Abstract
Due to the importance of neutrophils and proinflammatory cytokines in schistosomal liver damage, we analyzed the mechanisms underlying neutrophil and proinflammatory responses in murine schistosomiasis japonica. We found that granulomatous inflammation around parasite eggs in the liver was greater in Schistosoma japonicum-infected IL-4−/− IL-13−/− (double-knockout [DKO]) mice than in infected wild-type (WT) mice at 6 weeks, but not at 8 weeks, postinfection, suggesting the importance of Th2 responses in these typical hepatic lesions. Infected DKO mice also showed increased neutrophil infiltration accompanying more severe pathology, as shown by the enhanced necrosis of hepatocytes. This was not likely due to a Th1/Th2 imbalance, because there was no detectable increase in gamma interferon (IFN-γ) production in these DKO mice. mRNA expression of interleukin-17A (IL-17A), proinflammatory cytokines, and the neutrophil chemoattractant CXCL2 in liver was higher in infected DKO mice than in WT mice. However, in IL-4−/− IL-13−/− IL-17A−/− (triple-knockout [TKO]) mice, the absence of IL-17A was associated with only marginal differences in schistosomal liver damage, suggesting that IL-17A is only partially responsible for neutrophil-driven hepatic damage. Furthermore, the expression of mRNAs encoding proinflammatory cytokines was not under the control of IL-17A in TKO mice. These findings indicate that IL-4 and IL-13 suppress excessive neutrophil recruitment, proinflammatory cytokine production, and hepatic damage during the acute stage of S. japonicum infection, suggesting that neutrophils and proinflammatory cytokines are mainly responsible for hepatocyte damage during acute murine schistosomiasis japonica. However, neutrophil induction and the production of proinflammatory cytokines were not due solely to IL-17A.
INTRODUCTION
Schistosomiasis is a major tropical disease caused by trematode parasites of the genus Schistosoma. Currently more than 200 million people are infected, with 280,000 deaths reported annually (28). Although two of the three major species of human schistosomes, S. japonicum and S. mansoni, cause intestinal forms of schistosomiasis, their pathogenetic mechanisms have not been fully clarified. Although host Th responses to parasite eggs are essential for clinicopathological features (6), the quality and quantity of host responses differ for these two pathogens. For example, necrotic lesions are widespread in the livers of hamsters infected with S. japonicum but not in those of hamsters infected with S. mansoni (45). This may be due to differences in cells composing the cicrum oval lesions in the liver, with a higher ratio of neutrophils observed in animals infected with S. japonicum (23, 45) and a higher ratio of eosinophils in granulomas of animals infected with S. mansoni (23). Hepatic pathology is more severe in schistosomiasis japonica than in schistosomiasis mansoni, a difference related to necrosis (45). The accumulation of neutrophils may therefore be responsible for necrotic lesions in the liver (21), making it necessary to analyze mechanisms of neutrophil regulation during infection with S. japonicum.
The Th2 response is characteristic of allergies and helminth infections, including schistosomiasis (1, 6, 14, 39). In murine schistosomiasis mansoni, the interleukin-4 (IL-4)/IL-13-mediated pathway, which acts via the IL-4 receptor α chain (IL-4Rα) and STAT6 (31, 50), is important for the development of hepatic pathology, since granuloma formation was impaired in S. mansoni-infected IL-4−/− IL-13−/− (double-knockout [DKO]), IL-4Rα−/−, and STAT6−/− mice but not in IL-4−/− or IL-13−/− mice (11, 25, 27), and mortality rates were higher in DKO than in wild-type (WT) mice (11). IL-4/IL-13 induces alternatively activated macrophages (AAMs), which protect against hepatic damage through egg-induced inflammation, with a mortality rate of 100% in mice without AAMs (LysMcre IL-4Rα−/flox mice) following acute infection with S. mansoni (13, 19). Therefore, IL-4/IL-13 is essential for granuloma formation and host survival in acute murine schistosomiasis mansoni. IL-4 and IL-13 were recently shown to suppress excessive airway inflammation and neutrophil accumulation in ovalbumin-induced airway inflammation, as well as to downregulate excessive production of the proinflammatory cytokine IL-17A (18). Further, IL-4 has been found to suppress Th17 differentiation in vivo and in vitro (17, 32).
Although IL-4 and IL-13 are necessary for granuloma formation and host survival in murine schistosomiasis mansoni, the function of this pathway in murine schistosomiasis japonica remains unresolved. We hypothesized that IL-4 and IL-13 suppress hepatic granulomatous inflammation by downregulating excessive neutrophil accumulation associated with the production of proinflammatory cytokines during S. japonicum infection. We have therefore focused on mechanisms regulating neutrophil infiltration and the production of proinflammatory cytokines that are directly or indirectly related to IL-4/IL-13.
MATERIALS AND METHODS
Animals and parasite infection.
BALB/c (WT) mice were purchased from CLEA Japan (Tokyo, Japan). BALB/c IL-4−/− IL-13−/− (DKO) mice (35) and BALB/c IL-17A−/− mice have been previously described (37). IL-4−/− IL-13−/− IL-17A−/− (triple-knockout [TKO]) mice were produced by crossing DKO and IL-17A−/− mice. Six- to 9-week-old mice were percutaneously infected with 30 S. japonicum cercariae (Japanese Yamanashi strain, maintained in our laboratory using Onchomelania hupensis nosophora). At 6 and 8 weeks later, the mice were sacrificed and perfused from the portal vain, and recovered worms were counted. Liver tissue and spleens were dissected and used for analysis. Samples of liver and intestine were digested in 4% KOH at 37°C, and the numbers of eggs were quantified by microscopy, followed by calculation of the egg burden in tissues and egg production per female adult worm.
Animal care was in accordance with the guidelines of and approved by the Committee of Animal Ethics of Tokyo Medical and Dental University (0110038A).
Preparation of liver and spleen cells.
Spleen cells were prepared as described previously (3). Isolated liver tissue was briefly minced, passed through 200-μm steel mesh, and digested in 0.5 mg/ml collagenase (Wako Pure Chemical, Osaka, Japan) for 45 min at 37°C. This tissue was passed through 45-μm nylon mesh, and the cell pellet was resuspended in 35% Percoll and centrifuged at 2,000 rpm for 15 min at 4°C to remove hepatocytes and other debris (7). Erythrocytes were lysed with hemolysis buffer (0.017 M Tris, 0.16 M ammonium chloride, pH 7.4). The pellet was washed with phosphate-buffered saline (PBS), passed through 45-μm nylon mesh, centrifuged at 2,000 rpm for 3 min at 4°C, and resuspended in PBS. The dead cells were stained blue with trypan blue, and unstained living cells were counted on a hemocytometer.
Flow cytometry.
Cells were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-CCR3 monoclonal antibody (MAb) (R&D Systems, MN) and phycoerythrin (PE)-conjugated anti-Gr-1 MAb (eBioscience, San Diego, CA), followed by forward-scatter (FSC)/side-scatter (SSC) plotting (3). The cells were analyzed on a FACSCalibur flow cytometer (BD Bioscience, NJ) equipped with Cell Quest software. Eosinophils were defined as cells that were Gr-1+ CCR3+ SSChigh and neutrophils as cells that were Gr-1high CCR3− SSCint (16, 20, 36).
Pathology.
Liver specimens were fixed in 10% formalin, embedded in paraffin, and sectioned at 3 μm. The sections were stained with hematoxylin and eosin to measure the sizes of granulomas containing a single egg. Granuloma size was quantitated using ImageJ software (NIH). Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured with the Wako transaminase CII test (Wako Pure Chemicals).
Cytokine ELISA.
Spleen cells (5 × 106 cells/ml) were cultured in RPMI 1640 (Wako Pure Chemicals) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogen, CA), and 50 μM 2-mercaptoethnaol (ME) (Invitrogen) and stimulated with 25 μg/ml soluble egg antigen (SEA) from S. japonicum at 37°C for 72 h in 5% CO2. Supernatants were harvested after centrifugation and stored at −20°C until analyzed. The cytokines IL-4, IL-5, IL-13, IL-17A, and gamma interferon (IFN-γ) were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (eBioscience).
Real-time PCR.
Liver samples were minced with a Biomusher (Nippi Research Institute of Biomatrix, Ibaraki, Japan), and total RNA was extracted using TRIzol reagent (Invitrogen). Aliquots of total RNA (5 μg) were reverse transcribed using Superscript III (Invitrogen) and random primers (Invitrogen), followed by real-time PCR using the primers shown in Table 1 (3). Relative quantities of PCR products were determined using a Kapa SYBR Fast quantitative PCR (qPCR) kit (Kapa Biosystems, MA) and a LightCycler 480 (Roche, Mannheim, Germany) and by the comparative threshold cycle method (LightCycler 480 SW1.5; Roche). The quantity of each mRNA in each sample was normalized relative to β-actin expression and expressed relative to controls. If control gene expression levels were below the detection limit, the y axis showed target per reference.
Table 1.
Primers used in this study
| Primer | Sequence |
|
|---|---|---|
| Forward | Reverse | |
| β-Actin | GCTCTAGACTTCGAGCAGGAGA | AGGCAGCTCATAGCCCTTCT |
| IL-4 | CATCGGCATTTTGAACGAG | CGAGCTCACTCTCTGTGGTG |
| IL-5 | ACATTGACCGCCAAAAAGAG | ATCCAGGAACTGCCTCGTC |
| IL-13 | GGTCCACACAGGGCAACT | AATAAGATCAAGAAGAAATGTGCTCAA |
| IFN-γ | GGAGGAACTGGCAAAAGGAT | TTCAAGACTTCAAAGAGTCTGAGG |
| IL-10 | CAGAGCCACATGCTCCTAGA | TGTCCAGCTGGTCCTTTGTT |
| IL-17A | CAGGGAGAGCTTCATCTGTGT | GCTGAGCTTTGAGGGATGAT |
| IL-17F | CAAGAAATCCTGGTCCTTCG | GAGCATCTTCTCCAACCTGAA |
| IL-22 | TTTCCTGACCAAACTCAGCA | TCTGGATGTTCTGGTCGTCA |
| IL-6 | GCTACCAAACTGGATATAATCAGGA | CCAGGTAGCTATGGTACTCCAGAA |
| TNF-α | TGCCTATGTCTCAGCCTCTTC | GAGGCCATTTGGGAACTTCT |
| G-CSF | GCTGCTGGAGCAGTTGTG | GGGATCCCCAGAGAGTGG |
| CXCL2 | AAAATCATCCAAAAGATACTGAACAA | CTTTGGTTCTTCCGTTGAGG |
| S100A8 | TGCGATGGTGATAAAAGTGG | GGCCAGAAGCTCTGCTACTC |
| S100A9 | CACCCTGAGCAAGAAGGAAT | TGTCATTTATGAGGGCTTCATTT |
Statistical analyses.
Groups were compared using the two-tailed Student t test and analysis of variance (ANOVA) with Statcel3 software (OMS Publishing Inc., Saitama, Japan). Results were considered significant at a P value of <0.05.
RESULTS
Recruitment of neutrophils is higher in DKO mice than in WT mice at 6 weeks after infection with S. japonicum.
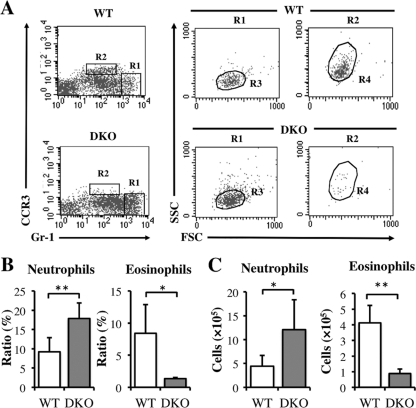
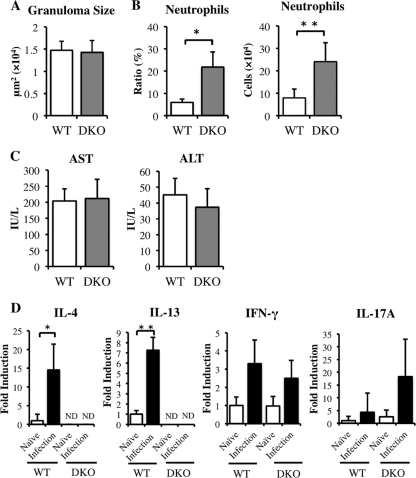
Hepatic granulomatous inflammation in murine schistosomiasis japonica is characterized by the infiltration of neutrophils and eosinophils. To examine whether the absence of IL-4/IL-13 affects neutrophil and eosinophil infiltration, we assayed liver cells of WT and IL-4−/− IL-13−/− (DKO) mice stained with antibodies to Gr-1 and CCR3, with Gr-1high CCR3− SSCint cells defined as neutrophils and Gr-1+ CCR3+ SSChigh cells as eosinophils (Fig. 1A). At 6 weeks postinfection, the average ratio and number of neutrophils were significantly higher, and the ratio and number of eosinophils were lower, in infected DKO mice than in WT mice (Fig. 1B and C), indicating that IL-4/IL-13 suppressed excessive neutrophil recruitment in the liver during acute S. japonicum infection.
Fig 1.
Upregulation of neutrophil recruitment and downregulation of eosinophil recruitment in DKO mice at 6 weeks after infection with S. japonicum. (A) Liver cells isolated from WT and DKO mice at 6 weeks after infection were stained with anti-Gr-1 and anti-CCR3 MAbs, followed by gating on FSC/SSC parameters. R1 shows Gr-1high CCR3−, and R2 shows Gr-1+ CCR3+. Gr-1high CCR3− SSCint (R3) cells represent neutrophils, and Gr-1+ CCR3+ SSChigh (R4) cells represent eosinophils. (B and C) Average populations (B) and numbers (C) of neutrophils or eosinophils from infected WT and DKO mice. Means and standard deviations (SD) are shown (n = 4 to 6). Naïve, noninfected mice; infection, infected mice. *, P < 0.05; **, P < 0.01.
Hepatic granulomatous inflammation is more severe in infected DKO mice than in WT mice with acute schistosomiasis japonica.
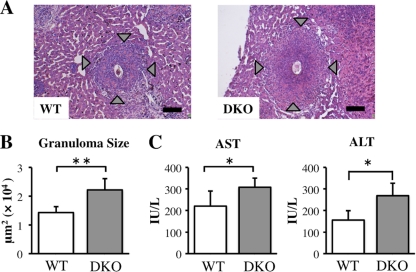
To determine whether excessive neutrophil infiltration in the livers of DKO mice correlated with hepatic inflammation, we analyzed tissue samples histologically. Granulomatous inflammation was more severe in DKO mice than in WT mice, with more severe necrosis observed in the granulomas of DKO mice (Fig. 2A). Moreover, the average granuloma size, including necrotic areas, was larger in DKO than in WT mice (Fig. 2B), and serum concentrations of AST and ALT, both markers of hepatocyte damage, were higher in DKO than in WT mice (Fig. 2C). In contrast, although hepatic damage was more severe in DKO than in WT mice, their mortality rates did not differ (data not shown). In addition, the number of recovered worms and the hepatic egg burden were similar in the two strains (data not shown). These results suggested that IL-4/IL-13 suppressed excessive liver damage caused by hepatic inflammation following S. japonicum infection.
Fig 2.
Severity of hepatic inflammation in infected DKO and WT mice at 6 weeks after S. japonicum infection. (A) Histopathology of S. japonicum-infected mice. Liver sections stained with hematoxylin-eosin show a granuloma surrounding a parasite egg. Original magnification, ×100; scale bar, 100 μm. Arrowheads indicate the margins of hepatic granulomatous inflammation. (B) Average granuloma sizes in DKO and WT mice. (C) Serum AST and ALT concentrations in infected WT and DKO mice. Means and SD are shown (n = 4 to 6). *, P < 0.05; **, P < 0.01.
The Th2 response is decreased but the Th1 response is unaffected in liver and splenocytes from DKO mice during acute infection.
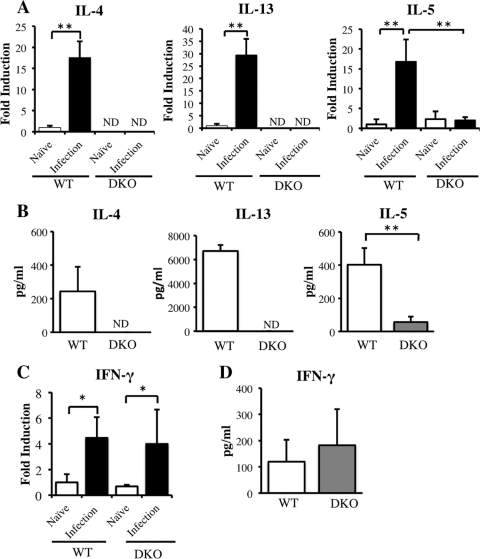
To explain the differences in hepatic pathology observed in WT and DKO mice, we measured the concentrations of Th1 and Th2 cytokines in these animals at 6 weeks postinfection. We found that the expression of mRNAs encoding the Th2 cytokines IL-4, IL-5, and IL-13 was enhanced in the livers of S. japonicum-infected WT mice (Fig. 3A). In contrast, expression of IL-5 mRNA was impaired in infected DKO mice and was significantly lower than that in infected WT mice. Similarly, SEA-stimulated spleen cells from infected WT mice showed elevated production of IL-4, IL-5, and IL-13, but the production of IL-5 was lower in SEA-stimulated spleen cells from infected DKO mice than in those from infected WT mice (Fig. 3B).
Fig 3.
Expression of Th1 and Th2 cytokines in WT and DKO mice at 6 weeks postinfection. (A) Expression of mRNAs encoding the Th2 cytokines IL-4, IL-5, and IL-13 in livers of infected mice. (B) Production of IL-4, IL-5, and IL-13 by SEA-stimulated splenocytes collected from infected WT and DKO mice. (C and D) Expression of IFN-γ mRNA in livers (C) and IFN-γ production by SEA-stimulated splenocytes (D) from infected WT and DKO mice. Means and SD are shown (n = 4 to 6). Naïve, uninfected mice; infection, infected mice; ND, not detected. *, P < 0.05; **, P < 0.01.
To determine the effect of IL-4/IL-13 knockout on the Th1/Th2 balance in infected mice, we measured the levels of IFN-γ mRNA in their livers. Infection increased the levels of IFN-γ mRNA in both WT and DKO livers, but their expression levels did not differ (Fig. 3C). Moreover, splenocyte production of IFN-γ did not differ in infected WT and DKO mice (Fig. 3D).
Absence of IL-4/IL-13 promotes the production of IL-17A and expression of Th17-related cytokine mRNAs during acute infection.
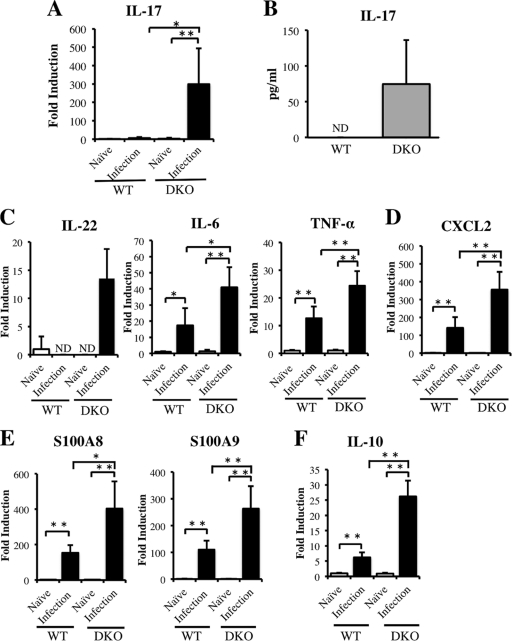
To determine the mechanism underlying the enhanced neutrophil infiltration and severe hepatic inflammation in DKO mice, we focused on IL-17A, since this cytokine is involved in the induction of neutrophils and the production of proinflammatory cytokines (4, 5, 29, 48). We found that the level of mRNA in liver and the splenocyte production of IL-17A protein were higher in infected DKO mice than in infected WT mice (Fig. 4A and B). We also found that liver expression of IL-22 mRNA, which is produced by Th17 cells (49), was higher in infected DKO mice than in infected WT mice (Fig. 4C). In addition, the expression of message encoding proinflammatory cytokines, such as IL-6 and tumor necrosis factor alpha (TNF-α) (Fig. 4C), CXCL2, a neutrophil chemokine enhanced by IL-17A (Fig. 4D) (48), and S100A8 and S100A9, which are enhanced by IL-17A and IL-22 (Fig. 4E), was greater in infected DKO mice than in infected WT mice (30). These results suggested that, at 6 weeks after infection, IL-4/IL-13 may suppress the Th17 response, which induces the expression in liver of mRNAs encoding the proinflammatory cytokines CXCL2, S100A8, and S100A9. Interestingly, mRNA encoding IL-10, which suppresses the secretion of Th17 cytokines, was more highly expressed in DKO than in WT mice (Fig. 4F) (15).
Fig 4.
Expression of mRNAs encoding Th17-related cytokines, proinflammatory cytokines, neutrophil chemokines, and S100 family proteins in infected DKO and WT mice at 6 weeks postinfection. (A) Expression of IL-17A mRNA in liver. (B) Production of IL-17A by SEA-stimulated splenocytes from infected mice. (C) Liver expression of mRNAs encoding the Th17-related and proinflammatory cytokines, IL-22, IL-6, and TNF-α. (D) Expression of mRNA encoding the neutrophil chemokine CXCL2 in liver. (E) Expression of S100A8 and S100A9 mRNAs in liver. (E) Expression of IL-10 mRNA in liver. Means and SD are shown (n = 4 to 6). Naïve, uninfected mice; infection, infected mice; ND, not detected. *, P < 0.05; **, P < 0.01.
Hepatic damage at 8 weeks after infection does not differ in WT and DKO mice.
Although hepatic damage was observed in both WT and DKO mice at 8 weeks after infection, their granuloma sizes did not differ (Fig. 5A), nor did their serum concentrations of AST and ALT (Fig. 5C). However, the neutrophil ratio and number were higher in DKO than in WT mice (Fig. 5B). Although IL-4 and IL-13 mRNA levels remained higher in WT mice, IFN-γ and IL-17A mRNA levels did not differ significantly (Fig. 5D). These findings therefore indicated that IL-4 and IL-13 do not suppress hepatic inflammation and damage at 8 weeks postinfection.
Fig 5.
Hepatic granulomatous inflammation and mRNAs of Th1, Th2, and Th17 cytokines in WT and DKO mice at 8 weeks postinfection. (A) Average granuloma sizes in WT and DKO mice. (B) Average population and numbers of neutrophils from infected WT and DKO mice. (C) Serum concentrations of AST and ALT in infected WT and DKO mice. (D) Levels of expression of IL-4, IL-13, IFN-γ, and IL-17A mRNAs in WT and DKO mice. Means and SD are shown (n = 3 or 4). Naïve, uninfected mice; infection, infected mice; ND, not detected. *, P < 0.05; **, P < 0.01.
Induction of hepatic inflammation and neutrophil infiltration in DKO mice by IL-17A-dependent and -independent mechanisms during acute S. japonicum infection.
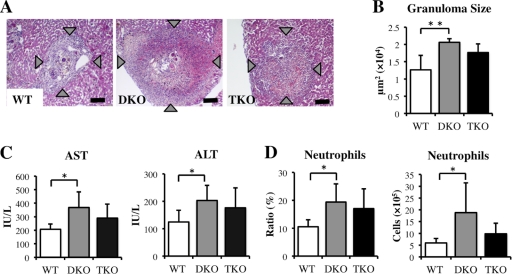
To investigate the role of IL-17A in neutrophil recruitment and severe hepatic inflammation in the absence of IL-4 and IL-13, IL-4−/− IL-13−/− IL-17A−/− (TKO) mice were infected with S. japonicum. We found that hepatic inflammation in TKO mice was intermediate between those in WT and DKO mice (Fig. 6A and B). Moreover, serum AST and ALT concentrations and the number and ratio of neutrophils in TKO mice did not differ significantly from those in WT and DKO mice (Fig. 6C and D). Eosinophil infiltration was impaired in both DKO and TKO mice (data not shown), whereas the number of recovered worms and the hepatic egg burden did not differ in WT, DKO, and TKO mice (Table 2). These findings indicate that hepatic damage and neutrophil infiltration in DKO mice could not be explained solely by the effects of IL-17A.
Fig 6.
Hepatic inflammation in WT, DKO, and TKO mice at 6 weeks after infection with S. japonicum. (A) Histopathology of S. japonicum-infected mice. Liver sections stained with hematoxylin/eosin show granuloma surrounding parasite eggs. Original magnification, ×100; scale bar, 100 μm. (B) Average granuloma sizes in WT, DKO, and TKO mice. (C) Serum AST and ALT concentrations in infected WT, DKO, and TKO mice. (D) Ratio and number of neutrophils in the livers of naïve and infected WT, DKO, and TKO mice. Means and SD are shown (n = 4 to 6). *, P < 0.05; **, P < 0.01.
Table 2.
Parasitological dataa
| Group | Worm burden | Fecundity (eggs/female, ×104) | Tissue eggs (×103) in: |
|
|---|---|---|---|---|
| Liver | Intestine | |||
| WT | 17.4 ± 4.3 | 23.9 ± 4.2 | 72.3 ± 43.7 | 59.3 ± 35.2 |
| DKO | 18.8 ± 3.9 | 23.8 ± 1.7 | 102.4 ± 22.2 | 65.0 ± 24.0 |
| TKO | 19.3 ± 4.3 | 24.0 ± 5.6 | 95.0 ± 25.2 | 57.1 ± 13.3 |
All data are expressed as mean ± standard deviation.
Expression of mRNAs encoding proinflammatory cytokines, neutrophil chemoattractant, and S100 family proteins is independent of IL-17A.
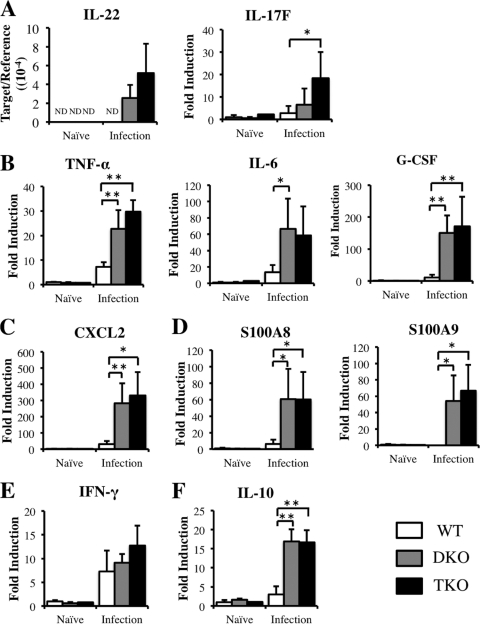
DKO mice infected with S. japonicum showed enhanced expression of mRNAs encoding IL-22, proinflammatory cytokines, CXCL2, and S100 family proteins. To determine whether the absence of IL-17A affects the expression of these mRNAs, we measured their expression in the livers of TKO mice. We found that the levels of mRNAs encoding the Th17-related cytokines IL-22 and IL-17F and the proinflammatory cytokines IL-6, TNF-α, and granulocyte colony-stimulating factor (G-CSF) did not differ in infected DKO and TKO mice (Fig. 7A and B), nor did the levels of CXCL2, S100A8, and S100A9 mRNAs, all of which are induced by IL17A (Fig. 7C and D). In addition, the expression of mRNA encoding the Th1 cytokine IFN-γ did not differ among WT, DKO, and TKO mice (Fig. 7E), and the levels of mRNA encoding the anti-inflammatory cytokine IL-10 were similar in DKO and TKO mice (Fig. 7F). Taken together, these results suggested that IL-17A may not affect the production of other Th17-related cytokines and of the proinflammatory cytokines CXCL2, S100A8, and S100A9.
Fig 7.
Effect of IL-17A on the expression of IL-22 and IL-17F (A), TNF-α, IL-6, and G-CSF (B), CXCL2 (C), S100A8 and S100A9 (D), IFN-γ (E), and IL-10 (F) mRNAs in the livers of WT, DKO, and TKO mice at 6 weeks postinfection, as determined by real-time PCR. Means and SD are shown (n = 4 to 6). *, P < 0.05; **, P < 0.01. Naïve, uninfected mice; infection, infected mice; ND, not detected. The y axis in the graph of IL-22 expression is the ratio of IL-22 mRNA to β-actin mRNA, since IL-22 mRNA in naïve controls was below the level of detection.
DISCUSSION
Less is known about the immunopathology of hepatic lesions in S. japonicum than about that in S. mansoni infection. Due to the importance of IL-4 and IL-13 in the development of hepatic lesions in S. mansoni infection (11), we analyzed the roles of IL-4 and IL-13 in S. japonicum-infected mice. Examination of hepatic lesions in infected DKO mice indicated that IL-4/IL-13 downregulates neutrophil infiltration and the production of proinflammatory cytokines. IL-4/IL-13 has been shown to suppress inflammation and neutrophil recruitment in a model of ovalbumin (OVA)-induced airway inflammation (18). We found that at 6 weeks after S. japonicum infection, the ratio and number of neutrophils in the liver were higher, while the ratio and number of eosinophils were lower, in DKO mice than in WT mice, suggesting the importance of IL-4/IL-13 in the suppression of excessive neutrophil recruitment and the induction of eosinophil recruitment in granulomatous inflammation of murine schistosomiasis japonica.
It was reported that IL-4 and IL-13 share the IL-4 receptor α chain (IL-4Rα) and activate STAT6 via IL-4Rα (31, 50). In murine schistosomiasis mansoni, egg-granuloma formation is promoted by IL-4/IL-13 and the IL-4Rα-STAT6 pathway (11, 25, 27), whereas in the absence of IL-4/IL-13, hepatic granulomatous inflammation and eosinophil infiltration are impaired (11). Eosinophil infiltration in S. japonicum-infected DKO mice was also impaired, as shown by the reduced production of IL-5 (10). Unexpectedly, however, we found that hepatic inflammatory foci were more severe in DKO than in WT mice infected with S. japonicum, since granulomas accompanying broadly necrotic areas were larger in DKO mice. Moreover, hepatic lesions, including necrotic areas, were larger and serum AST and ALT concentrations were higher in DKO than in WT mice at 6 weeks after infection. Neutrophils have been shown to induce hepatic necrosis in mice at 2 weeks after implantation of S. japonicum eggs (21). Our results indicate that the more severe pathology observed in DKO than in WT mice may be related to the excess neutrophil recruitment in the former. Thus, IL-4/IL-13 may downregulate severe hepatic inflammation in schistosomiasis japonica by suppressing excessive neutrophil accumulation.
Although neutrophil infiltration was higher in S. japonicum- and S. mansoni-infected DKO mice, granuloma formation was reduced in DKO mice infected with S. mansoni but not in those infected with S. japonicum almost 2 weeks after the commencement of egg laying (data not shown) (8, 11). In addition, unlike in S. mansoni infection, the mortality rates of S. japonicum-infected WT and DKO mice were similar (data not shown). Although IL-4/IL-13 suppressed excessive neutrophil infiltration during infection with both schistosomes, their activities largely differed, which may be partly attributed to differences in the roles of neutrophils in mice infected with these two schistosomes (11, 19, 21, 23). Thus, moderate neutrophil infiltration may be required in S. japonicum infection, since depletion of neutrophils causes high mortality and excessive infiltration of neutrophils may be associated with severe hepatic pathology (21).
Several mechanisms may be involved in neutrophil induction. The Th1 response has been reported to be involved in neutrophil induction in schistosomiasis japonica (6, 22). Although IFN-γ production was enhanced in S. mansoni-infected DKO mice (11), it did not differ in S. japonicum-infected DKO and WT mice, a finding contrary to our expectation and indicating that the increased neutrophil infiltration observed in S. japonicum-infected DKO mice was not due to increased IFN-γ production.
Th17 cells have been shown to produce IL-17A and IL-22 (9, 49) and to be involved in autoimmune diseases and the induction of proinflammatory cytokines such as IL-6 and TNF-α (5, 18, 21). IL-17A has been shown to induce the recruitment of neutrophils and the expression of CXCL2, a chemokine for neutrophil recruitment (9, 48). Since IL-4 has been found to downregulate Th17 differentiation in vivo and in vitro (17, 32), we investigated whether IL-17A production was enhanced in S. japonicum-infected DKO mice at 6 weeks postinfection. We found that IL-17A production by SEA-stimulated splenocytes and IL-17A mRNA expression in the liver were significantly higher in DKO than in WT mice. Moreover, the levels of mRNAs encoding IL-6, TNF-α, and CXCL2 were higher in the livers of DKO mice than in those of WT mice, as was the expression of mRNAs encoding the Ca2+-binding proteins S100A8 and S100A9 (40). The expression of S100A8 and S100A9 is enhanced by IL-17A and/or IL-22 (30), with both of these proteins playing a role in neutrophil recruitment and the production of proinflammatory cytokines (42, 44). S100A8 has been reported to localize to areas of neutrophil accumulation in S. japonicum-infected livers (7), suggesting that IL-17A production was upregulated in the absence of IL-4/IL-13 and may be responsible, at least in part, for the excessive neutrophil infiltration, production of proinflammatory cytokines, and more severe inflammation observed in infected DKO mice than in infected WT mice at 6 weeks after infection. Interestingly, the mRNA level of IL-10, which suppresses the production of Th17 cytokines, was also higher in DKO than in WT mice (15), suggesting that elevated IL-17A production was not due to the anti-inflammatory activity of IL-10.
When we analyzed these mice at 8 weeks after infection, we found that although the ratio and number of neutrophils were higher in DKO than in WT mice, the two strains did not differ significantly in the number and severity of hepatic lesions or in their levels of IL-17A and other proinflammatory cytokines, such as IL-6 and TNF-α, (data not shown). These results suggested that IL-4/IL-13 may play an anti-inflammatory role against hepatic inflammation only during the acute stage of infection and that neutrophils may contribute to severe pathology in the acute stage, as in the egg implant model (21).
Since IL-17A has been shown to induce severe pathology in schistosomiasis mansoni and to exaggerate OVA-induced airway inflammation (6, 18, 41, 46), we analyzed the function of IL-17A in the absence of IL-4/IL-13 in acute murine schistosomiasis japonica. We found that the hepatic pathology in IL-4−/− IL-13−/− IL-17A−/− (TKO) mice infected with S. japonicum was intermediate between those in WT and DKO mice and that this moderate hepatic damage was coincident with moderate neutrophil infiltration. These results suggest that severe hepatic inflammation in DKO mice was related to excessive neutrophil infiltration, which was partially regulated by IL-17A.
Since IL-17A regulates the production of IL-6, TNF-α, G-CSF, CXCL2, S100A8, and S100A9, we hypothesized that the expression of mRNAs encoding these molecules would be downregulated in TKO mice infected with S. japonicum (5, 26, 30, 34, 43, 47). Our results for infected TKO mice suggested, however, that IL-17A may not induce their expression.
We then considered whether another factor could induce the production of proinflammatory cytokines, CXCL2, and S100 family proteins. Possible candidates included the proinflammatory cytokines IL-17F and IL-22, which are produced by Th17 cells (2, 12, 24, 30, 33, 47) and enhance the expression of G-CSF, CXCL2, S100A8, and S100A9, all of which induce neutrophils infiltration (4, 30, 38, 42). Moreover, S100A8 and S100A9 are secreted as a complex, and the S100A8/A9 heterodimer has been shown to enhance the production of proinflammatory cytokines (40, 44). We found that the expression of IL-17F and IL-22 mRNAs was similar in S. japonicum-infected TKO and DKO mice but was higher than that in infected WT mice. Although we did not analyze the function of IL-17F and IL-22, our results suggest that these two cytokines may be responsible for the inflammation and neutrophil recruitment observed in S. japonicum-infected DKO and TKO mice.
Unexpectedly, in the absence of IL-4/IL-13, the IL-10 level was increased in S. japonicum infection, but this result was opposite to that in the case of S. mansoni infection (11). It may be caused by the species difference of the parasites tested. Our findings indicated that in the absence of IL-4/IL-13, IL-10 did not seem to play an anti-inflammatory role in S. japonicum infection. However, drastic reduction of AAM induction in the absence of IL-4/IL-13 might result in increased hepatic pathology at 6 weeks after S. japonicum infection, as AAMs protect against hepatic damage in S. mansoni infection (19). Fizz-1, an AAM marker, was lower, whereas classically activated inducible nitric oxide synthase (iNOS), a macrophage (CAM) marker, was higher in DKO and TKO mice than in WT mice (data not shown) (19). Therefore, additional studies are needed to clarify the anti-inflammatory mechanism of IL-4/IL-13 in acute murine schistosomiasis japonica.
In conclusion, we found that IL-4 and IL-13 regulate excessive hepatic inflammation, proinflammatory cytokine production, and neutrophil recruitment in acute murine schistosomiasis japonica. Enhanced expression of mRNAs encoding IL-17A and proinflammatory cytokines may be involved in the more severe pathology observed in infected DKO mice than in WT mice. Although IL-17A has a proinflammatory role (5, 22, 25), it is not totally responsible for the excessive hepatic inflammation, neutrophil recruitment, and proinflammatory cytokine production observed in infected DKO mice.
ACKNOWLEDGMENTS
We thank A. N. McKenzie (MRC Center, Cambridge, United Kingdom) and H. Karasuyama (Tokyo Medical and Dental University, Japan) for providing the IL-4−/− IL-13−/− mice and N. Akao and K. Obata for informative discussion.
This study was supported in part by Grants-in-Aid for Scientific Research, the Ministry of Education, Culture, Sports, and Technology, Japan (21406008 and 22790393), and a grant from the Ministry of Health, Welfare, and Labor, Japan (H23-Kokui-004).
Footnotes
Published ahead of print 28 October 2011
REFERENCES
- 1. Abbas AK, Murphy KM, Sher A. 1996. Functional diversity of helper T lymphocytes. Nature 383: 787–793 [DOI] [PubMed] [Google Scholar]
- 2. Andoh A, et al. 2005. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 129: 969–984 [DOI] [PubMed] [Google Scholar]
- 3. Anyan WK, et al. 2010. Schistosome eggs have a direct role in the induction of basophils capable of a high level of IL-4 production: comparative study of single- and bisexual infection of Schistosoma mansoni in vivo. Trop. Med. Health 38: 13–22 [Google Scholar]
- 4. Aujla SJ, et al. 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14: 275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bettelli E, Oukka M, Kuchroo VK. 2007. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8: 345–350 [DOI] [PubMed] [Google Scholar]
- 6. Burke ML, et al. 2009. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 31: 163–176 [DOI] [PubMed] [Google Scholar]
- 7. Burke ML, et al. 2010. Temporal expression of chemokines dictates the hepatic inflammatory infiltrate in a murine model of schistosomiasis. PLoS Negl. Trop. Dis. 4: e598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheever AW, Poindexter RW, Wynn TA. 1999. Egg laying is delayed but worm fecundity is normal in SCID mice infected with Schistosoma japonicum and S. mansoni with or without recombinant tumor necrosis factor alpha treatment. Infect. Immun. 67: 2201–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dong C. 2006. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat. Rev. Immunol. 6: 329–333 [DOI] [PubMed] [Google Scholar]
- 10. Drazen JM, Arm JP, Austen KF. 1996. Sorting out the cytokines of asthma. J. Exp. Med. 183: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. 2000. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J. Immunol. 164: 2585–2591 [DOI] [PubMed] [Google Scholar]
- 12. Geboes L, et al. 2009. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 60: 390–395 [DOI] [PubMed] [Google Scholar]
- 13. Gordon S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3: 23–35 [DOI] [PubMed] [Google Scholar]
- 14. Grzych JM, et al. 1991. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J. Immunol. 146: 1322–1327 [PubMed] [Google Scholar]
- 15. Gu Y, et al. 2008. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur. J. Immunol. 38: 1807–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gurish MF, et al. 2002. CCR3 is required for tissue eosinophilia and larval cytotoxicity after infection with Trichinella spiralis. J. Immunol. 168: 5730–5736 [DOI] [PubMed] [Google Scholar]
- 17. Harrington LE, et al. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6: 1123–1132 [DOI] [PubMed] [Google Scholar]
- 18. He R, et al. 2009. Exaggerated IL-17 response to epicutaneous sensitization mediates airway inflammation in the absence of IL-4 and IL-13. J. Allergy Clin. Immunol. 124: 761–770.e761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herbert DR, et al. 2004. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20: 623–635 [DOI] [PubMed] [Google Scholar]
- 20. Hestdal K, et al. 1991. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J. Immunol. 147: 22–28 [PubMed] [Google Scholar]
- 21. Hirata M, Hara T, Kage M, Fukuma T, Sendo F. 2002. Neutropenia augments experimentally induced Schistosoma japonicum egg granuloma formation in CBA mice, but not in C57BL/6 mice. Parasite Immunol. 24: 479–488 [DOI] [PubMed] [Google Scholar]
- 22. Hirata M, et al. 2001. Schistosoma japonicum egg granuloma formation in the interleukin-4 or interferon-gamma deficient host. Parasite Immunol. 23: 271–280 [DOI] [PubMed] [Google Scholar]
- 23. Hsü SY, Hsü HF, Davis JR, Lust GL. 1972. Comparative studies on the lesions caused by eggs of Schistosoma japonicum and Schistosoma mansoni in livers of albino mice and rhesus monkeys. Ann. Trop. Med. Parasitol. 66: 89–97 [DOI] [PubMed] [Google Scholar]
- 24. Ikeuchi H, et al. 2005. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 52: 1037–1046 [DOI] [PubMed] [Google Scholar]
- 25. Jankovic D, et al. 1999. Schistosome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J. Immunol. 163: 337–342 [PubMed] [Google Scholar]
- 26. Jovanovic DV, et al. 1998. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J. Immunol. 160: 3513–3521 [PubMed] [Google Scholar]
- 27. Kaplan MH, Whitfield JR, Boros DL, Grusby MJ. 1998. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J. Immunol. 160: 1850–1856 [PubMed] [Google Scholar]
- 28. King CH. 2010. Parasites and poverty: the case of schistosomiasis. Acta Trop. 113: 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Komiyama Y, et al. 2006. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177: 566–573 [DOI] [PubMed] [Google Scholar]
- 30. Liang SC, et al. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203: 2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin JX, et al. 1995. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity 2: 331–339 [DOI] [PubMed] [Google Scholar]
- 32. Lubberts E, et al. 2000. IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion. J. Clin. Invest. 105: 1697–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ma HL, et al. 2008. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Invest. 118: 597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McAllister F, et al. 2005. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J. Immunol. 175: 404–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. 1999. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J. Exp. Med. 189: 1565–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagase H, et al. 2001. Regulation of chemokine receptor expression in eosinophils. Int. Arch. Allergy Immunol. 125 (Suppl. 1): 29–32 [DOI] [PubMed] [Google Scholar]
- 37. Nakae S, et al. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17: 375–387 [DOI] [PubMed] [Google Scholar]
- 38. Oda N, et al. 2005. Interleukin-17F induces pulmonary neutrophilia and amplifies antigen-induced allergic response. Am. J. Respir. Crit. Care Med. 171: 12–18 [DOI] [PubMed] [Google Scholar]
- 39. Pearce EJ, MacDonald AS. 2002. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2: 499–511 [DOI] [PubMed] [Google Scholar]
- 40. Rammes A, et al. 1997. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J. Biol. Chem. 272: 9496–9502 [DOI] [PubMed] [Google Scholar]
- 41. Rutitzky LI, Lopes da Rosa JR, Stadecker MJ. 2005. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J. Immunol. 175: 3920–3926 [DOI] [PubMed] [Google Scholar]
- 42. Ryckman C, et al. 2003. Role of S100A8 and S100A9 in neutrophil recruitment in response to monosodium urate monohydrate crystals in the air-pouch model of acute gouty arthritis. Arthritis Rheum. 48: 2310–2320 [DOI] [PubMed] [Google Scholar]
- 43. Schwarzenberger P, et al. 2000. Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J. Immunol. 164: 4783–4789 [DOI] [PubMed] [Google Scholar]
- 44. Sunahori K, et al. 2006. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res. Ther. 8: R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Von Lichtenberg F, Erickson DG, Sadun EH. 1973. Comparative histopathology of schistosome granulomas in the hamster. Am. J. Pathol. 72: 149–178 [PMC free article] [PubMed] [Google Scholar]
- 46. Wilson MS, et al. 2007. Immunopathology of schistosomiasis. Immunol. Cell Biol. 85: 148–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang XO, et al. 2008. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 205: 1063–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ye P, et al. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194: 519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zheng Y, et al. 2007. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445: 648–651 [DOI] [PubMed] [Google Scholar]
- 50. Zurawski SM, Vega F, Huyghe B, Zurawski G. 1993. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J. 12: 2663–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]