-PCR in bone marrow neutrophils (polymorphonuclear leukocytes) of noninfected mice (Control) and in BALF polymorphonuclear leukocytes at 20 h postinjection of P. aeruginosa (106 CFU/mice). Data shown are from one experiment of two performed with similar results. *, P < 0.05 versus control (two-tailed Student's t test; n = 5).
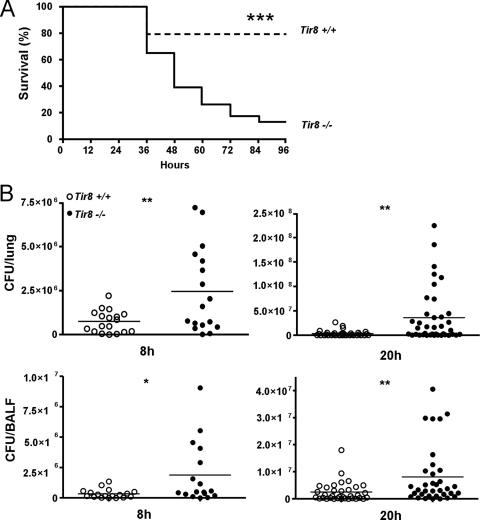
Next, Tir8+/+ and Tir8−/− mice were infected i.t. with 106 PAO1 CFU. This dose caused mortality in 22% of Tir8+/+ mice over a 96-h period. As shown in Fig. 2A, PAO1 infection in Tir8−/− mice resulted in increased mortality (61% in 48 h and 87% in 96 h). As reported in Fig. 2B, showing the results from three pooled experiments with 6 to 12 mice per group, increased susceptibility of Tir8−/− mice was associated with significantly higher lung and BALF bacterial loads, both at 8 h and 20 h postinjection. In particular, in the lungs of Tir8−/− mice, the bacterial load was 10-fold higher at 20 h postinfection (3.4 × 106 ± 1.02 × 106 CFU versus 35.78 × 106 ± 8.8 × 106 CFU in Tir8+/+ and Tir8−/−, respectively [mean ± SE]; P = 0.001) and 5-fold higher at 36 h postinfection (4.8 × 107 ± 1.8 × 107 CFU versus 2.5 × 108 ± 8.0 × 107 CFU, in Tir8+/+ and Tir8−/−, respectively; P = 0.03, one experiment performed; n = 8).
Fig 2.
Increased susceptibility to P. aeruginosa lung infection of Tir8 deficient mice. (A) Survival of Tir8+/+ and Tir8−/− mice infected i.t. by P. aeruginosa (106 CFU/mice). Data shown were pooled from three experiments performed with 8 to 10 mice per group per experiment. ***, P < 0.0001 (two-tailed log rank test). (B) Bacterial load. Lungs and BALF from Tir8+/+ (open symbols) and Tir8−/− (filled symbols) mice were collected at 8 and 20 h after injection and processed for CFU counts. Data shown were pooled from two experiments performed at 8 h or three experiments at 20 h with 8 to 12 mice per group. The mean value is shown. **, P < 0.01; *, P < 0.05 (two-tailed Student's t test).
We next measured local and systemic levels of proinflammatory cytokines and chemokines. In noninfected mice, pulmonary levels of IL-1β, IL-6, TNF-α, MIP-2α/CXCL2, KC/CXCL1, and JE/CCL2 were similar in Tir8+/+ and Tir8−/− mice. At 8 h after infection, levels of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) and chemokines (KC/CXCL1, MIP-2α/CXCL2, and JE/CCL2) in lung homogenates were elevated compared to levels in noninfected mice, but they were similar in Tir8+/+ and Tir8−/− mice. At 20 h, the levels of IL-1β, IL-6, KC/CXCL1, MIP-2α/CXCL2, and JE/CCL2 were reduced compared to the levels observed at 8 h postinfection, but in Tir8−/− mice they were significantly higher than in Tir8+/+ mice (Table 1). At 20 h, higher levels of IFN-γ, IL-23, MIP-1α/CCL3, RANTES/CCL5, eotaxin/CCL11, and IL-10 were also observed in Tir8−/− homogenate compared to levels in Tir8+/+ homogenate. In contrast, no differences were observed in IL-2, IL-4, IL-12p70, or TGF-β levels. At the systemic level, TNF-α, IL-6, KC/CXCL1, and MIP-2α/CXCL2 levels were significantly higher in Tir8−/− mice than in Tir8+/+ mice (Table 2).
Table 1.
Cytokine and chemokine levels in P. aeruginosa lung infection
| Cytokine or chemokine | Amt (ng/ml) in lung homogenate of:a |
P valueb | |
|---|---|---|---|
| Tir8+/+ mice | Tir8−/− mice | ||
| Proinflammatory cytokines | |||
| IFNγ | 0.17 ± 0.03 | 0.30 ± 0.03 | * |
| TNF-α | 3.95 ± 0.78 | 8.66 ± 0.83 | *** |
| IL-1β | 9.96 ± 2.00 | 19.69 ± 1.70 | ** |
| IL-6 | 2.45 ± 0.61 | 6.04 ± 0.52 | *** |
| IL-2 | 0.12 ± 0.05 | 0.11 ± 0.04 | NS |
| IL-12p70 | 0.20 ± 0.05 | 0.21 ± 0.04 | NS |
| IL-23 | 0.15 ± 0.07 | 0.83 ± 0.14 | *** |
| Th2 cytokines | |||
| IL-4 | 0.44 ± 0.04 | 0.41 ± 0.03 | NS |
| IL-5 | 0.06 ± 0.01 | 0.05 ± 0.01 | NS |
| Regulatory cytokines | |||
| IL-10 | 0.24 ± 0.05 | 0.54 ± 0.08 | ** |
| TGF-β | 4.16 ± 0.76 | 4.49 ± 0.67 | NS |
| Chemokines | |||
| KC/CXCL1 | 5.49 ± 1.04 | 13.68 ± 1.35 | *** |
| MIP-2α/CXCL2 | 8.06 ± 2.39 | 19.55 ± 2.04 | ** |
| IP-10/CXCL10 | 7.06 ± 0.39 | 7.95 ± 0.35 | NS |
| JE/CCL2 | 0.52 ± 0.06 | 0.91 ± 0.12 | ** |
| MIP-1α/CCL3 | 1.17 ± 0.26 | 1.94 ± 0.16 | * |
| RANTES/CCL5 | 5.39 ± 0.42 | 7.65 ± 0.88 | * |
| EOTAXIN/CCL11 | 0.75 ± 0.14 | 1.98 ± 0.15 | *** |
Cytokine and chemokine levels were analyzed 20 h after i.t. injection of P. aeruginosa (106 CFU/mice). Data shown are from one experiment of two performed with similar results. Values are means ± SE (n = 10 per group).
Significance was determined by a two-tailed Student's t test, as follows: ***, P < 0.0001; **, P < 0.005; *, P < 0.05; NS, nonsignificant.
Table 2.
Systemic cytokine and chemokine levels in P. aeruginosa lung infection
| Cytokine or chemokine | Amt (ng/ml) in serum of:a |
P valueb | nc | |
|---|---|---|---|---|
| Tir8+/+ mice | Tir8−/− mice | |||
| Proinflammatory cytokines | ||||
| TNF-α | 13.23 ± 2.6 | 20.26 ± 3.1 | * | 7 |
| IL-6 | 1.1 ± 0.22 | 3.1 ± 0.63 | * | 15 |
| Chemokines | ||||
| KC/CXCL1 | 2.94 ± 0.84 | 8.01 ± 2.45 | † | 10 |
| MIP-2α/CXCL2 | 0.09 ± 0.03 | 0.33 ± 0.09 | * | 10 |
Cytokine and chemokine levels were analyzed at 8h (TNF-α) or 20 h after i.t. injection of P. aeruginosa (106 CFU/mice). Values are means ± SE.
Significance was determined by a Student's t test as follows: *, P < 0.05, two-tailed test; †, P < 0.05, one-tailed test.
n, number of mice.
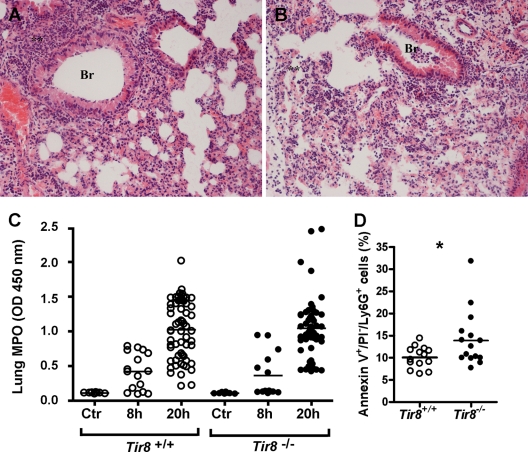
Histologically, we observed diffuse signs of pneumonia in both Tir8−/− and Tir8+/+ mice. At 8 h as well as at 20 h and 36 h after inoculation with 106 P. aeruginosa CFU, lungs appeared inflamed and hemorrhagic, showed hyperemia, perivasal edema, and an important interstitial and alveolar infiltrate, composed mostly of neutrophils (Fig. 3A and B and data not shown), but we did not observe differences in the severity of lung lesions or in the intensity and composition of the inflammatory infiltrate in the two groups, and the scores were comparable. Consistently, the injection of P. aeruginosa induced similar increased MPO activity in the lungs after 8 h and 20 h in both groups (Fig. 3C).
Fig 3.
Histological analysis of P. aeruginosa-infected lungs. Representative H&E-stained lung sections of Tir8+/+ (A) and Tir8−/− (B) mice 20 h after infection by P. aeruginosa (106 CFU/mice). Lungs were fixed in 4% PFA overnight at 4°C and paraffin embedded. BR, acute bronchitis; **, acute granulocytic alveolitis (original magnification, ×40). (C) MPO activity in the lungs of Tir8+/+ and Tir8−/− untreated mice (Ctr) and 8 and 20 h after i.t. injection of P. aeruginosa (106 CFU/mice). Data shown (means ± SE) were pooled from two to four experiments performed with 5 to 12 mice per group, at each time point. (D) Percentage of annexin V+/PI−/Ly6G+ cells in BALF 20 h after the infection in Tir8+/+ and Tir8−/− mice. Data are from three pooled experiments. *, P < 0.05 (two-tailed Student's t test).
To analyze the pneumonia-associated edema, we measured the lung wet weight and the protein content in BALF of Tir8+/+ and Tir8−/− mice. P. aeruginosa-induced pneumonia caused a severe increase in the wet lung weight (60% after 8 h and 100% after 20 h) and vascular leakage in both groups of mice compared to noninfected mice. However, we did not observe significant differences between Tir8+/+ and Tir8−/− mice for either parameter (not shown).
Total and differential cell counts performed on BALF at 8 h postinjection showed an important leukocyte recruitment, composed mostly of neutrophils, which further increased at 20 h postinjection. However, total and differential cell counts were similar in Tir8+/+ and Tir8−/− mice at both time points analyzed (data not shown). In contrast, we observed increased annexin V+/PI−/Ly6G+ cells in Tir8−/− BALF by FACS analysis (10.08% ± 0.6% versus 13.9% ± 1.5% in Tir8+/+ and Tir8−/− mice, respectively; values are mean ± SE of 15 mice from three experiments; P = 0.04) (Fig. 3D).
Involvement of IL-1RI-dependent inflammation in the increased susceptibility of Tir8−/− mice to P. aeruginosa.
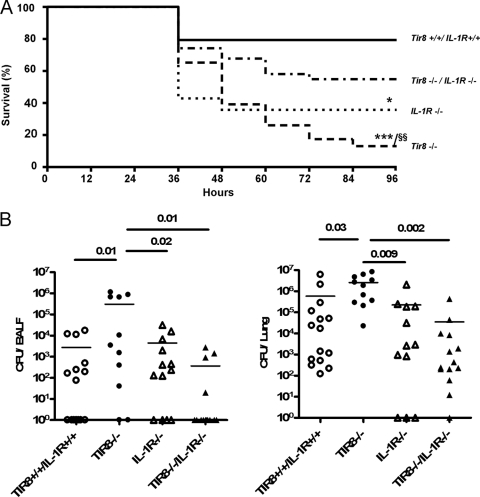
TIR8 is a member of the ILR family. To assess the actual role of IL-1 in the phenotype observed during P. aeruginosa lung infection, we generated Tir8−/− IL-1R−/− mice and infected them with P. aeruginosa i.t. As shown in Fig. 4A, the susceptibility to mortality of Tir8−/− mice was significantly reduced by the absence of IL-1RI (46% mortality in Tir8−/− IL-1R−/− mice versus 87% in Tir8−/− mice; P < 0.005) in a 96-h period.
Fig 4.
IL-1RI deficiency rescues the increased mortality and impaired bacterial clearance of Tir8−/− mice. (A) Survival of Tir8+/+ IL-1R+/+, Tir8−/−, IL-1R−/−, and Tir8−/− IL-1R−/− mice infected i.t. with P. aeruginosa (106 CFU/mice). Data shown were pooled from three experiments performed with 10 mice per group. ***, P < 0.0001; *, P < 0.05 versus Tir8+/+ IL-1R+/+; §§, P < 0.005 versus Tir8−/− IL-1R−/− (two-tailed log rank test). (B) Bacterial load in lungs and BALF of Tir8+/+ IL-1R+/+, Tir8−/−, IL-1RI−/−, and Tir8−/− IL-1R−/− mice 20 h after i.t. injection by P. aeruginosa (106 CFU/mice). Data shown were pooled from two experiments performed with 5 to 8 mice per group. Mean values are shown. A two-tailed Student's t test was used.
Moreover, as shown in Fig. 4B, at 20 h postinjection the bacterial load in BALF and lungs of Tir8−/− IL-1R−/− mice was significantly reduced compared to that of Tir8−/− mice and was comparable to bacterial loads of Tir8+/+ IL-1R+/+ and IL-1R−/− mice (P is nonsignificant). In particular, the mean lung CFU count of Tir8−/− IL-1R−/− mice was 3.5 × 104 ± 3 × 104 (± SE), whereas the mean lung CFU count of Tir8−/− mice was 2.6 × 106 ± 0.8 × 106 (± SE; P = 0.002).
We next analyzed cytokine and chemokine levels in lungs and serum of the four groups of mice at 20 h postinfection. As reported in Table 3, the deficiency of IL-1RI abated the excessive production of proinflammatory cytokines and chemokines observed in Tir8−/− mice. In particular, TNF-α, IL-1β, IL-6, KC/CXCL1, MIP-2α/CXCL2, and JE/CCL2 levels were significantly lower in Tir8−/− IL-1R−/− lung homogenates than in those of Tir8−/− mice. At the systemic level, IL-1RI deficiency reduced the excessive production of IL-1β, IL-6, KC/CXCL1, MIP-2α/CXCL2, and JE/CCL2, thus suggesting that IL-1 plays a major role in the systemic inflammatory response of Tir8−/− mice to the infection. Moreover, IL-6, MIP-2α/CXCL2, and JE/CCL2 systemic levels were dramatically lower in IL-1R−/− and also in Tir8−/− IL-1R−/− mice than in wild-type mice, suggesting that, during the infection, the production of these cytokines is IL-1RI dependent (Table 4).
Table 3.
Effect of IL-1RI-deficiency on lung cytokine and chemokine levels in Tir8−/− mice
| Cytokine or chemokine | Amt (ng/ml) in lung homogenate of:a |
|||
|---|---|---|---|---|
| Tir8+/+ IL-1R+/+ mice | Tir8−/− mice | IL-1R−/− mice | Tir8−/−IL-1R−/− mice | |
| Proinflammatory cytokines | ||||
| TNF-α | 4.74 ± 1.23 | 13.19 ± 3.70* | 10.03 ± 3.84 | 5.62 ± 1.78§ |
| IL-1β | 5.33 ± 1.20 | 10.99 ± 2.55* | 4.50 ± 1.09§ | 5.04 ± 1.33§ |
| IL-6 | 1.40 ± 0.41 | 7.17 ± 2.46* | 0.90 ± 0.37§ | 0.76 ± 0.22† |
| IL-17 | 0.47 ± 0.14 | 0.97 ± 0.16* | 0.59 ± 0.12 | 0.80 ± 0.16 |
| IL-23 | 0.45 ± 0.18 | 1.04 ± 0.19* | 0.61 ± 0.12 | 1.29 ± 0.11 |
| Chemokines | ||||
| KC/CXCL1 | 11.93 ± 3.38 | 30.91 ± 5.14** | 9.52 ± 1.9‡ | 10.36 ± 2.8‡ |
| MIP-2α/CXCL2 | 3.97 ± 0.62 | 9.87 ± 2.29* | 1.97 ± 0.4 | 2.96 ± 0.32† |
| JE/CCL2 | 0.49 ± 0.14 | 1.81 ± 0.42* | 0.80 ± 0.32 | 0.35 ± 0.04† |
Cytokine and chemokine levels were analyzed by ELISA in lung homogenates at 20 h following P. aeruginosa lung injection (106 CFU/mice). Data shown are from two pooled experiments. Values are means ± SE (n = 10 to 18 per group). Significance was determined by a two-tailed Student's t test as follows: *, P < 0.05; **, P < 0.005 versus Tir8+/+ IL-1-R+/+ group; §, P < 0.05; †, P < 0.005; ‡, P < 0.0001 versus Tir8−/− group.
Table 4.
Effect of IL-1RI-deficiency on systemic levels of proinflammatory mediators in Tir8−/− mice
| Cytokine or chemokine | Amt (ng/ml) in serum of:a |
|||
|---|---|---|---|---|
| Tir8+/+ IL-1R+/+ mice | Tir8−/− mice | IL-1R−/− mice | Tir8−/−IL-1R−/− mice | |
| Proinflammatory cytokines | ||||
| IL-1β | 0.16 ± 0.08 | 0.56 ± 0.19* | 0.14 ± 0.04 | 0.22 ± 0.04§ |
| IL-6 | 2.37 ± 1.24 | 4.74 ± 1.63 | 0.32 ± 0.19§ | 0.03 ± 0.02† |
| Chemokines | ||||
| KC/CXCL1 | 0.89 ± 0.23 | 5.49 ± 1.35** | 4.29 ± 2.34 | 0.51 ± 0.21‡ |
| MIP-2α/CXCL2 | 1.18 ± 0.78 | 7.30 ± 1.66** | 0.04 ± 0.03† | 0.03 ± 0.02‡ |
| JE/CCL2 | 1.23 ± 0.59 | 5.03 ± 2.19* | 0.54 ± 0.3 | 0.10 ± 0.03§ |
Cytokine and chemokine serum levels analyzed by ELISA 20 h following P. aeruginosa lung injection (106 CFU/mice). Data represent means ± SE of 5 to 10 mice per group. Significance was determined by a two-tailed Student's t test as follows: *, P < 0.05; **, P < 0.005 versus Tir8+/+ IL-1R+/+ group; §, P < 0.05; †, P < 0.005; ‡, P < 0.0001 versus Tir8−/− group.
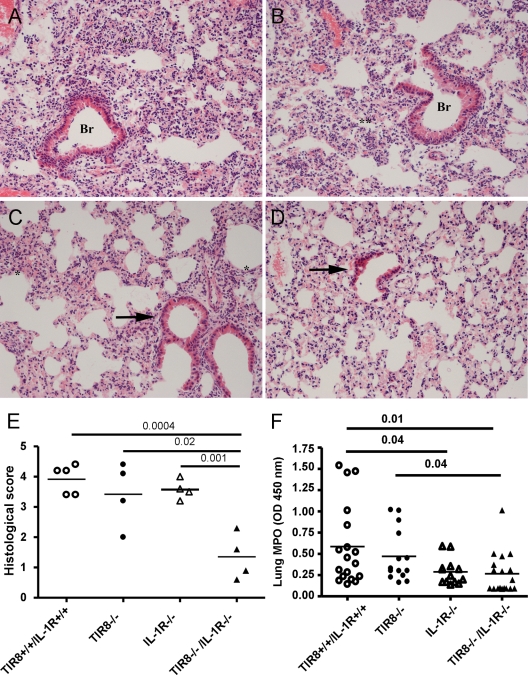
As reported above (Fig. 3A and B), diffuse and severe acute granulocytic alveolitis and bronchitis were observed in Tir8+/+ and Tir8−/− mice (Fig. 5A and B, respectively). In Tir8−/− IL-1R−/− lungs (Fig. 5D and E), alveolitis and bronchitis scores were significantly lower than in the wild-type, Tir8−/−, and IL-1R−/− mice. In agreement, the MPO activity of Tir8−/− IL-1R−/− mice was significantly lower than that observed in wild-type or Tir8−/− mice (Fig. 5F). Similarly, the wet weights of Tir8−/− IL-1R−/− lungs were significantly lower than those of wild-type and Tir8−/− mice (data not shown).
Fig 5.
Effect of IL-1RI-deficiency on lung pathology of Tir8−/− mice. (A to D) H&E-stained lung sections of Tir8+/+ IL-1R+/+ (A), Tir8−/− (B), IL-1R−/− (C), and Tir8−/− IL-1R−/− (D) mice at 20 h after i.t. injection of P. aeruginosa (106 CFU/mice). Lungs were fixed in 4% PFA overnight at 4°C and paraffin embedded. **, diffuse and severe acute granulocytic alveolitis; *, small and focal areas of granulocytic alveolar inflammation; Br, bronchitis; arrow, normal bronchus (original magnification, ×40). (E) Histological score obtained analyzing the five lobes of four mice per group at 20 h after the infection. Alveolitis and bronchitis were scored on a scale from 0 to 3. A two-tailed Student's t test was used. (F) MPO activity in the lung of Tir8+/+ IL-1RI+/+, Tir8−/−, IL-1RI−/−, and Tir8−/− IL-1RI−/− mice 20 h after infection. Data shown were pooled from three experiments performed with 10 mice per group, respectively. A two-tailed Student's t test was used.
DISCUSSION
In the present work, we examined the role of TIR8/SIGIRR, a negative regulator of the ILR/TLR superfamily, in innate resistance to acute P. aeruginosa lung infections. We found that Tir8 deficiency caused an increased susceptibility to acute P. aeruginosa lung infection, in terms of mortality and bacterial load, which was associated with an exaggerated production of proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IFN-γ) and chemokines (CXCL1, CXCL2, and CCL2) within the pulmonary compartment and systemically. IL-1RI deficiency reverted these phenotypes and was associated with significantly less severe lung lesions than those observed in Tir8−/− mice. These findings suggest that mortality of Tir8−/− mice was caused by an excessive local and systemic inflammatory response and that the unregulated IL-1RI-dependent signaling played a major role in the deleterious effect of Tir8 deficiency.
The susceptibility to P. aeruginosa infection and lung injury are due either to the toxic effect of a virulence factor or to the severe inflammation resulting from the response to virulence factors, including type III secreted toxins (TTSTs), lipopolysaccharide (LPS), phospholipase C, and flagellin (5, 23, 46, 55, 61). The relevance of the different players involved in the pathogenesis of the infection may differ depending on the strain involved. In the present study, we used the PAO1 reference strain, whose LPS structure and virulence are similar to those of clinical strains isolated at the early stage of CF chronic infection (7, 10).
In the innate response to P. aeruginosa, TLR2 and TLR4, recognizing LPS, and TLR5, responsible for flagellin sensing (38, 40), are involved in the production of inflammatory mediators, playing nonredundant roles in the infection (39). In agreement, the development of an early host response to P. aeruginosa lung infection is critically dependent on MyD88 in mice (36) and on NF-κB (9). On the other hand, TLR2- and TLR4-dependent signaling has been implicated in impairing neutrophil effector functions, such as migration to inflammatory sites through CXCR2 internalization (2), phagocytosis, and bacterial killing, as well as in inducing apoptosis and bone marrow neutrophil exhaustion (32, 43, 44). In particular, inhibition of neutrophil migration and induction of neutrophil apoptosis are two ways in which TLR agonists exert detrimental effects in sepsis, enhancing bacterial proliferation and mortality (2, 44). Altogether, these studies provide support to anti-TLR treatment strategies for Gram-negative sepsis (24, 44). We speculate that some of the phenotypes observed in Tir8−/− mice in this study could be related to excessive TLR2 and TLR4 activation, in particular, defective bacterial clearance, neutrophil lung recruitment comparable to that of wild-type mice despite high chemokine levels, and increased neutrophil apoptosis.
Proinflammatory cytokines are key mediators of innate defense in pneumonia models, such as Klebsiella pneumoniae or Streptococcus pneumoniae (48). However, several experiments have demonstrated that some of these mediators play a deleterious role in the host defense during Pseudomonas pneumonia, inhibiting bacterial clearance. Actually, decreased susceptibility to the infection was observed in mice deficient in IFN-γ receptor (50), type 1 TNF receptor (TNFR1) (53), or IL-18 (48), whereas IL-10 played a protective role (27, 47). The role of IL-1β in P. aeruginosa infection has been widely studied in the context of chronic (41) and acute (49) infections. Reiniger et al. (41) found that a lack of IL-1R led to increased lung colonization upon oral inoculation of P. aeruginosa, suggesting that a moderate amount of IL-1β might play a beneficial role during a chronic infection. In contrast, Schultz et al. reported that the absence of the IL-1R-dependent signal improved the host defense against acute lung infection with P. aeruginosa, decreased influx of neutrophils in bronchoalveolar lavage fluids, and reduced levels of cytokines (TNF-α and IL-6) and chemokines (MIP-2 and KC) in the lungs (49).
Several studies support these data and suggest that the IL-1 system potentially plays a detrimental effect in P. aeruginosa infection and sepsis (11, 58). P. aeruginosa triggers activation of the acid sphingomyelinase and the release of ceramide in sphingolipid-rich rafts, which are required to internalize the pathogen, induce apoptosis, and regulate cytokine production. Failure to generate ceramide-enriched membrane platforms in infected cells results in an unabated inflammatory response, massive release of IL-1, and septic death of mice, which are prevented by IL-1β neutralizing antibodies (19, 56). In the same line, a deficiency in the transcriptional repressor Gfi1 results in an uncontrolled release of TNF-α and IL-1 β, leading to P. aeruginosa-induced septic shock, which was inhibited by TNF-α and IL-1 neutralization (20).
A rapid innate response is directed specifically at P. aeruginosa bacteria expressing a translocation-competent type three secretion system (TTSS), a virulence factor involved in inflammasome activation (22). This response, which requires host caspase-1 and is accompanied by increased IL-1β production, is associated with the recruitment of neutrophils to the airways and results in rapid bacterial clearance (60). Indeed, IPAF and ASC are critical regulators of IL-1β and IL-18 production by P. aeruginosa-infected alveolar macrophages (13, 46, 55, 58). However, the P. aeruginosa TTSS has been associated with increased virulence in murine pneumonia models and with worse clinical outcomes in human patients with ventilator-associated pneumonia (23, 45). Our results are in line with all of these studies suggesting that excessive inflammatory responses may be responsible for increased susceptibility to this infection, impaired neutrophil effector functions, and inhibited microbial clearance.
That the histological score observed in Tir8−/− IL-1R−/− mice was better than that of other experimental groups suggests that the pathways controlled by TIR8/SIGIRR and the IL-1RI-dependent pathway do not overlap. Indeed, since TIR8 controls both IL-1R- and TLR-dependent signaling, we speculate that increased TLR activity combined with inhibited IL-1RI-dependent inflammation may be beneficial for the host in this model.
Some of the phenotypes presented in this study, such as increased local and systemic cytokine production and the beneficial effect of blocking IL-1, are in agreement with those observed in the model of pulmonary tuberculosis, where Tir8 deficiency was associated with increased inflammatory responses leading to death despite normal control of the bacterial load (15). Signaling through IL-1R and TNFR1 is essential in regulating granuloma formation and persistence and necessary for host resistance to Mycobacterium tuberculosis (26, 42). However, the phenotype of Tir8-deficient mice could be reverted by blocking IL-1 and TNF-α, suggesting that Tir8 plays a nonredundant role in the control of the balance between protective immune responses and immunopathology in pulmonary tuberculosis (3, 15). In the same vein, Tir8-deficient mice showed increased susceptibility to fungal infections due to Candida albicans or Aspergillus fumigatus, which was associated with increased fungal growth and heightened harmful Th17 cell responses (6, 21, 34, 62). In the absence of Tir8, this abnormal polarization was dependent on increased IL-1 signaling, in line with the evidence that IL-1 plays a key role in the differentiation of Th17 cells from naïve precursors (1).
In the absence of TIR8/SIGIRR, the exacerbated inflammatory response to P. aeruginosa lung infection was associated with high levels of proinflammatory cytokines, which contribute significantly to lethality in septic shock syndrome (TNF-α, IL-1, IL-6, IL-17, IL-23, IFN-γ), whereas the lung histological score did not suggest increased tissue damage. In IL-1RI- and Tir8-deficient mice, we observed a general reduction of most of all these proinflammatory mediators, suggesting that the deficiency of IL-1R-dependent signaling prevented the deleterious effect of these mediators. Interestingly, in a P. aeruginosa sepsis model, administration of IL-23-neutralizing antibody was sufficient to reduce mortality and decrease circulating levels of the pathogenetic cytokines, TNF-α and IFN-γ (4).
These results strongly suggest that in this model of severe infection by P. aeruginosa, the lack of a negative regulator of TLR and IL-1R results in exacerbated inflammation, which is detrimental for the host, and that an IL-1-dependent inflammatory cascade plays a critical role in the balance between protective innate responses and excessive local and systemic inflammatory responses.
ACKNOWLEDGMENTS
The contributions of the European Commission (European Research Council project HIIS, MUGEN LSHG-CT-2005-005203, MUVAPRED LSH-CT-2003-503240 [http://ec.europa.eu/contracts_grants/index_en.htm]), Ministero dell'Istruzione, dell'Università de della Ricerca (progetto FIRB RBLA039LSF [www.miur.it]), and the Italian Cystic Fibrosis Research Foundation (http://www.fibrosicisticaricerca.it) are gratefully acknowledged.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 24 October 2011
REFERENCES
- 1. Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. 2007. Interleukins 1β and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8: 942–949 [DOI] [PubMed] [Google Scholar]
- 2. Alves-Filho JC, et al. 2009. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc. Natl. Acad. Sci. U. S. A. 106: 4018–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bekker LG, et al. 2000. Immunopathologic effects of tumor necrosis factor alpha in murine mycobacterial infection are dose dependent. Infect. Immun. 68: 6954–6961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belladonna ML, et al. 2006. IL-23 neutralization protects mice from Gram-negative endotoxic shock. Cytokine 34: 161–169 [DOI] [PubMed] [Google Scholar]
- 5. Bomberger JM, et al. 2011. A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog. 7: e1001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bozza S, et al. 2008. Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection. J. Immunol. 180: 4022–4031 [DOI] [PubMed] [Google Scholar]
- 7. Bragonzi A, et al. 2009. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am. J. Respir. Crit. Care Med. 180: 138–145 [DOI] [PubMed] [Google Scholar]
- 8. Bulek K, et al. 2009. The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. J. Immunol. 182: 2601–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen SM, et al. 2008. The nuclear factor kappa-B pathway in airway epithelium regulates neutrophil recruitment and host defence following Pseudomonas aeruginosa infection. Clin. Exp. Immunol. 153: 420–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cigana C, et al. 2009. Pseudomonas aeruginosa exploits lipid A and muropeptides modification as a strategy to lower innate immunity during cystic fibrosis lung infection. PLoS One 4: e8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dinarello CA. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27: 519–550 [DOI] [PubMed] [Google Scholar]
- 12. Dubin PJ, Kolls JK. 2007. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 292: L519–L528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a. European Economic Community 1987. EEC Council Directive 86/609, OJ L 358,1, 12 December 1987, on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. European Economic Community, Brussels, Belgium [Google Scholar]
- 13. Franchi L, et al. 2007. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur. J. Immunol. 37: 3030–3039 [DOI] [PubMed] [Google Scholar]
- 14. Garlanda C, Anders HJ, Mantovani A. 2009. TIR8/SIGIRR: an IL-1R/TLR family member with regulatory functions in inflammation and T cell polarization. Trends Immunol. 30: 439–446 [DOI] [PubMed] [Google Scholar]
- 15. Garlanda C, et al. 2007. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling. J. Immunol. 179: 3119–3125 [DOI] [PubMed] [Google Scholar]
- 16. Garlanda C, et al. 2004. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc. Natl. Acad. Sci. U. S. A. 101: 3522–3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garlanda C, et al. 2007. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res. 67: 6017–6021 [DOI] [PubMed] [Google Scholar]
- 18. Gomez MI, Prince A. 2007. Opportunistic infections in lung disease: Pseudomonas infections in cystic fibrosis. Curr. Opin. Pharmacol. 7: 244–251 [DOI] [PubMed] [Google Scholar]
- 19. Grassme H, et al. 2003. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat. Med. 9: 322–330 [DOI] [PubMed] [Google Scholar]
- 20. Grassme H, et al. 2006. Regulation of pulmonary Pseudomonas aeruginosa infection by the transcriptional repressor Gfi1. Cell Microbiol. 8: 1096–1105 [DOI] [PubMed] [Google Scholar]
- 21. Harrington LE, et al. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6: 1123–1132 [DOI] [PubMed] [Google Scholar]
- 22. Hauser AR. 2009. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol. 7: 654–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hauser AR, et al. 2002. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit. Care Med. 30: 521–528 [DOI] [PubMed] [Google Scholar]
- 24. Hedayat M, Netea MG, Rezaei N. 2011. Targeting of Toll-like receptors: a decade of progress in combating infectious diseases. Lancet Infect. Dis. 11: 702–712 [DOI] [PubMed] [Google Scholar]
- 25. Huang X, Hazlett LD, Du W, Barrett RP. 2006. SIGIRR promotes resistance against Pseudomonas aeruginosa keratitis by down-regulating type-1 immunity and IL-1R1 and TLR4 signaling. J. Immunol. 177: 548–556 [DOI] [PubMed] [Google Scholar]
- 25a. (a)Italian Ministry of Health 1992. Guidelines for the ethical use of animals. Decreto Legislativo 4, n. 116. Gazzetta Ufficiale 40 (Suppl): 18 February 1992 [Google Scholar]
- 26. Juffermans NP, et al. 2000. Interleukin-1 signaling is essential for host defense during murine pulmonary tuberculosis. J. Infect. Dis. 182: 902–908 [DOI] [PubMed] [Google Scholar]
- 27. Kurahashi K, et al. 1999. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J. Clin. Invest. 104: 743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lech M, et al. 2009. Resident dendritic cells prevent postischemic acute renal failure by help of single Ig IL-1 receptor-related protein. J. Immunol. 183: 4109–4118 [DOI] [PubMed] [Google Scholar]
- 29. Lech M, et al. 2008. Tir8/Sigirr prevents murine lupus by suppressing the immunostimulatory effects of lupus autoantigens. J. Exp. Med. 205: 1879–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liew FY, Xu D, Brint EK, O'Neill LA. 2005. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5: 446–458 [DOI] [PubMed] [Google Scholar]
- 31. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 32. Navarini AA, et al. 2009. Innate immune-induced depletion of bone marrow neutrophils aggravates systemic bacterial infections. Proc. Natl. Acad. Sci. U. S. A. 106: 7107–7112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Noris M, et al. 2009. The Toll-IL-1R member Tir8/SIGIRR negatively regulates adaptive immunity against kidney grafts. J. Immunol. 183: 4249–4260 [DOI] [PubMed] [Google Scholar]
- 34. Park H, et al. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6: 1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Polentarutti N, et al. 2003. Unique pattern of expression and inhibition of IL-1 signaling by the IL-1 receptor family member TIR8/SIGIRR. Eur. Cytokine Netw. 14: 211–218 [PubMed] [Google Scholar]
- 36. Power MR, Peng Y, Maydanski E, Marshall JS, Lin TJ. 2004. The development of early host response to Pseudomonas aeruginosa lung infection is critically dependent on myeloid differentiation factor 88 in mice. J. Biol. Chem. 279: 49315–49322 [DOI] [PubMed] [Google Scholar]
- 37. Qin J, Qian Y, Yao J, Grace C, Li X. 2005. SIGIRR inhibits interleukin-1 receptor- and Toll-like receptor 4-mediated signaling through different mechanisms. J. Biol. Chem. 280: 25233–25241 [DOI] [PubMed] [Google Scholar]
- 38. Ramphal R, Balloy V, Huerre M, Si-Tahar M, Chignard M. 2005. TLRs 2 and 4 are not involved in hypersusceptibility to acute Pseudomonas aeruginosa lung infections. J. Immunol. 175: 3927–3934 [DOI] [PubMed] [Google Scholar]
- 39. Ramphal R, et al. 2008. Control of Pseudomonas aeruginosa in the lung requires the recognition of either lipopolysaccharide or flagellin. J. Immunol. 181: 586–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Raoust E, et al. 2009. Pseudomonas aeruginosa LPS or flagellin are sufficient to activate TLR-dependent signaling in murine alveolar macrophages and airway epithelial cells. PLoS One 4: e7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reiniger N, et al. 2007. Resistance to Pseudomonas aeruginosa chronic lung infection requires cystic fibrosis transmembrane conductance regulator-modulated interleukin-1 (IL-1) release and signaling through the IL-1 receptor. Infect. Immun. 75: 1598–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roach DR, et al. 2001. Secreted lymphotoxin-alpha is essential for the control of an intracellular bacterial infection. J. Exp. Med. 193: 239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roger T, Calandra T. 2009. TLR2-mediated neutrophil depletion exacerbates bacterial sepsis. Proc. Natl. Acad. Sci. U. S. A. 106: 6889–6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roger T, et al. 2009. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc. Natl. Acad. Sci. U. S. A. 106: 2348–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roy-Burman A, et al. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183: 1767–1774 [DOI] [PubMed] [Google Scholar]
- 46. Sadikot RT, Blackwell TS, Christman JW, Prince AS. 2005. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 171: 1209–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sawa T, et al. 1997. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J. Immunol. 159: 2858–2866 [PubMed] [Google Scholar]
- 48. Schultz MJ, et al. 2003. Interleukin-18 impairs the pulmonary host response to Pseudomonas aeruginosa. Infect. Immun. 71: 1630–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schultz MJ, et al. 2002. Role of interleukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 282: L285–290 [DOI] [PubMed] [Google Scholar]
- 50. Schultz MJ, Rijneveld AW, Speelman P, van Deventer SJ, van der Poll T. 2001. Endogenous interferon-gamma impairs bacterial clearance from lungs during Pseudomonas aeruginosa pneumonia. Eur. Cytokine Netw. 12: 39–44 [PubMed] [Google Scholar]
- 51. Sims JE, Smith DE. 2010. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10: 89–102 [DOI] [PubMed] [Google Scholar]
- 52. Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. 2004. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J. Immunol. 172: 3377–3381 [DOI] [PubMed] [Google Scholar]
- 53. Skerrett SJ, et al. 1999. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am. J. Physiol. 276: L715–L727 [DOI] [PubMed] [Google Scholar]
- 54. Stover CK, et al. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406: 959–964 [DOI] [PubMed] [Google Scholar]
- 55. Sutterwala FS, et al. 2007. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 204: 3235–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Teichgraber V, et al. 2008. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat. Med. 14: 382–391 [DOI] [PubMed] [Google Scholar]
- 57. Thomassen E, Renshaw BR, Sims JE. 1999. Identification and characterization of SIGIRR, a molecule representing a novel subtype of the IL-1R superfamily. Cytokine 11: 389–399 [DOI] [PubMed] [Google Scholar]
- 58. van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. 2011. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 32: 110–116 [DOI] [PubMed] [Google Scholar]
- 59. Wald D, et al. 2003. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat. Immunol. 4: 920–927 [DOI] [PubMed] [Google Scholar]
- 60. Wangdi T, Mijares LA, Kazmierczak BI. 2010. In vivo discrimination of type 3 secretion system-positive and -negative Pseudomonas aeruginosa via a caspase-1-dependent pathway. Infect. Immun. 78: 4744–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wieland CW, et al. 2002. Pulmonary inflammation induced by Pseudomonas aeruginosa lipopolysaccharide, phospholipase C, and exotoxin A: role of interferon regulatory factor 1. Infect. Immun. 70: 1352–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zelante T, et al. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 37: 2695–2706 [DOI] [PubMed] [Google Scholar]