Abstract
Because epithelial cells are the major cell type productively infected with Chlamydia during genital tract infections, the overall goal of our research was to understand the contribution of infected epithelial cells to the host defense. We previously showed that Toll-like receptor 3 (TLR3) is the critical pattern recognition receptor in oviduct epithelial (OE) cells that is stimulated during Chlamydia infection, resulting in the synthesis of beta interferon (IFN-β). Here, we present data that implicates TLR3 in the expression of a multitude of other innate-inflammatory immune modulators including interleukin-6 (IL-6), CXCL10, CXCL16, and CCL5. We demonstrate that Chlamydia-induced expression of these cytokines is severely disrupted in TLR3-deficient OE cells, whereas Chlamydia replication in the TLR3-deficient cells is more efficient than in wild-type OE cells. Pretreatment of the TLR3-deficient OE cells with 50 U of IFN-β/ml prior to infection diminished Chlamydia replication and restored the ability of Chlamydia infection to induce IL-6, CXCL10, and CCL5 expression in TLR3-deficient OE cells; however, CXCL16 induction was not restored by IFN-β preincubation. Our findings were corroborated in pathway-focused PCR arrays, which demonstrated a multitude of different inflammatory genes that were defectively regulated during Chlamydia infection of the TLR3-deficient OE cells, and we found that some of these genes were induced only when IFN-β was added prior to infection. Our OE cell data implicate TLR3 as an essential inducer of IFN-β and other inflammatory mediators by epithelial cells during Chlamydia infection and highlight the contribution of TLR3 to the inflammatory cytokine response.
INTRODUCTION
Chlamydia trachomatis is a Gram-negative obligate-intracellular bacterium and is the most common cause of bacterial sexually transmitted infections in both industrialized and developing countries (38, 68). Chronic infection with urogenital serovars of C. trachomatis in the upper female reproductive tract can cause pelvic inflammatory disease (PID), scarring, and infertility (40, 44, 66). The ability of Chlamydia to cause a persistent infection suggests that the organism is able to effectively evade immune surveillance in some individuals (4, 9, 35, 47, 50, 58, 62). The initial innate immune response to Chlamydia infections results in the production of a plethora of cytokines and chemokines. The cytokines and chemokines produced are required for the recruitment of T cells and other inflammatory cells needed to initiate adaptive immunity.
Infected epithelial cells are the primary sources of cytokine production, resulting in local inflammation and tissue remodeling caused during acute Chlamydia disease (60). Cytokines and chemokines secreted during Chlamydia infection are due to the stimulation of pattern recognition receptors (PRRs) located internally and on the surface of epithelial cells. In mammalian cells, the Toll-like receptor (TLR) families are membrane-bound PRRs that recognize microbial pathogen-associated molecular patterns (PAMPs) (6). Engagement of TLRs by the bacterial, viral, and fungal PAMPs can lead to the activation of phagocytosis and the production of acute inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and granulocyte-macrophage colony-stimulating factor (GM-CSF), as an important step prior to the switch from an innate immunity and the onset of an adaptive immune response (1, 23, 24, 36).
Using Chlamydia muridarum and a murine infection model as a surrogate for C. trachomatis infections of humans, we previously identified TLR2 (and its heterocomplexes with either TLR1 or TLR6) as the key TLRs stimulated early during infection of epithelial cells lining the lumen of the oviduct. Our data using cloned oviduct epithelial (OE) cells show that TLR2 stimulation by C. muridarum results in the secretion of acute-phase inflammatory cytokines including GM-CSF, IL-6, and TNF-α (15). Other findings from that investigation demonstrated that C. muridarum infection also induces production of beta interferon (IFN-β). We subsequently demonstrated that the Chlamydia-induced IFN-β synthesis in OE cells was largely MyD88-independent and sensitive to disruption of TRIF and IRF3 signaling (13). We also showed that IFN-β synthesis was TLR3-dependent in OE cells, but was not TLR3 dependent in bone marrow-derived macrophages from TLR3-deficient mice (14).
TLR3 is a receptor for double-stranded RNA (dsRNA) and is known to activate transcription of IFN-β via the adaptor protein Toll-IL-1 receptor (TIR) domain-containing adaptor molecule 1 (TICAM-1; also called TIR-domain-containing adapter inducing IFN-β [TRIF]) (2, 33). Studies using human fibroblasts and epithelial cells indicate that TLR3 is expressed both intracellularly and on the cell surface (33). In contrast, other cell types, including human and mouse monocyte-derived immature dendritic cells (DCs), macrophages, and peripheral blood DCs, express intracellular TLR3 exclusively, and TLR3 signaling is localized to mature endosomes in these cells (22, 30, 32). TLR3 has been identified as the major MyD88-independent PRR stimulated in the type I IFN responses to many different viral infections due to its intracellular localization (16, 19, 20, 41, 54, 64). A defined role for TLR3 in bacterial infection has not been clearly established; however, the TLR3 agonist poly(I·C) has been successfully used to provide protection against the intracellular bacterial pathogen Francisella tularensis (48). In these studies, the investigators present poly(I·C) as a potential therapeutic agent against inhaled F. tularensis, and they hypothesized that TLR3 signaling via poly(I·C) provided a boost in host immunity prior to or soon after F. tularensis exposure.
In the present study, we further investigated the role of TLR3 in Chlamydia-induced pathogenesis. Based on our previous observations that the IFN-β response to Chlamydia infection is largely TLR3-dependent in OE cells, we hypothesize that TLR3 plays a critical role in the innate response to Chlamydia infection and genital tract pathogenesis. Herein, we demonstrate that TLR3 plays a role in the synthesis of other inflammatory mediators during Chlamydia infection (in addition to IFN-β), and we show that the Chlamydia induced IFN-β regulates the synthesis of a subset of some (but not all) of these other inflammatory mediators. We demonstrate that Chlamydia replication in TLR3-deficient OE cells is more efficient than in wild-type OE cells and that Chlamydia replication is attenuated in TLR3-deficient cells pretreated with IFN-β prior to infection. We propose that TLR3 and TLR3-induced IFN-β play a critical role in the inflammatory immune response during Chlamydia infection.
MATERIALS AND METHODS
Mice.
B6129SF2/J (control) and B6.129S1-Tlr3tm1Flv/J (TLR3-deficient) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The TLR3-deficient mice and the control B6129SF2/J mice are C57BL/6J × 129S1/SvImJ F1 hybrids that were self-crossed to generate F2 hybrids identified as homozygous for H-2Kb, with or without the TLR3 deletion. The homozygous mice are maintained at The Jackson Laboratory by breeding descendants of the original homozygous F2 hybrids. All mice were housed in Indiana University Purdue University-Indianapolis specific-pathogen-free facilities. Age-matched mice were used at age 8 to 12 weeks for the experiments in the present study. The Indiana University Institutional Animal Care and Utilization Committee (IACUC) approved all experimental protocols.
Reagents.
Recombinant murine IFN-β was purchased from R&D Systems (Minneapolis, MN). The lyophilized IFN-β was suspended in phosphate-buffered saline (PBS) supplemented with 0.1% bovine serum albumin (BSA), divided into aliquots, and frozen at −80°C until use. Recombinant murine IFN-β was thawed and diluted to 50 U/ml in fresh epithelial cell media immediately before use.
Cells, plasmids, and bacteria.
The cloned OE cell lines OE129TLR3(−/−)C19 and OE129WT (14) were grown at 37°C in a 5% CO2 humidified incubator and maintained in epithelial cell medium, 1:1 Dulbecco modified Eagle medium-F12K (Sigma), supplemented with 10% characterized fetal bovine serum (HyClone), 2 mM l-alanyl-l-glutamine (Glutamax I; Gibco/Invitrogen, Carlsbad, CA), 5 μg of bovine insulin/ml, and 12.5 ng of recombinant human FGF-7 (keratinocyte growth factor; Sigma)/ml as previously described (14, 23).
Mycoplasma-free C. muridarum Nigg, previously known as C. trachomatis strain MoPn, was grown and titered in McCoy cells (American Type Culture Collection) as described previously (23, 56).
C. muridarum infection of wild-type and TLR3-deficient mice.
Infections of mice were done as described previously (12) with some minor modifications. Mice were treated with 2.5 mg of Depo-Provera (medroxyprogesterone acetate; Pfizer, New York, NY) in 0.1 ml 1 week before vaginal infection with 105 inclusion-forming units (IFU) of C. muridarum (approximately 100 times the 50% inhibitory concentration) in 10 μl of SPG (sucrose-phosphate-glutamic acid) buffer. Infection was monitored by swabbing the vaginal vault and cervix with a calcium alginate swab (Spectrum Medical Industries, Los Angeles, CA), and titers (IFU) of C. muridarum collected on the swabs were determined on McCoy cell monolayers as described previously (23, 56). Each mouse strain (both wild type and TLR3 deficient) was infected in groups of five, and each experiment was repeated three times.
In vitro infection of OE cells.
OE129WT and OE129TLR3(−/−)C19 cells were plated in 48-well tissue culture plates and used when confluent. For all experiments, the cells were infected with 10 IFU of C. muridarum/cell in 900 μl of culture medium as described previously (13, 14). IFN-β pretreatments were performed as described in reference 46 with modifications: 1 h prior to infection, the medium was carefully removed, and fresh medium containing 50 U of recombinant murine IFN-β/ml was added to the cells in minimal volumes needed for Chlamydia infection. The media containing recombinant murine IFN-β remained on the cells throughout the course of the infection. Untreated cells and SPG buffer-only treatment were used as uninfected and mock-infected controls, respectively.
Analysis of chlamydial growth in OE cells.
OE129WT and OE129TLR3(−/−)C19 cells were plated in 24-well tissue culture plates and either mock infected, infected with 5 IFU of C. muridarum/ml, or pretreated with 50 U of recombinant IFN-β/ml and then infected with 5 IFU of C. muridarum/ml as described above. At 30 h postinfection, the cells were harvested by scraping mechanically with a pipette tip in 500 μl of SPG buffer and frozen at −70°C until further processed. In order to study infectivity, the collected infected cell samples were vortexed and sonicated for 15 min in a water bath, and 50 μl of the sample was passaged onto a fresh layer of McCoy cells for titering as described previously (23, 56).
Collection of genital tract secretions for cytokine analysis.
Vaginal secretions for measurement of IL-6 and CCL5 were collected by the aseptic sponge technique (12). Aseptic surgical sponges (ear wicks, 2 by 5 mm) (DeRoyal, Powell, TN) were inserted into the vaginas of anesthetized Chlamydia-infected and mock-infected mice each day during the first 10 days postinfection. Each sponge was retrieved 30 min later and individually collected in microfuge tubes for storage at −80°C.
Quantitative real-time PCR array.
Total RNA was purified from the small interfering RNA-transfected Bm1.11 cells using an RNeasy kit (Qiagen, Valencia, CA). During purification, all RNA samples were treated with RNase-free DNase I (Qiagen) to remove genomic-DNA contamination. The RNA was quantified by spectrophotometric analysis, and RNA integrity was confirmed by agarose gel electrophoresis prior to PCR array. A PCR array was performed using the SYBR green-based RT2 Profiler system (SA Biosciences, Frederick, MD) according to the manufacturer's instructions. The Mouse Inflammatory Cytokines and Receptors Array (PAMM-011) is a pathway focused array that contains a set of 84 related genes involved in the inflammatory immune response. This particular assay also contains five housekeeping genes and three other reaction controls to assess genomic DNA contamination, RNA quality, and general PCR performance. The PCR was performed on an ABI Prism 7500 sequence detection system (Applied Biosystems, Norwalk, CT), and the results were measured using Sequence Detector 2.2 software. Each assay was performed a total of three times for proper statistical calculation. The data analysis was performed using a company-provided program and is based on the ΔΔCT method, with normalization of the raw data to either housekeeping genes or an external RNA control.
Quantitative real-time PCR to measure TLR2 gene expression was conducted with the diluted cDNA and primers according to the protocol outlined in the iTaq SYBR green Supermix with ROX kit (Bio-Rad, Hercules, CA) as described previously (13), using the primers described in reference 14. Dissociation curves were recorded after each run to ensure primer specificity.
Semiquantitative reverse transcription-PCR (RT-PCR).
Total RNA was isolated from untreated and IFN-β-treated OE129TLR3(−/−)C19 cells using RNeasy minicolumns (Qiagen). During purification, all RNA samples were treated with RNase-free DNase I (Qiagen) to remove genomic-DNA contamination and then quantified by spectrophotometric analysis. Optimized primer pairs for TLR2 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were designed using Vector NTI Suite (Infomax, Inc., Frederick, MD) and are described elsewhere (14). Reactions were performed using 1 μg of total RNA and only 25 of the 40 cycles of the PCR protocol described in reference 13.
Determination of cytokine production.
Vaginal sponges were removed from storage at −80°C, and the sponges were soaked in 300 μl of sterile resuspension buffer (PBS supplemented with 0.5% BSA and 0.05% Tween 20) for 1 h on ice and filtered through a preblocked 0.2-μm-pore-size cellulose acetate filter by centrifugation in a Spin-X microfuge tube (Fisher Scientific, Pittsburgh, PA) prior to enzyme-linked immunosorbent assay (ELISA). The amounts of CCL5 and IL-6 secreted into the vaginas of the mice during infection were measured by the ELISA (R&D Systems) according to the manufacturer's protocol and as described in reference 15, respectively.
Confluent OE129WT and OE129TLR3(−/−)C19 cell monolayers grown in 48-well tissue culture-treated plates were either mock infected, infected with 10 IFU of C. muridarum/cell, or infected with 10 IFU of C. muridarum/cell after 1 h pretreatment with recombinant murine IFN-β. Supernatants were harvested at 24 h postinfection and analyzed for cytokine content using customized ELISAs for IL-6 as described in reference 15 and for CCL5, CXCL10, and CXCL16 using commercially available ELISA kits as described by the manufacturer (R&D Systems). The lower range of ELISA sensitivity for IL-6 was 50 pg/ml, and the lower ranges of assay sensitivities for CXCL10, CXCL16, and CCL5 were 25, 10, and 50 pg/ml; respectively.
For antibody blocking studies, confluent OE129TLR3(−/−)C19 cell monolayers that were grown in 48-well tissue culture-treated plates had supernatants removed and washed once with PBS. PBS was removed and was replaced with either fresh medium, fresh medium containing 10 μg of anti-mouse CD282 (TLR2) monoclonal antibody (clone T2.5)/ml, or fresh medium containing 10 μg of mouse IgG2a(κ) isotype control antibody/ml (eBioscience, San Diego, CA). Cells were returned to 37°C in a 5% CO2 humidified incubator for 2 h before being stimulated with 5 μg of peptidoglycan (PGN-EC)/ml from Escherichia coli serotype O111:B4 (125 endotoxin units (EU)/mg; Invivogen, San Diego, CA). After the addition of PGN, cells were incubated at 37°C in a 5% CO2 humidified incubator for an additional 6 to 8 h before the supernatants were analyzed for IL-6 expression by ELISA. All experiments were repeated at least three times, and the significance was determined by using the statistical analyses described below.
Flow cytometric analyses.
Two hours prior to cell surface antibody staining, confluent monolayers of mock-treated or IFN-β-treated TLR3-deficient OE cells were dislodged from tissue culture plastic using a Hanks salt-based, enzyme-free cell dissociation buffer (Sigma-Aldrich). The cells were stained for 20 min on ice in PBS–2% BSA with either phycoerythrin (PE)-coupled anti-mouse CD282 (TLR2) monoclonal antibody or mouse IgG2a(κ) isotype control antibody (eBioscience). Cells were washed three times with ice-cold PBS–2% BSA and immediately analyzed with a FACSCalibur cytometer (BD Biosciences).
Statistical methods.
Unless otherwise stated, figures for each experimental investigation are presented as a “pooled” means with their associated standard deviations. Figure legends indicate the number of independent experiments pooled to generate each figure. The mean concentration of each analyte was summarized for each experiment, infected status, and cell type. For each experiment and each infected status, the mean concentration was modeled using a linear model with a fixed effect for cell type and a random effect for the date of the experiment. The random date effect incorporates into the model the correlations of samples run on the same date. Residual plots were examined to assess possible violation of model assumptions of normality and homogeneity of variance. For some experiments, there was a deviation from these assumptions, so the data were transformed to a log scale and reanalyzed. Pairwise comparison of the log mean concentration was made using a Sidak adjustment. The Sidak adjustment controls the type 1 error rate.
For vaginal cytokine levels, we compared individual mouse groups on each day of the experiment. The model for this analysis included fixed effects for mouse group, day, and mouse group-day interaction. There was no random effect in this model. Residual plots were examined, and there was some evidence of nonconstant variance; thus, the data analyzed on a log scale. A Sidak adjustment was used to control the type 1 error rate.
RESULTS
C. muridarum-induced inflammatory cytokine production is diminished in the absence of TLR3.
We previously reported that C. muridarum-induced IFN-β synthesis was TLR3 dependent in OE cells by showing that the IFN-β response was severely diminished in OE cells derived from TLR3-deficient mice (14). In an effort to identify other genes and cellular pathways that are aberrantly regulated in TLR3-deficient OE cells compared to wild-type OE cells, we conducted a small-scale PCR array using the commercially available RT2 Profiler system. Total RNA was isolated from mock-infected and Chlamydia-infected OE129WT and OE-TLR3(−/−)C19 cells; equal amounts were converted to cDNA and then subjected to pathway-focused gene expression profiling using real-time PCR.
We selected a mouse inflammatory cytokine and receptor array which examines the expression of 84 different inflammatory response genes, plus several housekeeping genes to assess genomic DNA contamination, RNA quality, and general PCR performance. Table 1 show comparative results between C. muridarum-infected wild-type and TLR3-deficient OE cells, outlining the genes that were differentially expressed in this particular PCR array. As indicated, 33 of the 84 inflammatory mediators tested were significantly upregulated in the OE129WT cells (versus mock infected); this list includes genes involved in T-cell and B-cell chemotaxis, various interleukins, complement, and other factors such as caspase-1 that have been recently implicated in apoptosis via cleavage of sphingosine kinase-2 (65). Our data indicate that both CCR10 and CXCL15 gene expressions were negatively regulated (compared to mock-infected controls), suggesting that their expression was downregulated in the C. muridarum-infected OE129WT cells.
Table 1.
PCR array of inflammatory mediator synthesis in C. muridarum-infected OE cells
| Gene identification | Fold change in cell transcript levela |
|||
|---|---|---|---|---|
| OE129WT + C. muridarum | TLR3 deficient + C. muridarum | TLR3 deficient + C. muridarum/IFN-β | TLR3 deficient + IFN-β only | |
| Chemokines (C-C) | ||||
| CCL2 | 57.3 | – | 22.7 | 11.7 |
| CCL3 | 48.4 | – | – | – |
| CCL4 | 3,373 | 37.4 | 240.5 | – |
| CCL5 | 2,745 | 24.7 | 237.2 | 123 |
| CCL7 | 41.4 | – | 19.3 | 17.7 |
| CCL8 | 34.7 | – | 84.5 | 82.8 |
| CCL9 | 31 | – | 18.9 | 17.9 |
| CCL11 | 12.7 | – | 16.3 | – |
| CCL12 | 12.6 | – | 10.2 | – |
| CCL20 | 427.4 | – | 6.7 | – |
| CCL22 | 48.4 | – | – | – |
| CCL24 | 44.3 | – | – | – |
| Chemokines (C-X-C) | ||||
| CXCL1 | 43.6 | – | 10.1 | – |
| CXCL5 | 163.5 | – | – | – |
| CXCL9 | 44.7 | – | 15.8 | – |
| CXCL10 | 6,854 | 72.3 | 4,067 | 3,853 |
| CXCL11 | 2,223 | – | 455.4 | 255 |
| CXCL13 | – | – | – | 8.8 |
| CXCL15 | –43.6 | –34.3 | –25.6 | – |
| Chemokine receptors | ||||
| CCR1 | 54.3 | – | – | – |
| CCR2 | 33.6 | – | – | – |
| CCR4 | 28.4 | – | – | – |
| CCR6 | 15.6 | – | – | – |
| CCR9 | – | 8.7 | – | – |
| CCR10 | –10.2 | – | – | |
| Interleukins/receptors | ||||
| IL-10 | 10.4 | – | – | – |
| IL-11 | 10.7 | – | – | – |
| IL-13 | 50.3 | – | – | – |
| IL-15 | 8.8 | – | – | – |
| IL-1α | 125.6 | – | – | – |
| IL-1F6 | 8.3 | – | – | – |
| IL-1F8 | 10.4 | – | – | – |
| IL-1R2 | 11.6 | – | – | – |
| Others | ||||
| Casp1 | 16.5 | – | 10.9 | 8.7 |
| Itgam | 28.3 | – | – | – |
| Spp1 | 18.3 | – | – | – |
| Lta | – | 5.8 | 5.8 | – |
| TNF | 263 | – | – | – |
Values represent the fold change in transcript levels versus mock-treated OE cells (P< 0.05). –, not significantly different between infected and uninfected OE cells.
In comparative analyses, PCR array results of the C. muridarum-infected OE cells derived from TLR3-deficient mice showed significant upregulation in the expression of only 5 of the 84 inflammatory mediators (Table 1). As indicated, the inflammatory mediators were significantly upregulated (compared to mock-infected controls); however, the level of upregulation was attenuated compared to C. muridarum-infected wild-type OE cells. Because TLR3-deficient OE cells are severely impaired in the ability to synthesize IFN-β in response to Chlamydia infection, we hypothesized that IFN-β plays a role in the regulation and subsequent synthesis of other inflammatory mediators. To determine whether TLR3-dependent IFN-β plays a role in the synthesis of other inflammatory mediators, the same PCR array was performed on TLR3-deficient OE cells that were pretreated with recombinant murine IFN-β 1 h prior to infection. In these (and subsequent) experiments, we pretreated OE cells with purified, recombinant murine IFN-β that was diluted in media at the concentration of 50 U/ml. The 50-U/ml IFN-β concentration used was determined by IFN-β-specific ELISA to be an approximation of the amount of IFN-β secreted into the media of C. muridarum-infected wild-type OE cells during the course of a 24-h infection (data not shown). As shown, the addition of recombinant murine IFN-β restored upregulation in the synthesis of an additional 10 inflammatory mediators (compared to the results of C. muridarum-infected TLR3-deficient OE cells with no IFN-β pretreatment). TLR3-deficient OE cells that were pretreated with IFN-β but were not infected with Chlamydia demonstrated significant upregulation in the expression of only CCL2, CCL5, CCL7, CCL8, CCL9, CXCL10, CXCL11, CXCL13, and Casp1. The data indicate that TLR3-induced IFN-β does play a role in mediating the synthesis of some (but not all) of the inflammatory mediators tested in this particular PCR array.
Defective infection-induced inflammatory cytokine and chemokine production is restored by IFN-β pretreatment in TLR3-deficient OE cells.
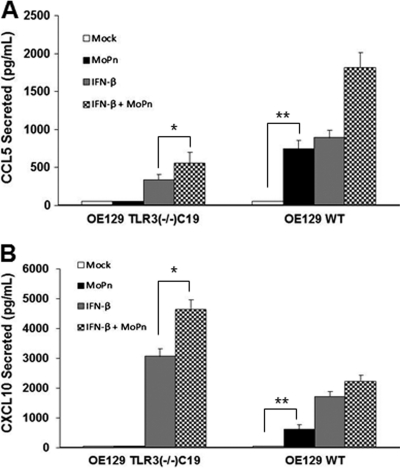
To validate our PCR array data indicating that the absence of IFN-β was linked to the decreased expression of some of these downstream inflammatory mediators, ELISA was used to measure the synthesis of specific chemokines after recombinant murine IFN-β was supplied exogenously to both OE cell types 1 h prior to infection with C. muridarum. We selected CCL5 and CXCL10 as candidate inflammatory chemokines that were both highly induced during C. muridarum infection of wild-type OE cells (Table 1), and both are commonly associated with T-lymphocyte recruitment (reviewed in references 27 and 67). As shown in Fig. 1, C. muridarum-induced CCL5 and CXCL10 syntheses were severely diminished in the TLR3-deficient OE cells, corroborating the gene-expression data obtained in the PCR array. Synthesis of these two chemokines (which are known to be regulated by IFN-β [18]) was restored in TLR3-deficient OE cells in which the medium was supplemented with recombinant IFN-β prior to C. muridarum infection. As indicated, IFN-β pretreatment induced synthesis of these chemokines in the absence of Chlamydia; however, the syntheses of these chemokines were significantly higher when the cells were also infected with Chlamydia.
Fig 1.
Chlamydia-induced CCL5 (A) and CXCL10 (B) synthesis in OE cells isolated from wild-type and TLR3-deficient mice. ELISA was used to measure infection-induced CCL5 and CXCL10 secreted into the supernatants of mock-infected and C. muridarum-infected OE129WT and OE129TLR3(−/−)C19 cells. The data presented are representative from four different experiments. Significance was determined using the Student t test (*, P < 0.05; **, P < 0.005).
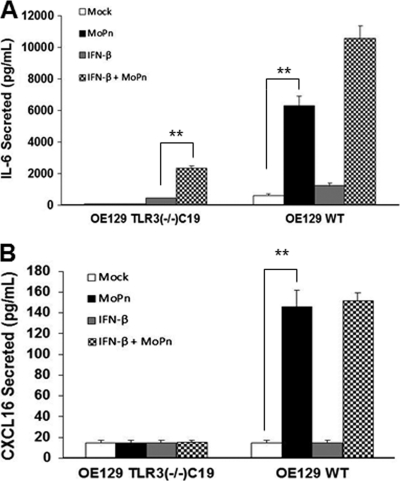
We previously reported that C. muridarum infection induces high-level expression of the acute inflammatory cytokines IL-6 and CXCL16, and we hypothesized that these factors are involved in polarization of the immune response and genital tract scarring in response to Chlamydia infection, respectively (23). We then showed that much of the IL-6 synthesis was MyD88 dependent and that it was induced in OE cells via a TLR2-dependent mechanism (15). However, as shown in Fig. 2, OE cells lacking TLR3 were also defective in synthesizing these inflammatory mediators in response to C. muridarum infection. Surprisingly, OE-TLR3(−/−)C19 cells pretreated with exogenous IFN-β prior to C. muridarum infection seemed to modestly restore synthesis of IL-6 in these cells (compared to OE129WT cells); however, the addition of IFN-β did not restore CXCL16 synthesis in these cells. Neither cell type treated with IFN-β alone secreted large amounts of CXCL16 or IL-6 in the absence of Chlamydia; however, CXCL16 and IL-6 are not known to be directly regulated by IFN-β (17, 69). The results of the CXCL16 ELISA suggest that there are other factors in addition to IFN-β that regulate synthesis of CXCL16, and these factors and/or pathways are defective in TLR3-deficient OE cells. Collectively, the data indicate that the OE-TLR3(−/−)C19 cells were deficient in the C. muridarum-induced synthesis of these selected inflammatory cytokines and chemokines (compared to the OE129WT cells). As indicated, there appeared to be an additive and/or synergistic enhancement in synthesis of IL-6, CCL5, and CXCL10 in all cell types when treated with IFN-β prior to infection (compared to either infection or IFN-β treatment alone). Because the levels of expression of IL-6, CCL5, and CXCL10 are higher in the infected cells pretreated with exogenous IFN-β than in either treatment condition individually, the data suggest that the OE cells may have a more potent immune response to C. muridarum infection in the presence of IFN-β.
Fig 2.
Chlamydia-induced IL-6 (A) and CXCL16 (B) synthesis in OE cells isolated from wild-type and TLR3-deficient mice. ELISA was used to measure infection-induced IL-6 and CXCL16 secreted into the supernatants of mock-infected and C. muridarum-infected OE129WT and OE129TLR3(−/−)C19 cells. The data presented are representative from four different experiments. Significance was determined using the Student t test (*, P < 0.05; **, P < 0.005).
Inflammatory mediator synthesis is diminished in the genital tracts of C. muridarum-infected TLR3-deficient mice.
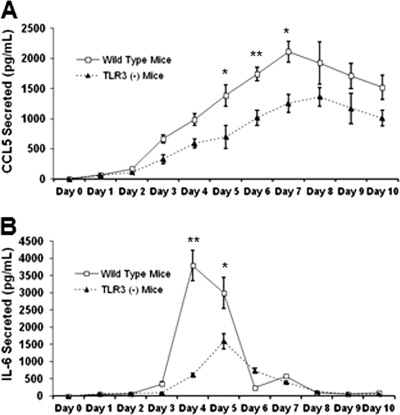
To determine the functional significance that the loss of TLR3 has on the genital tract levels of innate immune cytokines and chemokines in vivo, we infected groups of five B6129SF2/J control and five B6;129S1-Tlr3tm1Flv/J mice vaginally with 105 IFU of C. muridarum. Figure 3 shows the combined results of three independent experiments in which we measured inflammatory mediators secreted during the first 10 days postinfection. As shown in Fig. 3A, the CCL5 secreted into the genital tracts peaked at day 7 in the wild-type mice, and there was a statistically significant lower amount of CCL5 secreted into the genital tracts of the TLR3-deficient mice at days 5, 6, and 7. As shown in Fig. 3B, IL-6 synthesis peaked at day 4, and there were statistically significantly lower levels of syntheses of IL-6 in the TLR3-deficient mice on days 4 and 5. However, by day 6, IL-6 levels between wild-type and TLR3-deficient mice were virtually identical. Collectively, data from both the in vitro and in vivo experiments indicate that wild-type mice have a more potent inflammatory response to C. muridarum infection than TLR3-deficient mice.
Fig 3.
CCL5 and IL-6 in the genital tracts of Chlamydia-infected WT and TLR3-deficient mice. Genital tract secretions were obtained during the first 10 days postinfection and analyzed for CCL5 (A) and IL-6 (B) secretion via ELISA. Pooled data from three independent experiments are presented. Significance was determined using the Student t test (*, P < 0.05; **, P < 0.005).
C. muridarum replication is more robust in the absence of TLR3.
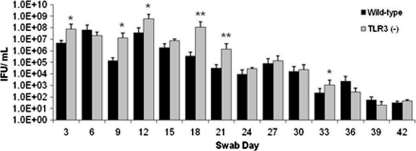
We previously reported that TLR3-deficient mice had diminished levels of IFN-β secreted into the genital tracts during C. muridarum infection, but that clearance kinetics were quite similar in both strains and that both mouse strains cleared Chlamydia by week seven (14). In this report, we show a similar trend with CCL5 and IL-6 synthesis in the TLR3-deficient mice being substantially less than that of wild-type mice during C. muridarum infection (Fig. 3). However, close examination of Chlamydia titers between days 9 and 24 postinfection showed TLR3-deficient mice shed more Chlamydia than wild-type mice, with a >300-fold difference on day 18 (Fig. 4). The chlamydial titers for both strains were virtually the same after day 27 and for the remainder of infection, and again the actual clearance kinetics were similar between both strains. It is unclear whether higher titers between days 9 and 24 in the TLR3-deficient mice results in more C. muridarum-induced pathology in these mice, and this requires further investigation.
Fig 4.
C. muridarum replication in the genital tracts of wild-type and TLR3-deficient mice. Genital tract infections were performed and swabs were collected every third day as described in Materials and Methods for a period of 7 weeks. The swabs were then quantified for C. muridarum replication by determining the titers of sonicated suspensions on McCoy cells. Pooled data from three independent experiments with n = 5 in each group are shown. IFU, inclusion-forming units. Significance was determined using the Student t test (*, P < 0.05; **, P < 0.005).
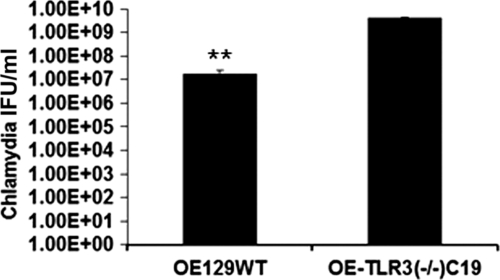
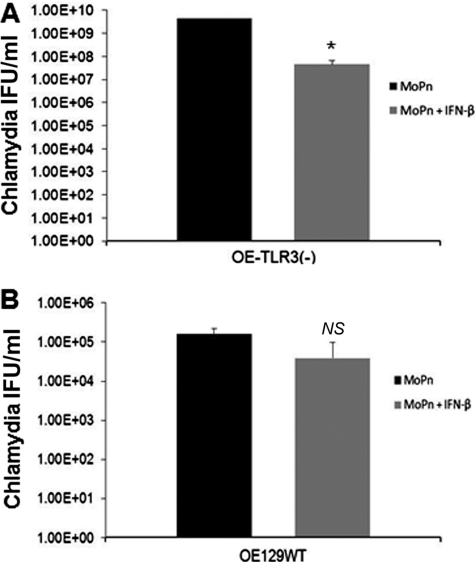
To examine whether C. muridarum growth in OE cells reflect the genital tract results, we next assessed C. muridarum replication in OE cells derived from wild-type and TLR3-deficient mice. As shown in Fig. 5, C. muridarum replication was greater in the TLR3-deficient cells compared to the wild-type, suggesting that stimulation of TLR3 by C. muridarum results in an immune response that negatively affects Chlamydia growth. To examine the effect that IFN-β has on Chlamydia replication in TLR3-deficient OE cells, we measured C. muridarum replication in TLR3-deficient OE cells and compared it to experiments in which we infected TLR3-deficient OE cells that were preincubated with exogenous IFN-β 1 h before infection. As shown in Fig. 6A, preincubation of the TLR3-deficient OE cells with IFN-β decreased the bacterial growth in these cells. The results using wild-type OE cells that were preincubated with exogenous IFN-β were similar but showed a less dramatic reduction and were not statistically significant (Fig. 6B). These data indicate that IFN-β is detrimental to C. muridarum growth and suggest that its synthesis during Chlamydia infection may beneficial to the host in the context of TLR3 deficiency.
Fig 5.
Chlamydia replication in OE cells derived from wild-type and TLR3-deficient mice. OE cells were infected with 5 IFU of C. muridarum/cell. Lysates were collected, sonicated, and titered on McCoy cell monolayers. The data presented are representative from three different experiments. Significance was determined using the Student t test (**, P < 0.005). IFU, inclusion-forming units.
Fig 6.
Effect of IFN-β on Chlamydia replication in OE cells derived from TLR3-deficient mice. OE129TLR3(−/−)C19 cells (A) and OE129WT cells (B) were infected with 5 IFU of C. muridarum/cell in the presence or absence of 50 U of recombinant IFN-β/ml. Lysates were collected, sonicated, and titered on McCoy cell monolayers. The data presented are representative from three different experiments. Significance was determined using the Student t test (*, P < 0.05; NS, not statistically significant). IFU, inclusion-forming units.
IFN-β upregulates TLR2 gene expression in oviduct epithelial cells.
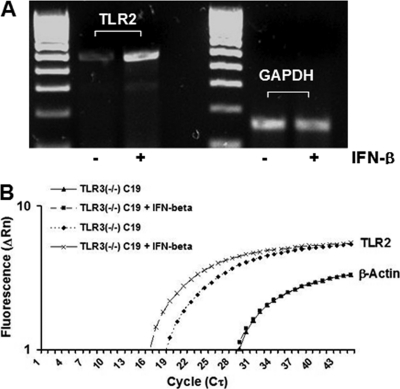
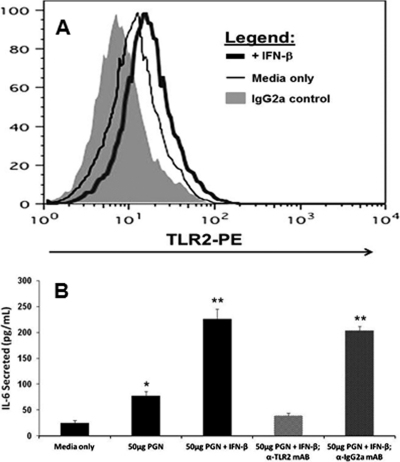
Our data indicate that the addition of exogenous IFN-β to TLR3-deficient OE cells prior to C. muridarum infection restores the expression of several different inflammatory mediators, including IL-6 and CXCL10, that were shown to be TLR2-dependent in OE cells and other cell types (5, 15). This observation proposes the hypothesis that the TLR3-dependent IFN-β synthesized during C. muridarum infection plays in role regulating the inflammatory immune response in part by modulating the gene expression of components found in the TLR2 signaling pathway. One possible mechanism to modulate TLR2 signaling would involve increasing sensitivity to TLR2 PAMPs by upregulating TLR2 mRNA and subsequent protein expression. To ascertain whether TLR3-induced IFN-β is a secreted factor that can enhance the sensitivity of TLR2 to chlamydial PAMPs, we performed semiquantitative RT-PCR and real-time PCR analyses to determine whether IFN-β has any effect on TLR2 mRNA synthesis. As shown in Fig. 7A, TLR2 mRNA expression was increased in uninfected TLR3-deficient OE cells that were treated with media containing IFN-β for 1-h prior to RNA isolation. These data were corroborated via quantitative real-time PCR, which indicated that TLR2 gene expression was increased 6.8-fold in this particular experiment (Fig. 7B). To ascertain whether the increased expression of TLR2 mRNA resulted in a corresponding increase in TLR2 protein expression, we examined the cell surface TLR2 expression levels on TLR3-deficient OE cells. As shown in Fig. 8A, there was a slight (but significant) shift in the peak representing the fluorescent intensity of the OE cells that were pretreated with IFN-β for 2 h prior to staining with PE-conjugated TLR2-specific monoclonal antibody. The increase in TLR2 protein expression levels in TLR3-deficient OE cells that were pretreated with recombinant IFN-β was confirmed by immunoblotting with TLR2-specific monoclonal antibody (data not shown).
Fig 7.
IFN-β induces TLR2 gene expression in TLR3-deficient OE cells. (A) Total cell RNA was extracted from OE129TLR3(−/−)C19 cells and was reverse transcribed and amplified with the TLR2 and GAPDH control primers for only 25 PCR cycles as described in Materials and Methods. The reverse transcription reactions were performed in the presence (+) or absence (−) of exogenously added IFN-β as described in the text. (B) Quantitative real-time PCR to measure TLR2 mRNA was performed on total-cell RNA isolated from OE129TLR3(−/−)C19 cells in both presence or absence of exogenously added IFN-β. Control reactions were set up with primers specific for β-actin to ensure that equal amounts of RNA were used. The representative data presented, from one of three independent experiments, show a 6.8-fold increase in TLR2 mRNA in the IFN-β-treated cells.
Fig 8.
IFN-β enhances TLR2 protein function. (A) Histogram data of TLR3-deficient OE cells that were either untreated or treated with 50 U of recombinant IFN-β/ml prior to staining with PE-conjugated TLR2-specific or IgG2a isotype control monoclonal antibody. (B) IL-6 synthesis was measured in the supernatants of mock-treated and IFN-β-pretreated TLR3-deficient OE cells stimulated with the peptidoglycan (PGN) after being untreated or blocked for 2 h with either TLR2-specific or IgG2a isotype control antibody. The results are representative of three independent experiments. Significance was calculated comparing test condition samples to the medium-only control using the Student t test (*, P < 0.05; **, P < 0.005).
To determine whether the increase in TLR2 protein expression caused by pretreatment with recombinant IFN-β can also result in an enhanced sensitivity to TLR2 PAMPS, we assessed the efficacy of a TLR2-specific agonist in its ability to induce IL-6 production in untreated and IFN-β-treated TLR3-deficient OE cells. We previously reported that exposure of the OE cell lines to E. coli peptidoglycan (PGN) caused marked secretion of IL-6 into the supernatants of treated cells in a dose-dependent manner (15). Figure 8B shows that PGN induces the synthesis of IL-6 in TLR3-deficient OE cell when added at a concentration of 5 μg/ml; however, the synthesis of IL-6 increased >2-fold when TLR3-deficient OE cells were pretreated with IFN-β prior to the addition of PGN to the cells. As shown in Fig. 8B, the ability of IFN-β to enhance the sensitivity of TLR2 to PGN was significantly attenuated by TLR2-specific neutralizing antibody but was not affected by the IgG2a isotype control. These results confirm our previous findings that the PGN induced IL-6 synthesis in a TLR2-specific manner in OE cells and supports our presumption that IFN-β can enhance the sensitivity of TLR2 to chlamydial PAMPs. Collectively, these data support our overall hypothesis that IFN-β can modulate TLR2-dependent inflammatory mediator synthesis and provides an interesting model for a possible mechanism of IFN-β-elicited immunomodulation of the inflammatory response to C. muridarum infection in OE cells.
DISCUSSION
The immune response to Chlamydia in infected epithelial cells represents the initial stage of Chlamydia pathogenesis and the onset of the disease process (60). We previously reported on the contributions of TLRs 2, 1, and 6 in the initiation of the acute inflammatory response of epithelial cells lining the murine oviduct tissue, and we showed that IFN-β secreted during infection was TLR3 dependent (14). Here, we found that TLR3 also has a role in the upregulation of mRNAs for a multitude of cytokines and chemokines (in addition to IFN-β), and that the absence of IFN-β in TLR3-deficient OE cells negatively affects synthesis of a subset of these inflammatory mediators. We showed that C. muridarum replication is more robust in TLR3-deficient OE cells compared to wild-type OE cells and that TLR3-deficient mice generally had higher C. muridarum titers secreted into the genital tract between days 9 and 24 postinfection. Our data show that TLR3-deficient OE cells have lower Chlamydia titers if recombinant murine IFN-β was added to the cell supernatants prior to infection, suggesting that IFN-β has a role in controlling Chlamydia replication. Our findings represent the first such report that directly links TLR3 to the pathogenesis of Chlamydia infection.
As a dsRNA receptor, TLR3 is a major PRR associated with type I IFN responses to a multitude of viral infections (reviewed in references 34 and 70). The link between TLR3 and other inflammatory mediators has been presented in the investigations of Wang et al., who demonstrated the important role of TLR3 in infection by West Nile virus. In that study, TLR3-deficient mice had impaired inflammatory cytokine production and enhanced viral load in the peripheral blood (63). In other RNA viral infections, such as respiratory syncytial virus, influenza A virus, and phlebovirus infections, inflammatory cytokine and chemokine production is impaired in the absence of TLR3, therefore affecting the virus-induced pathology and host survival (19, 20, 28, 54, 55). Our data showing decreased C. muridarum-induced inflammatory cytokine and chemokine synthesis in the absence of TLR3 propose that TLR3 plays a role in the pathogenesis of Chlamydia infections and that its presence in target epithelial cells likely has an effect on Chlamydia survival in the host. Our data also imply that C. muridarum infection provides a PAMP that binds to and stimulates TLR3, and studies are under way to identify that unknown chlamydial or infection-induced cellular PAMP.
Although the apparent link between pathogen-induced TLR3 stimulation and synthesis of inflammatory mediators (other than IFN-β) has been described in various viral infection models, the transcriptional mechanisms that lead to the synthesis of these other factors involved in the respective innate immune responses have not been elucidated. TLR3-induced IFN-β transcriptional induction is part of a potent feed-forward mechanism, amplifying the induction of several other genes and making it difficult to distinguish between direct and indirect targets of TLR3-activated transcription modules (11). The example of the feed-forward mechanism of IFN-β inducing other genes was observed in our experiments whereby we showed that TLR3-deficient OE cells were defective in the C. muridarum-induced synthesis of several inflammatory mediators (compared to wild-type OE cells), unless IFN-β was added to the media prior to infection. However, there were several factors that were not affected by the addition of IFN-β to TLR3-deficient cells, and the link between TLR3 and these unaffected inflammatory factors remains unresolved and requires further investigation.
We hypothesize that there are other immunomodulatory cytokines in addition to IFN-β that are responsible for regulating the synthesis of inflammatory mediators that are unaffected by the addition of IFN-β alone. In addition, we postulate that the expression these additional comodulatory factors are also defective in C. muridarum-infected TLR3-deficient OE cells. One possible modulatory or comodulatory cytokine that is also disrupted in TLR3-deficient OE cells is TNF-α. The role of TNF-α as an immunomodulatory cytokine has been described in experiments demonstrating TNF-α's direct role in regulating the synthesis of inflammatory mediators, including TGF-β, IL-12p40, and IL-10, in the guinea pig model for tuberculosis infection (25, 31). Others have described a comodulatory role for TNF-α by demonstrating that TNF-α works synergistically with IFN-β in order to sufficiently induce the synthesis of indoleamine 2,3-dioxygenase (IDO) production during Haemophilus ducreyi infection of human DCs (29). In this example, the induction of IDO in human DCs is hypothesized to contribute to bacterial persistence through the suppression of anti-H. ducreyi immune responses. The investigators in that study hypothesize that IDO-expressing dendritic downregulate anti-H. ducreyi T cell responses through inhibition of T cell proliferation, induction of T cell death, and the expansion of FOXP3+ Treg cells via immune modulation.
Our data implicate IFN-β as a factor in modulating the immune response to C. muridarum infection in OE cells, and the induction of IDO as an actual mechanism that IFN-β uses to perform this function is one possibility. An example of how induction of IDO expression affects cytokine production during Chlamydia infection was observed in Chlamydia psittaci infection of human monocyte-derived macrophages (10, 45). In those studies, the investigators describe a mechanism whereby IFN-β and IFN-α work synergistically to induce IDO expression as a part of the normal host cell response to control intracellular infection. It is not known whether IFN-β interacts with other immunomodulatory cytokines to induce IDO as a mechanism to regulate inflammatory mediator expression in C. muridarum-infected OE cells, and the role these putative regulatory mechanisms in TLR3-deficient OE cells will be investigated in future studies. We hypothesize that IDO induction (if present) will likely represent only a part of the mechanisms invoked by IFN-β in response to C. muridarum infection of OE cell, and we describe another putative mechanism in subsequent passages of this discussion.
Our data indicate that the TLR3 had the most dramatic effect on the induction of CCL4, CCL5, and CCL20 in C. muridarum-infected OE cells, with each showing 400-fold (or greater) induction in their expression levels in the C. muridarum-infected wild-type OE cells versus mock-treated controls. CCL4 and CCL5 were only modestly induced in the TLR3-deficient cells (<40-fold), but the addition of IFN-β increased the syntheses of these factors >240-fold over mock-treated controls. A similar trend was observed in the C. muridarum-induced synthesis of CXCL10 and CXCL11, but the effect of IFN-β was even more dramatic. We hypothesize that Chlamydia infection stimulates the synthesis of IFN-β in a TLR3-dependent manner, and the induction of these specific cytokines by TLR3-dependent IFN-β are tantamount to characterization of IFN-β as a master regulator in the synthesis of various factors that recruit NK cells, T cells, and other cell types to the site of infection (21, 52). This hypothesis proposes a beneficial role for IFN-β in the control of Chlamydia infection in vivo and is supported by our data showing that C. muridarum titers were actually lower between days 9 and 24 postinfection in wild-type mice (Fig. 4).
Interestingly, results from the PCR array indicate that CXCL15 synthesis was decreased in both C. muridarum-infected wild-type and TLR3-deficient OE cells (compared to mock-treated controls). CXCL15 was initially reported to be strongly expressed in the adult lung of the inbred mouse strains BALB/c and C57BL/6 but was not expressed in lymphoid organs such as the spleen (53). CXCL15 was shown in those studies to be upregulated in response to multiple inflammatory stimuli, including an ovalbumin-induced model of asthma and in Nippostrongylus brasiliensis or Aspergillus infection models. Because CXCL15 is also known to be a chemoattractant for neutrophils, the exact reason for CXCL15 downregulation in OE cells in response to Chlamydia infection is somewhat of an enigma. It is widely accepted that the neutrophil is viewed as a professional phagocyte whose sole function in immunity is to engulf, kill, and clear bacteria in response to infection. However, neutrophils have been implicated in host tissue damage in Chlamydial infections, including guinea pig ocular chlamydial infection models (51). Recent investigations into the role of neutrophil involvement in Chlamydia pathogenesis revealed that neutrophil depletion dramatically decreased ocular pathology both clinically and histologically and that there was an associated alteration in adaptive immunity (26). Our data imply that there is not a significant recruitment of neutrophils elicited by the C. muridarum-infected OE cells; however, the in vivo role of neutrophil involvement in genital tract infections between wild-type and TLR3-deficient mice remains to be investigated. A similar reduction in CXCL15 expression was also observed in two different models of murine colitis as an inflammatory response to Helicobacter infection (57). The exact mechanism and role(s) for CXCL15 downregulation in response to Helicobacter infection in the murine colitis remains to be elucidated; however, the mild-to-moderate Gr-1+ neutrophil infiltrate (compared to Helicobacter-associated gastritis model) implies that those neutrophils were recruited by chemokines other than CXCL15.
Uncovering the link between TLR3 stimulation and the synthesis of inflammatory mediators would provide significant insight into our understanding of the innate immune response to Chlamydia infection in epithelial cells. One hypothesis is that TLR3 through some unknown factor enhances sensitivity of the other TLRs to Chlamydia PAMPs, resulting in increased inflammatory mediator synthesis. We previously reported an upregulation in the expression of several TLR genes in response to C. muridarum infection in OE cells (15). Our current data showing that IFN-β enhances synthesis of IL-6 that was severely diminished in TLR3-deficient cells is an interesting observation. We showed in our previous studies that IL-6 was heavily dependent on TLR2 and was induced via the MyD88 signaling pathway during C. muridarum infection of OE cells (15). Therefore, we were somewhat surprised to see that its synthesis was also diminished in the TLR3-deficient OE cells, allowing us to postulate a possible role for TLR3-dependent IFN-β in regulating TLR2-dependent acute inflammatory cytokine synthesis. The data from Fig. 7 shows that exogenously added IFN-β can increase the expression of TLR2 mRNA in uninfected OE cells. The increased TLR2 mRNA synthesis results in a corresponding increase in TLR2 protein expression, which ultimately enhances sensitivity of the OE cells to TLR2 PAMPs (Fig. 8).
Our data from the experiments using the commercially purified peptidoglycan as a TLR2 PAMP allow us to extrapolate that IFN-β, induced during infection, is required to elevate TLR2's sensitivity to C. muridarum PAMPs during the course of infection in the OE cells. This phenomenon is highlighted in the experiments demonstrating that TLR3-deficient mice, which are defective in their synthesis of IFN-β during C. muridarum infection, are also defective in the synthesis of TLR2-dependent IL-6. It is not known whether supplementing TLR3-deficient mice with exogenous IFN-β will restore synthesis of IL-6 and other TLR2-dependent inflammatory mediators, as demonstrated in vitro in the OE cell lines, but the investigations into whether this phenomenon is also observed in vivo are under way.
These preliminary experiments support our hypothesis of a TLR3-dependent factor synthesized during Chlamydia infection being able to upregulate TLR gene expression. Our data implicate IFN-β as that C. muridarum-induced factor in OE cells and suggest a possible mechanism for the increased inflammatory mediator synthesis in TLR3-deficient OE cells after being pretreated with IFN-β 1 h prior to infection. This mechanism was previously presented in earlier studies who show that type I IFNs enhanced TLR responsiveness in macrophages by upregulating the expression of TLR3, TLR4, and TLR7 (59). Others have shown in investigations using cells endogenous to the CNS (astrocytes and microglia cells) that the synthetic TLR3 PAMP poly(I·C) effectively induces innate antiviral responses by enhancing the gene expression of TLRs 3, 7, 8, and 9 (61). These data suggest that the type I IFNs secreted in response to poly(I·C) play a role in regulating TLR expression as part of their function in modulating the innate and adaptive immune response to viral infection. Further studies will be required to completely elucidate this likely novel homeostatic biology linking TLR3-induced IFN-β to inflammatory cytokine synthesis through TLR2-dependent signaling pathways in C. muridarum-infected OE cells.
Type I IFNs have been shown to have different effects regarding the outcomes of infection for different intracellular bacterial pathogens (3, 37, 43). Early studies by Byrne and Krueger (8) showed that type I IFNs had some inhibitory effect on Chlamydia replication in mouse fibroblasts and that the inhibition was reversed in the presence of antibodies specific for IFN-γ. The exact role of the IFN-β and its contributions to the overall immune response to Chlamydia infection have not been conclusively elucidated; however, experiments conducted in mice defective in the IFN-α/β receptor (IFNAR) suggest that type I IFNs are detrimental to the host in both the genital tract and lung infection models. Nagarajan et al. show that the loss of type I IFNs actually decreases C. muridarum genital tract disease in IFN-α receptor-deficient IFNAR−/− mice, through an inhibition of the Chlamydia-specific CD4 T-cell response (42). In that study, the authors showed reductions in Chlamydia shedding, duration of infection, and oviduct pathology in the IFNAR−/− mice. Qiu et al. reported that IFNAR−/− mice were significantly more resistant to C. muridarum infection, thus showing less bacterial burden and bodyweight loss and milder pathological changes in the lung infection model of infection (49). Our results do not coincide with the findings of Nagarajan and Qiu, and our data present an alternative hypothesis that the TLR3-induced IFN-β may actually be beneficial to the host if it is produced during Chlamydia infection.
In contrast, we report that TLR3-deficient OE cells produce greater yields of Chlamydia progeny versus wild-type OE cells, likely because they are deficient in IFN-β synthesis. In addition, we show in Fig. 5 that C. muridarum replicates more efficiently in TLR3-deficient OE cells (that are defective in C. muridarum-induced IFN-β synthesis) and that adding IFN-β to these cells prior to infection decreases the yield of infectious progeny (Fig. 6). Because IFNAR affects expression of all type-I IFNs (reviewed in references 7 and 39), it is possible that the observed deleterious effects attributed to type I IFNs in the IFNAR-deficient mouse experiments of Nagarajan and Qiu are due to the expression of type I IFNs other than IFN-β. We present the alternative theory that the deleterious effects of these other type I IFNs observed in wild-type mice may be diminished in knockout mouse due to reduced expression levels in the INFAR-deficient mice. Our experiments using TLR3-deficient OE cells have not demonstrated significant reductions in other type I IFNs such as IFN-α, and we propose that it is production of these other type I IFNs that can potentially be detrimental to the host and perhaps contribute to the observed differences between our data and that of Nagarajan and Qiu. However, it is critical to point out that the increased production of TLR2-dependent acute inflammatory cytokines such as IL-6 and TNF-α in the wild-type OE cells (compared to the TLR3-deficient OE cells) may result in increased oviduct pathology despite decreased replication and diminished Chlamydia shedding in wild-type OE cells and mice. In this scenario, there will likely be a diminished inflammatory response to Chlamydia infection in the TLR3-deficient mice, resulting in less pathology and thereby corroborating the observations of Nagarajan and Qiu. Investigations are under way to ascertain the role of TLR3 in actual oviduct pathology, and we are hopeful to address the direct role of IFN-β in these future studies.
The identification of TLR3 as a PRR that is stimulated during Chlamydia infection of OE cells represents a novel observation in Chlamydia pathogenesis. In addition, this observation may be cell type specific since TLR3 appears to be dispensable in IFN-β and IL-6 production in bone marrow-derived macrophages derived from TLR3-deficient mice (14). Although it is somewhat of an enigma that TLR3 has a role in Chlamydia pathogenesis due to the lack of a known dsRNA component, it is not surprising that the epithelial cells would take advantage of PRRs reserved for viral intracellular pathogens to combat an intracellular pathogen such as Chlamydia. As we delve further into the understanding of innate immunity to pathogenic organisms, we further uncover additional and redundant roles for previously overlooked cellular pathways. It is clear that these pathways and mechanisms are overlooked and not believed to have a role in the pathogenesis of a particular organism only because these mechanisms are not invoked in all target cell types. The culmination of our investigations of C. muridarum infections in OE cells showing some mechanistic contrasts to investigations in cells of hematopoietic origin (macrophages and myeloid dendritic cells) underscores the importance of examining the pathogenesis of infection in all major target cell types. It is our belief that Chlamydia research must continue to proceed using all major cell types infected in order to get the most complete understanding of host immunity to Chlamydia infection.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grant 1K22AI072072 (W.A.D.).
We thank James Williams and the staff at the Indiana University infectious disease diagnostic laboratory for assistance with real-time PCR. We also acknowledge the thoughtful manuscript critiques provided by Stanley Spinola, Raymond Johnson, and Randy Brutkiewicz.
Footnotes
Published ahead of print 17 October 2011
REFERENCES
- 1. Aderem A. 2001. Role of Toll-like receptors in inflammatory response in macrophages. Crit. Care Med. 29: S16–S18 [DOI] [PubMed] [Google Scholar]
- 2. Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413: 732–738 [DOI] [PubMed] [Google Scholar]
- 3. Al Moussawi K, et al. 2010. Type I interferon induction is detrimental during infection with the Whipple's disease bacterium, Tropheryma whipplei. PLoS Pathog. 6: e1000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barlow RE, et al. 2001. The prevalence of Chlamydia trachomatis in fresh tissue specimens from patients with ectopic pregnancy or tubal factor infertility as determined by PCR and in-situ hybridization. J. Med. Microbiol. 50: 902–908 [DOI] [PubMed] [Google Scholar]
- 5. Barrenschee M, Lex D, Uhlig S. 2010. Effects of the TLR2 agonists MALP-2 and Pam3Cys in isolated mouse lungs. PLoS One 5: e13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barton GM, Medzhitov R. 2002. Toll-like receptors and their ligands. Curr. Top. Microbiol. Immunol. 270: 81–92 [DOI] [PubMed] [Google Scholar]
- 7. Bekisz J, Schmeisser H, Hernandez J, Goldman ND, Zoon KC. 2004. Human interferons alpha, beta, and omega. Growth Factors 22: 243–251 [DOI] [PubMed] [Google Scholar]
- 8. Byrne GI, Krueger DA. 1983. Lymphokine-mediated inhibition of Chlamydia replication in mouse fibroblasts is neutralized by anti-gamma interferon immunoglobulin. Infect. Immun. 42: 1152–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell LA, et al. 1993. Detection of Chlamydia trachomatis deoxyribonucleic acid in women with tubal infertility. Fertil Steril. 59: 45–50 [PubMed] [Google Scholar]
- 10. Carlin JM, Borden EC, Byrne GI. 1989. Interferon-induced indoleamine 2,3-dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J. Interferon Res. 9: 329–337 [DOI] [PubMed] [Google Scholar]
- 11. Cavalieri D, et al. 2010. DC-ATLAS: a systems biology resource to dissect receptor specific signal transduction in dendritic cells. Immunome Res. 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Darville T, et al. 2003. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J. Immunol. 171: 6187–6197 [DOI] [PubMed] [Google Scholar]
- 13. Derbigny WA, Hong SC, Kerr MS, Temkit M, Johnson RM. 2007. Chlamydia muridarum infection elicits a beta interferon response in murine oviduct epithelial cells dependent on interferon regulatory factor 3 and TRIF. Infect. Immun. 75: 1280–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Derbigny WA, Johnson RM, Toomey KS, Ofner S, Jayarapu K. 2010. The Chlamydia muridarum-induced IFN-beta response is TLR3-dependent in murine oviduct epithelial cells. J. Immunol. 185: 6689–6697 [DOI] [PubMed] [Google Scholar]
- 15. Derbigny WA, Kerr MS, Johnson RM. 2005. Pattern recognition molecules activated by Chlamydia muridarum infection of cloned murine oviduct epithelial cell lines. J. Immunol. 175: 6065–6075 [DOI] [PubMed] [Google Scholar]
- 16. Ebihara T, Shingai M, Matsumoto M, Wakita T, Seya T. 2008. Hepatitis C virus-infected hepatocytes extrinsically modulate dendritic cell maturation to activate T cells and natural killer cells. Hepatology 48: 48–58 [DOI] [PubMed] [Google Scholar]
- 17. Fahy OL, Townley SL, McColl SR. 2006. CXCL16 regulates cell-mediated immunity to Salmonella enterica serovar Enteritidis via promotion of gamma interferon production. Infect. Immun. 74: 6885–6894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujita H, Asahina A, Tada Y, Fujiwara H, Tamaki K. 2005. Type I interferons inhibit maturation and activation of mouse Langerhans cells. J. Invest. Dermatol. 125: 126–133 [DOI] [PubMed] [Google Scholar]
- 19. Gowen BB, et al. 2006. TLR3 deletion limits mortality and disease severity due to phlebovirus infection. J. Immunol. 177: 6301–6307 [DOI] [PubMed] [Google Scholar]
- 20. Guillot L, et al. 2005. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280: 5571–5580 [DOI] [PubMed] [Google Scholar]
- 21. Huber JP, Farrar JD. 2011. Regulation of effector and memory T-cell functions by type I interferon. Immunology 132: 466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang W, Pisetsky DS. 2008. The induction of HMGB1 release from RAW 264.7 cells by transfected DNA. Mol. Immunol. 45: 2038–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson RM. 2004. Murine oviduct epithelial cell cytokine responses to Chlamydia muridarum infection include interleukin-12-p70 secretion. Infect. Immun. 72: 3951–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kopp EB, Medzhitov R. 1999. The Toll-receptor family and control of innate immunity. Curr. Opin. Immunol. 11: 13–18 [DOI] [PubMed] [Google Scholar]
- 25. Kramp JC, McMurray DN, Formichella C, Jeevan A. 2011. The in vivo immunomodulatory effect of recombinant tumour necrosis factor-alpha in guinea pigs vaccinated with Mycobacterium bovis bacille Calmette-Guerin. Clin. Exp. Immunol. 165: 110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lacy HM, et al. 2011. Essential role for neutrophils in pathogenesis and adaptive immunity in Chlamydia caviae ocular infections. Infect. Immun. 79: 1889–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee EY, Lee ZH, Song YW. 2009. CXCL10 and autoimmune diseases. Autoimmun. Rev. 8: 379–383 [DOI] [PubMed] [Google Scholar]
- 28. Le Goffic R, et al. 2006. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2: e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li W, Katz BP, Spinola SM. 2011. Haemophilus ducreyi lipooligosaccharides induce expression of the immunosuppressive enzyme indoleamine 2,3-dioxygenase via type I interferons and tumor necrosis factor alpha in human dendritic cells. Infect. Immun. 79: 3338–3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lien E, Zipris D. 2009. The role of Toll-like receptor pathways in the mechanism of type 1 diabetes. Curr. Mol. Med. 9: 52–68 [DOI] [PubMed] [Google Scholar]
- 31. Ly LH, Jeevan A, McMurray DN. 2009. Neutralization of TNFα alters inflammation in guinea pig tuberculous pleuritis. Microbes Infect. 11: 680–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matsumoto M, et al. 2003. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J. Immunol. 171: 3154–3162 [DOI] [PubMed] [Google Scholar]
- 33. Matsumoto M, Kikkawa S, Kohase M, Miyake K, Seya T. 2002. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem. Biophys. Res. Commun. 293: 1364–1369 [DOI] [PubMed] [Google Scholar]
- 34. Matsumoto M, Seya T. 2008. TLR3: interferon induction by double-stranded RNA including poly(I:C). Adv. Drug Deliv. Rev. 60: 805–812 [DOI] [PubMed] [Google Scholar]
- 35. McCormack WM, et al. 1979. Fifteen-month follow-up study of women infected with Chlamydia trachomatis. N. Engl. J. Med. 300: 123–125 [DOI] [PubMed] [Google Scholar]
- 36. Medzhitov R, Janeway C., Jr 2000. The Toll receptor family and microbial recognition. Trends Microbiol. 8: 452–456 [DOI] [PubMed] [Google Scholar]
- 37. Mielke ME, Peters C, Hahn H. 1997. Cytokines in the induction and expression of T-cell-mediated granuloma formation and protection in the murine model of listeriosis. Immunol. Rev. 158: 79–93 [DOI] [PubMed] [Google Scholar]
- 38. Miller WC, et al. 2004. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA 291: 2229–2236 [DOI] [PubMed] [Google Scholar]
- 39. Mogensen KE, Lewerenz M, Reboul J, Lutfalla G, Uze G. 1999. The type I interferon receptor: structure, function, and evolution of a family business. J. Interferon Cytokine Res. 19: 1069–1098 [DOI] [PubMed] [Google Scholar]
- 40. Moller BR, et al. 1979. Chlamydia trachomatis infection of the Fallopian tubes: histological findings in two patients. Br. J. Vener. Dis. 55: 422–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muller U, et al. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264: 1918–1921 [DOI] [PubMed] [Google Scholar]
- 42. Nagarajan UM, et al. 2008. Type I interferon signaling exacerbates Chlamydia muridarum genital infection in a murine model. Infect. Immun. 76: 4642–4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ottenhoff TH, Verreck FA, Hoeve MA, van de Vosse E. 2005. Control of human host immunity to mycobacteria. Tuberculosis (Edinb.) 85: 53–64 [DOI] [PubMed] [Google Scholar]
- 44. Paavonen J. 1998. Pelvic inflammatory disease. From diagnosis to prevention. Dermatol. Clin. 16: 747–756, xii [DOI] [PubMed] [Google Scholar]
- 45. Paguirigan AM, Byrne GI, Becht S, Carlin JM. 1994. Cytokine-mediated indoleamine 2,3-dioxygenase induction in response to Chlamydia infection in human macrophage cultures. Infect. Immun. 62: 1131–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pasquinelli AE. 2002. MicroRNAs: deviants no longer. Trends Genet. 18: 171–173 [DOI] [PubMed] [Google Scholar]
- 47. Patton DL, et al. 1994. Detection of Chlamydia trachomatis in Fallopian tube tissue in women with postinfectious tubal infertility. Am. J. Obstet. Gynecol. 171: 95–101 [DOI] [PubMed] [Google Scholar]
- 48. Pyles RB, Jezek GE, Eaves-Pyles TD. 2010. Toll-like receptor 3 agonist protection against experimental Francisella tularensis respiratory tract infection. Infect. Immun. 78: 1700–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qiu H, et al. 2008. Type I IFNs enhance susceptibility to Chlamydia muridarum lung infection by enhancing apoptosis of local macrophages. J. Immunol. 181: 2092–2102 [DOI] [PubMed] [Google Scholar]
- 50. Rahm VA, Gnarpe H, Odlind V. 1988. Chlamydia trachomatis among sexually active teenage girls: lack of correlation between chlamydial infection, history of the patient and clinical signs of infection. Br. J. Obstet. Gynaecol. 95: 916–919 [DOI] [PubMed] [Google Scholar]
- 51. Rank RG, Whittimore J, Bowlin AK, Dessus-Babus S, Wyrick PB. 2008. Chlamydiae and polymorphonuclear leukocytes: unlikely allies in the spread of chlamydial infection. FEMS Immunol. Med. Microbiol. 54: 104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reiter Z. 1993. Interferon: a major regulator of natural killer cell-mediated cytotoxicity. J. Interferon Res. 13: 247–257 [DOI] [PubMed] [Google Scholar]
- 53. Rossi DL, et al. 1999. Lungkine, a novel CXC chemokine, specifically expressed by lung bronchoepithelial cells. J. Immunol. 162: 5490–5497 [PubMed] [Google Scholar]
- 54. Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. 2005. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J. Virol. 79: 3350–3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rudd BD, et al. 2006. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J. Immunol. 176: 1937–1942 [DOI] [PubMed] [Google Scholar]
- 56. Schachter J. (ed). 1980. Chlamydiae (psittacosis-lymphogranuloma venereum-trachoma group), 3rd ed. American Society for Microbiology, Washington, DC [Google Scholar]
- 57. Schmitz JM, McCracken VJ, Dimmitt RA, Lorenz RG. 2007. Expression of CXCL15 (Lungkine) in murine gastrointestinal, urogenital, and endocrine organs. J. Histochem. Cytochem. 55: 515–524 [DOI] [PubMed] [Google Scholar]
- 58. Shepard MK, Jones RB. 1989. Recovery of Chlamydia trachomatis from endometrial and fallopian tube biopsies in women with infertility of tubal origin. Fertil. Steril. 52: 232–238 [DOI] [PubMed] [Google Scholar]
- 59. Siren J, Pirhonen J, Julkunen I, Matikainen S. 2005. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J. Immunol. 174: 1932–1937 [DOI] [PubMed] [Google Scholar]
- 60. Stephens RS. 2003. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 11: 44–51 [DOI] [PubMed] [Google Scholar]
- 61. Suh HS, et al. 2009. TLR3 and TLR4 are innate antiviral immune receptors in human microglia: role of IRF3 in modulating antiviral and inflammatory response in the CNS. Virology 392: 246–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van den Brule AJ, et al. 2002. Prevalence and persistence of asymptomatic Chlamydia trachomatis infections in urine specimens from Danish male military recruits. Int. J. STD AIDS 13(Suppl 2): 19–22 [DOI] [PubMed] [Google Scholar]
- 63. Wang T, et al. 2004. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 10: 1366–1373 [DOI] [PubMed] [Google Scholar]
- 64. Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80: 5059–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Weigert A, et al. 2010. Cleavage of sphingosine kinase 2 by caspase-1 provokes its release from apoptotic cells. Blood 115: 3531–3540 [DOI] [PubMed] [Google Scholar]
- 66. Westrom L, Mardh PA. 1984. Current views on the concept of pelvic inflammatory disease. Aust. N Z J. Obstet. Gynaecol. 24: 98–105 [DOI] [PubMed] [Google Scholar]
- 67. Wong MM, Fish EN. 2003. Chemokines: attractive mediators of the immune response. Semin. Immunol. 15: 5–14 [DOI] [PubMed] [Google Scholar]
- 68. Wyrick PB. 2000. Intracellular survival by Chlamydia. Cell Microbiol. 2: 275–282 [DOI] [PubMed] [Google Scholar]
- 69. Yang X, Brunham RC. 1998. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J. Immunol. 161: 1439–1446 [PubMed] [Google Scholar]
- 70. Zhang SY, et al. 2008. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol. Rev. 226: 29–40 [DOI] [PubMed] [Google Scholar]