Abstract
Infection with Salmonella spp. is a significant source of disease globally. A substantial proportion of these infections are caused by Salmonella enterica serovar Typhimurium. Here, we characterize the role of the enterobacterial common antigen (ECA), a surface glycolipid ubiquitous among enteric bacteria, in S. Typhimurium pathogenesis. Construction of a defined mutation in the UDP-N-acetylglucosamine-1-phosphate transferase gene, wecA, in two clinically relevant strains of S. Typhimurium, TML and SL1344, resulted in strains that were unable to produce ECA. Loss of ECA did not affect the gross cell surface ultrastructure, production of lipopolysaccharide (LPS), flagella, or motility. However, the wecA mutant strains were attenuated in both oral and intraperitoneal mouse models of infection (P < 0.001 for both routes of infection; log rank test), and virulence could be restored by complementation of the wecA gene in trans. Despite the avirulence of the ECA-deficient strains, the wecA mutant strains were able to persistently colonize systemic sites (spleen and liver) at moderate levels for up to 70 days postinfection. Moreover, immunization with the wecA mutant strains provided protection against a subsequent lethal oral or intraperitoneal challenge with wild-type S. Typhimurium. Thus, wecA mutant (ECA-negative) strains of Salmonella may be useful as live attenuated vaccine strains or as vehicles for heterologous antigen expression.
INTRODUCTION
Salmonella enterica serovars are enteropathogens that display a broad range of host specificities and are common causes of gastroenteritis worldwide (47, 48). In the United States alone, nontyphoidal Salmonella isolates cause an estimated 1.4 million cases of salmonellosis annually (12, 48), which results in up to $50 million per year in medical expenses and work absences (9). More than 2,500 different serotypes of Salmonella have been implicated in diarrheal disease (4, 12, 58); however, the majority of enteric salmonellosis cases are caused by a small subset of these serotypes (12, 16, 58), including Salmonella enterica serovar Typhimurium. Infection with S. Typhimurium can result in a debilitating inflammatory diarrhea that is often accompanied by fever, malaise, vomiting, muscle aches, and abdominal cramps (47).
Salmonella serotypes that cause diarrheal disease are ingested in contaminated food and water and colonize the small intestine during passage through the digestive tract. Entry into the host intestinal tissue is thought to occur preferentially via antigen-sampling microfold (M) cells, although these pathogens can also invade enterocytes (28). Prior to M-cell entry, however, Salmonella must first survive/evade host defenses, such as the low pH of the stomach, bile salts, and various other innate immune mechanisms (16).
A subset of the molecules that permit survival of bacteria in these initial steps in pathogenesis are located on the bacterial cell surface. The expression of these bacterial surface components has also been associated with virulence. Multiple reports have demonstrated that Salmonella is less virulent in the absence of cell surface proteins, such as OmpS, lipoproteins, and flagella (10, 13, 17, 34, 36, 54). Similarly, attenuation of virulence also occurs in the absence of bacterial surface polysaccharides, such as lipopolysaccharide (LPS) (20). Recently, these studies have been corroborated utilizing a screen for genes that contribute to the ability of S. Typhimurium to establish an infection; several of the genes identified are involved in cell surface polysaccharide biosynthesis (14). Moreover, the role of LPS in resistance to antimicrobial effector molecules, systemic disease, and induction of proinflammatory cytokines has been well characterized (8, 19, 22, 25, 35, 53, 56). In contrast, the contributions of other major cell surface polysaccharides to Salmonella virulence have not been as clearly delineated.
The cell surface of Gram-negative enteric bacteria contains an additional glycolipid that is ubiquitous among members of the family Enterobacteriaceae, the phosphoglyceride-linked enterobacterial common antigen (ECAPG) (52). The carbohydrate component of ECAPG is a heterotrimeric repeat, →3-α-d-Fuc4NAc-(1→4)-β-d-ManNAcA-(1→4)-α-d-GlcNAc, where Fuc4NAc is 4-acetamido-4,6-dideoxy-d-galactose, ManNAcA is N-acetyl-d-mannosaminuronic acid, and GlcNAc is N-acetyl-d-glucosamine (18, 51). ECA polysaccharide chains are linked to diacylglycerol through phosphodiester linkage through the potentially reducing terminal GalNAc residue, and the phosphoglyceride anchors the molecules in the outer leaflet of the outer membrane (52). ECAPG accounts for approximately 0.2% of the cellular dry weight of the bacterial cell (52). Two related forms (ECALPS and ECACYC) have also been described (18), but ECALPS, which is linked to the lipid A core region, is present on only a subset of enteric bacteria, and ECACYC is not surface expressed (18, 52). The genes involved in ECAPG biosynthesis are chromosomally encoded in the wec gene cluster (also known as rfe) (32, 39, 52) and include wecA through wecG, as well as the wzx, wzy, and wzz genes, which are involved in the addition of the ECA polysaccharide chains to the lipid carrier and transfer of ECAPG to the bacterial cell surface (6, 49). Although the structure and biochemical composition of ECAPG has been well characterized, relatively few studies have investigated the biological function of this major cell surface glycolipid, and a conclusive role for ECAPG in vivo has yet to be elucidated.
Previous studies suggest that ECAPG (referred to below as ECA) may contribute to organic acid resistance in Shiga toxin-producing Escherichia coli O157:H7 (7). In addition, ECA may play a role in the virulence of Yersinia enterocolitica (64) and was shown to be linked to pustule formation in Haemophilus ducreyi infections (5). In the context of Salmonella, several intriguing observations have been made: (i) Mayer and Schmidt (37) showed that S. Typhimurium ECA does not elicit endotoxin-like activity, (ii) passive immunization with anti-ECA antibodies does not protect against salmonellosis (1), and (iii) ECA plays a role in the resistance of bacteria to host bile salts (41). Moreover, studies in the 1970s, using poorly defined mutations, reported that the virulence of ECA mutant strains of S. Typhimurium is attenuated in mice (61). Ramos-Morales et al. (49) described a role for two ECA-specific loci (wecA and wecD) in bile resistance, as well as virulence. Most recently, a comprehensive study by Chaudhuri et al. (14), which identified S. Typhimurium genes required for infection of BALB/c mice, showed that among transposon mutations that were ranked by the severity of their attenuation, several genes within the ECA biosynthesis (wec) gene cluster, including wecA, wecB, wecE, wecD, and wecC, were found to have high attenuation scores (14). Collectively, these data suggest that ECA may play a broad role in bacterial virulence, and taken together with the studies presented here, they suggest that ECA is important for Salmonella pathogenesis.
The results presented in this paper describe a thorough in vitro and in vivo characterization of ECA mutant strains (wecA::Gm) of S. Typhimurium. Our studies demonstrate that disruption of the wecA locus results in abrogation of ECA production and that the resulting strains are attenuated in vivo. Importantly, these wecA-null strains are not cleared by the host but establish a persistent infection. Moreover, the wecA mutants can protect against a subsequent lethal challenge in a well-established murine model of salmonellosis. Our results highlight the possibility of using ECA-negative strains of Salmonella as live attenuated vaccine strains. Furthermore, the capacity of the ECA mutants to establish a persistent infection suggests the possibility of using ECA-negative strains of S. Typhimurium as vehicles for heterologous antigen delivery.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. Bacterial stocks were maintained in LB broth plus 40% glycerol at −80°C. Bacteria were routinely grown as indicated at 37°C in LB or Trypticase soy (Difco) broth (TSB) or on LB or Trypticase soy agar containing 12.5 μg/ml tetracycline and/or 20 μg/ml gentamicin (Gm) where applicable. Plasmids and PCR products were purified using Qiagen reagents. Growth kinetics were measured in either TSB or minimal N medium (42) supplemented with 0.1% Casamino Acids.
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Plasmids | ||
| pTOPO-TA | Cloning vector | Invitrogen |
| pTA-BRABR1 | pTOPO-TA::wecA | This study |
| pBR322 | Cloning vector | Promega |
| pRL140 | pBR322::wecA | This study |
| pUC-Gm | pUC::Gm | This study |
| pRL144 | pBR322::wecA::Gm | This study |
| pMAK705ts | Temperature-sensitive vector; Cmr | This study |
| pRL145 | pMAK705ts::wecA::Gm | This study |
| pACYC184 | Complementation vector; Tetr | 55 |
| pGEM-T Easy | Commercial cloning vector; Ampr | Promega |
| pDSM643 | pGEM-T Easy::wecA; Ampr | This study |
| pDSM647 | pACYC184::wecA; Tetr | This study |
| E. coli strains | ||
| Top10 | Cloning strain | Laboratory strain |
| DH5α | Cloning strain | Laboratory strain |
| Top10F′ | Cloning strain; F+ | Laboratory strain |
| PR4131 | E. coli DH5α(pRL140) | This study |
| PR4135 | E. coli DH5α(pRL144) | This study |
| PR4136 | E. coli Top10F′(pRL145) | This study |
| S. enterica strains | ||
| DSM644 | Wild-type S. Typhimurium TML | 23 |
| DSM645 | TML ΔwecA; Gentr | This study |
| DSM649 | TML ΔwecA (pDSM647) Gentr Tetr | This study |
| DSM753 | Wild-type S. Typhimurium SL1344 | 62 |
| DSM754 | SL1344 ΔwecA; Gentr | This study |
| DSM732 | SL1344 ΔwecA (pDSM647) Gentr Tetr | This study |
Construction of wecA::Gm mutant strains.
S. Typhimurium ECA mutant strains (wecA::Gm) were constructed in both TML and SL1344 wild-type backgrounds. A 1.5-kb fragment containing the wecA gene was PCR amplified from genomic DNA from the wild-type TML strain using primers BRA (5′-TACCCGTAAAGAAGAGCTGCTCACC-3′) and BR (5′-CAGCCCATAAAGTACGAAACAACCC-3′). This product was cloned into pTOPO-TA to yield plasmid pTA-BRABR1. Insertion of the wecA gene was confirmed by restriction digestion and sequencing. The 1.5-kb fragment containing the wecA gene was liberated from pTA-BRABR1 by EcoRI digestion and subsequently cloned into the EcoRI site of pBR322 to yield plasmid pRL140. An 800-bp Gm resistance cassette was liberated from plasmid pUC-GM using XbaI and then cloned into an SpeI site within the coding region of the wecA gene contained within pRL140 to create pRL144 (wecA::Gm). A 3.07-kb fragment containing the wecA::Gm cassette was then digested from pRL144 using PstI and ClaI and subsequently cloned into the PstI and ClaI sites in pMAK705ts to create plasmid pRL145. pRL145 was electroporated into TML and SL1344, and transformants were selected on LB containing Gm at 30°C. Single-colony Gmr transformants were subcultured in Gm and incubated overnight at 44°C to promote plasmid loss; growth would occur only if the wecA::Gm cassette had recombined into the bacterial chromosome. Gentamicin-resistant strains were passaged multiple times at 44°C to ensure complete loss of the vector pMAK705ts::wecA::Gm. Gmr/Cms colonies were then selected and screened by PCR to confirm the mutation of wecA. To ensure that insertion of the gentamicin resistance cassette into wecA did not abrogate expression of the downstream gene (wzzE), we performed semiquantitative reverse transcription PCR for both TML and SL1344 wild-type and wecA mutant strains. Expression of wzzE in the wecA mutant strains was similar to that in the respective wild-type strains (data not shown). Mutant strains were further characterized as described below.
Complementation of the wecA mutation.
The wecA gene and promoter from the wild-type strain TML was PCR amplified using high-fidelity Phusion enzyme (Finnzymes, Espoo, Finland) and primers WecAF1 (5′-gcggccgcCCTGACTATCATCGCGACGGC-3′) and WecAR1 (5′-ccgcggCATTTTCAGCGCTCACCGCGCG-3′), which incorporate a NotI and SacII site, respectively (the restriction enzyme recognition sites are indicated by lowercase letters). The 1,715-bp product was gel purified, 3′ A overhangs were added, and the full-length fragment was subcloned into pGemT-Easy (Promega, Madison, WI) to create plasmid pDSM643. The correct insert size was confirmed by EcoRI digestion, and the digested product was subsequently cloned into the EcoRI site of pACYC184 (55) to create plasmid pDSM647. The complementation plasmid pDSM647 was transformed into wecA-null strains DSM645 and DSM754 in order to create strains DSM649 and DSM732, respectively. Transformants were selected on 12.5 μg/ml tetracycline, and complementation of ECA production was verified as described below.
In vitro characterization of wecA::Gm and wecA complementation strains.
Serotyping of wild-type and wecA mutant strains was performed with Salmonella strain-specific O:4 and O:9 antisera (Difco Laboratories, BD Systems, Sparks, MD) according to the manufacturer's instructions. ECA phenotypes were characterized by passive hemagglutination and immunoblotting. Passive hemagglutination was conducted using a 1:320 dilution of anti-ECA antibody as previously described (50). ECA immunoblotting and LPS/O-antigen silver staining were conducted as follows. Whole-cell lysates were prepared as previously described (27). Briefly, 2 optical density (OD) units/ml of overnight culture were pelleted, resuspended in 200 μl of lysis buffer (0.065 M Tris-HCl [pH 6.8], 2% [wt/vol] SDS 5% [vol/vol] β-mercaptoethanol, 10% [vol/vol] glycerol, 0.05% [wt/vol] bromophenol blue), and incubated at 95°C for 15 min. The lysates were cooled to room temperature and treated with 20 μg proteinase K (Ambion) at 60°C for 1 h. Equal volumes of each lysate were subjected to 12% SDS-PAGE using a 4% stacking layer and subsequently stained using the method of Tsai and Frasch (60). For preparation of immunoblots, non-proteinase K-treated lysates were prepared as described above and transferred at a constant current of 200 mA for 3 h using a semidry transfer apparatus (Thermo Scientific, Rochester, NY). Membranes were probed with a 1:10,000 dilution of murine anti-ECA monoclonal antibody 898 (1, 27). ECA glycoconjugates were detected using a 1:20,000 dilution of goat anti-mouse IgG conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA). Blots were developed using the SuperSignal West Pico chemiluminescent substrate kit (Thermo Scientific/Pierce) and imaged using a LAS-3000 Intelligent Dark Box with LAS-3000 Lite capture software (Fujifilm, Stamford, CT).
Electron microscopy.
Transmission electron microscopy was performed at the Biomedical Instrumentation Center (BIC) of the Uniformed Services University of the Health Sciences (USUHS). Overnight stationary-phase cultures were harvested by centrifugation, fixed with 2% electron microscopy (EM) grade glutaraldehyde fixation solution, and examined using a Phillips CM100 electron microscope without staining.
Motility assays.
Swimming and swarming motility assays were performed as previously described (11, 59). Briefly, LB plates containing 0.25% (swimming motility) or 0.5% (swarming motility) agar were inoculated with each strain, as indicated. For swimming motility assays, a single colony of each strain was inoculated in the center of each plate and incubated overnight at room temperature. To analyze swarming motility, the LB-based plates contained 0.5% glucose, and each strain was inoculated in the center of each plate from liquid cultures and incubated overnight at 37°C.
In vivo characterization experiments.
Animal experiments were approved by the USUHS Animal Care and Use Committee and were performed in accordance with guidelines set forth by the National Institutes of Health (NIH). S. Typhimurium strains were cultured overnight at 37°C in TSB with shaking at 225 rpm. Overnight cultures were diluted ∼1:100 and grown to exponential phase (optical density at 600 nm, ∼0.6). Bacterial cultures were harvested by centrifugation, standardized to a density of 0.6 OD unit/ml in isotonic saline, and subsequently diluted to the appropriate dosage as indicated in the text. Doses of bacterial suspensions were administered in isotonic saline at a volume of 0.2 ml for oral gavage or at a volume of 0.5 ml for intraperitoneal (i.p.) injection. Serial dilutions of each inoculum were plated to confirm the dose. Female C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were used for all experiments. For oral infections only, animals were fasted for 4 h prior to inoculation and for 1 h postinoculation. The animals were monitored daily for mortality and onset of end-stage disease symptoms.
Bacterial loads at systemic sites.
To assess the bacterial loads at systemic sites, mice were inoculated with 1 × 104 CFU of wild-type or wecA-null strains as indicated. Spleen and liver tissues were harvested at days 3, 5, 7, 10, 14, 21, 28, 35, 45, 48, 56, 63, and 70 postinfection. Each tissue was homogenized, and an aliquot was serially diluted and plated to determine bacterial counts.
In vivo attenuation and complementation of the wecA mutation.
Mice were inoculated with the TML wild-type, wecA mutant, or wecA complemented strain (DSM644, DSM645, and DSM649, respectively) at a dose of 1 × 105, 1 × 106, or 1 × 107 CFU by oral gavage. The i.p. infections were performed using 1 × 102 CFU of each bacterial strain, as indicated. Animals were monitored daily for mortality and onset of end-stage disease symptoms. Where indicated, the mean time to death (MTD) was calculated as follows: MTD is equal to the sum of all x values divided by the total number of mice dead, where x is the number of days postinfection multiplied by the number of mice that died that day.
Immunization and lethal challenge studies.
Immunizations with wecA mutant strains were administered by oral gavage or i.p. injection and were given either 30 or 60 days prior to lethal challenge, as detailed in the text for each experiment. Vaccination doses were as indicated in the text for each individual experiment. The postvaccination oral and i.p. lethal challenges were performed as described above.
Statistical analyses.
Statistical analyses for colonization experiments were performed using a two-tailed Student's t test. The statistical significance of differences in percent survival for oral and i.p. infection experiments was determined using the Kaplan-Meier method and a log rank test as indicated in the text.
RESULTS
In vitro characterization of S. Typhimurium wecA mutant strains.
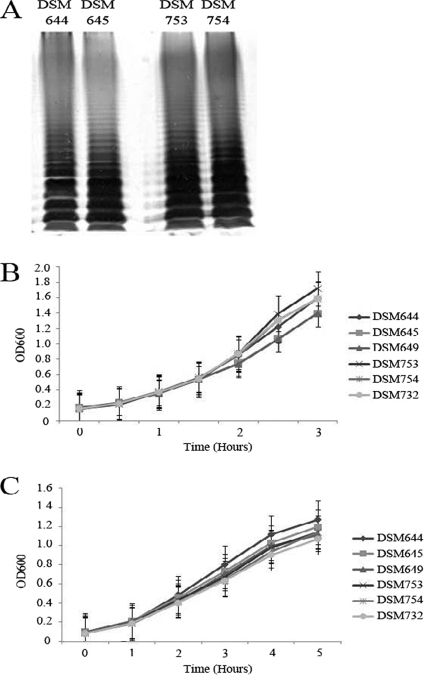
Previous studies implicated ECA as a virulence factor of S. Typhimurium and indicated that wecA mutants are attenuated in vivo (49, 61). However, the mutant strains used in those studies were poorly defined (11) or the in vivo experiments with those strains were conducted only in competition with the wild-type strain (49). Furthermore, complementation of the mutant strains was not attempted in any of these studies. Thus, the observed mutant phenotypes could not be conclusively linked solely to the absence of ECA expression. Therefore, to clearly delineate the role of ECA as a virulence factor, we analyzed well-defined ECA mutants in a series of in vitro and in vivo studies. We created isogenic wecA mutant strains in two clinically relevant S. Typhimurium strain backgrounds, TML (DSM644) (23) and SL1344 (DSM753) (62), as described above. The wecA gene encodes a UDP-N-acetylglucosamine-1-phosphate transferase and is the first gene in a gene cluster that also encodes the downstream enzymatic components of the ECA biosynthetic pathway. Since WecA catalyzes the first step in ECA biosynthesis, we created a nonpolar mutation in the gene to eliminate the possibility of changes in the bacterial cell due to the build-up of ECA intermediates. Deletion of the wecA gene resulted in an ECA-negative phenotype, as determined by passive hemagglutination (Table 2) and immunoblotting (data not shown). Because components of the ECA biosynthetic pathway are also known to be involved in LPS synthesis in some bacteria (52, 64) and because LPS is important for Salmonella colonization (33, 43), we wanted to ensure that non-ECA cell surface polysaccharides were unaltered in our wecA mutant strains. To this end, DSM645 (TML wecA::Gm) and DSM754 (SL1344 wecA::Gm) were serotyped using S. Typhimurium O:4 antisera to ensure that our S. Typhimurium strains retained expression of the wild-type O:4 antigen (Table 2). In addition, the LPS/O-antigen profile of the wecA mutant and wild-type strains was evaluated by silver staining. As depicted in Fig. 1A, the wecA mutant strains (DSM645 and DSM754) retained LPS profiles indistinguishable from those of the respective parental wild-type strains. Furthermore, analysis of cell surface structure by transmission electron microscopy revealed no gross abnormalities in the wecA mutant strains (see Figure S1 in the supplemental material).
Table 2.
Serotyping and ECA passive hemagglutination
| Strain | Genotype | ECAa | PHAb | Serotypingc |
|
|---|---|---|---|---|---|
| O:4 | O:9 | ||||
| Saline control | NA | − | − | − | |
| TML | |||||
| DSM644 | WT | + | + | + | − |
| DSM645 | wecA::Gm | − | − | + | − |
| DSM649 | wecA::Gm Complemented | + | + | NT | NT |
| SL1344 | |||||
| DSM753 | WT | + | + | + | − |
| DSM754 | wecA::Gm | − | − | + | − |
| DSM732 | wecA::Gm Complemented | + | + | NT | NT |
NA, not applicable; +, positive; −, negative.
PHA, passive hemagglutination; +, hemagglutination; −, no hemagglutination.
NT, not tested; +, expressed; −, not expressed.
Fig 1.
In vitro characterization of wecA mutant and complementation strains of S. enterica serovar Typhimurium. (A) LPS/O-antigen profiles of TML wild-type (DSM644), TML wecA mutant (DSM645), SL1344 wild-type (DSM753), and SL1344 wecA mutant (DSM754) strains. Silver staining was performed as described in Materials and Methods. (B and C) Growth kinetics of TML wild-type (DSM644), TML wecA mutant (DSM645), and TML wecA complementation (DSM649) strains and growth kinetics of SL1344 wild-type (DSM753), SL1344 wecA mutant (DSM754), and SL1344 wecA complementation (DSM732) strains in TSB (B) and minimal N medium (C). Overnight cultures were diluted to an optical density of ∼0.1 and incubated at 37°C with shaking. Growth was monitored by the OD at 600 nm (OD600). The data points shown are the averages of 2 or 3 independent experiments, and the error bars represent the standard errors of the mean.
To ensure that the ECA-negative phenotype of DSM645 and DSM754 was due to mutation of the wecA gene, wecA complementation strains were constructed as described in Materials and Methods. Functional complementation of the ECA-negative phenotype was observed in both DSM649 (TML wecA::Gm with or without pACYC184::wecA) and DSM732 (SL1344 wecA::Gm with or without pACYC184::wecA) by both passive hemagglutination (Table 2) and immunoblotting (data not shown). The in vitro growth of the wecA mutant and complementation strains was comparable to that of the parental wild-type strains (Fig. 1B and C), with the exception of a slight but reproducible difference in the growth of the TML wild-type and mutant strains in rich media; however, the growth kinetics were more similar in minimal media, which may more accurately represent in vivo growth conditions.
Recently, ECA biosynthesis was shown to be a checkpoint for flagellar biosynthesis in another enteric bacterium, Serratia marcescens (11). Since previous studies have shown a link between the presence of flagella and virulence in S. Typhimurium (10), we sought to determine whether ECA expression was linked to S. Typhimurium flagellar biosynthesis. To this end, we determined whether the S. Typhimurium wecA mutant strains displayed any defects in production of flagella or motility. As shown in Fig. S1 in the supplemental material, the wecA mutant strains (DSM645 and DSM754) appear to retain normal numbers and localization of flagella. Furthermore, the wecA mutant strains appear to retain wild-type levels of swimming motility (see Fig. S2 in the supplemental material). Similarly, the mutant and complementation strains retained the ability to swarm (see Fig. S2 in the supplemental material). In some replicates of the assay, the swarming speeds of the mutant strains appeared slightly slower than those of the parental wild-type strains when assessed at early time points (data not shown). However, after 24 h of growth, the mutant strains were able to swarm to the edge of the plate as well as the wild-type strains. Collectively, our in vitro data suggest that the wecA-null strains are both genetically and functionally isogenic with their respective parental wild-type strains, except for the absence of ECA.
wecA mutant strains are attenuated in vivo.
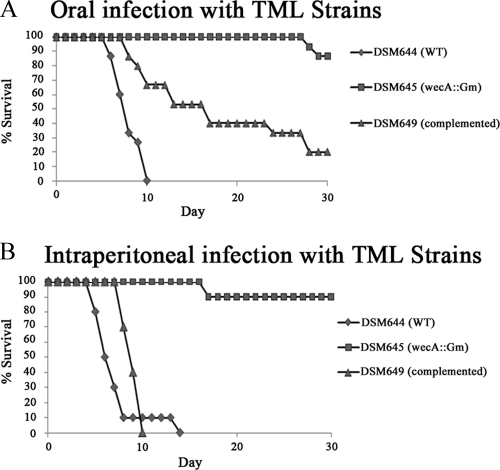
While previous reports implied that ECA may play a role in S. Typhimurium virulence (49, 61), those studies utilized poorly defined mutants, competitive indices, or short-term infection studies. Although the reports were suggestive, we wished to definitively establish an in vivo role for ECA using the more robust single-strain model of infection. To this end, we inoculated five C57BL/6 mice per group with 1 × 105, 1 × 106, or 1 × 107 CFU of the TML wild-type (DSM644) or wecA mutant (DSM645) strain by oral gavage. As depicted in Fig. 2A, all 15 of the mice infected with the wild-type TML strain (DSM644) succumbed to infection by day 10, with an MTD of 7.1 days. The MTDs for animals infected with 1 × 105, 1 × 106, and 1 × 107 CFU were 7.4, 7.4, and 6.4 days, respectively (see Fig. S3 in the supplemental material). These data are similar to those shown previously with the same strain of S. Typhimurium and the same strain of mice (44). In contrast, only 2 of the 15 mice infected with the wecA mutant strain DSM645 succumbed to infection (∼87% survival), with an MTD of 27.5 days (1 animal infected with 1 × 106 CFU died on day 28, and 1 animal infected with 1 × 107 CFU died on day 27 [see Fig. S3 in the supplemental material]). This difference in percent survival was highly significant (P < 0.001; log rank test). In addition, complementation of the wecA mutation in trans partially restored virulence; 20% of the mice infected with the wecA complementation strain (DSM649) survived for the duration of the experiment, with an MTD of 13.6 days (Fig. 2A). The MTDs for animals infected with 1 × 105, 1 × 106, and 1 × 107 CFU of DSM649 were 11.5, 18.6, and 12.2 days, respectively. The difference in percent survival between the wecA mutant and wecA complementation strains was also significant (P < 0.001; log rank test). In addition, the difference in percent survival between the wild-type and complemented strains was also significant (P < 0.001; log rank test), which highlights the fact that only partial complementation was achieved.
Fig 2.
Attenuation and complementation of the TML wecA mutant strain. (A) Groups of 5 C57BL/6 mice were orally inoculated with 105, 106, or 107 CFU of the TML wild-type (DSM644), TML wecA mutant (DSM645), or TML wecA complementation (DSM649) strain. The percent survival data for each strain represent combined data from all inoculation doses tested. (B) Groups of 8 to 10 C57BL/6 mice were intraperitoneally inoculated with 102 CFU of the TML wild-type (DSM644), TML wecA mutant (DSM645), or TML wecA complementation (DSM649) strain. The percent survival data for each strain represent combined data from two biologically independent experiments. The differences in percent survival between the wild-type and wecA mutant strains, and wecA mutant and complementation strains, are statistically significant (P < 0.0001; log rank test) for both panels A and B. The percent survival for each of the individual doses is shown in Fig. S3 in the supplemental material.
We next tested the virulence of the wecA mutant strain (DSM645) in the murine single-strain i.p. infection model. C57BL/6 mice were inoculated with 1 × 102 CFU of either the wild-type strain DSM644 or the wecA mutant strain DSM645 (Fig. 2B). Whereas all of the mice infected with the wild-type strain succumbed to infection (MTD, 5.4 days), only 1 mouse infected with the wecA mutant strain died at day 16 postinfection (90% survival). These results were similar to those in the oral infection experiments, where the differences in percent survival were highly significant (P < 0.001; log rank test). Once again, complementation of wecA in trans restored virulence; all mice infected with DSM649 succumbed to infection (MTD, 7.3 days). The difference in survival between the wecA mutant and complemented strains was significant (P < 0.001; log rank test). Similar to the oral infection experiments, the difference in percent survival between the wild-type and complemented strains was also significant (P = 0.008; log rank test), indicating partial complementation. Collectively, these data indicate that the wecA mutant strain is significantly attenuated in both the oral and i.p. single-strain infection models and that the attenuation of virulence is due to the loss of ECA expression, since virulence could be at least partially restored upon complementation of the wecA mutation.
wecA mutant strains establish a persistent infection.
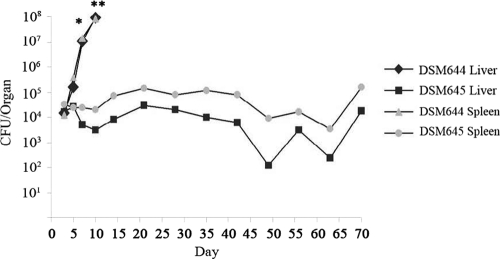
A key component of Salmonella virulence is the ability to establish colonization and persist within the host (16, 24, 46). Given the dramatic changes in lethality we observed with the ECA mutant strain, we next sought to determine whether the wecA mutant strain retained the ability to efficiently disseminate and colonize within the host. Groups of mice received 1 × 104 CFU of the TML wild-type (DSM644) or wecA mutant (DSM645) strain by oral gavage and were sacrificed at 3- to 5-day intervals postinfection (Fig. 3). The spleen and liver were harvested from each mouse, and aliquots of homogenized tissue were serially diluted and plated to determine bacterial counts. Mice infected with the wild-type TML (DSM644) bacteria succumbed to infection within 10 days postinfection. In contrast, mice infected with the wecA mutant strain (DSM645) survived until the experiment was terminated at day 70 postinfection. Moreover, bacterial counts from mice infected with the wild-type bacteria (DSM644) rapidly increased until host death, whereas mice infected with the wecA mutant strain (DSM645) displayed a moderate level of persistent infection throughout the experiment (Fig. 3). This persistent infection was accompanied by splenomegaly and hepatomegaly (data not shown), which often typify persistent S. Typhimurium colonization (41). These data demonstrate that the wecA mutant strain of S. Typhimurium is able to efficiently colonize mice and establish a persistent infection.
Fig 3.
Bacterial loads at systemic sites of infection. C57BL/6 mice were orally inoculated with 104 CFU of the TML wild type (DSM644) or TML wecA mutant (DSM645). Groups of 7 or 8 mice were sacrificed on days 3, 5, 7, 10, 14, 21, 28, 35, 45, 48, 56, 63, and 70 postinfection. Bacterial counts from liver and spleen were determined by plating serially diluted tissue homogenates and are shown as the geometric mean log10 CFU per organ. Student's t test was used to compare the colonization levels of TML and TML wecA mutant strains for each tissue site. *, P < 0.05; **, not enough animals remained to do statistical tests for the time point.
Oral immunization protects against a subsequent oral challenge.
Since the virulence of the wecA mutant strain was significantly attenuated but the strain was still able to establish a persistent infection, we next sought to determine whether immunization/vaccination with the mutant strains could induce a protective immune response. To this end, mice were orally immunized with 1 × 102, 1 × 103, 1 × 104, 1 × 105, 1 × 106, or 1 × 107 CFU of the TML wecA mutant strain DSM645. On day 30 postimmunization, the mice were challenged orally with 1 × 103 CFU of the wild-type TML strain DSM644 (Table 3). Of the 78 total mice vaccinated, 66 survived until the day of challenge; of the 66 mice that were challenged with wild-type S. Typhimurium, 41 mice (62.1%) were protected for the duration of the experiment (30 days).
Table 3.
Oral immunization with ECA mutants and subsequent oral challenge at day 30 with wild-type S. Typhimurium strainsa
| Background | Immunization |
Challenge |
|||||
|---|---|---|---|---|---|---|---|
| Strain | Dose | No. survived/total | Strain | Dose | No. survived/total | % Survival | |
| Control | Saline | NA | 7/7b | DSM644 | 1.0 × 103 | 0/7b | 0 |
| Saline | NA | 4/4 | DSM644 | 1.7 × 105 | 0/4 | 0 | |
| Saline | NA | 4/4 | DSM644 | 1.7 × 106 | 0/4 | 0 | |
| TML | DSM645 | 1.0 × 102 | 3/3 | DSM644 | 1.0 × 103 | 3/3 | 100 |
| DSM645 | 1.0 × 103 | 14/15b | DSM644 | 1.0 × 103 | 7/14b | 50 | |
| DSM645 | 1.0 × 104 | 13/15b | DSM644 | 1.0 × 103 | 9/13b | 69.2 | |
| DSM645 | 1.0 × 105 | 14/15b | DSM644 | 1.0 × 103 | 8/14b | 57.1 | |
| DSM645 | 1.0 × 106 | 11/15b | DSM644 | 1.0 × 103 | 7/11b | 63.6 | |
| DSM645 | 1.0 × 107 | 11/15b | DSM644 | 1.0 × 103 | 7/11b | 63.6 | |
| Total | 66/78 | 41/66 | 62.1 | ||||
| SL1344 | DSM754 | 1.3 × 104 | 8/8 | DSM753 | 1.7 × 105 | 4/8 | 50 |
| DSM754 | 1.3 × 104 | 8/8 | DSM753 | 1.7 × 106 | 5/8 | 62.5 | |
| DSM754 | 1.3 × 105 | 7/7 | DSM753 | 1.7 × 105 | 4/7 | 57.1 | |
| DSM754 | 1.3 × 105 | 8/8 | DSM753 | 1.7 × 106 | 6/8 | 75 | |
| DSM754 | 1.3 × 106 | 10/10 | DSM753 | 1.7 × 105 | 6/10 | 60 | |
| DSM754 | 1.3 × 106 | 10/10 | DSM753 | 1.7 × 106 | 6/10 | 60 | |
| Total | 51/51 | 31/51 | 60.7 | ||||
| Total | 117/129 | 72/117 | 61.5 | ||||
DSM644, TML wild type; DSM645, TML wecA mutant; DSM753, SL1344 wild type; DSM754, SL1344 wecA mutant.
Combined results from 2 independent experiments.
To ensure that the capacity of the wecA mutant strain to protect mice against a subsequent lethal challenge with wild-type bacteria was not S. Typhimurium strain dependent, we conducted a similar experiment with the S. Typhimurium wild-type strain SL1344. Mice were orally immunized with 1 × 104, 1 × 105, or 1 × 106 CFU of the SL1344 wecA mutant strain DSM754; 30 days postimmunization, groups of vaccinated mice were challenged orally with 1 × 105 or 1 × 106 CFU of the SL1344 wild-type strain DSM753. As shown in Table 3, all 51 mice immunized with the SL1344 wecA mutant strain survived until the day of challenge. Moreover, 31 of the 51 challenged mice were fully protected against subsequent lethal challenge; the percent survival was similar across all dosage regimens. Although a higher percentage of mice vaccinated with the SL1344 mutant strain survived until the day of challenge, the degree of protection was similar to that of groups immunized with the TML wecA mutant: 62.1% versus 60.7%.
Since the potential to use the wecA mutants as live vaccines or vehicles for heterologous antigen delivery would be enhanced if the bacteria persisted in the host for longer than 30 days, we next considered whether the immunity induced by the wecA mutant strain would provide protection for longer periods. To address this question, mice were orally immunized with 1 × 102, 1 × 103, 1 × 104, 1 × 105, 1 × 106, or 1 × 107 CFU of the TML wecA mutant strain DSM645 on day 0. On day 45 postimmunization, the mice were orally challenged with 1 × 104 CFU of the wild-type TML strain DSM644 (Table 4). Twenty-four of the 30 immunized mice (80%) survived until the day of challenge. Of the 24 mice challenged on day 45, 12 survived (50%); the highest degree of protection was seen in the mice immunized with 1 × 106 or 1 × 107 CFU (100%). Taken together, these data indicate that oral immunization with a wecA mutant strain of S. Typhimurium can induce a protective immune response and that protection may last for at least 45 days postimmunization.
Table 4.
Oral immunization with the TML ECA mutant and subsequent oral challenge at day 45 with wild-type TML strain mutant
| Immunization |
Challenge |
|||
|---|---|---|---|---|
| Dose | No. survived/total | Dose | No. survived/total | % Survival |
| Saline | 5/5 | 1.0 × 104 | 0/5 | 0 |
| 1.0 × 102 | 4/5 | 1.0 × 104 | 0/4 | 0 |
| 1.0 × 103 | 5/5 | 1.0 × 104 | 1/5 | 20 |
| 1.0 × 104 | 5/5 | 1.0 × 104 | 3/5 | 60 |
| 1.0 × 105 | 3/5 | 1.0 × 104 | 1/3 | 33 |
| 1.0 × 106 | 4/5 | 1.0 × 104 | 4/4 | 100 |
| 1.0 × 107 | 3/5 | 1.0 × 104 | 3/3 | 100 |
| Totala | 24/30 | Total: | 12/24 | 50 |
The totals exclude animals immunized with saline.
Intraperitoneal immunization protects against subsequent intraperitoneal or oral challenge.
To determine whether i.p. immunization with the wecA mutant strain could also induce protective immunity, mice were injected with 1 × 101, 1 × 102, 1 × 103, or 1 × 104 CFU of DSM645. On day 30 postimmunization, the surviving mice were challenged with either an i.p. lethal dose (1 × 102 CFU) or an oral lethal dose (1 × 104 CFU) of the wild-type TML strain (DSM644) (Table 5). Thirty-nine of the 40 mice immunized i.p. with either 1 × 101 or 1 × 102 CFU survived until the day of challenge (97.6%); 32 of the 39 mice challenged were then fully protected (82.1%) against an intraperitoneal or oral challenge of 1 × 102 or 1 × 104 CFU, respectively. An immunizing dose of 1 × 103 or 1 × 104 CFU of the wecA mutant strain was more virulent than the lower immunization doses; only 6 of the 14 mice immunized at these doses survived (42.9%). Clearly, the wecA mutant can still be lethal if the immunizing dose is too high.
Table 5.
Intraperitoneal immunization with the TML ECA mutant and subsequent challenge at day 30 with wild-type TML strain
| Immunization |
Challenge |
|||||
|---|---|---|---|---|---|---|
| Route | Dose | No. survived/total | Route | Dose | No. survived/total | % Survival |
| i.p. | Saline | 10/10 | i.p. | 1.5 × 102 | 0/10 | 0 |
| i.p. | Saline | 9/10 | Oral | 1.0 × 104 | 0/9 | 0 |
| i.p. | 1.4 × 101 | 10/10 | i.p. | 1.5 × 102 | 7/10 | 70 |
| i.p. | 1.4 × 102 | 9/10 | i.p. | 1.5 × 102 | 8/9 | 88.9 |
| i.p. | 1.4 × 103 | 6/9 | i.p. | 1.5 × 102 | 2/6 | 30 |
| i.p. | 1.4 × 104 | 0/5 | i.p. | N/Aa | NAa | NA |
| i.p. | 1.0 × 101 | 10/10 | Oral | 1.0 × 104 | 8/10 | 80 |
| i.p. | 1.0 × 102 | 10/10 | Oral | 1.0 × 104 | 9/10 | 90 |
| Totalb | 45/54 | 34/45 | 75.5 | |||
NA, not applicable.
Totals exclude animals immunized with saline.
DISCUSSION
These studies were undertaken to more clearly understand the biological role of one of the major glycolipids on the surface of Gram-negative bacteria, ECAPG. Compared with the long history of structure and function studies of other major bacterial surface glycolipids, such as LPS, definitive studies of ECA function are rare. Although a clear physiological role for the enterobacterial common antigen has not been determined, ECA has been linked to virulence in several species of bacteria (5, 7, 49, 61). Despite this link to pathogenesis, the function of ECA seems to differ in each species. For example, whereas ECA production in S. marcescens is linked to flagellar assembly and motility (11), ECA imparts resistance to organic acids in pathogenic E. coli (7). Additionally, while ECA is involved in the swarming motility of S. marcescens (11), this phenomenon has not been reported in other Gram-negative bacterial species. Since each of these functions is essential for the survival of these organisms in their respective niches, these data perhaps suggest that although ECA is present in many bacteria, each species has evolved unique ways to utilize ECA or ECA biosynthesis in a manner that is most conducive to survival of the species. Thus, it is possible that Gram-negative bacteria that target different host sites may utilize ECA in different ways. This speculation is supported by studies that indicate ECA is important in the pathogenesis of bacterial species that colonize very different sites within the host (3, 5, 7, 11, 31, 49). As such, we were interested in determining the role of ECA in S. Typhimurium biology.
An early report indicated that an ECA mutant strain of S. Typhimurium was attenuated in an intraperitoneal infection model (61); however, due to the limitations of genetic techniques at that time, the mutations responsible for the ECA-negative phenotype could not be confirmed as completely isogenic, and restoration of virulence by complementation was not achieved. Nevertheless, these data imply a role for ECA in the virulence of S. Typhimurium. The role of this molecule in virulence is further supported by the work of Chaudhuri et al. and Ramos-Morales et al., who independently reported that ECA-negative strains were attenuated in competitive tail vein and both oral and intraperitoneal short-term infection models (14, 49). Intrigued by these findings, we sought to further evaluate the role of ECA in S. Typhimurium pathogenesis.
To this end, we created genetically defined ECA mutant (wecA::Gm) and complementation (ΔwecA plus pACYC184::wecA) strains in both the TML and SL1344 strain backgrounds of S. Typhimurium (Table 1). In vitro characterization of these strains showed that disruption of the wecA gene resulted in the complete loss of ECA production and that ECA production was fully restored in the complementation strains (Table 2 and data not shown). Further in vitro characterization showed no major changes in the growth kinetics (Fig. 1B and C), O-antigen production (Table 2), LPS/O-antigen profile (Fig. 1A), or motility (see Fig. S2 in the supplemental material) of the ECA-negative strains. Conversely, we found that the ECA mutant strain was severely attenuated in vivo; significantly fewer animals were killed by the ECA mutant strain than by the wild type in both the oral and intraperitoneal routes of infection (Fig. 2A and B). Finally, we determined that either oral or intraperitoneal vaccination with the ECA mutant strain provided protection against subsequent lethal challenge with wild-type S. Typhimurium.
Interestingly, Ramos-Morales et al. previously reported that the intraperitoneal competitive defect seen with an ECA mutant strain of S. Typhimurium was modest compared to the defect seen during oral infections (49). These results suggested that ECA performs an essential function during oral infection that is less crucial during intraperitoneal infection or that the presence of ECA on the wild-type bacteria is able to partially complement the ECA mutant phenotype during intraperitoneal, but not oral, infection. The authors went on to show a role for S. Typhimurium ECA in bile resistance and suggested that bile sensitivity may be responsible for the oral defect (49). Surprisingly, we found that in single-strain infections, the ECA mutant strain displayed comparable virulence defects regardless of the route of infection (Fig. 2A and B). Moreover, we were unable to show increased bile sensitivity with either of our defined wecA mutant strains (data not shown). While the reason(s) for the discrepancies between the studies remains unclear, they may be due to the models employed (competitive versus single-strain infections) or subtle differences among the S. Typhimurium strains used (strain 14028 versus strains TML and SL1344). Regardless, the data clearly indicate that ECA is important for Salmonella colonization and virulence, though the actual biological function of ECA remains open to debate.
A key component of S. Typhimurium pathogenesis in the murine model of salmonellosis is the ability of the pathogen to disseminate and colonize systemic sites. During oral infection, this process requires that bacteria efficiently cross the intestinal barrier, establish and survive in an intracellular niche, and then disseminate to peripheral sites. The results of our in vitro analyses showed no evidence that ECA expression plays a role in bacterial uptake; wild-type and ECA mutant strains showed similar levels of internalization into INT 407 cells in vitro (data not shown). As a consequence of these observations, we postulated that the attenuation of ECA mutant strains was due to the inability of the bacteria to disseminate throughout the host. However, as illustrated in Fig. 3, the ECA mutant strain was not only able to colonize systemic sites, such as the liver and spleen, but was also able to persist for up to 70 days postinfection. Thus, attenuation of the ECA mutants is not due to an inability to properly disseminate within the host but may be a reflection of the ECA mutants' inability to attain high numbers within the host (Fig. 3).
Previous studies have shown that mutations in other genes also lead to the ability of S. Typhimurium to establish persistent infections in vivo. These genes include (but are not limited to) those associated with metabolic pathways (e.g., aroD, aroA, purA, purE, and surA) and regulatory factors (ompR, rpoS, rpoE, and phoP). Many strains with mutations in such genes have been considered potential live-vaccine candidates (13, 15, 17, 21, 26, 40, 54, 57). However, for many of these strains, the ability to achieve persistence requires the use of an intravenous, intraperitoneal, or highly concentrated oral (1 × 108-CFU) dose; thus, utilizing these strains as vaccine candidates may be challenging. In contrast, the ECA mutant strain was able to establish a persistent infection with a moderate oral dose of 104 CFU; colonization levels for the ECA mutant were approximately 103 to 104 CFU/spleen and approximately 104 to 105 CFU/liver (Fig. 3). These colonization values are slightly higher than those reported in the studies using aroA, purA, and clpP mutant strains (36, 45, 63). Given these findings, we hypothesized that higher numbers of persistent bacteria within these organs could result in increased or prolonged immune stimulation within the host and that the ECA mutant might serve as a viable vaccine to protect against subsequent S. Typhimurium challenge. Indeed, we found that ECA mutants could provide protection against subsequent lethal challenge by both the oral and i.p. routes of infection. Moreover, this protection lasted as long as 45 days postvaccination and could be achieved with both the TML- and SL1344-derived ECA mutant strains.
A major consideration in the future development of any live-attenuated vaccine strain is the ability to maintain the delicate balance between attenuation of virulence and optimal immunogenicity. While definitive in vivo studies of the role of ECA have been limited, our work clearly demonstrates that ECA mutant strains of S. Typhimurium are significantly attenuated in a murine model of infection. In addition, the data presented here also indicate that ECA-negative strains of S. Typhimurium can persist for over 2 months and induce a protective immune response, as indicated by the survival of animals immunized with ECA-negative S. Typhimurium strains after a lethal challenge (Tables 3, 4, and 5). However, the precise mechanism of attenuation remains unclear. Molecules expressed on the surfaces of bacterial cells have been shown to serve as pathogen-associated molecular patterns (PAMPs) and are known to act as ligands for immune signaling receptors (2, 29, 38). It is possible that the lack of ECA on the surfaces of the wecA mutant strains alters the initial steps in activation of the host immune response and results in a failure to clear the organisms from systemic sites. Since ECA itself has been shown to be minimally immunogenic (30, 52), ECA mutant strains should retain a degree of immunogenicity similar to that of wild-type strains. Therefore, given the results presented here, we propose that ECA mutant strains of S. enterica serovar Typhimurium may be good candidates for the development of a live attenuated vaccine against salmonellosis. Furthermore, the ability of the ECA mutant strain to persist at systemic sites suggests the potential to use ECA-negative strains as vehicles for heterologous antigen delivery. Future work will focus on characterization of the host immune response to ECA-negative strains of S. Typhimurium and exploration of the efficiencies of these strains as delivery vehicles for heterologous protective antigens.
Supplementary Material
ACKNOWLEDGMENTS
We thank Tony Maurelli for the gift of the pACYC184 complementation vector, Suzanne Häubler for the gift of the anti-ECA antibody, Arif Rahman and Gretchen Guelde for technical expertise, and Cara Olsen for help with statistical analyses.
This work was supported by R073N0, USUHS (E.S.M.); AI42287, NIAID (M.J.S. and E.S.M.); and AI065529, NIAID (D.S.M.). J. Gilbreath is supported by the Robert D. Watkins Graduate Research Fellowship from the American Society for Microbiology and the Koniag Education Foundation.
The contents of this work are solely our responsibility and do not necessarily represent the official views of the NIH or the Department of Defense.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print 24 October 2011
REFERENCES
- 1. Albertson TE, et al. 2003. Multicenter evaluation of a human monoclonal antibody to Enterobacteriaceae common antigen in patients with Gram-negative sepsis. Crit. Care Med. 31: 419–427 [DOI] [PubMed] [Google Scholar]
- 2. Anderson KV. 2000. Toll signaling pathways in the innate immune response. Curr. Opin. Immunol. 12: 13–19 [DOI] [PubMed] [Google Scholar]
- 3. Bahrani-Mougeot FK, et al. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45: 1079–1093 [DOI] [PubMed] [Google Scholar]
- 4. Bangtrakulnonth A, et al. 2004. Salmonella serovars from humans and other sources in Thailand, 1993–2002. Emerg. Infect. Dis. 10: 131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Banks KE, et al. 2008. The enterobacterial common antigen-like gene cluster of Haemophilus ducreyi contributes to virulence in humans. J. Infect. Dis. 197: 1531–1536 [DOI] [PubMed] [Google Scholar]
- 6. Barr K, Klena J, Rick PD. 1999. The modality of enterobacterial common antigen polysaccharide chain lengths is regulated by o349 of the wec gene cluster of Escherichia coli K-12. J. Bacteriol. 181: 6564–6568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barua S, et al. 2002. Involvement of surface polysaccharides in the organic acid resistance of Shiga Toxin-producing Escherichia coli O157:H7. Mol. Microbiol. 43: 629–640 [DOI] [PubMed] [Google Scholar]
- 8. Beaty CD, Franklin TL, Uehara Y, Wilson CB. 1994. Lipopolysaccharide-induced cytokine production in human monocytes: role of tyrosine phosphorylation in transmembrane signal transduction. Eur. J. Immunol. 24: 1278–1284 [DOI] [PubMed] [Google Scholar]
- 9. Buzby JC, Roberts T, Lin CJ, MacDonald JM. 1996. Bacterial foodborne disease: medical costs and productivity losses. U.S. Department of Agriculture, Washington, DC. http://hdl.handle.net/10113/34242 [Google Scholar]
- 10. Carsiotis M, Weinstein DL, Karch H, Holder IA, O'Brien AD. 1984. Flagella of Salmonella typhimurium are a virulence factor in infected C57BL/6J mice. Infect. Immun. 46: 814–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castelli ME, et al. 2008. Enterobacterial common antigen integrity is a checkpoint for flagellar biogenesis in Serratia marcescens. J. Bacteriol. 190: 213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention 1994. Salmonella surveillance report—annual summary—1990. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 13. Chatfield SN, Dorman CJ, Hayward C, Dougan G. 1991. Role of ompR-dependent genes in Salmonella typhimurium virulence: mutants deficient in both ompC and ompF are attenuated in vivo. Infect. Immun. 59: 449–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaudhuri RR, et al. 2009. Comprehensive identification of Salmonella enterica serovar Typhimurium genes required for infection of BALB/c mice. PLoS Pathog. 5: e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curtiss R, III, Kelly SM. 1987. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect. Immun. 55: 3035–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Darwin KH, Miller VL. 1999. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin. Microbiol. Rev. 12: 405–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dorman CJ, Chatfield S, Higgins CF, Hayward C, Dougan G. 1989. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect. Immun. 57: 2136–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Erbel PJ, et al. 2003. Identification and biosynthesis of cyclic enterobacterial common antigen in Escherichia coli. J. Bacteriol. 185: 1995–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Erridge C, Bennett-Guerrero E, Poxton IR. 2002. Structure and function of lipopolysaccharides. Microbes Infect. 4: 837–851 [DOI] [PubMed] [Google Scholar]
- 20. Fadl AA, et al. 2005. Attenuation of Salmonella enterica serovar Typhimurium by altering biological functions of murein lipoprotein and lipopolysaccharide. Infect. Immun. 73: 8433–8436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fang FC, et al. 1992. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc. Natl. Acad. Sci. U. S. A. 89: 11978–11982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freudenberg MA, et al. 2008. Lipopolysaccharide sensing an important factor in the innate immune response to Gram-negative bacterial infections: benefits and hazards of LPS hypersensitivity. Immunobiology 213: 193–203 [DOI] [PubMed] [Google Scholar]
- 23. Giannella RA, Formal SB, Dammin GJ, Collins H. 1973. Pathogenesis of salmonellosis: studies of fluid secretion, mucosal invasion, and morphologic reaction in the rabbit ileum. J. Clin. Invest. 52: 441–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haraga A, Ohlson MB, Miller SI. 2008. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 6: 53–66 [DOI] [PubMed] [Google Scholar]
- 25. Heumann D, Roger T. 2002. Initial responses to endotoxins and Gram-negative bacteria. Clin. Chim Acta 323: 59–72 [DOI] [PubMed] [Google Scholar]
- 26. Hoiseth SK, Stocker BA. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291: 238–239 [DOI] [PubMed] [Google Scholar]
- 27. Ilg K, et al. 2009. O-antigen-negative Salmonella enterica serovar Typhimurium is attenuated in intestinal colonization but elicits colitis in streptomycin-treated mice. Infect. Immun. 77: 2568–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones BD, Ghori N, Falkow S. 1994. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 180: 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kopp EB, Medzhitov R. 1999. The Toll-receptor family and control of innate immunity. Curr. Opin. Immunol. 11: 13–18 [DOI] [PubMed] [Google Scholar]
- 30. Kuhn HM, Meier-Dieter U, Mayer H. 1988. ECA, the enterobacterial common antigen. FEMS Microbiol. Rev. 4: 195–222 [DOI] [PubMed] [Google Scholar]
- 31. Lawlor MS, Hsu J, Rick PD, Miller VL. 2005. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol. Microbiol. 58: 1054–1073 [DOI] [PubMed] [Google Scholar]
- 32. Lew HC, Nikaido H, Makela PH. 1978. Biosynthesis of uridine diphosphate N-acetylmannosaminuronic acid in rff mutants of Salmonella typhimurium. J. Bacteriol. 136: 227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Licht TR, et al. 1996. Role of lipopolysaccharide in colonization of the mouse intestine by Salmonella typhimurium studied by in situ hybridization. Infect. Immun. 64: 3811–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lindgren SW, Stojiljkovic I, Heffron F. 1996. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc. Natl. Acad. Sci. U. S. A. 93: 4197–4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Loppnow H, Brade H, Rietschel ET, Flad HD. 1994. Induction of cytokines in mononuclear and vascular cells by endotoxin and other bacterial products. Methods Enzymol. 236: 3–10 [DOI] [PubMed] [Google Scholar]
- 36. Matsui H, et al. 2003. Oral immunization with ATP-dependent protease-deficient mutants protects mice against subsequent oral challenge with virulent Salmonella enterica serovar typhimurium. Infect. Immun. 71: 30–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mayer H, Schmidt G. 1979. Chemistry and biology of the enterobacterial common antigen (ECA). Curr. Top. Microbiol. Immunol. 85: 99–153 [DOI] [PubMed] [Google Scholar]
- 38. Medzhitov R, Janeway C., Jr 2000. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 173: 89–97 [DOI] [PubMed] [Google Scholar]
- 39. Meier U, Mayer H. 1985. Genetic location of genes encoding enterobacterial common antigen. J. Bacteriol. 163: 756–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller SI, Kukral AM, Mekalanos JJ. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. U. S. A. 86: 5054–5058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Monack DM, Bouley DM, Falkow S. 2004. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization. J. Exp. Med. 199: 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nelson DL, Kennedy EP. 1972. Transport of magnesium by a repressible and a nonrepressible system in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 69: 1091–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nevola JJ, Stocker BA, Laux DC, Cohen PS. 1985. Colonization of the mouse intestine by an avirulent Salmonella typhimurium strain and its lipopolysaccharide-defective mutants. Infect. Immun. 50: 152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O'Brien AD, Taylor BA, Rosenstreich DL. 1984. Genetic control of natural resistance to Salmonella typhimurium in mice during the late phase of infection. J. Immunol. 133: 3313–3318 [PubMed] [Google Scholar]
- 45. O'Callaghan D, Maskell D, Liew FY, Easmon CS, Dougan G. 1988. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attention, persistence, and ability to induce protective immunity in BALB/c mice. Infect. Immun. 56: 419–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ohl ME, Miller SI. 2001. Salmonella: a model for bacterial pathogenesis. Annu. Rev. Med. 52: 259–274 [DOI] [PubMed] [Google Scholar]
- 47. Pegues DA, Hohmann EL, Miller SI. 1995. Salmonella, including S. typhi, p. 785–809 In Blaser MJ. (ed.), Infections of the gastrointestinal tract. Raven Press, New York, NY [Google Scholar]
- 48. Rabsch W, Tschape H, Baumler AJ. 2001. Non-typhoidal salmonellosis: emerging problems. Microbes Infect. 3: 237–247 [DOI] [PubMed] [Google Scholar]
- 49. Ramos-Morales F, Prieto AI, Beuzon CR, Holden DW, Casadesus J. 2003. Role for Salmonella enterica enterobacterial common antigen in bile resistance and virulence. J. Bacteriol. 185: 5328–5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rick PD, Mayer H, Neumeyer BA, Wolski S, Bitter-Sueermann D. 1985. Biosynthesis of enterobacterial common antigen. J. Bacteriol. 162: 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rick PD, et al. 1998. Characterization of the lipid-carrier involved in the synthesis of enterobacterial common antigen (ECA) and identification of a novel phosphoglyceride in a mutant of Salmonella typhimurium defective in ECA synthesis. Glycobiology 8: 557–567 [DOI] [PubMed] [Google Scholar]
- 52. Rick PD, et al. 1995. Enterobacterial common antigen and capsular polysaccharides, p. 104–112 In Neidhardt FC, et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC [Google Scholar]
- 53. Rietschel ET, et al. 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8: 217–225 [DOI] [PubMed] [Google Scholar]
- 54. Rodriguez-Morales O, et al. 2006. Salmonella enterica serovar Typhimurium ompS1 and ompS2 mutants are attenuated for virulence in mice. Infect. Immun. 74: 1398–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rose RE. 1988. The nucleotide sequence of pACYC184. Nucleic Acids Res. 16: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schletter J, Heine H, Ulmer AJ, Rietschel ET. 1995. Molecular mechanisms of endotoxin activity. Arch. Microbiol. 164: 383–389 [DOI] [PubMed] [Google Scholar]
- 57. Sydenham M, et al. 2000. Salmonella enterica serovar typhimurium surA mutants are attenuated and effective live oral vaccines. Infect. Immun. 68: 1109–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tauxe RV. 1996. An update on Salmonella. Health Environ. Digest 10: 1–4 [Google Scholar]
- 59. Toguchi A, Siano M, Burkart M, Harshey RM. 2000. Genetics of swarming motility in Salmonella enterica serovar typhimurium: critical role for lipopolysaccharide. J. Bacteriol. 182: 6308–6321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tsai CM, Frasch CE. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119: 115–119 [DOI] [PubMed] [Google Scholar]
- 61. Valtonen MV, Larinkari UM, Plosila M, Valtonen VV, Makela PH. 1976. Effect of the enterobacterial common antigen on mouse virulence of Salmonella typhimurium. Infect. Immun. 13: 1601–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wray C, Sojka WJ. 1978. Experimental Salmonella typhimurium infection in calves. Res. Vet. Sci. 25: 139–143 [PubMed] [Google Scholar]
- 63. Yamamoto T, et al. 2001. Disruption of the genes for ClpXP protease in Salmonella enterica serovar Typhimurium results in persistent infection in mice, and development of persistence requires endogenous gamma interferon and tumor necrosis factor alpha. Infect. Immun. 69: 3164–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang L, Radziejewska-Lebrecht J, Krajewska-Pietrasik D, Toivanen P, Skurnik M. 1997. Molecular and chemical characterization of the lipopolysaccharide O-antigen and its role in the virulence of Yersinia enterocolitica serotype O:8. Mol. Microbiol. 23: 63–76 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.